Chapter 34 Laparoscopic cholecystectomy and choledocholithotomy
Overview
Gallstones are an extremely common condition, occurring in approximately 10% to 20% of the adult population. It has long been recognized that the treatment for symptomatic gallstones is cholecystectomy, one of the most commonly performed abdominal surgical procedures. In developed countries, most cholecystectomies are performed laparoscopically. To give an example, 90% of cholecystectomies in the United States are performed laparoscopically (Csikesz et al, 2009). Laparoscopic cholecystectomy is considered the gold standard for the surgical treatment of gallstone disease, because it results in less postoperative pain, better cosmesis, shorter hospital stays, and less disability when compared with open cholecystectomy (Soper et al, 1992c). The early experience with laparoscopic cholecystectomy was associated with an increased risk for common bile duct (CBD) injuries. However, through advances in education and training, this risk has been decreased. In the hands of properly trained surgeons, laparoscopic cholecystectomy can be safely performed in the majority of patients.
Over the past several years, much investigation has been done on techniques to improve outcomes from laparoscopic cholecystectomy. One approach has been to use smaller diameter ports and instruments to perform the operation (Gurusamy et al, 2010); however, the limited variety of 2 mm diameter instruments and their lack of robustness have hindered this approach. More recently, there has been an upsurge in interest in the performance of laparoscopic cholecystectomy using a single incision at the umbilicus or via natural orifice translumenal endoscopic surgery (NOTES). Up to this time, no study has definitely proven the advantage of either smaller incisions or fewer incisions compared with standard multiport laparoscopic cholecystectomy, other than the potential for improved cosmesis. This chapter discusses the history and current status of laparoscopic cholecystectomy and the laparoscopic management of CBD stones.
History
Gallbladder disease has plagued humanity since antiquity, and gallstones have been found in Egyptian mummies dating from more than 2000 years ago (Beal, 1984). Some have even conjectured that the death of Alexander the Great was caused by acute cholecystitis. Although the presence of the gallbladder was acknowledged by anatomists beginning in the fifth century, the gallbladder’s true function was not elucidated until relatively recently. The potential for elective surgical treatment of gallstones was limited by the crude diagnostic techniques available, but the first successful cholecystolithotomy likely occurred in 1676, when Joenisius apparently extracted gallstones from a biliary fistula of the abdominal wall (Beal, 1984). By the eighteenth century, physicians increasingly realized that cholelithiasis could cause abdominal pain and jaundice. As surgical treatment was not considered to be feasible, numerous treatments were described, including purgatives, therapeutic bleeding, and emetics. It was not until 1867 that John S. Bobbs of Indianapolis performed an elective cholecystotomy for hydrops of the gallbladder under chloroform anesthesia (Cutter, 1928). Various other surgeons then described techniques for performing cholecystolithotomy in this era, as Listerian principles were being adopted around the world.
Carl Langenbuch of Berlin performed the first elective cholecystectomy in 1882 on a patient who had been suffering from symptomatic cholelithiasis for more than 10 years. Although the patient was found “smoking a cigar” the following day, he was not discharged from the hospital for two months. Dr. Langenbuch stated, “My opinion is that a cholecystectomy is suitable for those cases in which both the patient and physician have reached the end of their patience” (Langenbuch, 1882). The first cholecystectomy was not performed in the United States until 1886, by Justus Ohage of Minnesota. For the next 30 years, considerable debate followed at surgical meetings as to whether a cholecystolithotomy or cholecystectomy would be preferable for gallstone disease. By the 1920s it was generally accepted that cholecystectomy was most suitable, except in the face of severe inflammation, rendering the dissection hazardous to the patient.
Over the ensuing 6 decades, open cholecystectomy was the procedure of choice for patients with symptomatic cholelithiasis. Great strides were made in developing diagnostic methods for demonstrating the presence of gallstones, using either cholecystography or ultrasound (US). Throughout much of this time, many of the operations were done by family physicians not well trained in surgical techniques, and thus bile duct injuries were seen relatively frequently. In Christopher’s Textbook of Surgery from 1936 it states, “Frank hemorrhage from the cystic artery and injury to the CBD are the two great dangers in performing cholecystectomy. Cholecystectomy will not only be easier but safer if it is begun at the cystic duct, since the circulation will be controlled at the outset.” Later, other surgeons suggested that a top-down or fundus-first technique for performing cholecystectomy would be less likely to result in CBD injury. Various surgical debates included whether to perform intraoperative cholangiography on a routine or selective basis and the appropriate timing for performing cholecystectomy in the face of acute cholecystitis. Some surgeons advocated performing cholecystectomy via a small incision (minicholecystectomy) to minimize the discomfort and disability brought about by larger incisions during gallbladder removal (Basu et al, 2006).
By the 1980s, the rates of morbidity and mortality for open cholecystectomy were quite acceptable. In published reports of large series taken from population-based studies or from single institutions, mortality rates ranged from 0.1% to 0.6%, and overall morbidity was 10% to 15% with bile duct injuries occurring in 0.1% to 0.2% of patients. It was also shown clearly that complications occurring with open cholecystectomy were directly related to the condition of both the patient and the gallbladder at the time of operation. Postoperative hospital length of stay for open cholecystectomy varies widely, but most elective cases are discharged within 4 days of operation (Hall, 1987).
Medical Therapy for Gallstone Disease
The prevailing public perception of open cholecystectomy as a procedure that results in pain, disability, and a disfiguring scar engendered many attempts in the 1980s and 1990s at nonoperative treatment of gallstones (Schoenfield & Lachin, 1981; Schoenfield et al, 1990). In addition, an understanding of the chemistry of gallstones led to numerous attempts to develop agents that could dissolve cholesterol gallstones. By the mid-1980s, ursodeoxycholic acid was made commercially available for oral administration, and its use was described in several clinical studies. Unfortunately, the indications for this agent were limited, and the 40% rate of complete gallstone dissolution in this highly selected group was disappointing (Roda et al, 1982). In the late 1980s gastroenterologists and interventional radiologists tried to increase this dissolution rate by attempting direct contact dissolution of gallstones. The biliary tree was accessed percutaneously, and methyl tert-butyl ether (MTBE) was instilled in the gallbladder. This therapy was invasive and had significant side effects, thus it was rapidly abandoned (Thistle et al, 1989).
In the early 1980s, extracorporeal shock wave lithotripsy (ESWL) was shown to be efficacious therapy for kidney stones. Others extrapolated that shock wave therapy might also break gallstones into smaller fragments that could then be more effectively dissolved. A brief flurry of interest and activity in the use of ESWL for gallstones followed, but the selection criteria limited this to fewer than 20% of gallstone patients. Despite strict selection criteria, the efficacy of treatment at 6 months was only 22%, and the gallstone recurrence rate of 10% per year for the first 5 years left much to be desired (Sackmann et al, 1990).
Laparoscopic Cholecystectomy
The technique of laparoscopy (lapara, the flank; skopein, to examine) was initially developed near the outset of the twentieth century. However, until the 1960s, its use was strictly limited to diagnostic procedures within the peritoneal cavity. Gynecologists first used laparoscopic techniques to perform tubal ligation. By that time, the laparoscopic optics, light sources, and carbon dioxide insufflators had been markedly improved, but the eyes of the surgeon were wedded to the eyepiece of the laparoscope during performance of the operation. Despite this limitation, Dr. Kurt Semm of Kiehl, Germany, the father of “pelviscopy,” performed the first laparoscopic appendectomy in 1980 (Semm, 1982). By 1983, Semm stated that “laparoscopic cholecystectomy and the bowel anastomosis under laparoscopic vision had moved into the domain of the possible” (Manegold, 1985). Two years later, a German surgeon named Eric Muhe working in Böblingen, West Germany, performed the first laparoscopic cholecystectomy (Litynski, 1996). He used a modified operating laparoscope placed at the umbilicus after establishing pneumoperitoneum. Muhe later changed his technique to perform cholecystectomies through a small, open tube placed in the right upper quadrant without pneumoperitoneum. Despite presenting his initial work at the congress of the German Surgical Society in 1986, Muhe’s approach was rejected by German surgeons and largely forgotten. In 1987 a French surgeon, Phillipe Mouret of Lyon, France, performed laparoscopy on a woman with both gallstones and a gynecologic disorder. He performed the first video laparoscopic cholecystectomy by using a camera attached to the laparoscope, thereby allowing the entire operating team to view the operative field. As he was in private practice, few other surgeons learned of his work initially, but word spread within France. The following year both François DuBois in Paris and Jacques Perissat in Bordeaux, working in academic centers, performed laparoscopic cholecystectomy. Relatively soon thereafter, two groups of general surgeons in the southern United States performed laparoscopic cholecystectomy. They used a laser to dissect the gallbladder away from the liver bed, as it was thought that monopolar electrocautery used within the closed abdomen was potentially a hazard for combustion. The first publication of the “laparoscopic laser cholecystectomy” was published in the industry publication “Laser Practice Report” by Reddick in December 1988, whereas DuBois and colleagues published their first 36 cases (in English) in 1990.
All of these early pioneers in laparoscopic cholecystectomy used a four-port technique with the laparoscope placed at the umbilicus, albeit the French surgeons stood between the patient’s legs, and the Americans worked from the patient’s left side. Word of this new laparoscopic operation spread relatively rapidly after its introduction at the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) meeting in Louisville in April 1989, and the subsequent American College of Surgeons meeting in Atlanta in October 1989. Given that cholecystectomy makes up nearly a third of the work in many general surgeons’ practices, surgeons in the United States feverishly attempted to learn this technique for a competitive advantage. Numerous courses sprang up across the United States and Europe. Many of these courses were sponsored by industry and provided little hands-on experience for surgeons who generally were poorly trained in basic laparoscopic techniques. It must be remembered that laparoscopy differs significantly from traditional open surgery in several important ways: 1) a satisfactory pneumoperitoneum of carbon dioxide gas must be established in the abdominal cavity; 2) the laparoscopic image of the operative field is controlled by someone other than the surgeon, and this image is highly magnified and two-dimensional; 3) the surgeon views a video screen, rather than the operative field itself; and 4) the laparoscopic ports act as a fulcrum, so the tips of the instruments move in the direction opposite the surgeon’s hands. Given these and other differences, it is not surprising that a learning curve effect was quickly recognized, whereby the incidence of bile duct injuries was much higher in a surgeon’s early cases compared with later in his or her experience (Meyers & Southern Surgeons Club, 1991).
Going from an unheard of procedure in 1988 to one that had become the subject of numerous published case series by 1992, the adoption rate of this technology in the United States was unprecedented. In 1992, the National Institutes of Health (NIH) organized a consensus development conference titled “Gallstones and Laparoscopic Cholecystectomy,” which was sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases and the NIH office of Medical Applications of Research. At the conclusion of the conference, it was determined that laparoscopic cholecystectomy “provides a safe and effective treatment for patients with symptomatic cholelithiasis” and “provides distinct advantages over open cholecystectomy” but that “the outcome of laparoscopic cholecystectomy is influenced greatly by the training, experience, skill, and judgment of the surgeon performing the procedure” (Gollen et al, 1993).
It was also in 1992 that the first of several small prospective randomized trials comparing laparoscopic to open cholecystectomy was published. Each of these showed that the laparoscopic approach was associated with less pain, shorter hospitalization, and a more rapid return to full activity (Barkun et al, 1992). Coincidentally, 1992 was also the year in which a publication of a large series of laparoscopic cholecystectomies first suggested that this technique may become the new gold standard for performance of the operation (Soper et al, 1992b). By contrast, a relatively large randomized trial comparing laparoscopic with open cholecystectomy using a minilaparotomy showed no difference in many standard outcome measures (Majeed et al, 1996).
Over the ensuing years, a number of trials confirmed what had previously been shown in the open cholecystectomy literature. The aphorism “get it while it’s hot” could be used to describe the preferred approach to laparoscopic cholecystectomy for acute cholecystitis: prospective randomized trials showed that early cholecystectomy resulted in fewer complications and shorter hospitalization than interval cholecystectomy (Lo et al, 1998). Intraoperative cholangiography was performed on a selective basis by the majority of practicing surgeons, despite several population-based studies showing decreased rates of bile duct injury when performed by surgeons using cholangiography routinely during laparoscopic cholecystectomy (Flumm & Massarweh, 2007). Numerous reports were published concerning the causes of bile duct injury during laparoscopic cholecystectomy and the means by which these injuries could be minimized, including dissecting “the critical view of safety” before clipping or dividing the cystic structures (Strasberg et al, 1995).
Indications
An increase in the number of cholecystectomies performed has been documented since the introduction of laparoscopic cholecystectomy (Escarce et al, 1995; Legorreta et al, 1993; Nenner et al, 1994; Steiner et al, 1994). It is unclear whether patients are more willing to undergo a laparoscopic procedure, rather than to endure biliary colic, or whether the indications for cholecystectomy have become more liberal with the advent of laparoscopic cholecystectomy.
The indications for laparoscopic cholecystectomy are, and should be, the same as the indications for open cholecystectomy (Table 34.1). Patients generally have documented cholelithiasis and symptoms attributable to a diseased gallbladder (see Chapter 30). Biliary colic is typically a severe and episodic right upper abdominal or epigastric pain that often radiates to the back. Attacks frequently occur postprandially, or they awaken the patient from sleep. Patients with asymptomatic gallstones have less than a 20% chance of ever developing symptoms, and the risks associated with prophylactic operation outweigh the potential benefit of surgery in most patients (Fendrick et al, 1993; Ransohoff & Gracie, 1993; Ransohoff et al, 1983). Prophylactic laparoscopic cholecystectomy for asymptomatic cholelithiasis may be justified for certain patients, however, such as those with sickle cell disease, those who require long-term total parenteral nutrition, and those who are therapeutically immunosuppressed after solid organ transplantation. Patients with sickle cell disease often have hepatic or vasoocclusive crises, which can be difficult to differentiate from acute cholecystitis (Tagge et al, 1994). In transplant patients, there is concern that immunosuppression would mask the signs and symptoms of inflammation until overwhelming infection had occurred (Hull et al, 1994). Recommendations in the literature range from mandatory screening and treatment of biliary disease before transplantation (Girardet et al, 1989) to prophylactic cholecystectomy 6 months after transplantation (Boline et al, 1991) to expectant management of all asymptomatic patients (Fendrick et al, 1993; Steck et al, 1991). Other possible indications for prophylactic laparoscopic cholecystectomy include known gallstones in patients who may not have access to modern health care facilities for an extended period, such as missionaries and military personnel, and patients who already are undergoing laparoscopic abdominal surgery for other indications (incidental laparoscopic cholecystectomy). Less commonly, individuals without gallstones but with typical biliary symptoms (i.e., acalculous cholecystitis or biliary dyskinesia) may be considered for the procedure (Soper, 1991). Other indications for laparoscopic cholecystectomy include gallstone pancreatitis and gallbladder polyps greater than 1 cm in size (see Chapter 49).
Contraindications
Absolute and relative contraindications to performing laparoscopic cholecystectomy (Table 34.2) have decreased since the 1990s, as minimally invasive surgical equipment and skills have improved. Absolute contraindications include the inability to tolerate general anesthesia or laparotomy, refractory coagulopathy, diffuse peritonitis with hemodynamic compromise, cholangitis, and potentially curable gallbladder cancer. Diffuse peritonitis with hemodyInamic compromise represents a surgical emergency in which attempted laparoscopic cholecystectomy is not prudent, because the cause of the peritonitis is unclear or uncertain. Standard open laparotomy allows rapid determination of the cause and more expeditious management of the disorder, and pneumoperitoneum may exacerbate hypotension by diminishing venous return. Suspicion of gallbladder malignancy generally mandates that standard open resection be undertaken; this is because of persistent concerns with adequacy of resection and reports of port-site metastases associated with the use of minimally invasive surgical techniques for the treatment of intraabdominal malignancies (see Chapter 49). Relative contraindications are dictated primarily by the surgeon’s philosophy and experience. These include previous upper abdominal surgery with extensive adhesions, cirrhosis, portal hypertension, severe cardiopulmonary disease, morbid obesity, and pregnancy.
Table 34.2 Contraindications to Laparoscopic Cholecystectomy
| Absolute |
Previous Abdominal Surgery
Intraabdominal adhesions secondary to previous abdominal surgery can tether underlying viscera and consequently increase the risk of hollow organ injury during placement of laparoscopic trocars (Wolfe et al, 1991). Previous lower abdominal operations typically add little difficulty in performing cholecystectomy, but patients with previous upper abdominal operations, especially in the right upper quadrant, present much more difficulty in gaining access to the area around the gallbladder. To diminish the risk of intraperitoneal injury, we routinely use the open Hasson technique for placement of the initial trocar. Other surgeons advocate the use of the Veress needle and percutaneous insertion of the initial trocar at a site remote from the abdominal wall scars. Once the initial trocar is placed, additional trocars are placed under direct laparoscopic visualization in an area free of adhesions. Some degree of laparoscopic adhesiolysis may be necessary to allow optimal port placement. Although early series demonstrated that previous abdominal surgery with the accompanying risk of adhesions was a predictor of conversion, it is clear that laparoscopic cholecystectomy may be performed safely on most of these patients.
Cirrhosis
Cirrhosis (see Chapter 70B) results in a brittle, friable, heavy liver that may be difficult to retract in the cephalad direction, limiting exposure of the porta hepatis and gallbladder. Cirrhosis also may be accompanied by decreased synthetic function of the liver, resulting in coagulopathy and portal hypertension. Coagulopathies should be reversed before performance of laparoscopic cholecystectomy.
The ability to achieve effective hemostasis laparoscopically is significantly compromised compared with that of open exposure. Portal hypertension and aberrant portosystemic venous collateralization may lead to exsanguinating hemorrhage from small veins in the liver bed and porta hepatis or from large veins in the abdominal wall (e.g., a recanalized umbilical vein) at risk for laceration during trocar puncture. Laparoscopic cholecystectomy may be attempted with care in these patients by experienced surgeons. Various hemostatic agents and energy sources need to be available in case bleeding develops; the argon cautery device can be quite useful. Prompt conversion to an open procedure is recommended in the face of unusual bleeding, regardless of the stage of the operation (Soper, 1993). Additionally, particular care should be taken in making watertight closures for all incisions to minimize postoperative ascitic leak (Poggio et al, 2000).
Cardiopulmonary Disease
Most patients undergoing laparoscopic cholecystectomy exhibit mildly elevated Pco2 (Liu et al, 1991). Chronic obstructive pulmonary disease may predispose patients to carbon dioxide retention disproportionate to the measured end-tidal value during laparoscopic cholecystectomy (Wittgen et al, 1991). Prudent measures in these patients include obtaining preoperative pulmonary function tests, making arterial blood gas determinations, and maximizing pulmonary function by smoking cessation and bronchodilator therapy. An intraoperative arterial catheter also should be placed to allow frequent measurement of Pco2 and pH. Hypercarbia may manifest as hypertension, tachycardia, or ventricular arrhythmias; these should be addressed by immediate evacuation of the pneumoperitoneum and stabilization. Gradual reestablishment of the pneumoperitoneum with a low pressure limit may be attempted, but the procedure should be terminated or converted to open if the hypercarbia recurs and is refractory to continued pneumoperitoneum.
Morbid Obesity
Other challenging factors associated with morbid obesity include the presence of an enlarged, friable, fatty liver and an increased amount of adipose tissue around the gallbladder and in the area of the triangle of Calot. The surgeon should not hesitate to place additional trocars if needed (Schirmer et al, 1992). Retrospective and prospective studies have shown modestly increased operative times with performance of minimally invasive procedures in obese patient groups (Schirmer et al, 1992; Underwood et al, 1998). Today, laparoscopic cholecystectomy is commonly performed in morbidly obese patients and seems to offer the same advantages as in nonobese patients; it may also offer advantages more specific to obese patients, such as a decrease in wound infections, incisional hernias, and thrombotic complications (Miles et al, 1992; Talamini & Gadacz, 1992). Rather than being contraindicated in morbidly obese patients, laparoscopic cholecystectomy may become the preferred mode of therapy for these patients.
Pregnancy
Studies have shown that laparoscopic cholecystectomy can be performed safely during pregnancy, but only with great care (Soper et al, 1992a); A retrospective cross-sectional analysis of hospital discharge data from the Healthcare Cost and Utilization Project Nationwide Inpatient Sample database was conducted to study outcomes after cholecystectomy during pregnancy (Kuy et al, 2009). In this study, 9714 pregnant women who underwent cholecystectomy experienced significantly higher surgical, maternal, and fetal complication rates, lengths of stay, and costs with open cholecystectomy compared with laparoscopic surgery (Kuy et al, 2009). This may reflect the reasons for choosing an open cholecystectomy—such as prior surgery, gangrenous gallbladder, or trimester of pregnancy—rather than a true difference in complication rates between the procedures. High-volume surgeons, defined as those providers above the 75th percentile based on the annual number of cholecystectomies performed, had significantly lower rates of surgical complications.
Preoperative Evaluation and Preparation
Laboratory Test and Imaging Studies
Patients undergoing elective laparoscopic cholecystectomy for biliary colic should have preoperative liver function tests (LFTs). Elevations in these tests suggest the presence or recent passage of CBD stones. In patients with cholecystitis, LFTs are not typically elevated, because the obstruction is limited to the gallbladder; if present, elevated LFTs should raise concerns about complicating conditions such as cholangitis, choledocholithiasis, or Mirizzi syndrome (a gallstone impacted in the distal cystic duct causing extrinsic compression of the CBD; see Chapter 35).
In a patient with typical biliary colic, the only diagnostic study necessary before laparoscopic cholecystectomy is an abdominal US revealing gallstones. US shows the size and number of stones, the thickness of the gallbladder wall, the presence or absence of pericholecystic fluid, and the diameter of the CBD and other components of the biliary ductal system (see Chapter 13). Other nonbiliary disorders, such as hepatic lesions or steatosis, masses in the pancreas, or renal tumors, also may be diagnosed. When US results are negative despite typical biliary symptoms, cholecystokinin-stimulated biliary scintigraphy showing a low gallbladder ejection fraction with or without pain reproduction suggests acalculous cholecystitis or gallbladder dyskinesia (Soper, 1993).
If a patient with gallstones has atypical symptoms, however, a more extensive workup that includes upper gastrointestinal contrast radiography or endoscopy, computed tomography (CT), or cardiac and pulmonary evaluation may be appropriate to rule out significant nonbiliary disease processes. Magnetic resonance cholangiopancreatography (MRCP) may be useful to evaluate the common duct in patients with mild elevations of their transaminases or mild CBD dilatation on US (see Chapter 17). Additionally, if a patient has a dilated CBD, CBD stones, or jaundice, consideration should be given to a preoperative endoscopic retrograde cholangiopancreatography (ERCP) with clearing of the stones followed by laparoscopic cholecystectomy (see Chapter 18).
Antibiotics
Routine preoperative antibiotic administration for elective laparoscopic cholecystectomy is controversial, with strong opinions on both sides. A recent meta-analysis of randomized controlled trials concluded that prophylactic antibiotics do not prevent infections in low-risk patients undergoing laparoscopic cholecystectomy; the usefulness of prophylaxis in high-risk patients—those older than 60 years and those with diabetes, acute colic within 30 days of operation, jaundice, acute cholecystitis, or cholangitis—remains uncertain (Choudhary et al, 2008). The most recent randomized, prospective study included in the above-mentioned meta-analysis showed no difference in the postoperative wound infection rate, although the control group had a 1.5% infection rate, and the antibiotic group had a 0.7% infection rate; however, because there was a total of 277 patients in the study, a type II error might have been made (Chang et al, 2006). Among papers that suggest a benefit of antibiotic prophylaxis is a recent randomized study that found fewer wound infections with ampicillin-sulbactam versus cefuroxime, particularly for infection caused by Enterococcus in the setting of high-risk patients undergoing elective cholecystectomy (Dervisoglou et al, 2006).
Operative Technique
Operating Room Setup
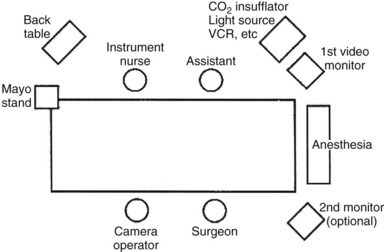
Using the “American” technique, the surgeon stands to the left of the patient, the first assistant stands to the patient’s right, and the laparoscopic video camera operator stands to the left of the surgeon (Fig. 34.1). In the “French” technique, the patient’s legs are abducted, and the surgeon stands between them. The camera operator always must maintain the proper orientation of the camera and keep the operating instruments in the center of the video image. Following all instruments as they enter or exit the operative field is a matter of surgeon preference. Sharp instruments should never be moved intracorporeally unless they are under direct videoscopic vision.
Pneumoperitoneum
A working space, generally provided by a pneumoperitoneum, is essential for the surgeon to both view and operate within the abdominal cavity. Carbon dioxide has the advantage of being noncombustible and rapidly absorbed from the peritoneal cavity; however, it may lead to hypercarbia in patients with significant cardiopulmonary disease (Fitzgerald et al, 1992). Pneumoperitoneum can be established by either a closed or an open technique. In the closed technique, carbon dioxide is insufflated into the peritoneal cavity through a Veress needle placed blindly into the abdominal cavity; the needle is subsequently replaced with a laparoscopic port. In the open technique, a laparoscopic port is inserted under direct vision into the peritoneal cavity via a small incision; pneumoperitoneum is established only after ensuring definitive and safe peritoneal entry. Both techniques have advantages and disadvantages, and surgeons performing laparoscopic cholecystectomy should learn both techniques and use them selectively.
Insufflator tubing is connected to the Veress needle, and carbon dioxide is insufflated at a low flow rate of approximately 1 L/min. The initial pressure of the abdomen is usually 2 to 6 mm Hg and should not increase appreciably during the early phase of insufflation. Asymmetric distension, a rapid increase in pressure with low insufflated volume, or an initial pressure greater than 10 mm Hg suggests that the needle is not in the proper position. The abdomen should be serially percussed to confirm symmetric tympany associated with insufflation. The abdomen is fully insufflated with the upper pressure limit set at 12 to 15 mm Hg; this usually requires 3 to 6 L of carbon dioxide, depending on the size of the abdominal cavity and degree of muscle relaxation. If intraabdominal pressures exceed 20 mm Hg, central venous pressures and blood pressures decrease because of decreased venous return and diminished cardiac output (Chui et al, 1993; Sharma et al, 1997). Studies also have shown direct negative effects on urine output relative to increased intraabdominal pressures (McDougall et al, 1996, 1997).
During the initial period of insufflation, the patient must be monitored closely for signs of gas embolism (hypotension, decreased oxygen saturation, decreased end-tidal carbon dioxide, “mill-wheel” heart murmur), vagal reaction (hypotension, bradycardia), ventricular arrhythmias, and hypercarbia with acidosis. Most of these complications require immediate treatment by desufflation followed by gradual reestablishment of the pneumoperitoneum after the patient’s condition has stabilized. Gas embolism results in an “air lock” right ventricular outflow obstruction with a dramatic decrease in end-tidal carbon dioxide concentration. When suspected, this problem should be treated by placing the patient in a steep Trendelenburg and left lateral decubitus position followed by insertion of a central venous catheter to aspirate the carbon dioxide from the apex of the right ventricle (Chui et al, 1993; Hanney et al, 1995; Lantz & Smith, 1994; Sharma et al, 1997).
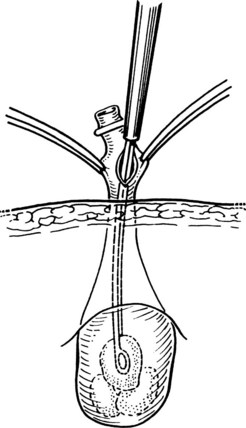
Using the open technique, the pneumoperitoneum is established similarly to an open diagnostic peritoneal lavage. A 1.5-cm skin incision is made in the infraumbilical or supraumbilical skin fold. Dissection of the subcutaneous tissue is performed at the base of the umbilical raphe to reach the fascia rapidly, even in obese patients, because this is the thinnest part of the abdominal wall. Kocher clamps are applied to both sides of the linea alba, and a small vertical incision is made to gain access to the peritoneal cavity. A finger is placed into the wound to ensure that the free peritoneal cavity has been entered and to sweep away any adhesions that may be present. If a Hasson trocar (Weck & Co, Research Triangle Park, NC) is available, two sutures are placed on both sides of the fascial incision and are tied to the wings of the wedge-tipped sheath after it is inserted into the peritoneal cavity under direct vision (Fig. 34.2). Alternatively, standard laparoscopic sheaths can be used after placing two concentric polypropylene purse-string stitches around the fascial incision. After removing the sheath at the conclusion of the procedure, the outer purse-string suture is removed, and the inner one is tied. Open insertion of the initial port takes a few minutes longer than its closed counterpart. Extraction of the gallbladder at the conclusion of the operation is easier, however, which equalizes the time differential of the two access techniques.
Port Placement and Exposure
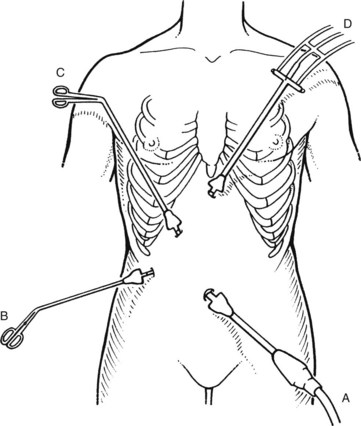
A final operating trocar/port is placed through an incision in the midline of the epigastrium. This trocar usually is inserted approximately 5 cm below the xiphoid process, but the precise position and angle depend on the location of the gallbladder and the size of the medial segment of the left lobe of the liver. When the appropriate position for this trocar is hard to determine, a Veress needle may be placed first at the proposed site to ascertain whether its location and angle of insertion seen through the laparoscope are optimal. The trocar is inserted in a controlled fashion, angling its tip just to the right of the falciform ligament, aiming toward the gallbladder. Dissecting forceps are inserted and directed toward the gallbladder neck. The orientation of the laparoscope is generally parallel to that of the cystic duct when the fundus is elevated, whereas the instruments placed through the other three ports enter the abdomen at right angles to this plane (Figs. 34.3 and 34.4).
Dissection
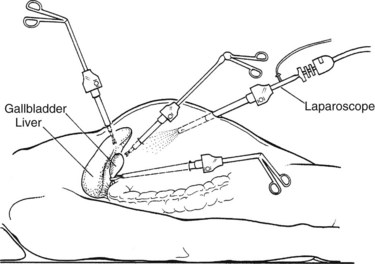
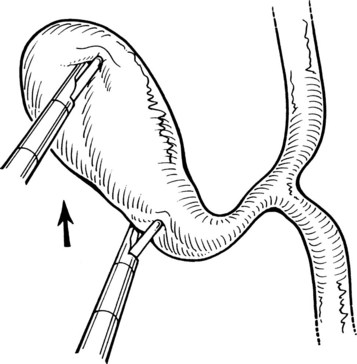
A grasping instrument on the infundibulum exerts traction on the gallbladder away from the CBD in a superior and lateral direction. Fine-tipped dissecting forceps are used to dissect away the overlying fibroareolar structures from the infundibulum of the gallbladder. This is done with a blunt stripping action, always starting on the gallbladder and stripping the tissue toward the porta hepatis (Fig. 34.5). The dissection should begin on a known structure (e.g., the gallbladder), rather than in an unknown area, to avoid damage to the underlying structures, such as the bile duct or the hepatic artery.
It is important to identify clearly the structures forming the sides of the triangle of Calot (i.e., cystic duct, cystic artery, and common hepatic duct), the standard ventral aspect, and its reverse (dorsal) aspect (Fig. 34.6). Distinction is made here with the hepatocystic triangle proper, which is the ventral aspect of the area bounded by the gallbladder wall and cystic duct, the liver edge, and the common hepatic duct; the cystic artery and the triangle of Calot lie within this space. The hepatocystic triangle is maximally opened and converted into a trapezoid shape by retracting the infundibulum of the gallbladder inferiorly and laterally, while maintaining the fundus under traction in a superior and medial direction. A lymph node usually lies adjacent to the cystic artery, and occasionally it is necessary to use a brief application of electrosurgical coagulation to obtain hemostasis as the lymph node is bluntly swept away. To expose the reverse of Calot’s triangle, the infundibulum of the gallbladder is pulled in a superior and medial direction (see Fig. 34.6).
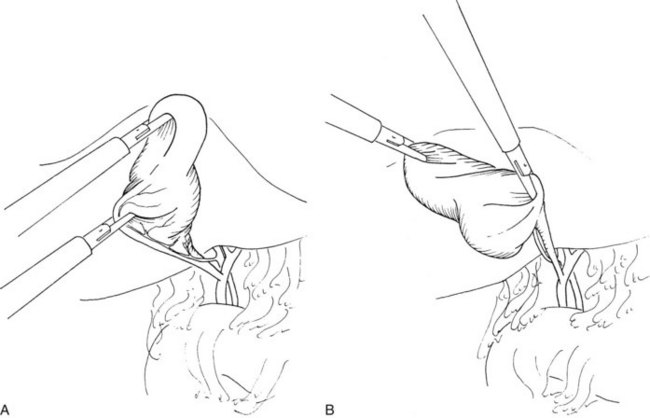
FIGURE 34.6 Proper gallbladder retraction for exposure of Calot’s triangle (A) and reverse Calot’s triangle (B).
(Courtesy Quality Medical Publishing, St Louis, MO.)
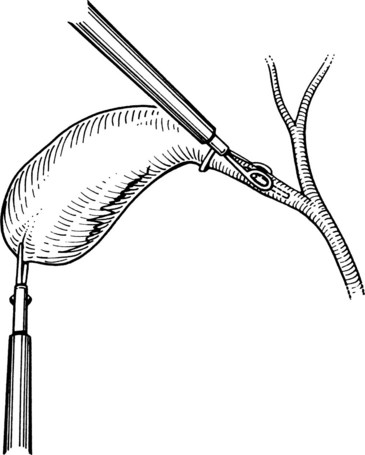
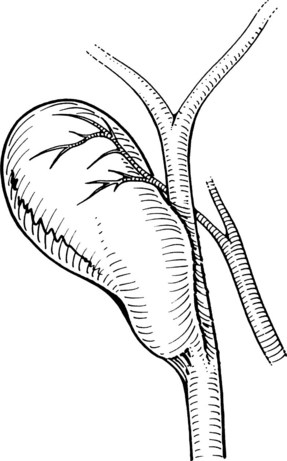
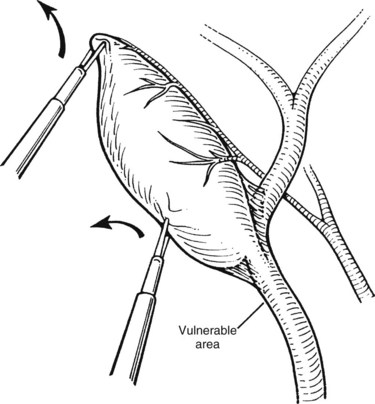
Next, the cystic artery is separated from the surrounding tissue by similar blunt dissection. If the cystic artery crosses anterior to the duct, the artery may require dissection and division before approaching the cystic duct. The neck of the gallbladder is dissected away from its liver bed, leaving only two structures entering the gallbladder: the cystic duct and artery. No structure should be divided until the cystic duct and cystic artery are unequivocally identified. This is the “critical view” of safety (Fig. 34.7) essential to prevent bile duct injury during laparoscopic cholecystectomy (Strasberg et al, 1995).
Intraoperative Evaluation for Choledocholithiasis
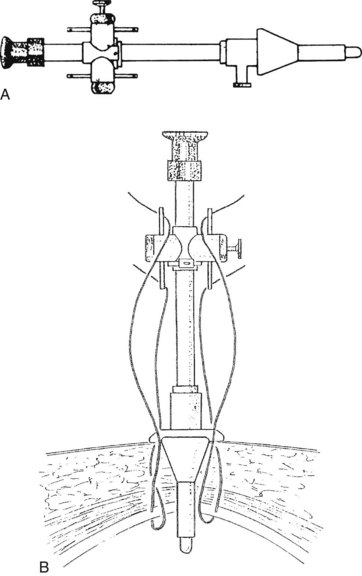
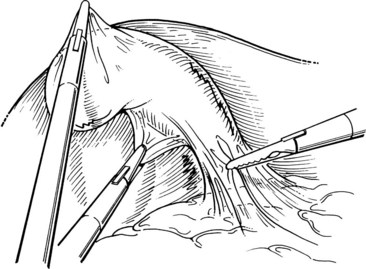
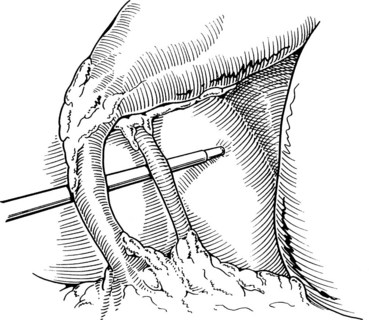
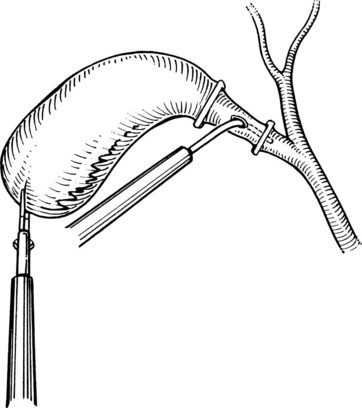
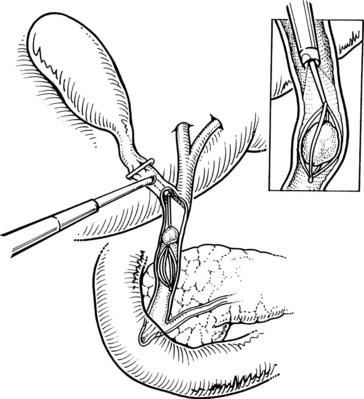
After initially dissecting the proximal cystic duct, the CBD should be imaged if there is any concern for choledocholithiasis or any question regarding the biliary anatomy. This can be achieved by radiographic intraoperative cholangiography (IOC) or intracorporeal LUS (see Chapter 21). Before either procedure, a clip is applied high on the cystic duct at its junction with the gallbladder to prevent stones from migrating down the duct. To perform IOC, the anterolateral wall of the cystic duct is incised, and dissecting forceps are used to compress the cystic duct gently and systematically back toward the gallbladder, “milking” stones away from the CBD and out the ductotomy (Figs. 34.8 and 34.9). A 4- to 5-Fr catheter is inserted into the duct through a hollow, 5-mm metal tube that has an appropriate gasket to prevent carbon dioxide leakage around the catheter itself. The cholangiography catheter is inserted into the cystic duct, and a clip is applied loosely to secure the catheter in place (Fig. 34.10). If the introducer has grasping jaws, it can be used to secure the catheter into the duct (Fig. 34.11). Alternatively, catheters equipped with balloons proximal to the tip may be used for fixation.
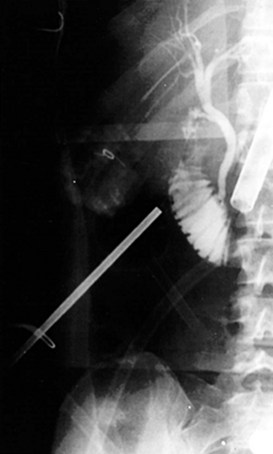
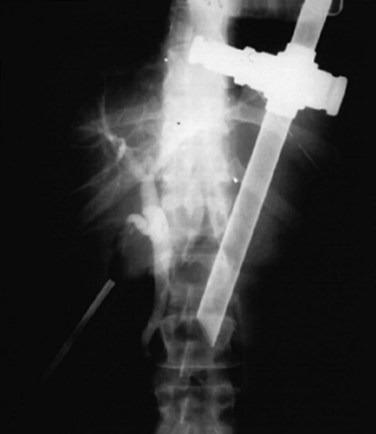
Cholangiography can be performed by either real-time fluoroscopy (dynamic IOC) or by obtaining two standard radiographs (static IOC) after injecting 5 mL and 10 mL of water-soluble contrast medium (Figs. 34.12 and 34.13). The films should be inspected for the following: 1) the length of cystic duct and location of its junction with the CBD, 2) the size of the CBD, 3) the presence of intraluminal filling defects, 4) free flow of contrast material into the duodenum, and 5) anatomy of the extrahepatic and intrahepatic biliary tree. After the cholangiocatheter is removed, the cystic duct is doubly clipped below the ductotomy, with care taken to avoid the wall of the CBD, and divided. The posterior jaw of the clip applier must be visualized before applying each clip to avoid injury to the surrounding structures. Great care should be taken so that the CBD is not “tented up” into the clip. If the cystic duct is particularly large or friable, it may be preferable to replace one of the clips with a hand-tied or preformed suture.
Evaluation of the CBD by LUS is an alternative to cholangiography. Several studies performed at open cholecystectomy reported LUS to be more accurate than IOC in assessing the CBD for stones (97% to 99% vs. 89% to 94%; Machi et al 1993a, 1993b; Orda et al, 1994a, 1994b). Although few surgeons have adopted US for this purpose, LUS has been used in several centers during laparoscopic cholecystectomy and is gaining popularity (Jakimowicz, 1993; John et al, 1994; McIntyre et al, 1994; Orda et al, 1994a, 1994b; Steigmann et al, 1994). With LUS, the transducer has a higher frequency with improved resolution compared with that used with transabdominal US. In experienced hands, LUS seems to be as accurate as cholangiography for showing choledocholithiasis, but it can be performed more rapidly (Steigmann et al, 1995). In a prospective multicenter trial with 209 laparoscopic cholecystectomy patients, the time to perform LUS (7 ± 3 min) was significantly less than that of IOC (13 ± 6 min; Steigmann et al, 1995). The study showed that LUS was more sensitive for detecting stones but that IOC was better in delineating intrahepatic anatomy and defining anatomic anomalies of the ductal system. The authors concluded that the two methods of ductal imaging were complementary. Despite these promising data, more clinical experience is needed to establish the appropriate role of LUS for the detection of choledocholithiasis during laparoscopic cholecystectomy (Soper, 1997; Wu et al, 1998a).
Completion of Cholecystectomy
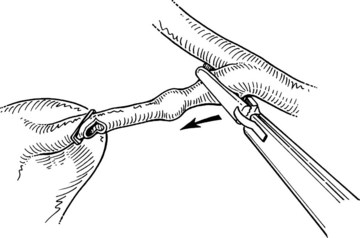
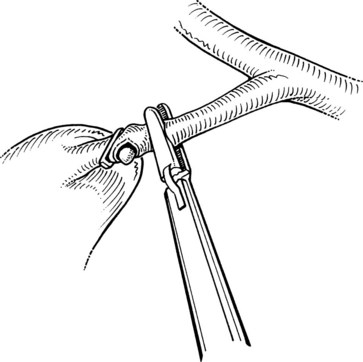
After clip ligation and division of the cystic duct, the cystic artery is dissected from the surrounding tissue for an adequate distance to permit placement of three clips. The surgeon must ascertain that the structure is the cystic artery and not the right hepatic artery, looping up onto the neck of the gallbladder, or an accessory or replaced right hepatic artery (see Chapter 1B). After an appropriate length of cystic artery has been dissected free, it is clipped proximally and distally before its transection. Electrocautery should not be used for this division, because the current may be transmitted to the proximal clips and lead to subsequent necrosis and hemorrhage. A common error is to dissect and divide the anterior branch of the cystic artery, mistaking it for the main cystic artery; this may result in hemorrhage from the posterior branch during dissection of the gallbladder fossa.
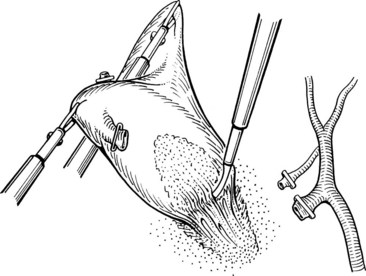
The ligated stumps of the cystic duct and the artery are examined to ensure that there is no leakage of either bile or blood and that the clips are placed securely; they should compress the entire lumen without impinging on adjacent tissues. A suction-irrigation catheter is used to remove any debris or blood that has accumulated during the dissection. Separation of the gallbladder from its hepatic bed is initiated using an electrosurgical probe to coagulate small vessels and lymphatics. While maintaining cephalad traction on the fundus of the gallbladder with the axillary forceps, the midclavicular forceps pulls the neck of the gallbladder anterosuperiorly and then alternatively medially and laterally to expose and place the tissue connecting the gallbladder to the fossa under tension (Fig. 34.14). An electrocautery spatula or hook is used in a gentle sweeping motion with low power (25 to 30 W) to coagulate and divide the tissue. Intermittent blunt dissection facilitates exposure of the proper plane.
If the gallbladder is not distended with bile or stones, it can be withdrawn with gentle traction. In most cases, a suction catheter introduced through an incision in the gallbladder neck is used to aspirate bile and small stones. Stone forceps also can be placed into the gallbladder to extract or crush calculi if necessary (Fig. 34.15). Occasionally, the fascial incision must be extended to extract larger stones or thick-walled gallbladders. After the gallbladder is extracted, the operator’s finger is used to occlude the port entry site. If any question concerning hemostasis or contamination of the right upper quadrant remains, the umbilical port can be replaced under direct vision, and the upper part of the abdomen can be copiously irrigated and aspirated again. If not, all laparoscopic ports are removed after allowing escape of the carbon dioxide.
Intraoperative Treatment of Common Bile Duct Stones
The reported incidence of CBD stones found during laparoscopic cholecystectomy ranges from 3% to 10% (see Chapters 30 and 34 through 37) (Barkun et al, 1993; Frazee et al, 1993). It is unclear whether uncomplicated and asymptomatic choledocholithiasis requires treatment. Autopsy series have shown that many patients with silent gallbladder stones also harbor silent CBD stones (Crump, 1931). Smaller stones are known to pass through the ampulla of Vater, as shown by stool analysis in patients with acute pancreatitis (see Chapter 53) or acute biliary colic (Acosta & Ledesma, 1974; Kelly, 1980). In one study, the rate of symptoms or complications in patients with retained CBD stones after cholecystectomy was 94%, with a mortality rate of 3% over 5 years (Hicken & McAllister, 1964). It is unclear what stone size precludes passage or which indicators predict complications of cholangitis (see Chapter 43) or pancreatitis if the stones are left in situ.
Laparoscopic CBD exploration can take the form of accessing the CBD through the cystic duct (transcystic technique) or directly incising and opening the CBD with stone retrieval (laparoscopic choledochotomy). In the transcystic duct approach, stones smaller than 4 mm often can be flushed through the ampulla into the duodenum, a technique that is facilitated by pharmacologic relaxation of the sphincter of Oddi using sublingual nitroglycerin or intravenous glucagon. When these simple methods fail, a helical stone basket can be passed over a guidewire through the cystic duct and into the CBD to extract stones under fluoroscopic guidance (Fig. 34.16) (Hunter, 1992).
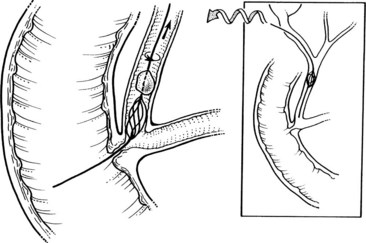
FIGURE 34.16 Transcystic retrieval of common bile duct stones using a helical basket under fluoroscopy.
If attempts at transcystic basket extraction fail, the next step is to insert a 7- to 8-Fr choledochoscope to remove the stones under direct vision. If the CBD stone is larger than the lumen of the cystic duct, the duct should be balloon dilated first to a maximum of 8 mm in diameter, but never larger than the internal diameter of the CBD (Hunter & Soper, 1992). The choledochoscope is passed into the peritoneal cavity through the midaxillary port, using a sheath to prevent damage to the scope by the port’s valve. The choledochoscope is inserted through the cystic duct into the CBD under direct vision. The lumen of the duct is optimally visualized by continuously infusing saline through the biopsy channel. Under visual guidance, the tip of a Segura-type stone basket is advanced beyond the stone and opened. As the basket is pulled backward and rotated, the stone is ensnared (Fig. 34.17; Jones & Soper, 1996).
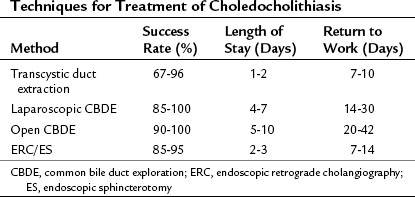
A completion cholangiogram should always be performed to ascertain clearance of the duct. Because of tissue edema secondary to ductal dilation and manipulation, the cystic duct stump is ligated, rather than clipped, for added security. Successful extraction has been reported in 67% to 96% of patients treated in most series (Table 34.3; Bagnato, 1993; DePaula et al, 1994; Dion et al, 1994; Ferzli et al, 1994; Franklin et al, 1994; Hunter, 1992; Petelin, 1993; Phillips et al, 1994). Complications such as infection and pancreatitis have been reported in 5% to 10% of patients, with a mortality rate of 1% to 2%. The duration of hospitalization after an uncomplicated transcystic duct stone extraction is the same as that for laparoscopic cholecystectomy alone, averaging 1 to 2 days. The main advantage of the transcystic approach is that it avoids the need to incise the CBD, with subsequent suture closure and placement of a T-tube. Poor candidates for transcystic extraction techniques are patients with large or multiple CBD stones, patients with stones in the proximal ductal system, and patients with small or tortuous cystic ducts.
If the transcystic approach fails, we recommend direct incision of the CBD laparoscopically. Indications for laparoscopic choledochotomy are stones that are multiple, large, or positioned within the proximal bile ducts in patients with a CBD diameter larger than 8 to 10 mm (Dion et al, 1994; Phillips et al, 1994). Stay sutures usually are placed on either side of the midline of the CBD wall to allow anterior traction on the duct. A longitudinal choledochotomy is made on the distal CBD, with adequate length to allow easy placement of a choledochoscope and removal of the largest stone. Stones are removed under endoscopic visualization, and an appropriate-size T-tube is placed in the duct. The ductotomy is closed with fine absorbable sutures using intracorporeal suturing techniques, and the T-tube is exteriorized through the lateral port site. The patient generally is discharged 2 to 4 days postoperatively and returns for a final T-tube cholangiogram and removal of the T-tube at 14 to 21 days postoperatively. Retained stones shown by T-tube cholangiography may be effectively removed percutaneously after allowing maturation of the T-tube tract. Percutaneous extraction is successful in more than 95% of patients with retained stones (Burhenne, 1980).
Overall, laparoscopic choledochotomy is successful in more than 90% of patients, with a minor morbidity rate of 5% to 10% and a mortality rate of 1% to 2% (see Table 34.3). Complications of this technique include laceration of the CBD, bile leakage, sewn-in T-tubes, and postoperative CBD strictures (Dion et al, 1994). Many surgeons have not mastered laparoscopic suturing to the point that they feel comfortable closing the choledochotomy, for fear of a resultant stricture. Nevertheless, advantages of the direct laparoscopic approach are that every portion of the ductal system can be accessed, and stone size is unimportant. The alternative approach of open operation with exploration of the CBD with or without choledochoduodenostomy should always be considered in difficult cases (see Chapters 29 and 35).
The possibility of finding CBD stones and the means by which they can be treated must be discussed with the patient before operation to formulate a plan of therapy. Many surgeons routinely leave CBD stones in place during laparoscopic cholecystectomy for planned postoperative endoscopic removal (see Chapter 27). Factors favoring postoperative endoscopic therapy include the presence of small, nonobstructing duct stones; a patient with significant comorbidities, making a prolonged operation risky; and a small CBD prone to postoperative stricture. In resorting to postoperative endoscopic retrograde cholangiography and sphincterotomy, the goals of minimally invasive surgery are maintained with a rapid return to full activity. Relying on postoperative endoscopic sphincterotomy (ES) subjects the patient to an additional procedure, with its associated morbidity and possibly a second operation if endoscopic stone extraction fails. In experienced hands, ES has an overall failure rate for stone clearance of 5% to 10% (Cotton, 1993).
Leaving a transcystic catheter in the CBD at the time of laparoscopic cholecystectomy may decrease the likelihood of failure by allowing a guidewire to be passed into the duodenum to facilitate cannulation of the CBD (Deslandres et al, 1993). It is reasonable to submit a patient to postoperative endoscopic stone extraction when the endoscopic expertise is high, whereas an open CBD exploration should be strongly considered when only an inexperienced endoscopist is available in the local setting. The expertise of the laparoscopist and the endoscopist are critical determinants of the means by which CBD stones are managed (Cotton, 1993).
Postoperative Management
Although laparoscopic cholecystectomy was initially thought of as a procedure that could reduce a typical 5-day hospital stay for an open cholecystectomy to 1 to 2 days, third-party payers in the United States have begun to push for discharge of the patient on the same day of surgery; the principal advantage is reduction of hospital costs. Nevertheless, not all patients are candidates for outpatient laparoscopic cholecystectomy. In a prospective analysis of 506 consecutive laparoscopic cholecystectomy cases, Saunders and colleagues (1995) argued that most complications, including life-threatening complications, were not apparent by 8 hours postoperatively and were clinically detected only 39% of the time at 24 hours after the procedure. These investigators recommended great caution in selecting patients for outpatient laparoscopic cholecystectomy. Patients who may benefit from an overnight stay include elderly patients, those with significant comorbid illnesses, those requiring substantial analgesia postoperatively, and patients with complications. For same-day discharge, patients should be healthy, reliable, and sensible; have undergone an uncomplicated operation; and have good home support. Written instructions are given to the patient and the family, with detailed advice concerning signs of potential problems. Routine postoperative follow-up at 2 to 4 weeks is advised for all patients.
Special Considerations
Acute Cholecystitis
Numerous reports indicate that laparoscopic cholecystectomy can be done safely for patients with acute cholecystitis (Prakash et al, 2002; Suter & Meyer, 2001). There is a higher rate of conversion in the setting of acute cholecystitis; in particular, after 72 hours, the rate of conversion increases significantly (Madan et al, 2002).
The timing of laparoscopic cholecystectomy for acute cholecystitis has been a long-standing matter of debate. Based on several prospective studies, early surgical intervention has economic, social, and medical benefits and is the preferred approach for experienced laparoscopic surgeons in the management of acute cholecystitis (Feldman et al, 2002). Our practice is to proceed with laparoscopic cholecystectomy immediately after the diagnosis of acute cholecystitis has been made. In a patient who is seen after 72 hours of symptoms, a laparoscopic cholecystectomy is attempted only if the patient has no preexisting medical conditions that preclude an open cholecystectomy. If the patient does have significant comorbid illnesses, we continue with antibiotic therapy and possibly a percutaneous cholecystostomy tube (see Chapter 32) and subsequent elective laparoscopic cholecystectomy 6 to 8 weeks later.
When acute inflammation has been present for several days or weeks before operation, the pericholecystic tissue planes may be obliterated by thick, “woody” tissue that is difficult to dissect bluntly. In these cases, some authors recommend attempting a fundus-first technique and possibly even a subtotal cholecystectomy (Beldi & Glattli, 2003; Mahmud et al, 2002). Subtotal cholecystectomy involves transecting the infundibulum as close to the cystic duct as possible and extracting all stones. The gallbladder neck is then sutured closed if possible, and a drain is placed in the gallbladder bed. This transection of an edematous, friable wall may be facilitated by using the ultrasonic shears. The surgeon must be comfortable in his or her ability to safely perform the procedure laparoscopically; however, the decision to convert to an open procedure should not be considered a complication but rather good surgical judgment based on the existing anatomy, local conditions, and the surgeon’s experience and confidence in his or her ability to complete the procedure using minimal-access techniques.
In a gallbladder densely adherent to the liver, where the plane between the two is obliterated, or in the presence of portal hypertension, it may be preferable to leave the back wall of the gallbladder intact and fully cauterize its mucosa (Beldi & Glattli, 2003). An active suction drain should be left beneath the liver if the integrity of the cystic duct closure is in question or with any suspicion of bile leakage from the gallbladder bed. We do not place a drain to monitor for bleeding.
Several authors have reported performing laparoscopic cholecystectomy in the face of acute inflammation with success, but with a higher conversion rate than for elective laparoscopic cholecystectomy (Cooperman, 1990; Hermann, 1990; Rattner et al, 1993; Reddick et al, 1991; Unger et al, 1991). Lo and colleagues (1996) reported in their prospective study that despite longer operative times and postoperative stays for early laparoscopic cholecystectomy in patients with acute cholecystitis (treatment within 5 days) versus delayed laparoscopic cholecystectomy (initial conservative treatment followed by laparoscopic cholecystectomy 3 to 4 months later), the advantage of early laparoscopic cholecystectomy was the reduction in the total hospital stay, from 15 to 7 days.
Rattner and associates (1993) retrospectively reviewed 20 patients who underwent attempted laparoscopic cholecystectomy for acute cholecystitis and examined factors that were predictive of a successful procedure. Seven of the 20 patients (35%) required conversion to open cholecystectomy. The interval from admission to cholecystectomy in the successful cases was 0.6 days versus 5 days in the cases requiring conversion to open cholecystectomy. Converted cases also had a significantly higher white blood cell count, alkaline phosphatase levels, and Acute Physiology and Chronic Health Evaluation (APACHE II) scores compared with cases involving successful laparoscopic cholecystectomy. US findings, such as gallbladder distension, wall thickness, and pericholecystic fluid, did not correlate with the success of laparoscopic cholecystectomy.
In a prospective study of 105 patients randomized to early laparoscopic cholecystectomy (within 24 hours of diagnosis of acute cholecystitis) versus delayed laparoscopic cholecystectomy (6 to 8 weeks later), no significant difference was found in conversion rate (early 21% vs. delayed 24%), postoperative analgesic requirement, or number of postoperative complications. The early group did have a longer operative time (123 minutes vs. 107 minutes, P = .04), but total hospitalization was shorter (8 days vs. 12 days, P = .001) (Lai et al, 1998). Laparoscopic cholecystectomy should be performed immediately after the diagnosis of acute cholecystitis. Delaying surgery allows inflammation to become more intense and neovascularized, increasing the technical difficulty of laparoscopic cholecystectomy.
Complications
Many complications related to laparoscopic removal of the gallbladder are similar to the complications that occur during traditional open cholecystectomy (Table 34.4) and include hemorrhage, bile duct injuries, bile leaks, retained stones, pancreatitis, wound infections, and incisional hernias (see Chapter 33). Other potential complications are pneumoperitoneum related (gas embolism, vagal reaction, ventricular arrhythmias, or hypercarbia with acidosis) and trocar related (injuries to the abdominal wall, intraabdominal organs, or major blood vessels). Most complications occur early in the surgeon’s experience. In a multivariate regression analysis of 8839 laparoscopic cholecystectomies, in which 15 bile duct injuries occurred, the only significant factor associated with an adverse outcome was the surgeon’s experience with the procedure (Moore & Bennett, 1995). The regression model predicted that a surgeon had a 1.7% chance of a bile duct injury occurring in the first case and 0.17% chance of a bile duct injury in the fiftieth case.
Conversion to Open Operation
Surgeons performing laparoscopic cholecystectomy should not think of conversion to open operation as a failure but rather as mature judgment. A surgeon should not hesitate to convert to a traditional open cholecystectomy if the anatomy is unclear, complications arise, or reasonable progress cannot be made in a timely manner (Table 34.5). It is better to open one too many than to open one too few, even if it means a longer hospital stay for the patient. Some complications requiring laparotomy are obvious, such as massive hemorrhage, bowel perforation, or major injury to the bile duct. Open laparotomy allows the additional tool of manual palpation and tactile sensation and should be performed when the anatomy cannot be delineated because of inflammation, adhesions, or anomalies. Fistulae between the biliary system and bowel are rare but may require laparotomy for optimal management. The demonstration of potentially resectable gallbladder carcinoma (see Chapter 49) also dictates an open exploration. Finally, when CBD stones cannot be removed laparoscopically and are unlikely to be extracted endoscopically owing to Billroth II anastomosis, previously failed ERCP, or an inexperienced endoscopist, the procedure should be converted to open operation without hesitation.
Table 34.5 When to Convert from Laparoscopic to Open Cholecystectomy
Anatomic Variations
One of the most frequent anomalies is a right hepatic artery that loops up onto the infundibulum of the gallbladder (see Chapter 1B). This anomaly may be misidentified as the cystic artery, leading to injury to the hepatic artery (Berci & Sackier, 1994). The surgeon also occasionally notices only a small cystic artery on the medial aspect of the gallbladder; when this occurs, the surgeon should be alert for a posterior branch leading onto the dorsal aspect of the gallbladder. If great care is not taken, brisk hemorrhage may occur.
A short cystic duct is seen frequently and may be draining into the right hepatic duct or a low-entry right sectoral hepatic duct (2%) or common hepatic duct (1%), or it may connect the infundibulum with the CBD by a duct of only a few millimeters in length in 5% to 6% of cases (Berci, 1992; Reid et al, 1986). During open cholecystectomy, this is relatively easy to recognize, especially if the cholecystectomy is performed in a fundus-first fashion. During laparoscopic cholecystectomy, these ducts are in danger of being mistaken for a normal cystic duct and divided (Fig. 34.18; Meyers et al, 1996). The most dangerous variant is when the cystic duct joins a low-lying aberrant right sectoral duct. These injuries are underreported, because occlusion of an aberrant duct may be asymptomatic and even unrecognized. In addition to anomalous biliary ducts, accessory ducts may drain into the cystic duct or from the liver directly into the gallbladder (ducts of Luschka). Since the widespread use of laparoscopic cholecystectomy, there has been an increased occurrence of bile leaks from the so-called ducts of Luschka. It is likely that many of these bile leaks are due to dissection into the liver substance with injury to a normal, albeit superficial, intrahepatic biliary radicle.
Intraoperative Gallbladder Perforation
Perforation of the gallbladder with bile or stone leakage can be a nuisance, but it should not ordinarily require conversion to open cholecystectomy. Perforation may occur secondary to traction applied by the grasping forceps or electrosurgical thermal injury during removal of the gallbladder from its bed. In our experience, almost one third of the patients have had intraoperative spillage of bile or stones (Jones et al, 1995). Patients with a bile leak have not experienced an increased incidence of infection, prolongation of hospitalization or postoperative disability, or adverse long-term complications (mean follow-up of 41 months in 250 consecutive laparoscopic cholecystectomy patients). The only difference between patients with and without bile leakage was that the operating time for patients with a gallbladder perforation was approximately 10 minutes longer, presumably owing to the time spent cleaning up the operative field. When perforation does occur, the bile should be aspirated completely, and irrigation should be used liberally. The hole in the gallbladder is best secured with a grasping instrument and sutured or tied with an endoloop, and the stones should be retrieved and removed. Gallbladder spillage, when treated in this manner, results in no adverse short-term or long-term complications.
Escaped stones composed primarily of cholesterol pose little threat of infection. Pigment stones frequently harbor viable bacteria, however, and may lead to subsequent infectious complications if allowed to remain in the peritoneal cavity (Deziel et al, 1993). The long-term complications of retained stones, either intraabdominally with resultant abscess formation or intramurally with resultant port-site abscess, have not been prospectively studied, but case reports and case series in the surgical literature document a clear potential for long-term infectious complications (Carlin et al, 1995; Horton & Florence, 1998; Parra-Davila et al, 1998; Shocket, 1995; Zamir et al, 1999). The relative infrequency of these complications probably does not justify conversion to open operation in the face of spilled stones, but vigilance in avoidance of perforation, a careful search for escaped stones, and liberal use of a plastic retrieval bag for large and friable gallbladders are recommended (Zamir et al, 1999).
Biliary Injury
Of all the potential complications, biliary injuries have received the most attention; these are discussed at length elsewhere in this text (see Chapter 42A). Most series quote major bile duct injury rates of 0.30% or less during open cholecystectomy, whereas the incidence of bile duct injuries during laparoscopic cholecystectomy is 0.40% or higher (Strasberg et al, 1995). These injuries can cause major morbidity, prolonged hospitalization (Cates et al, 1993), high costs, and litigation (Asbun et al, 1993). In addition to aberrant anatomy and the surgeon’s inexperience, many reports mention acute or chronic inflammation with dense scarring, operative bleeding obscuring the field, or fat in the portal area contributing to biliary injuries (Adams et al, 1993; Davidoff et al, 1992; Moosa et al, 1992; Soper et al, 1993). The classic biliary injury occurs, however, when the CBD or a right hepatic duct is mistaken for the cystic duct and is divided between clips. Many surgeons attribute this misidentification to the direction of traction of the gallbladder (i.e., pulling the CBD and the cystic duct into alignment, making them appear to be one). Other contributing factors to misidentification are a short cystic duct; a large stone in the Hartmann pouch, making retraction and display of the cystic duct difficult; or tethering of the infundibulum to the CBD by acute or chronic inflammation (Figs. 34.19 and 34.20). Constant awareness of these potential misidentifications and technical causes of biliary injuries is the best method of prevention. If a bile duct injury occurs, an immediate repair should be performed with no hesitation in asking for the help of a surgeon experienced in biliary repair.
A bile injury may also be unrecognized at the time of surgery but evident in the postoperative period. A postoperative bile leak occurs in less than 1% of cholecystectomies. Symptoms usually occur 4 to 10 days after surgery and consist of abdominal pain, fever, and abdominal distension. In this setting, it is mandatory to evaluate for biliary injury. Many postoperative bile leaks are considered minor biliary injuries, such as a cystic duct stump leak or an unclipped duct of Luschka. When a bile duct injury is discovered in the postoperative period, a coordinated effort by radiologists, endoscopists, and surgeons is necessary to optimize management (Strasberg et al, 1995). The initial diagnostic study should be US or CT of the abdomen. Fluid will typically be seen in the gallbladder fossa, hepatorenal recess, right gutter, and right subdiaphragmatic spaces. The biloma can be drained percutaneously, and the biliary anatomy should be evaluated with ERCP, which is preferred because it is both diagnostic and potentially theurapeutic. For example, a cystic duct stump leak can be treated successfully in the majority of cases with a temporary transsphincteric biliary stent.
Postcholecystectomy Syndrome
The term postcholecystectomy syndrome (PCS; see Chapter 38) describes the presence of symptoms after cholecystectomy. These symptoms can represent either the continuation of symptoms thought to be caused by the gallbladder or the development of new symptoms normally attributed to the gallbladder. PCS also includes the development of symptoms caused by removal of the gallbladder and is caused by alterations in bile flow resulting from loss of the reservoir function of the gallbladder. Two types of problems may arise: The first is continuously increased bile flow into the upper gastrointestinal (GI) tract, which may contribute to esophagitis and gastritis. The second is related to the lower GI tract, where diarrhea and colicky lower abdominal pain may result. PCS affects about 10% to 15% of patients; however, it is usually a temporary diagnosis, and an organic or functional diagnosis is established in most patients after a complete workup (Jaunoo et al, 2010). Postoperative symptoms may occasionally be caused by retained CBD stones or stones in the cystic duct remnant. Initial workup includes LFTs and US. MRCP or ERCP may be indicated if a retained CBD stone is suspected. A thorough preoperative evaluation is essential to minimize PCS, and patients should be warned of the possibility of postoperative symptoms. Although attempts to preoperatively identify patients at increased risk for PCS have largely been unsuccessful, the consensus opinion holds that the more secure the preoperative diagnosis, the lower the risk of PCS.
Results of Laparoscopic Cholecystectomy Series
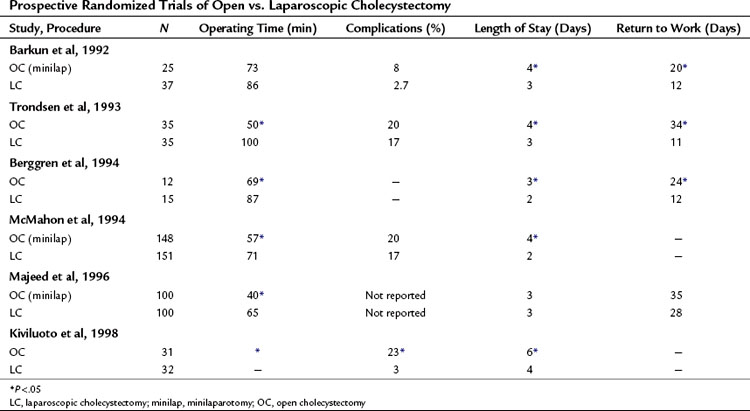
Several prospective, randomized trials have compared open cholecystectomy with laparoscopic cholecystectomy, and results have been mixed in showing advantages of laparoscopic cholecystectomy over an open approach in treating elective symptomatic cholelithiasis (Table 34.6). The largest study prospectively randomized 310 patients to laparoscopic cholecystectomy versus minilaparotomy open cholecystectomy with 155 patients in each group. Conversion to large-incision cholecystectomy was significantly more common with laparoscopic cholecystectomy (13% vs. 4%), and complications were significantly more frequent with laparoscopic cholecystectomy (9% vs. 3%). When laparoscopic cholecystectomy was successful, hospital stay was significantly shorter than for open cholecystectomy (2 days vs. 3 days), but overall the hospital stay was not significantly different. Postoperative analgesia requirements were reduced, and return to normal activities and to work was faster after laparoscopic cholecystectomy, but no significant cost difference was reported between the two procedures (McGinn et al, 1995).
Similarly, another study prospectively randomized 200 patients in a single-blinded fashion (identical wound dressings were applied in both groups so that caregivers would be blinded to the type of operation) to laparoscopic cholecystectomy versus minilaparotomy open cholecystectomy. Laparoscopic cholecystectomy took significantly longer than small-incision cholecystectomy (median, 65 min vs. 40 min; P < .001). No significant difference was reported in length of stay (median, 3 days for laparoscopic cholecystectomy vs. 3 days for small-incision open cholecystectomy; P = .74), return to work (median, 5 weeks vs. 4 weeks; P = .39), and time to full activity (median, 3 weeks vs. 3 weeks; P = .15) (Majeed et al, 1996).
A follow-up to this study prospectively randomized 200 additional patients to laparoscopic cholecystectomy or minilaparotomy open cholecystectomy. Postoperative pain, analgesic and antiemetic consumption, perceived health, and metabolic and respiratory responses were compared. Pain scores in both groups were low, but laparoscopic cholecystectomy was associated with lower postoperative pain scores and analgesic requirements compared with small-incision open cholecystectomy, however, the antiemetic requirements were greater after laparoscopic cholecystectomy. The duration of hospital stay and the perceived health after operation were the same, and both procedures were associated with a similar reduction of respiratory function. Twenty-four hours after operation, the inflammatory (C-reactive protein) response to laparoscopic cholecystectomy (22 ± 20 mg/L) was significantly lower than after small-incision open cholecystectomy (68 ± 30 mg/L), but the neuroendocrine (cortisol) response was similar (laparoscopic cholecystectomy, 475 ± 335 nmol/L compared with small-incision open cholecystectomy, 710 ± 410 nmol/L; Squirrell et al, 1998).
Numerous clinical series of laparoscopic cholecystectomy, accrued prospectively or retrospectively, also have been reported (Table 34.7). Among our personal series of more than 1,500 laparoscopic cholecystectomies performed in a teaching institution, the conversion rate was 2.1% with one perioperative death (<0.1%) owing to myocardial infarction at 1 week postoperatively. Significant postoperative complications occurred in 2.7%, including three minor duct injuries (0.2%). The mean operative time decreased from more than 100 minutes in the first 100 patients to 85 minutes in the most recent 100 patients. The postoperative course in most patients was uneventful, with more than 90% of patients discharged from the hospital within 24 hours of surgery; only 10% required parenteral narcotics after leaving the recovery room. Similarly, the duration of disability was minimal, with the average postoperative interval to return to full activity being 10 days. These results compared favorably with the results of traditional open cholecystectomy, in which the morbidity and mortality rates were similar, but hospitalization for 3 to 5 days and return to work at 1 month after surgery were standard (Wu et al, 1998b).
Our data mirror those from most series of laparoscopic cholecystectomy reported to date. Morbidity rates range from 1.5% to 8.6%, and bile duct injuries range from 0.2% to 0.7%. Death is rare after this procedure and usually is attributed to unrelated events. The conversion rates from laparoscopic to open operation in most series range from 1.8% to 7.8% and generally are greater early in the surgeon’s experience with the procedure. A nationwide study reported a conversion rate to open procedure of 5% to 10% (Livingston & Rege, 2004). In addition, studies have shown that advanced age, longer duration of procedure, and acute cholecystitis significantly increase postoperative morbidity and the length of stay (Lyass et al, 2000; Perraux et al, 2000).
As would be predicted for this excisional procedure, laparoscopic cholecystectomy effectively treats gallstone disease. Postcholecystectomy problems (see Chapter 38) occur in some patients, however. Kane and colleagues (1995) noted 89% of patients undergoing laparoscopic cholecystectomy had preoperative biliary-type pain, and only 10.6% of the patients had similar pain postoperatively. Similarly, Ure and colleagues (1995) found that the percentage of patients with biliary colic was reduced from 83% before laparoscopic cholecystectomy to 6.4% after laparoscopic cholecystectomy. Fenster and associates (1995) examined symptom relief in 225 patients who underwent laparoscopic cholecystectomy, followed postoperatively for 3 weeks to 3 months. They differentiated symptomatic gallstones from acalculous cholecystitis and discovered that 82% of patients with biliary colic and gallstones had complete relief of upper abdominal pain after the operation compared with only 52% of patients with acalculous cholecystitis (Fenster et al, 1995). These data are comparable to the data for open cholecystectomy, which showed that biliary-type pain persisted at follow-up (<1 year) in 9% to 34% of patients with symptomatic gallstones (Bates et al, 1991; Gilliland & Traverso, 1990; Scriven et al, 1993). In terms of cost-effectiveness, several studies have shown that laparoscopic cholecystectomy is less expensive than an open procedure (Bass et al, 1993; Fullarton & Bell, 1994), whereas others have shown the opposite (McMahon et al, 1994).
Emerging Techniques
Natural Orifice Translumenal Endoscopic Surgery Cholecystectomy
Over the past few years, many technical innovations have attempted to further minimize the field of laparoscopy. In 2004, peritoneoscopy was performed in an animal model by passing a flexible endoscope through the wall of the stomach and into the peritoneal cavity (Kalloo et al, 2004). This resulted in the concept of natural orifice translumenal endoscopic surgery (NOTES), with its general goal of minimizing trauma by eliminating incisions through the abdominal wall and utilizing natural orifices. Although a number of potential natural orifices can theoretically be used to access the abdominal cavity, clinical cases have thus far only used transgastric (TG) and transvaginal (TV) approaches. Human applications currently have been limited and have relied on laparoscopic-assisted or so-called hybrid techniques.
Single-Incision Laparoscopic Surgery Cholecystectomy
Single-incision laparoscopic surgery (SILS) cholecystectomy was first described in the Italian literature in the mid-1990s. Navarra and colleagues (1997) published the first case series of 30 patients who underwent what they described as “one-wound laparoscopic surgery.” Their method used three extracorporeal stay sutures and two 11-mm working ports in the umbilicus, and the incisions were connected at the end of the case to facilitate the removal of the gallbladder. The mean operative time was 123 minutes, and the mean postoperative stay was 1.8 days. These initial reports did not receive much attention, but recently a resurgence of interest in SILS has been seen with a concurrent rapid development of instrumentation.
Acosta JM, Ledesma CL. Gallstone migration as a cause of acute pancreatitis. N Engl J Med. 1974;290:484-487.
Adams DB, et al. Bile duct complications after laparoscopic cholecystectomy. Surg Endosc. 1993;7:79-83.
Airan M, et al. Retrospective and prospective multi-institutional laparoscopic cholecystectomy study organized by the Society of American Gastrointestinal Endoscopic Surgeons. Surg Endosc. 1992;6:169-176.
Asbun HJ, et al. Bile duct injury during laparoscopic cholecystectomy: mechanism of injury, prevention, and management. World J Surg. 1993;17:547-552.
Bagnato VJ. Laparoscopic choledochoscopy and choledocholithotomy. Surg Laparosc Endosc. 1993;3:164-166.
Barkun JS, et al. Randomized controlled trial of laparoscopic versus mini-cholecystectomy. Lancet. 1992;340:1116-1119.
Barkun JS, et al. Cholecystectomy without operative cholangiography: implications for common bile duct injury and retained common bile duct stones. Ann Surg. 1993;218:371-379.
Bass EB, et al. Cost-effectiveness of laparoscopic cholecystectomy versus open cholecystectomy. Am J Surg. 1993;165:466-471.
Basu S, et al. Feasibility of same day discharge after mini laparotomy cholecystectomy: a simulation study in a rural teaching hospital. Can J Rural Med. 2006;11:93-98.
Bates T, et al. Influence of cholecystectomy on symptoms. Br J Surg. 1991;78:964-967.
Beal JM. Historical perspective of gallstone disease. Surg Gynecol Obstet. 1984;158:181-189.
Beldi G, Glattli A. Laparoscopic subtotal cholecystectomy for severe cholecystitis. Surg Endosc. 2003;17:1427-1429.
Berci G. Biliary ductal anatomy and anomalies: the role of intraoperative cholangiography during laparoscopic cholecystectomy. Surg Clin North Am. 1992;72:1069-1077.
Berci G, Sackier JM. Laparoscopic cholecystectomy and laparoscopic choledocholithotomy. In: Blumgart, LH, editor. Surgery of the Liver and Biliary Tract. Edinburgh: Churchill Livingstone; 1994:633-662.
Berggren U, et al. Laparoscopic versus open cholecystectomy: hospitalization, sick leave, analgesia and trauma responses. Br J Surg. 1994;81:1362-1365.
Boline G, et al. Cholecystectomy in the potential heart transplant patient. J Heart Lung Transplant. 1991;10:269-274.
Brune IB, et al. Complications after laparoscopic and conventional cholecystectomy: a comparative study. HPB Surg. 1994;8:19-25.
Burhenne J. Percutaneous extraction of retained biliary stones: 661 patients. Am J Roentgenol. 1980;134:888-898.
Carlin CB, et al. Spilled gallstones: complications of abdominal wall abscesses. Surg Endosc. 1995;9:341-343.
Cates J, et al. Biliary complications of laparoscopic cholecystectomy. Am Surg. 1993;59:243-247.
Chang WT, et al. The impact of prophylactic antibiotics on postoperative infection complication in elective laparoscopic cholecystectomy: a prospective randomized study. Am J Surg. 2006;191:721-725.
Choudhary A, et al. Role of prophylactic antibiotics in laparoscopic cholecystectomy: a meta-analysis. J Gastrointest Surg. 2008;12:1847-1853.
Chui PT, et al. Anaesthesia for laparoscopic general surgery. Anaesth Intensive Care. 1993;21:163-171.
Collet D, et al. Conversions and complications of laparoscopic cholecystectomy: results of a survey conducted by the French Society of Endoscopic Surgery and Interventional Radiology. Surg Endosc. 1993;7:334-338.
Christopher F. Cholecystitis, Cholelithiasis, Choledocholithiasis, and Associated Diseases of the Liver: A Textbook of Surgery. Philadelphia: WB Saunders Company; 1936. 1319
Cooperman A. Laparoscopic cholecystectomy for severe acute, embedded, and gangrenous cholecystitis. J Laparoendosc Surg. 1990;1:37-40.
Cotton P. Endoscopic retrograde cholangiopancreatography and laparoscopic cholecystectomy. Am J Surg. 1993;165:474-478.
Croce E, et al. Laparocholecystectomy: 6,865 cases from Italian institutions. Surg Endosc. 1994;8:1088-1089.
Crump C. The incidence of gallstones in gallbladder disease. Surg Gynecol Obstet. 1931;53:447-455.
Csikesz NG, et al. Surgeon volume metrics in laparoscopic cholecystectomy. Dig Dis Sci. 2009;55:2398-2405.
Cuschieri A, et al. The European experience with laparoscopic cholecystectomy. Am J Surg. 1991;161:383-388.
Cutter IS. John Stough Bobbs and lithotomy of the gallbladder. Int Abst Surg. 1928;47:409-411.
Davidoff A, et al. Mechanisms of major biliary injury during laparoscopic cholecystectomy. Ann Surg. 1992;215:196-202.
DePaula A, et al. Laparoscopic management of choledocholithiasis. Surg Endosc. 1994;8:1399-1403.
Dervisoglou A, et al. The value of chemoprophylaxis against Enterococcus species in elective cholecystectomy: a randomized study of cefuroxime vs ampicillin-sulbactam. Arch Surg. 2006;141:1162-1167.
Deslandres E, et al. Intraoperative endoscopic sphincterotomy for common bile duct stones during laparoscopic cholecystectomy. Gastrointest Endosc. 1993;39:54-58.
Deveney KE. The early experience with laparoscopic cholecystectomy in Oregon. Arch Surg. 1993;128:627-632.
Deziel D, et al. Complications of laparoscopic cholecystectomy: a national survey of 4,292 hospitals and an analysis of 77,604 cases. Am J Surg. 1993;165:9-14.
Dion Y, et al. Common bile duct exploration: the place of laparoscopic choledochotomy. Surg Laparosc Endosc. 1994;4:419-424.
DuBois F, et al. Coelioscopic cholecystectomy: preliminary report of 36 cases. Ann Surg. 1990;211:60-62.
Escarce J, et al. Falling cholecystectomy thresholds since the introduction of laparoscopic cholecystectomy. JAMA. 1995;273:1581-1585.
Feldman LS, et al. Does a special interest in laparoscopy affect the treatment of acute cholecystitis? Surg Endosc. 2002;16:1697-1703.
Fendrick A, et al. Asymptomatic gallstones revisited: is there a role for laparoscopic cholecystectomy? Arch Fam Med. 1993;2:959-968.
Fenster L, et al. What symptoms does cholecystectomy cure? Insights from an outcome measurement project and review of literature. Am J Surg. 1995;169:533-538.
Ferzli G, et al. The utility of laparoscopic common bile duct exploration in the treatment of choledocholithasis. Surg Endosc. 1994;8:296-298.
Fitzgerald S, et al. Hypercarbia during carbon dioxide pneumoperitoneum. Am J Surg. 1992;163:186-190.
Flumm DR, Massarweh NN. Role of intraoperative cholangiography in avoiding bile duct injury. J Am Coll Surg. 2007;204:656-664.
Franklin M, et al. Laparoscopic common bile duct exploration. Surg Laparosc Endosc. 1994;4:119-124.
Frazee R, et al. Combined laparoscopic and endoscopic management of cholelithiasis and choledocholithiasis. Am J Surg. 1993;166:702-705.
Fullarton G, et al. Evaluation of the cost of laparoscopic and open cholecystectomy. Br J Surg. 1994;81:124-126.
Fullarton GM, Bell G. West of Scotland Laparoscopic Cholecystectomy Audit Group prospective audit of the introduction of laparoscopic cholecystectomy in the west of Scotland. Gut. 1994;35:1121-1126.
Gilliland T, Traverso L. Modern standards for comparison of cholecystectomy with alternative treatments for symptomatic gallstones with emphasis on long-term relief of symptoms. Surg Gynecol Obstetrics. 1990;170:39-44.
Girardet R, et al. Significance of asymptomatic biliary tract disease in heart transplantation recipients. J Heart Transplant. 1989;8:391-399.
Gollen JL, et al. NIH consensus development panel on gallstones and laparoscopic cholecystectomy. JAMA. 1993;269:1018-1029.
Gurusamy KS, et al, 2010: Mini port vs. standard ports for laparoscopic cholecystectomy. Cochrane Database Syst Rev 2010 March 17(3)CD006804.
Hall RC. Short hospital stay: two hospital days for cholecystectomy. Am J Surg. 1987;154:510-515.
Hanney RM, et al. Major vascular injury and laparoscopy. Aust N Z J Surg. 1995;65:533-535.
Hermann R. Surgery for acute and chronic cholecystitis. Surg Clin North Am. 1990;70:1263-1275.
Hicken N, McAllister A. Operative cholangiography as an aid in reducing the incidence of “overlooked” common bile duct stones: a study of 1,293 choledocholithotomies. Surgery. 1964;55:753-758.
Horton M, Florence MG. Unusual abscess patterns following dropped gallstones during laparoscopic cholecystectomy. Am J Surg. 1998;175:375-379.
Hull D, et al. Management of cholelithiasis in heart and lung transplant patients: with review of laparoscopic cholecystectomy. Conn Med. 1994;58:643-647.
Hunter J. Laparoscopic transcystic common bile duct exploration. Am J Surg. 1992;163:53-58.
Hunter J, Soper N. Laparoscopic management of common bile duct stones. Surg Clin North Am. 1992;72:1077-1098.
Jakimowicz J. Review: intraoperative ultrasonography during minimal access surgery. J R Coll Surg Edinb. 1993;38:231-238.
Jatzko GTR, et al. Multivariate comparison of complications after laparoscopic cholecystectomy and open cholecystectomy. Ann Surg. 1995;221:381-386.
Jaunoo SS, et al. Postcholecystectomy syndrome (PCS). Int J Surg. 2010;8:15-17.
John T, et al. Preliminary experience with intracorporeal laparoscopic ultrasonography using a sector scanning probe: a prospective comparison with intraoperative cholangiography in the detection of choledocholithiasis. Surg Endosc. 1994;8:1176-1180.
Jones D, Soper N. Advances in Surgery. Chicago: Mosby Year Book; 1996. 271-289
Jones D, et al. The influence of intraoperative gallbladder perforation on long-term outcome after laparoscopic cholecystectomy. Surg Endosc. 1995;9:977-980.
Kalloo AN, et al. Flexible transgastric peritoneoscopy: a novel approach to diagnostic and therapeutic interventions in the peritoneal cavity. Gastrointest Endosc. 2004;60(1):114-117.
Kane R, et al. The outcomes of elective laparoscopic and open cholecystectomy. J Am Coll Surg. 1995;180:136-145.
Kelly T. Gallstone pancreatitis: pathophysiology. Surgery. 1980;80:488-492.
Kimura T, et al. Laparoscopic cholecystectomy: the Japanese experience. Surg Laparosc Endosc. 1993;3:194-198.
Kiviluoto T, et al. Randomised trial of laparoscopic versus open cholecystectomy for acute and gangrenous cholecystitis. Lancet. 1998;351:321-325.
Kuy S, et al. Outcomes following cholecystectomy in pregnant and nonpregnant women. Surgery. 146, 2009. 358–198
Lai PBS, et al. Randomized trial of early versus delayed laparoscopic cholecystectomy for acute cholecystitis. Br J Surg. 1998;85:764-767.
Langenbuch C. Ein fall von Exstirpation der Gallenblase wegen chronischer Cholelithiasis. Berl Klin Wochenschr. 1882;48:725-727.
Lantz PE, Smith JD. Fatal carbon dioxide embolism complicating attempted laparoscopic cholecystectomy: case report and literature review. J Forensic Sci. 1994;39:1468-1480.
Larson GM, et al. Multipractice analysis of laparoscopic cholecystectomy in 1,983 patients. Am J Surg. 1992;163:221-226.
Legorreta A, et al. Increased cholecystectomy rate after introduction of laparoscopic cholecystectomy. JAMA. 1993;270:1429-1432.
Litwin DEM, et al. Laparoscopic cholecystectomy: trans-Canada experience with 2201 cases. Can J Surg. 1992;35:291-296.
Litynski GS. Highlights in the History of Laparoscopy. Barbara Bernard Verlag: Frankfort; 1996. 165-176
Liu SY, et al. Prospective analysis of cardiopulmonary responses to laparoscopic cholecystectomy. J Laparoendosc Surg. 1991;1:241-246.
Livingston EH, Rege RV. A nationwide study of conversion from laparoscopic to open cholecystectomy. Am J Surg. 2004;188:205-211.
Lo C, et al. Early versus delayed laparoscopic cholecystectomy for treatment of acute cholecystitis. Ann Surg. 1996;223:37-42.
Lo CM, et al. Prospective randomized study of early vs. delayed laparoscopic cholecystectomy for acute cholecystitis. Ann Surg. 1998;227:461-466.
Lyass S, et al. Laparoscopic cholecystectomy: what does affect the outcome? A retrospective multifactorial regression analysis. Surg Endosc. 2000;14:661-665.
Machi J, et al. Operative ultrasonography during hepatobiliary and pancreatic surgery. World J Surg. 1993;17:640-646.
Machi J, et al. Technique of ultrasound examination during laparoscopic cholecystectomy. Surg Endosc. 1993;7:545-549.
Madan AK, et al. How early is early for laparoscopic treatment of cholecystitis? Am J Surg. 2002;183:232-236.
Mahmud S, et al. Fundus-first laparoscopic cholecystectomy. Surg Endosc. 2002;16:581-584.
Majeed AW, et al. Randomised, prospective, single-blind comparison of laparoscopic versus small-incision cholecystectomy. Lancet. 1996;347:989-994.
Manegold BC. Diagnostische und therapeutische Möglichkeiten der Laparoskopie. In: Richter, H, editor. Chirurgische Endoskopie, Komplikationen bei Diagnostik und Therapie. Munich: Urban & Schwarzenberg; 1985:119-127.
McDougall EM, et al. The effect of prolonged pneumoperitoneum on renal function in an animal model. J Am Coll Surg. 1996;182:317-328.
McDougall EM, et al. Functional MR imaging of the porcine kidney: physiologic changes of prolonged pneumoperitoneum. J Soc Laparoendosc Surg. 1997;1:29-35.
McGinn FP, et al. Randomized trial of laparoscopic cholecystectomy and mini-cholecystectomy. Br J Surg. 1995;82:1374-1377.
McIntyre R, et al. Update on laparoscopic ultrasonography. Endosc Surg Allied Technol. 1994;2:149-152.
McMahon A, et al. Laparoscopic versus minilaparoscopic cholecystectomy: a randomized trial. Lancet. 1994;343:135-138.
Meyers WC, Southern Surgeons Club. A prospective analysis of 1518 laparoscopic cholecystectomies. N Engl J Med. 1991;324:1073-1078.
Meyers WC, et al. Low insertion of hepatic segmental duct VII–VIII is an important cause of major biliary injury or misdiagnosis. Am J Surg. 1996;171:187-191.
Miles RH, et al. Laparoscopy: the preferred method of cholecystectomy in the morbidly obese. Surgery. 1992;112:818-822.
Moore M, Bennett C. The learning curve for laparoscopic cholecystotomy. The Southern Surgeons Club. Am J Surg. 1995;170:55-59.
Moosa A, et al. Laparoscopic injuries to the bile duct. Ann Surg. 1992;215:203-208.
Navarra G, et al. One wound laparoscopic cholecystectomy. Br J Surg. 1997;234:695.
Nenner R, et al. Increased cholecystectomy rates among Medicare patients after the introduction of laparoscopic cholecystectomy. J Community Health. 1994;19:409-415.
Newman CL, et al. 1525 laparoscopic cholecystectomy without biliary injury: a single institution’s experience. Am Surg. 1995;61:226-228.
NIH Consensus Development Conference. Gallstones and laparoscopic cholecystectomy. JAMA. 1992;269:1018-1024.
Orda R, et al. Intraoperative ultrasonography as a routine screening procedure in biliary surgery. Hepatogastroenterology. 1994;41:61-64.
Orda R, et al. Routine laparoscopic ultrasonography in biliary surgery. Surg Endosc. 1994;8:1239-1242.
Orlando RIII, et al. The Connecticut Laparoscopic Cholecystectomy Registry: laparoscopic cholecystectomy—a statewide experience. Arch Surg. 1993;128:494-499.
Parra-Davila E, et al. Retroperitoneal abscess as a complication of retained gallstones following laparoscopic cholecystectomy. J Laparoendosc Adv Surg Tech A. 1998;8:89-93.
Perissat J, et al. Laparoscopic cholecystectomy: an analysis of 777 cases. Baillières Clin Gastroenterol. 1992;6:727-742.
Perraux P, et al. Laparoscopic cholecystectomy in the elderly: a prospective study. Surg Endosc. 2000;14:1067-1069.
Petelin JB. Laparoscopic approach to common duct pathology. Am J Surg. 1993;165:487-491.
Phillips E, et al. Laparoscopic trans–cystic-duct common bile duct exploration. Surg Endosc. 1994;8:1389-1394.
Poggio JL, et al. A comparison of laparoscopic and open cholecystectomy in patients with compensated cirrhosis and symptomatic gallstone disease. Surgery. 2000;127:405.
Prakash K, et al. Laparoscopic cholecystectomy in acute cholecystitis. Surg Endosc. 2002;16:180-183.
Ransohoff D, Gracie W. Treatment of gallstones. Ann Intern Med. 1993;119:606-619.
Ransohoff D, et al. Prophylactic cholecystectomy or expectant management for silent gallstones: a decision analysis to assess survival. Ann Intern Med. 1983;99:199-204.
Rattner D, et al. Factors associated with successful laparoscopic cholecystectomy for acute cholecystitis. Ann Surg. 1993;217:233-236.
Reddick EJ. Laparoscopic laser cholecystectomy. Clin Laser Monthly. 1988;6:400-401.
Reddick E, et al. Safe performance of difficult laparoscopic cholecystectomies. Am J Surg. 1991;161:377-381.
Reid S, et al. Anomalous hepatic duct inserting into the cystic duct. Am J Roentgenol. 1986;147:1181-1182.
Roda E, et al. Ursodeoxycholic acid vs. chenodeoxycholic acid as cholesterol gallstone dissolving agents: a comparative study. Hepatology. 1982;2:804-811.
Sackmann M, et al. Early gallstone recurrence rate after successful shock wave therapy. Gastroenterology. 1990;98:392-398.
Saunders C, et al. Is outpatient laparoscopic cholecystectomy wise? Surg Endosc. 1995;9:1263-1268.
Schirmer BD, et al. Laparoscopic cholecystectomy in the obese patient. Ann Surg. 1992;216:146-152.
Schlumpf R, et al. A nation’s experience in laparoscopic cholecystectomy. Surg Endosc. 1994;8:35-41.
Schoenfield I, Lachin J. Chenodiol (chenodeoxycholic acid) for dissolution of gallstones: the National Cooperative Gallstone Study. The Steering Committee TNCGSG. Ann Intern Med. 1981;95:257-282.
Schoenfield I, et al. The effect of ursodiol on the efficacy and safety of extracorporeal shockwave lithotripsy of gallstones. N Engl J Med. 1990;323:1239-1245.
Scott TR, et al. Laparoscopic cholecystectomy: a review of 12,397 patients. Surg Laparosc Endosc. 1992;2:191-198.
Scriven M, et al. Cholecystectomy: a study of patient satisfaction. J R Coll Surg Edinb. 1993;38:79-81.
Semm K. Advances in pelviscopic surgery. In: Current Problems in Obstetrics and Gynecology V. Chicago: Year Book Medical Publishers; 1982.
Sharma KC, et al. Laparoscopic surgery and its potential for medical complications. Heart Lung. 1997;26:52-64.
Shocket E. Abdominal abscess from gallstones spilled at laparoscopic cholecystectomy. Surg Endosc. 1995;9:344-347.
Soper N. Laparoscopic cholecystectomy. Curr Prob Surg. 1991;28:585-655.
Soper N, et al. Laparoscopic cholecystectomy during pregnancy. Surg Endosc. 1992;6:115-117.
Soper N, et al. Laparoscopic versus standard open cholecystectomy: comparison of early results. Surg Gynecol Obstet. 1992;174:114-118.
Soper NJ, et al. Laparoscopic cholecystectomy: the new “gold standard”? Arch Surg. 1992;127S:917-921.
Soper N, et al. Diagnosis and management of biliary complications of laparoscopic cholecystectomy. Am J Surg. 1993;165:663-669.
Soper NJ. Effect of nonbiliary problems on laparoscopic cholecystectomy. Am J Surg. 1993;165:522-526.
Soper NJ. The utility of ultrasonography for screening the common bile duct during laparoscopic cholecystectomy. J Laparoendosc Adv Surg Tech A. 1997;7:271-276.
Squirrell DM, et al. A randomized, prospective, blinded comparison of postoperative pain, metabolic response, and perceived health after laparoscopic and small incision cholecystectomy. Surgery. 1998;123:485-495.
Steck T, et al. Prevalence and management of cholelithiasis in heart transplant patients. J Heart Lung Transplant. 1991;10:1024-1032.
Steigmann G, et al. Laparoscopic intracorporeal ultrasound: an alternative to cholangiography? Surg Endosc. 1994;8:167-171.
Steigmann G, et al. Laparoscopic ultrasonography as compared with static or dynamic cholangiography at laparoscopic cholecystectomy. Surg Endosc. 1995;9:1269-1273.
Steiner C, et al. Surgical rates and operative mortality for open and laparoscopic cholecystectomy in Maryland. N Engl J Med. 1994;330:403-408.
Strasberg S, et al. An analysis of the problem of biliary injury during laparosopic cholecystectomy. J Am Coll Surg. 1995;180:101-125.
Suter M, Meyer A. A 10-year experience with the use of laparoscopic cholecystectomy for acute cholecystitis: is it safe? Surg Endosc. 2001;15:1187-1192.
Tagge E, et al. Impact of laparoscopic cholecystectomy on the management of cholelithiasis in children with sickle cell disease. J Pediatr Surg. 1994;29:209-212.
Talamini MA, Gadacz TR. Laparoscopic approach to cholecystectomy. Adv Surg. 1992;25:1-20.
Thistle JL, et al. Dissolution of cholesterol gallbladder stones by methyl tert-butyl ether administered by percutaneous transhepatic catheter. n Engl J Med. 1989;320:633-639.
Trondsen E, et al. Laparoscopic and open cholecystectomy: a prospective, randomized study. Eur J Surg. 1993;159:217-221.
Underwood R, et al. Prospective, randomized trial of bipolar electrocautery versus ultrasonic coagulation for division of short gastric vessels during laparoscopic Nissen fundoplication. Surg Endosc. 1998;12:509.
Unger S, et al. Laparoscopic treatment of acute cholecystitis. Surg Laparosc Endosc. 1991;1:14-16.
Ure B, et al. Long-term results after laparoscopic cholecystectomy. Br J Surg. 1995;82:267-270.
Wittgen CM, et al. Analysis of the hemodynamic and ventilatory effects of laparoscopic cholecystectomy. Arch Surg. 1991;126:997-1001.
Wolfe BM, et al. Endoscopic cholecystectomy: an analysis of complications. Arch Surg. 1991;126:1192-1196.
Wu J, et al. The utility of intracorporeal ultrasonography for screening of the bile duct during laparoscopic cholecystectomy. J Gastrointest Surg. 1998;2:50-59.
Wu JS, et al. The evolution and maturation of laparoscopic cholecystectomy in an academic practice. J Am Coll Surg. 1998;186:554-561.
Zamir G, et al. The fate of the dropped gallstones during laparoscopic cholecystectomy. Surg Endosc. 1999;13:68-70.