CHAPTER 29 Laser
INTRODUCTION
Although many patients benefit from surgical discectomy with or without a fusion, these open procedures are associated with known risks, including early or late failures secondary to symptomatic adhesions, pseudoarthrosis, and adjacent level instability.1 These complications have prompted the development and introduction of less invasive percutaneous intradiscal procedures that have the ability to chemically or mechanically remove intradiscal nuclear material. Chemonucleolysis using the intranuclear injection of chymopapain was introduced several decades ago and a variety of other intradiscal and endoscopic procedures have followed. Among the intradiscal procedures are endoscopic nucleotomy, nucleoplasty (coblation), percutaneous lumbar discectomy methods of decompression.
Most of the devices removed nuclear material using mechanical incision by surgical instruments or suction, but in 1986 Choy et al.2 introduced nonendoscopic percutaneous laser disc decompression and nucleotomy (PLDN) with the Nd:YAG laser (wavelength 1064 nm). Even though the 1064 nm wavelength Nd:YAG laser technique in this context is not to be understood as the use of soft laser technology,3 many consider this first introduction of intradiscal laser technology a pioneering achievement.
PRINCIPLES
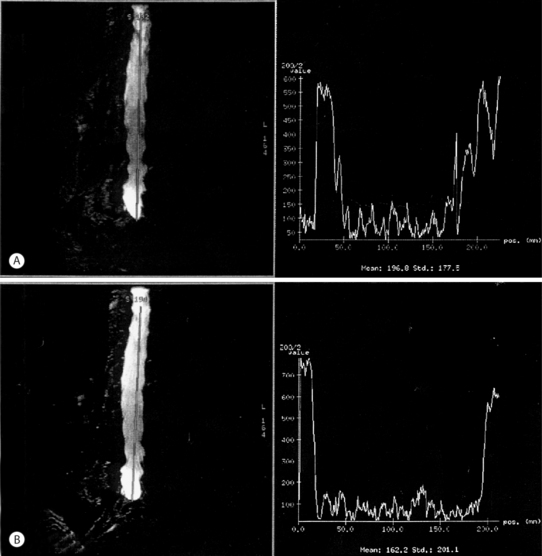
Several postulates have been proposed to explain why intradiscal laser decompression can help relieve pain caused by a herniated disc. The first and more apparent reason is the potential beneficial effect of lowering the intradiscal pressure by vaporizing nuclear material. The Nd:YAG 1064 nm irradiation of discal tissue creates a small vaporization defect lined with a carbonized margin (Fig. 29.1)4 and minimal ablation of disc tissue.5 Greater ablation is possible with the Nd:YAG laser 1320 nm with a pressure drop of up to 55.6% recorded (Fig. 29.2).6 This reduction in pressure appears to be independent of age or the degree of disc degeneration.7 Even though the amount of tissue removed and the ablation defect is less than that achieved with a mechanical discectomy, clinical results are similar using the Nd:YAG laser.
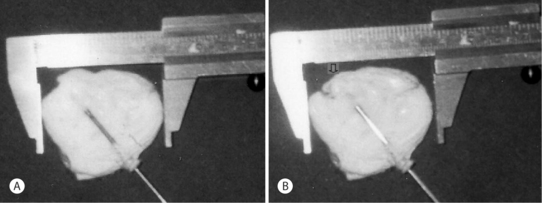
Fig. 29.1 Controlled vaporization of a disc. Note the zone of thermocoagulation surrounding the defect.
(Courtesy W. Siebert, M.D.)
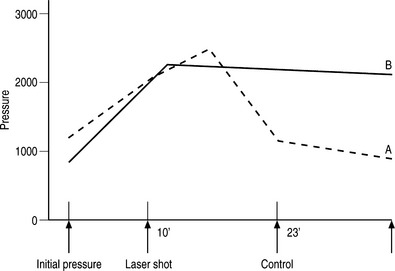
Fig. 29.2 Intradiscal pressure reduction after intradiscal irradiation by Nd:YAG laser
(modified from Choy et al.6).
Laser ablation may have other beneficial effects. Some believe that laser provides benefits other than mechanical decompression. One such potential beneficial effect is the shrinkage of collagen fibrils caused by laser-generated heat8–10 followed by a subsequent reduction of intradiscal volume. We postulated this mechanism after observing sudden shrinking of a resected meniscus irradiated by the Nd:YAG laser 1064 nm.8 Our basic studies showed a reduction in disc diameter up to 14% in explanted bovine discs, even though comparative studies8 using the holmium:YAG laser caused 1% or less diameter shrinkage. Consistent with our findings regarding disc shrinkage (Fig. 29.3), Turgut et al.11 demonstrated water loss, and proteoglycan and collagen changes in animal discs following laser treatment. Kosaka et al.12 showed size reduction of several millimeters of extruded discs by open surgical Nd: YAG laser disc decompression and intraoperative monitoring. As well, Mayer13 documented disc shrinkage on video during endoscopic Nd:YAG laser-assisted percutaneous discectomy and Grönemeyer14 with computed tomography (CT) intraoperatively.
Reductions in the density of disc protrusions and extrusions have been documented with CT scans performed on the first postoperative day following Nd:YAG PLDN.15 Brat et al.16 similarly documented reduced size of extrusions on postoperative magnetic resonance imaging (MRI) scans. Similar findings were seen on MRI myelograms performed on the first postoperative day17 where improved cerebrospinal fluid (CSF) flow was demonstrated at the site of the previously constricted dural sac (Fig. 29.4). The authors concluded that the improved CSF flow was the evidence of reduced venous congestion, reduced arteriole compression, and reduced dural sympathetic nerve fiber compression. Because only minimal venous congestion will adversely affect dorsal root neurons18 and compression often occurs at two adjacent segments,19 proximal and distal root decompression over two segments is recommended.
Contrary to open and most percutaneous surgical disc interventions, the Nd:YAG 1064 nm laser technique may not cause postoperative instability.20 On the contrary, Wittenberg and Steffen21 have even reported an increase in translation stability of spinal segments, which they felt was due to a change of collagen tissue formation.22 Scar tissue was seen at 6 weeks intradiscally, but takes a year to mature.10 Thal et al.22 reported a late shrinking effect of the intervertebral disc without any detectable increased segmental movement.
SAFETY OF USE
Siebert23 in the lumbar spine and Schmolke et al.24 in the cervical spine have provided safety data for the Nd:YAG 1064 nm laser system. At an irradiation time of one shot per second at 20 watts the laser beam will penetrate to a depth of 6 mm in disc tissue. When the laser beam was not directed at the endplate or within the spinal canal, temperatures above the coagulation threshold of proteins were not reached in either the endplate or the epidural tissue adjacent to the disc. Their findings prompted the recommendation that a posterolateral approach be used to access the dorsolateral third of the lumbar and thoracic disc and an anterior approach involving the ventral third of the cervical disc. Based on the experimental studies, the recommended maximal dose per disc is 1600 joules in the lumbar spine, 1000 joules in the thoracic spine, and 300–400 joules in the cervical spine.
Alternative laser technology
Carbon dioxide lasers have a wavelength that is very effective in shrinking the disc;25 however, using the Nd:YAG 1320 nm wavelength requires higher dosing to cause disc shrinkage comparable to the Nd:YAG 1064 nm laser. Such higher doses by 1320 nm can cause damage to both endplates in 8% of cases26 and thus its use in disc decompressions is limited.
The KTP laser is very similar to the Nd:YAG laser in both its mode of action and its effect,27,28 but basic research is significantly less than for the Nd:YAG 1064 nm laser. The diode laser uses 890–980 nm wavelengths and will shrink an intervertebral disc to the same degree as the Nd:YAG 1064 nm laser. Using a wavelength of 940 nm, we validated the shrinking effect of this laser29 and also showed that a reduced dose could provide sufficient decompression yet still avoid thermal damage to the endplates.
The holmium:YAG laser with a 2100 nm wavelength may not be suitable as a pulsed laser when used for nonendoscopic intradiscal use.30 The somewhat larger ablated volume compared to the Nd:YAG 1064 nm laser is less than 10 milligrams,31 and therefore of dubious clinical importance. Furthermore, there is 90% less disc shrinkage, and the apparent scattering may increase the risk of endplate damage.32
INDICATIONS AND PATIENT SELECTION
We define pain by its quality, quantity, topology, and chronology and classify discogenic pain as causing local, pseudoradicular, radicular, medullary, or autonomic symptoms. In addition, pain symptoms may be associated with neurological deficits such as dysesthesias, hypo- or hypersensitization, or even paralysis. The cause of these pain syndromes can be diagnosed by a characteristic history and physical examination substantiated by MRI or CT evidence of disc bulging, protrusions, extrusions, or sequestrations.33,34 Abnormal structural findings alone without corresponding symptoms are not, however, a reason for surgical intervention.35
Patients who fail 6 weeks of conservative care and continue to have disabling discogenic pain are first offered Nd:YAG PLDN before an open surgical procedure. Although progressive neurologic deficits, paralysis, conus medullaris, and cauda syndrome require immediate intervention, the severity of pain most often dictates when we determine that surgery will be offered. With the exception of a patient with a hemostatic disorder or untreated infection, there are few contraindications to discal laser intervention. Because shrinkable collagen fibers are always present in the fibrous ring, even age is not a contraindication. In fact, as long as we clinically believe the patient’s axial or extremity pain is due to a disc bulge, protrusion, or extrusion and it is seen on a CT or MRI scan, the patient will be offered laser decompression. In particular, laser decompression is not contraindicated when a disc protrusion is aggravating the pain of spinal instability or is contributing to stenosis.36 Furthermore, many patients who have unrelieved pain for over 6 weeks will begin to develop somatization symptoms, but unlike other authors20,37 we believe these symptoms are not a contraindication to surgery. In addition, although many physicians including Siebert20 and others16,37,38 prefer restricting percutaneous laser decompression to patients with monoradicular pain, the authors have shown that patients with monoradicular pain alone account for only 20% of patients presenting with discogenic pain.4 Consequently, the authors believe that axial pain alone is not a contraindication to the performance of laser disc decompression, similar to radiofrequency (RF) application. Finally, the authors do not routinely use discography to confirm the source of pain,39 but will use discography to help position the cannula during the operation. In practice, the majority of patients who are offered a percutaneous laser decompression have mono- or polyradicular pain associated with a disc bulge, protrusion, or contained and noncontained extrusions. When selection is limited to patients with radicular pain, patients who will eventually require an open procedure may be less than 10%4,40 and more than 90% would choose the procedure again for a recurrence in pain. In a consecutive prospective series, 85% of the operated patients had either protrusions or contained extrusions and 15% had noncontained extrusions with either caudal, cranial or buttonhole dislocations. The failure rate was 3% in the protrusion-contained extrusion group and even though the failure rate in the noncontained extrusions was 20%, open surgery was avoided in four of the five cases. Although most surgeons rarely operate on sequestered fragments that are free-floating in the epidural space, many sequestered fragments remain within the protrusion or trapped in the fibrous ring.41 Furthermore, when there is both a free-floating epidural sequestered fragment and a foraminal disc protrusion, decompression of the protrusion alone may provide enough radicular pain relief such that the patients are satisfied. In the authors’ series of 3970 lumbar Nd:YAG PLDN procedures between 1989 and 2002 there were seven patients with both protrusions and free-floating sequestered fragments. Six of the seven patients were satisfied with their outcome following discal decompression alone, and only three had residual local axial pain. Two patients with dorsiflexor foot weakness had full recovery.
Finally, most consider cauda equina syndrome as a contraindication for percutaneous disc decompression.20,42 The authors have, however, successfully performed Nd:YAG PLDN on 30 cases of cauda equina syndrome. Moreover, in only one case of a recurrent herniation with a free-floating sequestered fragment was open surgery necessary.43 While this chapter has emphasized lumbar disc disorders, it should be understood that laser decompression may also be considered for symptomatic disc herniations in the cervical and thoracic spine.44
TECHNIQUE
In the lumbar and thoracic spine, the patient is positioned in the lateral decubitus position with the painful side up, and a posterolateral approach is used. In the cervical spine, the patient’s neck is placed in hyperextension and access is made on the right side between the vessels and the trachea. Local anesthetic is infiltrated in the skin and subcutaneous muscles, and analgesic sedation is given by the anesthetist. Direct and continuous fluoroscopic visualization using intermittent anteroposterior (AP) and lateral projections is used during needle insertions. Because the 400–600 μ bare fiber of the Nd:YAG laser extends 2 mm beyond the cannula tip. The laser beam penetrates to a depth of 6 mm. Accurate and specific placement within the disc is important. Although rarely needed, puncture laser osteotomy45 through the edge of the vertebral body, osteophyte, or superior articular process will facilitate access to the intervertebral disc. On rare occasions, transdural puncture will be needed to access the L5–S1 disc.
Technical procedure for nonendoscopic percutaneous laser disc decompression and nucleotomy
Cervical
Thoracic
Lumbar
CLINICAL EXPERIENCE
A multicenter prospective study has been conducted. There was a total of 4977 patients, including 316 with cervical and 38 thoracic discogenic pain syndromes treated by laser between November 23, 1989, and January 12, 1999. Among the parameters recorded were pain symptomatology, clinical findings, neurological findings, image-based diagnosis and, increasingly, with the computerized spine motion test, integrated dorsal muscle electromyelogram (EMG). Patients with prior intervertebral disc surgery were also enrolled from the outset.46 The proportion of these patients remained unchanged at 20% over the years. Based on many years’ experience with open disc and spine surgery,47 the polysegmental use of nonendoscopic percutaneous laser disc decompression and nucleotomy with the Nd:YAG laser 1064 nm was introduced.44
Ninety percent of patients were followed up at 6 weeks, while the remaining 10% were questioned by telephone interview. This scheduled survey period of 6 weeks was considered the optimal timeframe48 as it allowed for scar formation of lacerated intervertebral discs.10
The results have remained consistently good over the years. Subjective outcome is positive in 80% of lumbar spine cases, 86.5% for cervical spine cases, and 90% for thoracic spine. Visual analogue pain ratings demonstrate a decrease of 80% (p < 0.01). Objectively, a 90% improvement in lumbar spine patients was observed, as evidenced by the change in the straight leg raising test from the first postoperative day onward. This remains an impressive testimony to the success of intradiscal laser treatments (p < 0.01). These findings have been confirmed in follow-up examinations at up to 4 years.4,40 More recently, these findings have been demonstrated in up to 8 years’ follow-up.49 Regression of paralysis in all spinal regions was noted to be over 90%. Computerized measurements of spinal mobility have also revealed marked improvement.4
COMPLICATIONS
The complication rate is an important criterion for the choice of spinal intervention. Therefore, complications need to be discussed. We will disclose our 15 years of experience of PLDN with the Nd:YAG 1064 nm combined with findings in the literature.50
Problems following the puncture of the discal region
In the lumbar spine region, five cases resulted in a prevertebral needle placement. No adverse consequences resulted from this complication. In over 8000 punctured discs, this equates to a complication rate of 0.062%. If access needs to be obtained in the pathological segment, laser osteotomy may be necessary in 0.25% of the cases. Bone injuries did not occur and never negatively influenced the results. When the L5–S1 disc was inaccessible from a posterolateral approach, three cases were performed using a transdural puncture without complications. No nerve root lesions were reported using this technique. Nerve root injury rate of more than 0.46% has been reported with percutaneous nucleotomy (PLD), percutaneous endoscopic discectomy (PED), percutaneous endoscopic laser discectomy (PELD), automated percutaneous lumbar discectomy (APLD), and percutaneous fenestration (PF). The diameter of these aforementioned puncture instruments utilized is significantly larger than the diameter of the puncture tube used for the PLDN (18 gauge to 2 mm exterior diameter). Retroperitoneal vessel damage from Nd:YAG laser was reported in 3%. In 0.15% of the cases, damage to the transverse process was described. In comparison to an open microscopic nucleotomy, nerve root damage occurs in up to 8% by open procedure while the rate of nerve root injury with Nd:YAG PLDN is estimated to be significantly lower.
Neurological complications
In comparison to other percutaneous operation procedures, nerve injuries were described in five of 3194 patients. Neurological injuries have been reported after chemonucleolysis, including paraplegia, conus medullaris, and cauda equina syndrome at a rate of 0.06%. In open procedures of the lumbar spine, different rates of neurological deficits have been described. It is important to recognize that conus medullaris and cauda equina syndromes, paraplegia, and other severe complications can occur.
DISCUSSION
One is an effect of the kind observed following open intraspinal decompression: mechanical relief of the compressed intraspinal structures such as the venous plexus, spinal arteries, radicular arteries, and neuronal structures such as the nerve root and long tracts. This mechanism of action is based on the combination of intradiscal pressure reduction by vaporization and the shrinking phenomenon with maximal pressure relief in the spinal canal. Relieving venous congestion is of paramount importance in this respect, since only slight increases cause changes in dorsal spinal ganglion synapses.18 Multisegmental decompression is preferred by the authors to reduce venous stasis. This has been substantiated by the studies of Porter and Ward19 on the significance of two-level pathology.
The second mechanism of action of the Nd:YAG laser is the destruction of the nociceptors in the posterior fibrous ring for pain relief. Equally as important is the destruction of the budding nerve fibers during neovascularization of the intervertebral disc tissue. The denaturation of pain-activating kinins from the torn intervertebral tissue is a factor that should not be underestimated. The experimental principles described in vitro, in vivo, and in clinical research now leave no doubt as to the efficacy of the Nd:YAG laser 1064 nm on the tissue of the intervertebral disc52 and thus on pathological manifestations in the form of clinical syndromes, despite a very mixed database. This confusion is due to incorrect quotations,42 combining results obtained with different types of lasers,53 and even incorrect scientific statements.23
It has been repeatedly asserted that there are psychological effects resulting from laser application that account for positive results. To date, no literature has substantiated this concept. Others question whether the procedure is genuinely effective for the patient or merely a ‘gimmick’ activity,54 placebo, or even nonsense. We believe that the clinical results of the megastudy and the evaluation of the meta-analysis impressively refute those claims.5
LASER SELECTION
The use of lasers naturally requires the necessary knowledge of laser physics and the ability to select an appropriate laser. Positive results can only be achieved if the right laser is used with a suitable wavelength. Experimental and clinical results have shown that the ideal choice is the Nd:YAG laser 1064 nm. The Nd:YAG laser 1320 nm also provides good results, but requires at least a threefold higher dose. Unfortunately, this higher dose can result in damage to the adjacent vertebrae. The holmium:YAG laser is less suitable for nonendoscopic operations because of the more mechanical effects, i.e. those performed only through percutaneous needle puncture.6 Based on our experimental studies and clinical experience, this technique should only be used in open or endoscopic intervertebral disc operations in an assistive mode and under visual control.30
The recently developed diode laser with wavelengths of around 940 nm (890–980 nm) has the greatest thermal effect with a very good shrinking mechanism. This has been shown by experimental research conducted by our own research group. In a prospective, randomized, single-blind clinical study, it provided the same results as the Nd:YAG laser 1064 nm without an increase in complications.29,55 Regrettably, cases in which much too high doses were used have also been reported, resulting in severe damage to the adjacent vertebrae. The same applies for the KTP laser which, when the correct fiber tip is chosen for straight shooting, provides good results without damaging the adjacent vertebrae.
SUMMARY
Since exact studies on the penetration depth of the Nd:YAG 1064 nm laser beam and heat convection have not produced any evidence of damage when using the correct dose, the Nd:YAG laser 1064 is currently the laser2,8,10,20,31,53,56,57 of choice for intradiscal intervertebral disc decompression and nucleotomy.49,53,57–60
1 Kiskimäki I, Seitsala S, Östermann H, et al. Reoperations after lumbar disc surgery. Spine. 2000;25:1500-1508.
2 Choy DSJ, Case RB, Ascher PW. Percutaneous laser ablation of lumbar disc. Ann Meet Orthop Res Soc. 1987;1:19.
3 Miriutora NF. Laser therapy in the treatment of discogenic neurological manifestations of spinal osteochondrosis. Vopr Kurortol Fizioter. 2000;3:30-33.
4 Kornelli H, Hellinger J. Der computerisierte Spine-motion-Test mit integrierte perkutane Rückenmuskel-EMG prä- und postoperativ nach perkutaner Laserdiskusdekompressio und nukleotomie. Schmerz. 1998;12(Suppl 1/98):63.
5 Hellinger J, Stern S. Nonendoskopische PLDN-Nd-YAG 1064 nm – Eine 10-Jahres-Bilanz als Megastudie und Metaanalyse. Newsletter Dornier Med Tech. 2000:S2.
6 Choy DSJ, Ascher PW, Saddekni S, et al. Percutaneous laser disc decompression. Spine. 1992;17:949-956.
7 Yonyzawa T, Matomura K, Atsumi K, et al. Laser nucleotomy: a preliminary study for vaporizing the degenerated nucleus. Laser in der Orthopädie, Symp. Hannover, Germany, 1991:19–20.09.
8 Hellinger J. Technical aspects of percutaneous cervical and lumbar laser disc decompression and nucleotomy. Neurol Res. 1999;21:99-102.
9 Hilbert J, Braun A, Papp J, et al. Erfahrungen mit der perkutanen Laserdiskusdekompression bei lumbalem Bandscheibenschaden. Orthop Prax. 1995;31:217-221.
10 Skuginna A, Reinicke J. Open discectomy after failed percutaneous laser disc decompression; histological results [abstract]. III. Barcelona: Kongress effort, 1997;581.
11 Turgut M, Acikgöz B, Klinc S, et al. Effects of Nd-YAG laser on experimental disc degeneration. Acta Neurochir (Wien). 1996;138:1348-1354.
12 Kosaka R, Onomura T, Yonyzawa T, et al. Laser nucleotomy – a case report of open procedures. J Japan Spine Res Soc. 1992;3:249.
13 Mayer HM. Percutaneous endoscopic laser discectomy. Internat. Symposium ‘New Developments in Knee and Spine Surgery.’ Munich, Germany, 1991:21–22.11.
14 Grönemeyer D. CT-guided lumbar laser nucleotomy [abstract]. Internat. Symposium ‘New Developments in Knee and Spine Surgery.’ Munich, Germany, 1991:21–22.1.
15 Hellinger J, Linke DR, Heller HJ. A biophysical explanation for Nd-YAG percutaneous laser disc decompression success. J Clin Laser Med Surg. 2001;19:235-238.
16 Brat H, Bouziane F, Lambert J, et al. CT-guided percutaneous laser-disc-decompression (PLDD): prospective clinical outcome. Laser Med Sci. 2003;18(Suppl 2):16.
17 Wuttge R, Hellinger J, Hellinger S. Pre- and postoperative MR-myelography of PLDN. In: Brock M, Schwarz W, Wille C, editors. Spinal surgery and related disciplines. Bologna: Monduzzi; 2000:895-898.
18 Sugawara O, Atsuta Y, Iwahara T, et al. The effects of mechanical compression and hypoxia on nerve root and dorsal root ganglia. Spine. 1996;21:2089-2094.
19 Porter RW, Ward D. The significance of two-level pathology. Spine. 1992;17:9-15.
20 Siebert W. Percutaneous laser disc decompression: the European experience. Spine. 1993;7:103-133.
21 Wittenberg RH, Steffen R. Minimal-invasive Therapie lumbaler Bandscheibenvorfälle. Bücherei d Orthop. 1997;68:23-24.
22 Thal DR, Werkmann K, Leheta F, et al. Effects of Nd-YAG laser radiation in cultured porcine vertebral tissue. SPIE. 1996;2623:212-231.
23 Siebert W. Corrigendum. Percutaneous laser discectomy of cervical disc: preliminary clinical results. J Clinical Laser Med Surg. 1996;14:354.
24 Schmolke S, Kirsch L, Barth A. Gosse: Infrarot-Thermographie und Bestimmung des Ablationsvolumens bei zervikaler Lasernukleotomie. In: Matzen KA, editor. Therapie des Bandscheibenvorfalls. München: Zuckschwerdt; 1998:193-201.
25 Kolarik J, Nadvornik B, Rozhold O. Photonucleolysis of intervertebral disc and its herniation. Zbl Neurochir. 1990;51:69-71.
26 Grasshoff H, Mahlfeld K, Kayser R. Komplikationen nach perkutaner Laser-diskusdekompression (PLDD) mit dem Nd-YAG-Laser. Lasermedizin. 1998;14:3-7.
27 Knight M, Patko JA, Wan S. KTP-523 laser disc decompression – 6 years experience [abstract]. Fifth Intern. Congr. IMLAS. Sevilla, Spain, 1998.
28 Liebler WA. Percutaneous laser disc nucleotomy. Clin Orthop. 1995;310:56-58.
29 Paul M, Hellinger J. Nd-YAG (1064) versus diode (940 nm) PLDN: a prospective randomised blinded study. In: Brock M, Schwarz W, Wille C, editors. Spinal surgery and related disciplines. Bologna: Monduzzi; 2000:555-558.
30 Hellinger J. Holmium-YAG-assistierte offene Nukleotomie. Laser Med Surg. 1995;11:86-87.
31 Schlangmann BA, Schmolke S, Siebert WE. Temperatur- und Ablationsmessungen bei der Laserbehandlung von Bandscheibengewebe. Orthopäde. 1996;25:3-9.
32 Casper GD. Results of a prospective clinical trial of the holmium-YAG laser disc decompression utilizing a side-firing fiber: four year follow-up [abstract]. Fifth Intern. Cong. IMLAS. Sevilla, Spain, 22–25.4, 1998.
33 Hellinger J, Manitz U. Vertebragene Syndrome bei degenerativen Wirbelsäulenerkrankungen. Med Akt. 1981;6:274-278.
34 Hellinger J. Zur Nosologie und Therapie zervikaler vertebraler Syndrome bei degenerativen Erkrankungen. Z Orthop. 1981;119:595-596.
35 Weishaupt D, Zanetti M, Hodler J, et al. Imaging of the lumbar spine: prevalence of intervertebral disk extrusion and sequestration, nerve root compression, endplate abnormalities and osteoarthritis of the joints in asymptomatic volunteers. Radiology. 1998;209:661-666.
36 Choy DS, Ngeow J. Percutaneous laser disc decompression in spinal stenosis. J Clin Laser Med Surg. 1998;16(2):123-125.
37 Ohnemeiss DD, Guyer RD, Hochschuler SH. Laser disc decompression. The importance of proper patient selection. Spine. 1994;19:2054-2058.
38 Gangi A, Dietemann JL, Ide C, et al. Percutaneous laser disk decompression under CT and fluoroscopic guidance: indications, technique and clinical experiment. Radiographics. 1996;16:89-96.
39 Grasshoff HR, Kayser N, Mahlfeld D. Diskographiebefund und Ergebnis der perkutanen Laserdiskusdekompression (PLDD). Fortschr Röntgenstr. 2001;173:191-194.
40 Evermann H, Stern S. Four years follow up non-endoscopic percutaneous laser disc decompression (PLDD) [abstract] Fifth Intern. Coug. IMLAS. Sevilla, Spain, 22–25.08, 1998.
41 Messing-Jünger AM, Bock WJ. Lumbale Nervenwurzelkompression. Ein cooperatives Projekt zur Qualitätssicherung in der Neurochirurgie. Zbl Neurochir. 1995;19:26-56.
42 Schmolke S, Gossè F, Rühmann O. Die perkutane Laser-Diskusdekompression. In: Matzen KA, editor. Therapie des Bandscheibenvorfalls. München: Zuckschwert; 1997:223-231.
43 Hellinger J. Borderline indications for lumbar Nd-YAG-PLDN. Intern. 21. Course for percutaneous endoscopic spinal surgery and complementary techniques. 23–24.01.03 Zürich, Schweiz, 2003.
44 Hellinger J. Nonendoskopische perkutane Laserdiskusdekompression und Nukleotomie. Med Bild. 1995;5:49-56.
45 Hellinger J. Die Laserosteotomie als Zugangsmöglichkeit zur lumbalen und zervikalen perkutanen Nukleotomie. Laser Med Surg. 1992;8:105.
46 Hellinger J. Nd-YAG-Laser-YAG PLDN in postnucletomy syndrome. In: Brock M, Schwarz W, Wille C, editors. Spinal surgery and related disciplines. Bologna: Monduzzi; 2000:277-280.
47 Kähn R, Hellinger J, Graner H, et al. Klinisch-neurologische, röntgenologische und elektrodiagnostische Nachuntersuchungsergebnisse des lumbalen Bandscheibenvorfalls. Beitr Orthop Traumatol. 1979;17:703-705.
48 Berendse GAM, Berg SGM, Kessels AHF, et al. Randomized controlled trial of percutaneous intradiscal radiofrequency thermocoagulation for chronic discogenic back pain. Spine. 2001;26:287-292.
49 Stern S. Eight years follow up non-endoscopic percutaneous laser disc decompression (PLDD) – high-tech tool for intradiscal pain therapy or placebo? Tenth Intern. Congr. IMLAS. Luxembourg, 19–21.6, 2003.
50 Hellinger J. Komplikationen der nonendoskopischen perkutanen Laserdiskusdekompression und Nukleotomie (PLDN) mit dem Neodym-YAG-Laser 1064 nm. Orthop Praxis. 2002;38:335-341.
51 Grumme TH, Kolodziejcyk D. Komplikationen in der Neurochirurgie. Berlin: Blackwell, 1994;121-202.
52 Anders JO, Pietsch S, Staupendahl G. Kritische Betrachtung der Indikationen des Holmium: YAG- und des Neodym: YAG-Lasers in der orthopädischen Chirurgie anhand einer In vitro-Studie. Biomed Tech (Berl). 1999;44:83-86.
53 Zweifel K, Panoussopoulos A. Laser und Bandscheibenchirurgie. In: Berlin HP, Müller G, Hrsg. Angewandte Lasermedizin. Landsberg [ecomed]. III-3.11.3:12–16, 1996.
54 Mayer HM, Müller G, Schwetlick G. Lasers in percutaneous disc surgery: beneficial technology or gimmick? Acta Orthop Scand. 1993;64(Suppl 251):38-44.
55 Menchetti PPM, Longo L. Diode laser treatment of migrated disc. Laser Med Sci. 2003;18(Suppl 2):17.
56 Choy DSJ, Altman P, Trokel SL. Efficiency of disc ablation with lasers of various wavelengths. J Clin Laser Med Surg. 1995;13:153-156.
57 Siebert W, Bise K, Breitner S, et al. Die Nucleus-pulposus-Vaporisation-Eine neue Technik zur Behandlung des Bandscheibenvorfalls? Orthop Praxis. 1988;12:73.
58 Berendsen B-T, Schlangmann B, Schmolke S. Percutaneous laser disc decompression (PLLD): fundamental experiments and clinical findings [letter]. Dornier. 1995;1:20-22.
59 Castro WHM, Halm H, Schinkel V. Neodymium-YAG-1064nm Laservaporisation von lumbalen Bandscheiben: Klinische Frühergebnisse. Aachen: Laser Shaker, 1992;187.
60 Hellinger J. Ein neuer Weg der Bandscheiben-Chirurgie. Ärztl Praxis. 1992;44:21-22.