Chapter 17 Lactation and Galactorrhea
PROLACTIN
Prolactin is a 198-amino acid polypeptide hormone secreted in pulsatile fashion from the anterior pituitary (adenohypophysis) by specific cells referred to as lactotrophs.1 These cells have a common origin with the growth hormone-secreting cells (i.e., somatotrophs). Both prolactin and growth hormone are classified as somatomammotropic hormones based on their structural similarities. Due to its structural similarity to growth hormone, prolactin was the last pituitary hormone to be isolated.
Prolactin has a short half-life of only 20 minutes and exists in heterogeneous forms, including big prolactin, a dimeric glycosylated form, and big big prolactin.2,3 Secretion has a circadian rhythm, with a higher average level of serum prolactin during sleep, especially during the rapid eye movement phase.
The prolactin receptor is a member of the cytokine receptor family. It is a single transmembrane polypeptide found in multiple tissues in addition to the mammary glands, including the liver, adrenal glands, lungs, testes, and ovaries.4 The effects of prolactin in many of these tissues remains unknown.5
Neuroendocrinology of Prolactin Secretion
The control of prolactin secretion by the anterior pituitary lies within the hypothalamus and is predominantly inhibitory. However, the hypothalamus releases both prolactin-inhibiting factors (PIF) and prolactin-releasing factors (PRF) that modulate the secretion of prolactin.6
Prolactin-inhibiting factors are released by the hypothalamus into the hypothalamo-hypophyseal portal system. Although other compounds have been shown to have inhibitory activity, dopamine appears to be the most important PIF.7 Interruption of the tuberoinfundibular tract and blocking dopamine receptors with “gene knockout” techniques both result in high prolactin levels.8 Gonadotropin-associated peptide and γ-aminobutyric acid are also PIFs.9
Prolactin secretion is regulated by a negative feedback loop wherein prolactin, acting via prolactin receptors in the median eminence, stimulates dopamine secretion.10 Dopamine acts via the adenylyl cyclase pathway to reduce prolactin secretion from the pituitary lactotrophs. The predominant dopamine receptor in the adenohypophysis is D2 (DRD2). Binding of dopamine to this receptor decreases cellular adenylyl cyclase activity and cyclic adenosine monophosphate.
Although the major control mechanism for prolactin is inhibitory, the hypothalamus also releases PRFs, which stimulate lactotrophs to secrete prolactin (Table 17-1). The primary physiologic PRF appears to be vasoactive intestinal peptide. However, there appear to be two more clinically relevant PRFs.11,12 The ability of thyrotropin-releasing hormone (TRH) to act as a PRF is believed to be the basis of the association between hypothyroidism and hyperprolactinemia, a condition that resolves with thyroid hormone replacement. Serotonin appears to be another PRF; use of any of the various selective serotonin reuptake inhibitors (SSRIs) is commonly associated with hyperprolactinemia.
| Thyrotropin-releasing hormone |
| Serotonin |
| Vasoactive intestinal peptide |
| Opioids |
| Growth hormone-releasing hormone |
| Gonadotropin-releasing hormone |
LACTATION
Breast Development
Prenatal
Normal development in utero requires fetal exposure to many hormones, including estrogens, progesterone, prolactin, insulin, cortisol, thyroxine, and growth hormone (Table 17-2). The endocrine system is essential for the proper development and function of the mammary glands.13,14 Gene knockout studies have demonstrated the importance of each of these hormones in fetal mammary gland development.15. These hormones work through normal hormone–receptor interactions using local growth factors in many cases.16,17
| Estrogen |
| Progesterone |
| Prolactin |
| Cortisol |
| Insulin |
| Thyroxine |
| Growth hormone |
Puberty
At puberty, estradiol is the major influence on breast development, and the breast contains both α and β estrogen receptors. However, multiple hormones are necessary for normal development, including all the hormones necessary for normal in utero development (see Table 17-2).
Under the influence of rising estradiol levels at puberty, the breast increases in size and the areola gains pigmentation.18 Most breast development at puberty is adipocyte differentiation under the influence of estrogens. Estrogens stimulate the lactiferous ducts to branch extensively. The terminal alveoli unit, however, is rudimentary.
The Mature Breast
Anatomy
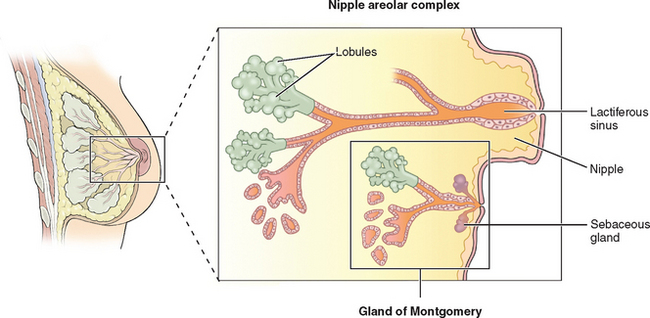
The breasts consist of glands, fat, and connective tissue. The basic glandular unit consists of ducts and secretory lobules (Fig. 17-1). Twenty or more lactiferous ducts converge on the nipple. Each large duct is connected to smaller branching ducts and eventually lobules. The ducts are lined by a cuboidal or columnar epithelium, at the base of which are myoepithelial cells. Each lobule contains alveoli, milk glands lined by epithelium.19,20 In a nonactive state, the ductules often have only rudimentary terminal alveoli that secrete milk.
Pregnancy
Breast development and lactogenesis during pregnancy occurs under the influence of multiple hormones, most notably prolactin, estrogen, and progesterone. Prolactin is secreted in large amounts by the decidua, and levels will be 5 to 10 times normal by the end of the first trimester.21 Under the influence of increased estrogen, the pituitary lactotrophs undergo hyperplasia. After delivery, prolactin declines to a normal baseline by 6 weeks postpartum and then pulses with breastfeeding.
Lactogenesis
Final differentiation of the breast into a lactating organ occurs during pregnancy. Lactogenesis is a three-stage process whereby mammary glands develop the ability to produce and secrete milk.22 Starting in early pregnancy and ending within a week of parturition, the mammary glands are hormonally transformed from an undifferentiated state to a fully differentiated state with an established supply of mature milk.
Stage I Lactogenesis
During this stage, mammary glands become competent to secrete milk. This stage begins early in gestation and is nearly complete by midpregnancy. As a result of rising levels of estrogen, progesterone, prolactin, and human placental lactogen, the terminal duct lobular units expand and secretory cells begin to differentiate. Prolactin induces the transcription of β-casein and lactalbumin genes.23
Stage II Lactogenesis
This stage begins at the time of birth and ends with the establishment of an appropriate milk supply. Delivery of the placenta results in a sudden drop in circulating progesterone levels. At the same time, both glucocorticoid and prolactin levels increase. This leads to increased synthesis of lactose. Lactose draws water by osmosis into the secretory vesicles. At the same time, synthesis of other milk components (e.g., nutrients and minerals) is increased under the influence of maternal insulin, growth hormone, cortisol, and parathyroid hormone. Secretion of the alveolar cells first leads to colostrum formation. Within the next 2 to 3 days, there is a relatively rapid onset of mature milk production.24
Breastfeeding
When human milk is unavailable, modern infant formulas are acceptable but incomplete substitutes. Most commonly made from cow’s milk or soybeans, formulas are similar to human milk in terms of water, fat, carbohydrates, and calories, but are devoid of antibodies and other bioactive factors. The exceptional benefits of human breast milk compared to formulas are so numerous that the American Academy of Pediatrics policy statement is that “pediatricians and other health care professionals should recommend human milk for all infants in whom breastfeeding is not specifically contraindicated.”26
Breastfeeding and Anovulation
As a result of these variables, the length of time a breastfeeding woman will have amenorrhea is highly variable, and women should not rely on lactation to prevent pregnancy. As many as 10% of breastfeeding women will ovulate within 10 weeks of giving birth.27 Two thirds of women who breastfeed for more than 9 months will resume ovulation and menstruation during that time. A small fraction of breastfeeding women will remain anovulatory and amenorrheic for more than 1 year.
GALACTORRHEA
Nonphysiologic galactorrhea is a common problem that has been estimated to occur in approximately one fourth of all previously parous women sometime during their reproductive life. The condition is most common in women between ages 20 and 35 and is less common in nulligravid women.28 The most common pathologic condition resulting in galactorrhea is hyperprolactinemia, which has several possible underlying causes (Tables 17-3 and 17-4).
Table 17-4 Drug Class Associated with Galactorrhea
The listed drugs are only examples and do not represent a complete list
Clinical Manifestations of Hyperprolactinemia
Hyperprolactinemia is known to be causally related to several pathologic conditions (see Table 17-3). As noted, a physiologic response to hyperprolactinemia is anovulation.29,30 High levels of prolactin inhibit the pulsatile release of GnRH, thereby reducing pituitary release of both luteinizing hormone (LH) and follicle-stimulating hormone (FSH). The LH surge is also inhibited.
There is evidence that high serum prolactin levels also have a direct effect on the ovary.28 Hyperprolactinemia inhibits androgen synthesis, thereby reducing substrate for estrogen formation, and may block aromatase activity. All of these effects lead to hypoestrogenism and explain the disrupted or absent menstrual cycles in women with high prolactin levels. Elevated serum prolactin levels have a luteolytic effect that can disrupt the cycle. Elevated prolactin levels should be considered in the differential diagnosis of luteal phase disorders.
ETIOLOGIES OF HYPERPROLACTINEMIA
Galactorrhea is most commonly a normal response of the breast to a normal or abnormal endocrine signal (i.e., hyperprolactinemia). Once elevated prolactin is detected, the goal of the clinician is to determine the underlying cause. After pregnancy is excluded, the clinician must assess the patient for drug causes, thyroid disease, anatomic breast or chest wall abnormalities, and pituitary disorders (see Table 17-3).
Physiologic Causes
Activities that Stimulate Prolactin Release
When evaluating a patient’s serum prolactin levels, it is important to be aware of activities that can stimulate a temporary increase in prolactin through normal physiologic mechanisms. These include sleeping, eating, sexual intercourse, breast or chest stimulation, and stress. For this reason, if a borderline elevation of serum prolactin is detected on initial evaluation, it is prudent to avoid these stimulants by repeating the prolactin determination in the morning after fasting remote from any breast stimulation to avoid expensive evaluation of a fallacious prolactin elevation.31
Central Nervous System Disorders
Pituitary Adenomas
Large tumors of the hypothalamus may also cause compression of the portal system that carries the prolactin inhibitors. Structural lesions such as empty sella syndrome or a cyst of Rathke’s pouch can also cause hyperprolactinemia and associated galactorrhea.32 The diagnosis and treatment of pituitary abnormalities are addressed in Chapter 22.
Hypothyroidism
Hypothyroidism, even if subclinical, may result in elevated prolactin levels. When levels of circulating thyroxine are low, the lack of negative feedback on the hypothalamus results in increased release of thyrotropin-releasing hormone (TRH) into the portal circulation. As thyrotrophs are stimulated to release thyrotropin, lactotrophs are stimulated to release prolactin. This cross-reactivity is thought to be related to the embryologic derivation of both of these cell types from the same precursor cells.33 Thyroid replacement will uniformly reduce both thyrotropin and prolactin release, and alleviate the associated galactorrhea.34
Chest Wall Lesions
Because chest stimulation can result in elevations of prolactin, it isn’t surprising that trauma or lesions of the chest wall may result in hyperprolactinemia and galactorrhea.35 Tumors, herpes zoster, spinal cord injury, and other lesions have been associated with hyperprolactinemia.36
Ectopic Prolactin Production
Rarely, malignant tumors have been reported to secrete prolactin, resulting in hyperprolactinemia. A list of some reported tumor types is given in Table 17-3.
Decreased Prolactin Metabolism
Because most prolactin is excreted by the kidneys, renal disease is associated with galactorrhea due to high prolactin levels resulting from decreased excretion. This effect disappears once normal renal function is restored via transplant.37 Elevated prolactin has long been associated with cirrhosis of the liver, although the mechanisms for this remain unclear.
Medications
Many psychotropic medications have been shown to cause galactorrhea via elevations of prolactin (see Table 17-4). The mechanism by which dopamine blocking agents (such as phenothiazines) increase prolactin is obvious, because they result in decreased exposure of lactotrophs to dopamine. It is less obvious how other centrally acting drugs increase prolactin levels, but this side effect is common with major neuroleptics such as haloperidol and antidepressants, including the tricyclics and the SSRIs.38–42 The SSRIs are probably the most frequent drug class associated with increased serum prolactin.
Other commonly used drugs associated with hyperprolactinemia include antihypertensive medications such as methyldopa and verapamil.43 Protease inhibitors used for the treatment of human immunodeficiency virus infections have also been associated with hyperprolactinemia.44 The list of other medications associated with hyperprolactinemia is extensive. For this reason, the clinician should consult the product information for any medications being taken by women found to have hyperprolactinemia to determine if they could be associated with the galactorrhea.
EVALUATION OF GALACTORRHEA
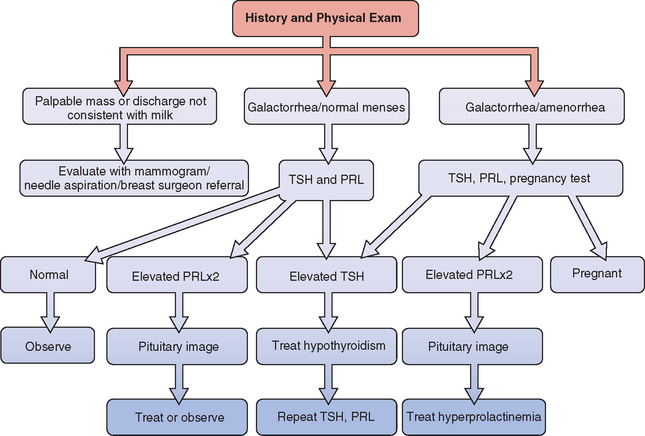
A general approach to the investigation and management of galactorrhea is outlined in Fig 17-2.
Diagnostic Tests
Imaging Studies
In the presence of reproducible hyperprolactinemia, evaluation of the pituitary and hypothalamus with magnetic resonance imagining is indicated to check for an intracranial lesion. Computed tomography can be used, but has the disadvantage of lower resolution. The most common brain tumor in women with hyperprolactinemia is a benign pituitary adenoma. These are classified as being either a microadenoma (<1 cm) or a macroadenoma (>1 cm). Their treatment and prognosis are discussed in Chapter 22.
TREATMENT FOR GALACTORRHEA RELATED TO HYPERPROLACTINEMIA
Indications for Treatment
Hypoestrogenemic Amenorrhea
Menstrual abnormalities in women with galactorrhea and hyperprolactinemia are often the result of hypoestrogenemia. The most significant long-term medical problem associated with this condition is decreased bone mineral density due to their hypoestrogenic state.45 Treatment to lower prolactin and thus increase estrogen will increase bone mass in these women.46 However, eumenorrheic women with galactorrhea demonstrate normal bone density and do not require treatment for this indication.47
Treatment Options
The treatment of hyperprolactinemia is covered fully in Chapter 22. In brief, elevated prolactin levels related to pituitary causes can usually be decreased to the normal range using a dopamine agonist. In most cases, this will result in resolution of galactorrhea, associated menstrual abnormalities, and infertility.
If infertility persists with normalized prolactin, ovulation induction medications may be used successfully in these patients.48 Conversely, if pregnancy is not desired, care must be taken to avoid pregnancy when hyperprolactinemia is corrected because a previously anovulatory patient has an 80% chance of becoming ovulatory after 6 months or less of treatment.49
If pregnancy is not desired, oral contraceptives can be used, especially if menstrual abnormalities persist. Oral contraceptives with higher levels of ethinyl estradiol (i.e., ≥50 μg) have been associated with galactorrhea and hyperprolactinemia.50 However, modern oral contraceptives (i.e., ≤35 μg) can be used in women with hyperprolactinemia with no measurable risk of enlargement of pituitary lesions.51
1 Veldhuis JD, Johnson L. Operating characteristics of the hypothalamo-pituitary-gonadal axis in men: Circadian, ultradian, and pulsatile release of prolactin and its temporal coupling with luteinizing hormone. J Clin Endocrinol Metab. 1988;67:116-123.
2 Garnier PE, Aubert ML, Kaplan ASL, et al. Heterogeneity of pituitary and plasma prolactin in man: Decreased affinity of “bigA” prolactin in a radioreceptor assay and evidence for its secretion. Endocrinology. 1978;47:1273-1279.
3 Whitaker MD, Klee GG, Kao PC, et al. Demonstration of biological activity of prolactin molecular weight variants in human sera. J Clin Endocrinol Metab. 1983;58:826-830.
4 Bole-Feysot C, Goffin V, Edery M, et al. Prolactin (PRL) and its receptor: Actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocrine Rev. 1998;19:225-268.
5 Ben-Jonathan N, Mershon JL, Allen DL, Steinmetz RW. Extrapituitary prolactin: distribution, regulation, functions, and clinical aspects. Endocrine Rev. 1996;17:639.
6 Lawrence RA, Lawrence RM. Breastfeeding: A Guide for the Medical Profession, 6th ed. St. Louis: Elsevier/CV Mosby, 2005.
7 Mogg RJ, Samson WK. Interactions of dopaminergic and peptidergic factors in the control of prolactin release. Endocrinology. 1990;126:728-735.
8 Kelly MA, Rubinstein M, Asa SL, et al. Pituitary lactotroph hyperplasia and chronic hyperprolactinemia in dopamine D2 receptor-deficient mice. Neuron. 1997;19:103-113.
9 Lamberts SWJ, Macleod RM. Studies on the mechanism of the GABA-mediated inhibition of prolactin secretion. Proc Soc Exp Biol Med. 1978;158:10-13.
10 Clemens JA, Meites J. Inhibition by hypothalamic prolactin implants of prolactin secretion, mammary growth and luteal function. Endocrinology. 1968;2:878-881.
11 Tashjian AHJr, Barowsky NJ, Jensen DK. Thyrotropin releasing hormone: Direct evidence for stimulation of prolactin production by pituitary cells in culture. Biochem Biophys Res Commun. 1971;43:516-623.
12 Clemens JA, Sawyer BD, Cerimele B. Further evidence that serotonin is a neurotransmitter involved in the control of prolactin secretion. Endocrinology. 1977;100:692-698.
13 Forsyth IA. Variation among species in the endocrine control of mammary growth and function: The roles of prolactin, growth hormone, and placental lactogen. J Dairy Sci. 1986;69:886-903.
14 Schams D, Kohlenberg S, Amselgruber W, et al. Expression and localization of oestrogen and progesterone receptors in the bovine mammary gland during development, function, and involution. J Endocrinol. 2003;177:305-317.
15 Grimm SL, Seagroves TN, Kabotyanski EB, et al. Disruption of steroid and prolactin receptor patterning in the mammary gland correlates with a block in lobuloalveolar development. Mol Endocrinol. 2002;16:2675-2691.
16 Hovey RC, Harris J, Hadsell DL, et al. Local insulin-like growth factor-II mediates prolactin-induced mammary gland development. Mol Endocrinol. 2002;17:460-471.
17 Stull MA, Rowzee AM, Loladze AV, Wood TL. Growth factor regulation of cell cycles progression in mammary epithelial cells. J Mammary Gland Biol Neoplasia. 2004;9:15-26.
18 Rosen JM, Humphreys R, Krnacik S, et al. The regulation of mammary gland development by hormones, growth factors and oncogenes. Prog Clin Biol Res. 1994;387:95-111.
19 McManaman JL, Neville MC. Mammary physiology and milk secretion. Advanced Drug Deliv Rev. 2003;55:629-641.
20 Clark R. Introduction and overview: Sex steroids in the mammary gland. J Mammary Gland Biol Neoplasia. 2000;5:245-250.
21 Tyson JE, Friesen HG. Factors influencing the secretion of human prolactin and growth hormone in menstrual and gestational women. Am J Obstet Gynecol. 1973;116:377-387.
22 Neville MC, Keller RP, Seacat J, et al. Studies in human lactation: Milk volumes in lactating women during the onset of lactation and full lactation. Am J Clin Nutr. 1988;48:1375-1386.
23 Schmitt-Ney M, Doppler W, Ball RK, Groner B. β-Casein gene promoter activity is regulated by the hormone mediated relief of transcriptional repression and a mammary-gland-specific nuclear factor. Mol Cell Biol. 1991;11:3745-3755.
24 Birkenfeld A, Kase NG. Functional anatomy and physiology of the female breast. Obstet Gynecol Clin North Am. 1994;21:433-445.
25 Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: Structure, function, and regulation of secretion. Physiol Rev. 2000;80:1523-1631.
26 Gartner LM, Morton J, Lawrence RA, et alfor the American Academy of Pediatrics Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics. 2005;115:496-506.
27 Campbell MR, Gray RH. Characteristics and determinants of postpartum ovarian function in women in the United States. Am J Obstet Gynecol. 1993;169:55.
28 Benjamin F. Normal lactation and galactorrhea. Clin Obstet Gynecol. 1994;37:887-897.
29 Sauder SE, Frager M, Case GD, et al. Abnormal patterns of pulsatile luteinizing hormone secretion in women with hyperprolactinemia and amenorrhea: Responses to bromocriptine. J Clin Endocrinol Metab. 1984;59:941-948.
30 Cheung CY. Prolactin suppresses luteinizing hormone secretion and pituitary responsiveness to luteinizing hormone-releasing hormone by a direct action at the anterior pituitary. Endocrinology. 1983;113:632-638.
31 Fujimoto VY, Clifton DK, Cohen NL, et al. Variability of serum prolactin and progesterone levels in normal women: The relevance of single hormone measurements in the clinical setting. Obstet Gynecol. 1990;76:71-87.
32 Simard MF. Pituitary tumor endocrinopathies and their endocrine evaluation. Neurosurg Clin North Am. 2003;14:41-54.
33 Jacobs LS, Snyder PJ, Wilber JF, et al. Increased serum prolactin after administration of synthetic thyrotropin releasing hormone (TRH) in man. J Clin Endocrinol Metab. 1974;39:6-17.
34 Tolino A, Nicotra M, Romano L, et al. Subclinical hypothyroidism and hyperprolactinemia. Acta Eur Fertil. 1991;22:275-277.
35 Boyd AE, Spare S, Bower B, et al. Neurogenic galactorrhea-amenorrhea. J Clin Endocrinol Metab. 1978;47:1374-1377.
36 Yarkony GM, Novick AK, Roth EJ, et al. Galactorrhea: A complication of spinal cord injury. Arch Phys Med Rehabil. 1992;73:878-880.
37 Lim VS, Kathpalia SC, Frohman LA. Hyperprolactinemia and impaired pituitary response to suppression and stimulation in chronic renal failure: Reversal after transplantation. J Clin Endocrinol Metab. 1979;48:101-107.
38 Pollock A, McLaren EH. Serum prolactin concentration in patients taking neuroleptic drugs. Clin Endocrinol. 1998;49:513-516.
39 Lawson DM, Gala RR. The influence of adrenergic, dopaminergic, cholinergic and serotoninergic drugs on plasma prolactin levels in ovariectomized, estrogen-treated rats. Endocrinology. 1975;96:313-318.
40 Molitch ME. Antipsychotic drug-induced hyperprolactinemia: Clinical implications. Endocrine Pract. 2000;6:479-481.
41 Slater SL, Lipper S, Schiling DJ, Murphy DL. Elevation of polasma prolactin by monoamine-oxidase inhibitors. Lancet. 1977;2:275-276.
42 Sherman L, Fisher A, Klass E, Markowitz S. Pharmacologic causes of hyperprolactinemia. Semin Reprod Endocrinol. 1984;2:31.
43 Gluskin LE, Strasberg B, Shah JH. Verapamil-induced hyperprolactinemia and galactorrhea. Ann Intern Med. 1981;95:66-67.
44 Luzzati R, Crosato IM, Mascioli M, et al. Galactorrhea and hyperprolactinemia associated with HIV postexposure chemoprophylaxis. AIDS. 2002;16:1306-1307.
45 Klibanski A, Neer RM, Beitins IZ, et al. Decreased bone density in hyperprolactinemic women. NEJM. 1982;303:1511-1514.
46 Klibanski A, Greenspan SL. Increase in bone mass after treatment of hyperprolactinemic amenorrhea. NEJM. 1986;315:542-546.
47 Ciccarelli E, Savino L, Carlevatto V, et al. Vertebral bone density in non-amenorrhoeic hyperprolactinaemic women. Clin Endocrinol. 1988;28:1-6.
48 Farine D, Dor J, Lupovici N, et al. Conception rate after gonadotropin therapy in hyperprolactinemia and normoprolactinemia. Obstet Gynecol. 1985;65:658-660.
49 Vance ML, Thorner MO. Prolactinomas. Endocrinol Metab North Am. 1987;16:731-753.
50 Luciano AA, Sherman BM, Chapler FK, et al. Hyperprolactinemia and contraception: A prospective study. Obstet Gynecol. 1985;65:506-510.
51 Testa G, Vegetti W, Motta T, et al. Two-year treatment with oral contraceptives in hyperprolactinemic patients. Contraception. 1998;58:69-73.
52 Powell DE, Stelling CB. The normal breast: Structure, function and epidemiology. In: The Diagnosis and Detection of Breast Disease. St. Louis: Mosby; 1994.