Laboratory Methods for Diagnosis of Parasitic Infections
Overview
1. State the specific diagnostic purpose for each test methodology.
2. Briefly describe the principle associated with the test method.
3. Determine specimen acceptability for parasite identification, including collection method, collection time/receipt time, number and/or quantity of specimen, and presence of interfering and contaminating substances.
4. Select appropriate preservatives for parasite specimens and explain the chemical principle and rationale for the preservative, including polyvinyl alcohol (PVA), formalin, and sodium acetate acetic acid formalin (SAF).
5. Select the appropriate method of detection and identification of parasites based on type of specimen.
• Protozoa (amebae, flagellates, ciliates, sporozoans, coccidia, microsporidia)
• Platyhelminthes, or flatworms (cestodes, trematodes)
• Pentastomids, or tongue worms
Identification of parasitic organisms depends on morphologic criteria; accurate depiction of these criteria, in turn, depends on correct specimen collection and adequate fixation. Improperly submitted specimens may result in failure to detect or misidentification of the organisms. Tables 47-1 to 47-3 present information on the various groups of parasites, those that may be recovered from various body sites, the most frequently used specimen collection approaches, and appropriate processing methods.
TABLE 47-1
Description of the More Common Groups of Human Parasites
| Parasite Group | Description |
| Protozoa, Intestinal | |
| Amebae | Single-celled organisms; pseudopodia (motility), trophozoite, and cyst stages in the life cycle. Exceptions: Some have no identified cyst. Fecal-oral transmission of the infective cyst. Entamoeba histolytica causes amebiasis and is the most significant organism in this group. |
| Flagellates | Protozoa with characteristic flagella. Fecal-oral transmission. Exceptions: Dientamoeba fragilis (internal flagella) and the genus Trichomonas have a trophozoite and no cyst stage. Reproduction by longitudinal binary fission. Examples: Giardia lamblia and D. fragilis. |
| Ciliates | Single-celled protozoa; cilia (motility), which beat in a coordinated, rhythmic pattern, moving the trophozoite in a spiral path. Trophozoite and cyst stages in the life cycle; both stages show a large macronucleus and a micronucleus. Fecal-oral transmission. Balantidium coli is the single human pathogen in the group. |
| Coccidia | Protozoa; asexual and sexual life cycles. Fecal-oral transmission via contaminated food and/or water. Infective stage (oocyst) containing sporocysts and/or sporozoites. Examples: Cryptosporidium spp., Cyclospora cayetanensis, Isospora belli, and Sarcocystis spp. |
| Microsporidia | Small (1-2.5 µm) intestinal protozoa. Transmission by ingestion, inhalation, or direct inoculation of spores. Nine genera cause disease in humans; the two most important are Encephalitozoon and Enterocytozoon. |
| Protozoa, Other Sites | |
| Amebae | Pathogenic, free-living organisms associated with warm freshwater environments. Except for Entamoeba gingivalis (found in the mouth), they have been isolated from the central nervous system (CNS), eye, and other body sites. Examples: Naegleria fowleri—acute CNS infection and death. Chronic CNS disease (Acanthamoeba spp., Balamuthia mandrillaris, and Acanthamoeba spp. can also cause keratitis). |
| Flagellates | Have flagella (long, proteinaceous organelles used for motility). Sexual transmission. Examples: Trichomonas vaginalis is located in the genitourinary system. Trichomonas tenax can be identified in the mouth and is considered nonpathogenic. |
| Coccidia | Obligate intracellular, spore forming. Transmission is typically fecal-oral through ingestion of contaminated materials or food. Examples: Cryptosporidium spp. and Toxoplasma gondii. |
| Microsporidia | Small (1-2.5 µm) spore-forming protozoa. Transmission is typically by ingestion of spores. Life cycles vary considerably; some have an asexual life cycle, whereas others are complex and have both asexual and sexual life cycles and multiple hosts. Examples: Encephalitozoon, Pleistophora, Trachipleistophora, and Brachiola spp. |
| Protozoa, Blood and Tissue | |
| Malaria, babesiosis | Arthropod vector–borne protozoa. Transmission via insect bite. Examples: Plasmodium spp. includes parasites that undergo exoerythrocytic and pigment-producing erythrocytic schizogony in vertebrates and a sexual stage followed by sporogony in mosquitoes. Babesia spp. are tick-borne and can cause severe disease in patients who have been splenectomized or otherwise immunologically compromised. |
| Flagellates (leishmaniae) |
Trypanosomatid protozoa; two morphologic forms—promastigotes (anterior flagellum) in the insect host and amastigote (no flagella) in the vertebrate host. Transmission is through an insect vector. Recovery and identification of the organisms are related to body site. Recovery of leishmanial amastigotes is limited to the site of the lesion in infections other than those caused by the Leishmania donovani complex (visceral leishmaniasis). |
| Flagellates (trypanosomes) |
Trypanosomatid protozoa; morphologic forms are identified based on the position, length, and attachment site of the flagella. At some time in their life cycle, these protozoa have the trypomastigote form with the typical undulating membrane and free flagellum at the anterior end. Transmission is typically through an insect vector. Some organisms cause African sleeping sickness (e.g., Trypanosoma brucei gambiense, T. b. rhodesiense). The etiologic agent of American trypanosomiasis is T. cruzi, which has amastigote and trypomastigote stages in the mammalian host and an epimastigote form in the arthropod host. |
| Nematodes, intestinal | Helminthic parasites; roundworms. Nematodes have separate sexes, are elongate-cylindrical and bilaterally symmetrical with a triradiate symmetry at the anterior end. Nematodes have an outer cuticle layer, no circular muscles, and a pseudocele that contains all systems (digestive, excretory, nervous, reproductive). Transmission is by ingestion of eggs or by skin penetration of larval forms from the soil. Examples: Ascaris, Enterobius, Trichuris, and Strongyloides spp. and hookworm. |
| Nematodes, tissue | Helminthic parasites; roundworms. Many of these organisms are rarely seen in the United States; however, some are important and are found worldwide. Diagnosis may be difficult if the only specimens are obtained through biopsy and/or autopsy, and interpretation must be based on examination of histologic preparations. Examples: Trichinella spp., visceral larva migrans (VLM), ocular larva migrans (OLM), cutaneous larva migrans (CLM). |
| Nematodes, filarial | Helminthic round worms. Transmission is via arthropods. Adult worms tend to live in the tissues or lymphatics of the vertebrate host. The diagnosis is made on the basis of recovery and identification of the larval worms (microfilariae) in the blood, other body fluids, or skin. Examples: Wuchereria, Brugia, Loa, and Onchocerca spp. |
| Cestodes, intestinal | Helminthic tapeworms. Adult tapeworm consists of a chain of egg-producing units called proglottids, which develop from the neck region of the attachment organ (scolex). Food is absorbed through the worm’s integument. The intermediate host contains the larval forms that are acquired through ingestion of the adult tapeworm eggs. Transmission is through the ingestion of larval forms in poorly cooked or raw meat or freshwater fish. Examples: Dipylidium caninum (infection is acquired by accidental ingestion of dog fleas). Hymenolepis nana and H. diminuta are transmitted via ingestion of certain arthropods (fleas, beetles). Also, H. nana can be transmitted through egg ingestion (life cycle can bypass the intermediate beetle host). Humans can serve as both the intermediate and definitive hosts in H. nana and Taenia solium infections. |
| Cestodes, tissue | Tissue tapeworms. Transmission is through ingestion of certain tapeworm eggs or accidental contact with certain larval forms, leading to tissue infection. Humans serve as the accidental intermediate host. Examples: Taenia solium, Echinococcus granulosus, and several other species. |
| Trematodes, intestinal | Flatworms that are exclusively parasitic. Except for the schistosomes (blood flukes), flukes are hermaphroditic. They may be flattened; most have oral and ventral suckers. Transmission: Intestinal trematodes require a freshwater snail to serve as an intermediate host; these infections are food borne (freshwater fish, mollusks, or plants). Example: Fasciolopsis buski, the giant intestinal fluke. |
| Trematodes, liver, lung | Transmission: Liver and lung trematodes require a freshwater snail to serve as an intermediate host; these infections are food borne (freshwater fish, crayfish or crabs, or plants). Examples: Public health concerns include cholangiocarcinoma associated with Clonorchis and Opisthorchis infections, severe liver disease associated with Fasciola infections, and misdiagnosis of tuberculosis in individuals infected with Paragonimus spp. |
| Trematodes, blood | Schistosomes; sexes are separate. Males are characterized by an infolded body that forms the gynecophoral canal in which the female worm is held during copulation and oviposition. Transmission: Infection is acquired by skin penetration by the cercarial forms that are released from freshwater snails. The adult worms reside in the blood vessels over the small intestine, large intestine, or bladder. Examples: Schistosoma mansoni, S. haematobium, and S. japonicum. |
TABLE 47-2
| Site | Parasites |
| Blood | |
| Red cells | Plasmodium spp. Babesia spp. |
| White cells | Leishmania spp. Toxoplasma gondii |
| Whole blood/plasma | Trypanosoma spp. Microfilariae |
| Bone marrow | Leishmania spp. Trypanosoma cruzi Plasmodium spp. |
| Central Nervous System | |
| Cutaneous ulcers | Taenia solium (cysticerci) Echinococcus spp. Naegleria fowleri Acanthamoeba spp. Balamuthia mandrillaris Sappinia diploidea Toxoplasma gondii Microsporidia Trypanosoma spp. |
| Intestinal tract | Leishmania spp. Acanthamoeba spp. Entamoeba histolytica Entamoeba dispar Entamoeba coli Entamoeba hartmanni Endolimax nana Iodamoeba bütschlii Blastocystis hominis Giardia lamblia Chilomastix mesnili Dientamoeba fragilis Pentatrichomonas hominis Balantidium coli Cryptosporidium spp. Cyclospora cayetanensis Isospora belli Microsporidia Ascaris lumbricoides Enterobius vermicularis Hookworm Strongyloides stercoralis Trichuris trichiura Hymenolepis nana Hymenolepis diminuta Taenia saginata Taenia solium Diphyllobothrium latum Clonorchis sinensis (Opisthorchis) Paragonimus spp. Schistosoma spp. Fasciolopsis buski Fasciola hepatica Metagonimus yokogawai Heterophyes heterophyes |
| Liver, spleen | Echinococcus spp. Entamoeba histolytica Leishmania donovani Microsporidia |
| Lung | Cryptosporidium spp.* Echinococcus spp. Paragonimus spp. Microsporidia |
| Muscle | Taenia solium (cysticerci) Trichinella spp. Onchocerca volvulus (nodules) Trypanosoma cruzi Microsporidia |
| Skin | Leishmania spp. Onchocerca volvulus Microfilariae |
| Urogenital system | Trichomonas vaginalis Schistosoma spp. Microsporidia Microfilariae |
| Eye | Acanthamoeba spp. Toxoplasma gondii Loa loa Microsporidia |
Note: This table does not include every possible parasite that can be found in a particular body site; the most likely organisms have been listed
TABLE 47-3
Specimens and/or Body Site: Specimen Options, Collection and Transport Methods, and Processing
| Specimens and/or Body Site | Specimen Options | Collection and Transport Methods | Specimen Processing | Comments |
| Stool for ova and parasite (O&P) examination | Fresh stool | 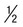 pint waxed container; 30 min if liquid, 60 min if semi formed, 24 h if formed; delivery to laboratory pint waxed container; 30 min if liquid, 60 min if semi formed, 24 h if formed; delivery to laboratory |
Direct wet smear (not on formed specimen), concentration, permanent stained smear | Stool specimens containing barium are unacceptable; intestinal protozoa may be undetectable for 5 to 10 days after barium use. Certain substances and medications also impede detection of intestinal protozoa: mineral oil, bismuth, antibiotics, antimalarial agents, and nonabsorbable antidiarrheal preparations. After administration of any of these compounds, parasitic organisms may not be recovered for a week to several weeks. Specimen collection should be delayed after barium or antibiotics are administered for 5 to 10 days or at least 2 weeks, respectively. |
| Preserved stool* | 5% or 10% formalin, MIF, SAF, Schaudinn’s, PVA, modified PVA, single vial systems, universal fixative | Concentration, permanent stained smear Depending on specimen (fresh or preserved) and patient’s clinical history, immunoassays may also be performed. |
||
| Stool for culture of nematodes | Fresh stool, entire stool specimen | 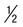 pint waxed container; immediate delivery to laboratory pint waxed container; immediate delivery to laboratory |
Filter paper strip, Petri dish, agar plate, charcoal cultures are all available. | Fresh stool (do not refrigerate) is required for these procedures. |
| Stool for recovery of tapeworm scolex | Preserved stool, entire stool specimen | 5% or 10% formalin (10% recommended) | The stool is filtered with a series of mesh screens and examined for the very small tapeworm scolex (proof of therapy efficacy) and/or proglottids (uncommon procedure but an option). | After treatment for tapeworm removal, the patient should be instructed to take a saline cathartic and to collect all stool material passed for the next 24 hr. The stool should be immediately placed in 10% formalin and thoroughly broken up and mixed with the preservative (1-gallon [3.8-liter] plastic jars are recommended, half full of 10% formalin). |
| Adult worms, worm segments | Saline, 70% alcohol | |||
| Cellophane tape preparation for pinworms | Surface sample from perianal skin; anal impression smear | Cellulose (Scotch) tape preparation or commercial sampling paddle or swab | Tape is lifted from a slide, a drop of xylene substitute is added, the tape is replaced, and the specimen is ready for examination under the microscope. | Specimens should be collected late at night after the person has been asleep for several hours or first thing in the morning before going to the bathroom or taking a shower. At least 4 to 6 consecutive negative tapes are required to rule out the infection. |
| Sigmoid colon | Sigmoidoscopy material, prepared as smears | Fresh or PVA or Schaudinn’s smears; specimen is taken with a spatula rather than cotton-tipped swabs; transported as smears in preservative | Direct wet smear, permanent stained smears | Material from the mucosal surface should be aspirated or scraped; it should not be obtained with cotton-tipped swabs. At least six representative areas of the mucosa should be sampled and examined (six samples, six slides). A parasitology specimen tray (containing Schaudinn’s fixative, PVA, and 5% or 10% formalin) should be provided, or a trained technologist should be available at the time of sigmoidoscopy to prepare the slides. Examination of sigmoidoscopy specimens does not take the place of routine O&P examinations. If the amount of material is limited, use of a fixative containing PVA is highly recommended. |
| Duodenum | Duodenal contents | Entero-Test or aspirates; string in Petri dish or tube; immediate transport to laboratory | The specimen may be centrifuged (10 min at 500× g) and should be examined immediately as a wet mount for motile organisms. Iodine also can be used. Direct wet smear of mucus; permanent stained smears can also be prepared. |
A fresh specimen is required; the amount may vary from < 0.5 mL to several milliliters of fluid. If the specimen cannot be completely examined within 2 hr after it is taken, any remaining material should be preserved in 5% to 10% formalin. |
| Entero-Test capsule (string) | Duodenal contents | Entero-Test (string test) in Petri dish (fresh) or preserved in PVA | Bile-stained mucus clinging to the yarn should be scraped off (mucus can also be removed by pulling the yarn between thumb and finger) and collected in a small Petri dish; disposable gloves are recommended. Usually 4 or 5 drops of material are obtained. The specimen should be examined immediately as a wet mount for motile organisms (iodine may be added later to facilitate identification of any organisms present). Organism motility is like that described previously for duodenal drainage. The pH of the terminal end of the yarn should be checked to ensure adequate passage into the duodenum (a very low pH means that it never left the stomach). The terminal end of the yarn should be yellow-green, indicating that it was in the duodenum (the bile duct drains into the intestine at this point). Permanent stained smears can also be prepared. |
If the specimen cannot be completely examined within 1 hr after removal of the yarn, the material should be preserved in 5% to 10% formalin or PVA-mucus smears should be prepared. |
| Urogenital tract | Vaginal discharge Urethral discharge Prostatic secretions |
Saline swab, transport swab (no charcoal), culture medium, plastic envelope culture, air-dried smear for FA |
Direct wet smear; fluorescence; urine must be centrifuged before examination. | Fresh specimens are required; an air-dried smear may be an option for fluorescence. Do not refrigerate swabs and/or culture containers at any time, because motility and/or ability to grow will probably be lost. |
| Urine | Single unpreserved specimen, 24-hr unpreserved specimen, early morning Nucleic acid-based testing media according to manufacturer’s instructions |
Examination of urinary sediment may be indicated in certain filarial infections. Administration of the drug diethylcarbamazine (Hetrazan) has been reported to enhance the recovery of microfilariae from the urine. The triple-concentration technique is recommended for the recovery of microfilariae. The membrane filtration technique can also be used with urine for the recovery of microfilariae. A membrane filter technique for the recovery of Schistosoma haematobium eggs has also been useful. | ||
| Sputum | Sputum | True sputum (not saliva) | Direct wet smear; permanent stained smears; fluorescence also available (Calcofluor for microsporidia). Sputum is usually examined as a wet mount (saline or iodine), using low and high dry power (×100 and ×400). The specimen is not concentrated before preparation of the wet mount. If the sputum is thick, an equal amount of 3% sodium hydroxide (NaOH) (or undiluted chlorine bleach) can be added; the specimen is thoroughly mixed and then centrifuged. NaOH should not be used if the examiner is looking for Entamoeba spp. or Trichomonas tenax. After centrifugation, the supernatant fluid is discarded, and the sediment can be examined as a wet mount with saline or iodine. If examination must be delayed for any reason, the sputum should be fixed in 5% or 10% formalin to preserve helminth eggs or larvae or in PVA fixative to be stained later for protozoa. |
True sputum is required; all specimens, especially induced specimens and BAL, should be delivered immediately to the laboratory (do not refrigerate). |
| Induced sputum | No preservative (10% formalin if time delay) | |||
| Bronchoalveolar lavage (BAL) | Sterile; immediate delivery to laboratory | |||
| Aspirates | Bone marrow | Sterile; immediate delivery to laboratory | Permanent stained smears; cultures can also be set (specifically designed for the recovery of blood parasites). | All aspirates for culture must be collected using sterile conditions and containers; this is mandatory for culture isolation of leishmaniae and trypanosomes. |
| Cutaneous ulcers | Sterile plus air-dried smears | |||
| Liver, spleen | Sterile, collected in 4 separate aliquots (liver) | |||
| Lung | ||||
| Transbronchial aspirate | Air-dried smears | |||
| Tracheobronchial aspirate | Air-dried smears | |||
| Central nervous system | Cerebrospinal fluid (CSF) | Sterile | Direct wet smear, permanent stained smears; culture for free-living amebae (Naegleria, Acanthamoeba spp.). | All specimens must be transported immediately to the laboratory (STAT procedure). |
| Biopsy | Intestinal tract | Routine histology | Direct wet smears, permanent stained smears; specimens to histology for routine processing. | The more material that is collected and tested, the more likely the organism is to be isolated and subsequently identified. Sterile collection is required for all specimens that will be cultured; bacterial and/or fungal contamination prevents isolation of parasites in culture. |
| Cutaneous ulcers | Sterile, nonsterile to histopathology (formalin acceptable) |
|||
| Eye | Sterile (in saline), nonsterile to histopathology | |||
| Scrapings | Sterile (in saline) | |||
| Cornea (scrapings) | Collected by physician, placed directly on microscope slide | Fixed using methyl alcohol and stained using Calcofluor white | Helpful in diagnosis of Acanthamoeba keratitis | |
| Liver, spleen | Sterile, nonsterile to histopathology | |||
| Lung | ||||
| Brush biopsy | Air-dried smears | |||
| Open lung biopsy | Air-dried smears | |||
| Muscle | Fresh, squash preparation, nonsterile to histopathology | |||
| Skin biopsy | Nonsterile to histopathology (formalin acceptable) |
|||
| Scrapings | Sterile (in saline), nonsterile to histopathology | |||
| Skin snip | Aseptic, smear or vial No preservative |
|||
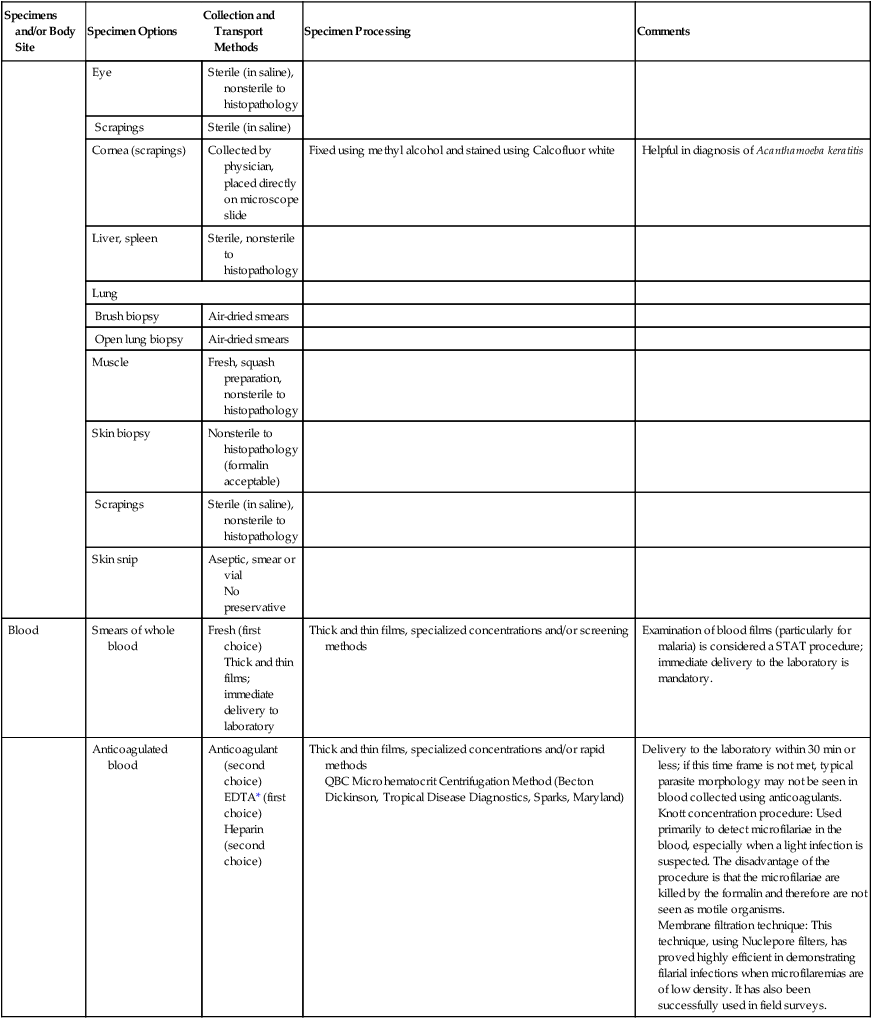
| Blood | Smears of whole blood | Fresh (first choice) Thick and thin films; immediate delivery to laboratory |
Thick and thin films, specialized concentrations and/or screening methods | Examination of blood films (particularly for malaria) is considered a STAT procedure; immediate delivery to the laboratory is mandatory. |
| Anticoagulated blood | Anticoagulant (second choice) EDTA* (first choice) Heparin (second choice) |
Thick and thin films, specialized concentrations and/or rapid methods QBC Microhematocrit Centrifugation Method (Becton Dickinson, Tropical Disease Diagnostics, Sparks, Maryland) |
Delivery to the laboratory within 30 min or less; if this time frame is not met, typical parasite morphology may not be seen in blood collected using anticoagulants. Knott concentration procedure: Used primarily to detect microfilariae in the blood, especially when a light infection is suspected. The disadvantage of the procedure is that the microfilariae are killed by the formalin and therefore are not seen as motile organisms. Membrane filtration technique: This technique, using Nuclepore filters, has proved highly efficient in demonstrating filarial infections when microfilaremias are of low density. It has also been successfully used in field surveys. |
|
†EDTA, Ethylenediaminetetraacetic acid; FA, fluorescent antibody; MIF, merthiolate-iodine-formalin; PVA, polyvinyl alcohol; SAF, sodium acetate–acetic acid–formalin.
*A number of new stool fixatives are available; some use a zinc sulfate base rather than mercuric chloride. Some collection vials can be used as a single-vial system; both the concentration and permanent stained smear can be performed from the preserved stool. However, not all single-vial systems (proprietary formulas) provide material that can be used for fecal immunoassay procedures. A universal fixative is now available (TOTAL-FIX), which contains no formalin, no mercury, and no PVA.
Modified from Garcia LS: Diagnostic medical parasitology, ed 5, Washington, DC, 2007, ASM Press.
Epidemiology
Parasites are transmitted from host to host through sexual means (venereal transmission) (Trichomonas vaginalis), from ingestion of infective forms in food or water (Giardia lamblia, Cryptosporidium spp., Ascaris lumbricoides), through skin penetration of infective larvae (Strongyloides stercoralis, hookworm), or through the bites of various arthropods (Plasmodium, Trypanosoma, Leishmania) (Table 47-4).
TABLE 47-4
Epidemiology of the More Common Groups of Human Parasites
| Parasite Group | Habitat (Reservoir) | Mode of Transmission | Prevention |
| Protozoa, Intestinal | |||
| Amebae | Single-celled organisms generally found in humans. Although certain animals harbor some of these organisms, they are not considered important reservoir hosts. | Humans acquire infections by ingesting food and water contaminated with fecal material containing the resistant, infective cyst stage of the protozoa. Various sexual practices have also been documented in transmission. | Preventive measures include increased attention to personal hygiene and sanitation measures; elimination of sexual activities that may involve fecal-oral contact. |
| Flagellates | The flagellates are generally found in humans. Although certain animals harbor some of these organisms, they are not considered important reservoir hosts; one exception may be animals, such as the beaver, that harbor Giardia lamblia. Contaminated water supplies are also a source. | Humans acquire infections by ingesting food and water contaminated with fecal material containing the resistant, infective cyst stage of the protozoa; in some cases (Dientamoeba fragilis), no cyst stage has been identified; the trophozoite forms may be transmitted from person to person in certain helminth eggs. | Preventive measures include increased attention to personal hygiene and sanitation measures; elimination of sexual activities that may involve fecal-oral contact; adequate water treatment (including filtration) is required; also awareness of environmental sources of infection. |
| Ciliates | Balantidium coli is generally found in humans, but it is also found in pigs. In some areas of the world, pigs are considered important reservoir hosts. | Humans acquire infections by ingesting food and water contaminated with fecal material containing the resistant, infective cyst stage of the protozoa. | Preventive measures include increased attention to personal hygiene and sanitation measures, as well as elimination of sexual activities that may involve fecal-oral contact. |
| Coccidia | Coccidia are found in humans. In some cases (e.g., cryptosporidiosis) animal reservoirs (cattle) can serve as important hosts. The muscle of various animals may contain sarcocysts that are infective for humans through the consumption of raw or poorly cooked meat. Numerous waterborne outbreaks with Cryptosporidium spp. have been reported throughout the world. Coccidian oocysts are extremely resistant to environmental conditions, particularly if they are kept moist. | These protozoa are acquired through ingestion of various meats or by fecal-oral transmission through contaminated food and/or water. The infective forms are called oocysts (Cryptosporidium spp., Isospora (Cystoisospora) belli, Cyclospora cayetanensis) or sarcocysts (Sarcocystis spp.), which are contained in infected meat. Cryptosporidia have also been implicated in nosocomial infections. | Preventive measures include increased attention to personal hygiene and sanitation measures; elimination of sexual activities that may involve fecal-oral contact. Adequate water treatment (including filtration) is mandatory; awareness of environmental sources of infection also is important. |
| Microsporidia | Microsporidia can infect every living animal, some of which probably serve as reservoir hosts for human infection. However, host specificity has not been well defined to date. The spores are environmentally resistant and can survive years if kept moist. | Infection with microsporidial spores usually occurs through ingestion; however, inhalation of spores and direct inoculation from the environment almost certainly occur. | Preventive measures include increased attention to personal hygiene and sanitation measures; increased awareness of environmental exposure possibilities; and adequate water treatment. |
| Protozoa, Other Sites | |||
| Amebae | Free-living amebae are associated with warm, freshwater environments; they are also found in soil. Although humans can harbor these organisms, person-to-person transfer is thought to be rare. Environmental sources are the primary link to human infection. Contaminated eye care solutions have been linked to organisms that cause keratitis. | Infection occurs through contact with contaminated water; organisms enter through the nasal mucosa and may travel via the olfactory nerve to the brain. Disease can be very severe and life-threatening; keratitis is also caused by these organisms, and infection can be linked to blindness or severe corneal damage. Eye infections can be linked to contaminated lens solutions or direct, accidental inoculation of the eye from environmental water and/or soil sources. | Avoidance of contaminated environmental water and soil sources; adequate care of contact lens systems. |
| Flagellates | Trichomonas vaginalis infection is found in a large percentage of humans; humans may present as symptomatic or asymptomatic. Person-to-person transfer is very common; reinfection is also common, particularly if sexual partners are not treated. | T. vaginalis is found in the genitourinary system and is usually acquired by sexual transmission. | Awareness of sexual transmission; treatment of all partners when infection is diagnosed in an individual patient. |
| Protozoa, Blood and Tissue | |||
| Malaria, babesiosis | Humans harbor the five species of malaria (Plasmodium vivax, P. ovale, P. malariae, P. knowlesi, and P. falciparum). Other animals can carry Babesia spp., and animal reservoir hosts play a large role in human transmission. | These organisms are arthropod-borne, Plasmodium spp. by the female anopheline mosquito and Babesia spp. by one or more genera of ticks. These infections can also be transmitted transplacentally, via shared needles, through blood transfusions, and from organ transplants. | Vector control; awareness of transmission through blood transfusions, shared drug needles, congenital infections, and organ transplants. Careful monitoring of the blood supply. Malaria prophylaxis if traveling to endemic areas. |
| Flagellates (leishmaniae) |
Some strains of leishmaniae have reservoir hosts (e.g., dogs for the Mediterranean strain of Leishmania donovani and wild rodents for the African strains of L. donovani.) L. tropica also has been linked to the same two animal reservoirs. | Transmission is through the bite of infected sandflies. Infection can also occur from person to person (cutaneous lesions), from blood transfusion, shared needles, and organ transplants. | Vector control; avoiding environmental sources (e.g., dogs, wild rodents); careful handling of all clinical specimens from infected patients. |
| Flagellates (trypanosomes) |
Humans are the only known hosts for Trypanosoma brucei gambiense (West African trypanosomiasis); Trypanosoma brucei rhodesiense (East African trypanosomiasis) infections are found in a number of antelope and other ungulates that act as reservoir hosts. Rodents and some mammals are reservoir hosts for Trypanosoma cruzi. | Transmission is through the bite of the infected tsetse fly and through blood transfusion, shared needles, and organ transplants. Transmission of T. cruzi is through the infected feces of the triatomid bug; the bug takes a blood meal, immediately defecates, and the human host scratches the infected feces into the bite site; bug saliva contains an irritant that stimulates scratching. |
Vector control; awareness of potential exposure/infection from blood sources (transfusions, shared needles, organ transplants). Laboratory accidents while handling infected blood have been reported. |
| Nematodes, intestinal | These roundworms generally do not have animal reservoirs relevant to human infection. One exception is the pig ascarid; human infections have been reported. These worms are found worldwide; Ascaris lumbricoides is probably the most common parasite of humans, although some would argue that Enterobius vermicularis is number one. Strongyloides stercoralis is particularly important as the causative agent of severe disease in the compromised host. | A. lumbricoides and Trichuris trichiura eggs must undergo development in the soil before they are infective; thus children who play in the dirt are a particularly high-risk group. Ingestion of food and water contaminated with infective eggs is the primary route of infection. Hookworm and S. stercoralis infections are initiated by larval penetration of the skin from contaminated soil. Pinworm infection (E. vermicularis) is acquired through ingestion of infective eggs from the environment (hand-to-mouth). |
Avoiding ingestion of contaminated soil and/or avoiding frequenting soil contaminated with hookworm eggs (pets, soil, water, warmth, warm weather); treatment for pinworm is recommended, but reinfection is common. |
| Nematodes, tissue | Trichinella spp. have a number of animal reservoir hosts, including bears, walruses, pigs, rodents, and other animals. Dog and cat hookworms cause cutaneous larva migrans (CLM), and the dog and cat ascarid, Toxocara spp., causes visceral and ocular larva migrans (VLM, OLM). These infections can be serious and cause severe disease if not treated. | Trichinella organisms are acquired by ingestion of raw or poorly cooked infected meat. CLM is caused by skin penetration of infective larvae from the soil; children should avoid sandboxes where dogs and cats are known to defecate. Larval migration is limited to the skin. VLM and OLM are caused by accidental ingestion of Toxocara spp. eggs from contaminated soil; larval migration occurs throughout the body, including the eyes. |
Adequate cooking of infected meat; awareness of possibility of contaminated soils for dog and cat hookworms and/or ascarids; covering of all sandboxes where pets have access to defecation and children play. |
| Nematodes, filarial | Wuchereria bancrofti, Loa loa, and Onchocerca volvulus have no animal reservoirs and are found only in humans, whereas Brugia spp. can also be found in cats and monkeys. Dracunculus medinensis can infect dogs, cats, and monkeys and also humans. | Filarial nematodes are transmitted through the bite of a blood-sucking arthropod (midges, mosquitoes, flies). Dracunculus infections are acquired through ingestion of water contaminated with small crustaceans, Cyclops spp., which contain infective larvae. | Vector control; protection of well-water sources. |
| Cestodes, intestinal | The human serves as the definitive host for beef (Taenia saginata) and pork (Taenia solium) tapeworms; cows/camels and pigs serve as intermediate hosts, respectively. Humans also serve as the intermediate host for T. solium (cysticercosis). Diphyllobothrium latum adult tapeworms can be found in a number of wild animals, the most important being dogs, bears, seals, and walruses, which serve as reservoir hosts; humans are the definitive host. Hymenolepis nana (dwarf tapeworm) can occur in rodents; humans can serve as both intermediate and definitive hosts, with development from the egg to adult worm occurring in the human intestine. | Human infection with the adult worm occurs through ingestion of raw or poorly cooked meat (beef, camel, pork) containing the intermediate forms, the cysticerci. Humans become the accidental intermediate host when eggs from an adult T. solium tapeworm are ingested. The cysticerci develop in the muscle and tissues of the human rather than the pig. Infection with the adult D. latum tapeworm occurs through ingestion of poorly cooked freshwater fish containing the sparganum or plerocercoid larval form. Infection with H. nana is primarily acquired through accidental ingestion of eggs from an adult tapeworm. | Adequate cooking of infected meat; treatment of patients harboring adult tapeworms (accidental ingestion of eggs can lead to infection). |
| Cestodes, tissue | Adult worms are found in a variety of animals; the human becomes the accidental intermediate host after ingestion of eggs from the adult worms. Reservoir hosts include dogs, cats, and rodents. | Ingestion of certain tapeworm eggs or accidental contact with certain larval forms can lead to tissue infection with Taenia solium, Echinococcus spp., and several others. | Preventive measures involve increased attention to personal hygiene and sanitation measures. |
| Trematodes, intestinal | Fish-eating wild and domestic animals serve as reservoir hosts. The definitive host of Fasciolopsis buski is the pig. | Ingestion of water chestnut and caltrop (raw, peeled with the teeth) is the source of infection; metacercariae are encysted on the plant material. Pig feces are used to fertilize various water plant crops. | Avoiding eating raw water plants that may contain encysted larval forms of the flukes; adequate waste disposal of farm animal feces (pigs). |
| Trematodes, liver, lung | Cats, dogs, and wild fish-eating mammals can serve as reservoir hosts for Opisthorchis spp., Clonorchis sinensis, and Paragonimus spp. Fasciola hepatica is normally a parasite of sheep, and F. gigantica is a parasite of cattle; humans are accidental hosts. | Infection occurs through ingestion of raw or poorly cooked fish, crabs, crayfish, and certain plants in or on which metacercariae are encysted. Infection with Fasciola spp. is not easily acquired (the parasite is not that well adapted to the human host). | Thorough cooking of potentially infected fish, crabs, crayfish; avoiding eating raw water plants that may contain encysted metacercariae. |
| Trematodes, blood | Schistosoma mansoni and S. haematobium appear to be restricted to the human host; S. japonicum can be found in cattle, deer, dogs, and rodents. The worms mature in the blood vessels, and eggs make their way outside the body in stool and/or urine. The freshwater snail is a mandatory part of the life cycle (contains developmental forms of schistosome). | Infection occurs through skin penetration by infected cercariae released from a freshwater snail containing the intermediate stages of the schistosome life cycle. Cercariae can be released from the snail intermediate host singly or in groups. | Protection from potentially contaminated water sources; awareness of mode of transmission; proper handling of human waste containing eggs (continued infection of snail intermediate hosts). |
Pathogenesis and Spectrum of Disease
It is important to understand the life cycle of parasites, in terms both of potential control and prevention and of infectivity and disease outcomes in normal and immunosuppressed hosts (Table 47-5). Table 47-6 lists mechanisms of pathogenesis and the spectrum of parasitic diseases. Specific guidelines for physician requests are presented in Table 47-7.
TABLE 47-5
Parasitic Infections: Clinical Findings in Normal and Compromised Hosts
| Organism | Normal Host | Compromised Host |
| Entamoeba histolytica | Asymptomatic to chronic-acute colitis; extraintestinal disease may also occur (primary site: right upper lobe of liver). | Diminished immune capacity may lead to extraintestinal disease |
| Free-living amebae Naegleria fowleri Acanthamoeba spp. Balamuthia mandrillaris Sappinia spp. |
Patients tend to have eye infections with Acanthamoeba spp. linked to poor lens care. | Primary amebic meningoencephalitis (PAM); granulomatous amebic encephalitis (GAE) |
| Giardia lamblia | Asymptomatic to malabsorption syndrome. | Certain immunodeficiencies tend to predispose an individual to infection. |
| Toxoplasma gondii | Approximately 50% of individuals have antibody and organisms in tissue but are asymptomatic. It is important to note that a developing fetus may be severally affected if the mother is infected; this is highly dependent on the time (trimester) when the infection occurs. | Disease in compromised hosts tends to involve the central nervous system (CNS), with various neurologic symptoms; it can mimic neurologic symptoms of infection with the human immunodeficiency virus (HIV). |
| Cryptosporidium spp. Cryptosporidium hominis (humans) Cryptosporidium parvum (humans and animals) |
Self-limiting infection with diarrhea and abdominal pain. | Because of the autoinfective nature of the life cycle, infection is not self-limiting and may produce fluid loss of more than 10 L/day; multisystem involvement may occur. No totally effective therapy is known. |
| Cyclospora cayetanensis | Self-limiting infection with diarrhea (3-4 days); relapses common. | Diarrhea may persist for 12 weeks or longer; biliary disease has also been reported in this group, particularly those with acquired immunodeficiency syndrome (AIDS). |
| Isospora (Cystoisospora) belli | Self-limiting infection with mild diarrhea or no symptoms. | May lead to severe diarrhea, abdominal pain, and possibly death (rare case reports); diagnosis occasionally may be missed because of failure to recognize the oocyst stage; is not seen when concentrated from polyvinyl alcohol (PVA) fixative. |
| Sarcocystis spp. | Self-limiting infection with diarrhea or mild symptoms. | Symptoms may be more severe and last for a longer period. |
| Microsporidia Anncaliia Nosema Brachiola Vittaforma Encephalitozoon Enterocytozoon Pleistophora Trachipleistophora “Microsporidium” |
Little is known about these infections in the normal host. Most infections have been identified as causing intestinal symptoms (Enterocytozoon, Encephalitozoon) or eye infections (Vittaforma, Encephalitozoon). | Organisms infect various parts of the body; diagnosis often depends on histologic examination of tissues; routine examination of clinical specimens (e.g., stool, urine) is becoming more common; infection can probably cause death. |
| Leishmania spp. | Asymptomatic to mild disease. Depending on species, infection can result in cutaneous, diffuse cutaneous, or mucocutaneous disease. | More serious manifestations of visceral leishmaniasis; some cutaneous species manifest visceral disease; infection is difficult to treat and manage; definite co-infection with AIDS. |
| Strongyloides stercoralis | Asymptomatic to mild abdominal complaints; can remain latent for many years because of low-level infection maintained by internal autoinfective life cycle. | Can result in disseminated disease (hyperinfection syndrome resulting from autoinfective nature of life cycle); abdominal pain, pneumonitis, sepsis-meningitis with Gram-negative bacilli, eosinophilia; distinct link to certain leukemias or lymphomas; can be fatal. |
| Crusted (Norwegian) scabies | Infections can range from asymptomatic to moderate itching. | Severe infection with reduced itching response; hundreds of thousands of mites on the body; infection is very easily transferred to others; secondary infection is common. |
TABLE 47-6
Pathogenesis and Spectrum of Parasitic Diseases
| Parasite Group | Pathogenesis | Spectrum of Disease |
| Protozoa, Intestinal | ||
| Amebae | Pathogens can cause severe disease; however, exposure does not always lead to disease; infection may be self-limiting; disease more likely in the compromised host. | Nonpathogens cause no disease, patients are asymptomatic; Entamoeba histolytica causes intestinal symptoms (bloody diarrhea) and the potential for amebic liver abscess; other tissues may be involved, especially in the immunocompromised patient. “Blastocystis hominis” comprises a number of strains, some considered pathogenic; patients’ conditions range from asymptomatic to severe diarrhea. |
| Flagellates | Not all patients are infected upon exposure; disease spectrum varies; some patients may remain asymptomatic; if nonexposed patients become infected, symptoms are much more likely to occur. | Nonpathogens cause no disease, patients are asymptomatic; Giardia lamblia (malabsorption syndrome) and Dientamoeba fragilis cause intestinal symptoms ranging from “indigestion” to nonbloody diarrhea, cramping, gas, and so on. |
| Ciliates | Balantidium coli infection is rare in the United States; people who have regular contact with pigs are much more likely to become infected; wide range of symptoms. | B. coli causes intestinal symptoms, including severe watery diarrhea, similar to coccidial and microsporidial infections. |
| Coccidia | All coccidia infective to humans can cause severe disease, particularly in the immunocompromised patient; infections in the immunocompetent patient tend to be self-limiting; Cryptosporidium spp. can maintain the infective cycle in the patient as a result of the autoinfective portion of the life cycle (the immunocompromised patient cannot produce antibody to limit this autoinfective cycle); huge waterborne outbreaks have been documented for Cryptosporidium spp.; infecting dose is low for Cryptosporidium spp. | Cryptosporidium spp., Cyclospora cayetanensis, and Isospora belli cause intestinal symptoms, including severe watery diarrhea; infections are more severe in immunocompromised patients. Life-threatening infections can be seen with Cryptosporidium spp.; the organisms can disseminate to other tissues, primarily the lung. Sarcocystis can cause intestinal symptoms and/or muscle pain, depending on the mode of infection (ingestion of oocysts or infected meat). |
| Microsporidia | A number of genera are pathogenic for humans and for animals; wide range of body sites; disease varies, depending on patient’s immune status; disease outcome is complicated by lack of treatment for some genera; albendazole is effective for Encephalitozoon spp. | Every human tissue may be infected; Enterocytozoon bieneusi and Encephalitozoon (Septata) intestinalis are the most common and are found in the intestinal tract; the latter can also disseminate to other tissues, including the kidneys. Eye infections have been seen in both healthy and compromised patients; severe corneal infections seen. |
| Protozoa, Other Sites | ||
| Amebae | Pathogenic for humans; disease ranging from acute meningoencephalitis to chronic encephalitis, cutaneous infections to keratitis, and the potential for other body sites; disease spectrum depends on patient’s immune capacity and the organism involved; disease can be mild (Acanthamoeba spp.) to fatal (Naegleria fowleri). | Infection occurs through contact with contaminated water; organisms enter through the nasal mucosa and may travel via the olfactory nerve to the brain. Disease caused by N. fowleri can be severe and life-threatening (primary amebic meningoencephalitis [PAM)]; chronic granulomatous amebic encephalitis (GAE) can be caused by Acanthamoeba spp. and Balamuthia mandrillaris; keratitis is also caused by these organisms, and infection can be linked to blindness or severe corneal damage. Eye infections can be linked to contaminated lens solutions or to direct, accidental inoculation of the eye from environmental water and/or soil sources. |
| Flagellates | Trichomonas vaginalis causes genitourinary disease, depending on vaginal pH, presence or absence of other organisms, sexual practices, and other factors. Disease can vary from mild to severe. | T. vaginalis is found in the genitourinary system and is usually acquired by sexual transmission. Disease can be asymptomatic in the male but can cause pain, itching, and discharge in females; some strains of drug-resistant Trichomonas have been documented. |
| Protozoa, Blood and Tissue | ||
| Malaria, babesiosis | Plasmodium vivax, P. falciparum, P. ovale, P. knowlesi, and P. malariae are pathogenic for humans; P. falciparum malaria is the leading cause of death in endemic areas; although the host can develop antibody, protection is strain specific and short-lived. | Malaria can cause a range of symptoms, with life-threatening illness caused by P. falciparum; symptoms include fever, chills, nausea, and central nervous system (CNS) symptoms; Babesia infections often mimic those seen with malaria but without the fever periodicity. |
| Flagellates (leishmaniae) | Leishmania donovani invades the spleen, liver, and bone marrow and can cause serious disease, particularly in compromised hosts. | Leishmaniasis can infect the skin and mucous membranes and the organs of the reticuloendothelial system; symptoms can be mild to life-threatening. |
| Flagellates (trypanosomes) | Humans are the only known hosts for Trypanosoma brucei gambiense (West African trypanosomiasis); Trypanosoma brucei rhodesiense (East African trypanosomiasis) infections are found in a number of antelope and other hoofed mammals that serve as reservoir hosts. Trypanosoma cruzi (American trypanosomiasis) can be found in rodents and chickens. | T. b. gambiense and T.b. rhodesiense cause African sleeping sickness, with eventual invasion of the CNS, leading to coma and death; Chagas disease (T. cruzi) causes acute to chronic problems, primarily linked to cardiac disease and diminished cardiac capacity; the muscles of the gastrointestinal (GI) tract are also infected, leading to loss of function in terms of movement of food through the GI tract. |
| Nematodes, intestinal | These worms can cause mild to severe disease, depending on the worm burden (original number of eggs ingested or infective larvae penetrating the skin); young children and debilitated patients are more likely to be symptomatic; severe infections are seen in hyperinfections caused by Strongyloides stercoralis (autoinfective life cycle and immunocompromised patients); outcome varies tremendously from patient to patient and depends on the original infective dose. | Ascaris lumbricoides, Trichuris trichiura, and hookworm symptoms range from none to diarrhea, pain, and so on, depending on the worm burden; anemia may be seen with severe hookworm infection; S. stercoralis infections can involve many body tissues (disseminated disease) in the immunocompromised patient and can cause death; pinworm infection (Enterobius vermicularis) symptoms range from none to anal itching, irritability, loss of sleep, and so on. |
| Nematodes, tissue | Depending on the infective dose, Trichinella spp. can cause mild to severe disease; both cutaneous larva migrans (CLM) (dog/cat hookworm larvae), and toxocariasis (visceral larva migrans [VLM], ocular larva migrans [OLM]) through ingestion of dog/cat ascarid eggs) cause serious disease if not treated; often CLM, VLM, and OLM are seen in children more than adults. | Trichinella spp. can cause eosinophilia, muscle aches and pains, and death, depending on the worm burden; CLM can cause severe itching and eosinophilia as a result of larval migration in the skin; VLM and OLM are caused by larval migration throughout the body, including the eyes (mimics retinoblastoma). |
| Nematodes, filarial | Wuchereria bancrofti, Loa loa, and Onchocerca volvulus cause human disease; however, some filarial infections are not well adapted to humans and require many years of exposure before disease is evident; some infections are not evident, some cause multiple disease manifestations. | Symptoms range from asymptomatic to elephantiasis, blindness, skin changes, lymphadenitis, lymphangitis; in some cases Loeffler’s syndrome also may be seen. |
| Cestodes, intestinal | The beef (Taenia saginata), pork (Taenia solium), and freshwater fish (Diphyllobothrium latum) tapeworms infect humans and are generally found in the intestine as a single worm. In the case of cysticercosis; ingestion of T. solium eggs can cause mild to severe disease, depending on the infecting dose and body site (muscle, CNS). Hymenolepis nana (dwarf tapeworm) infection can lead to many worms in the intestinal tract (autoinfective cycle; the organism can go from egg to larval form to adult in the human host). | Human infection with the adult tapeworm can cause no symptoms, or mild intestinal symptoms may occur. When the human becomes the accidental intermediate host for T. solium, CNS symptoms may occur, including epileptic seizures. Infection with the adult D. latum tapeworm can also cause intestinal symptoms, such as pain, diarrhea, and so on, but the patient may also be asymptomatic; a vitamin B12 deficiency may be seen. Infection with H. nana is primarily acquired from accidental ingestion of eggs from an adult tapeworm; symptoms may be absent, or diarrhea may be present. |
| Cestodes, tissue | Echinococcus spp. can cause severe disease, depending on the original infecting dose of tapeworm eggs; multiple organs can be involved, including brain, liver, lung, and bone; some cysts grow like a metastatic tumor; surgical removal can be very difficult, if not impossible. | Depending on the body site, hydatid cysts can cause pain, anaphylactic shock (fluid leakage), or CNS symptoms. The patient may be unaware of infection until a cyst begins to press on other body organs or a large fluid leak occurs. |
| Trematodes, intestinal | Many genera and species are pathogenic for humans; disease severity depends on the infective dose of metacercariae; some patients may be unaware of infection. | Intestinal trematodes can cause pain and diarrhea; intestinal toxicity can sometimes be seen in heavy infections with Fasciolopsis buski. |
| Trematodes, liver and lung | Many genera and species are pathogenic for humans; disease severity depends on the infective dose of metacercariae; some patients may be unaware of infection. | Paragonimus spp. infection in the lungs can be severe, resulting in coughing, shortness of breath, and other symptoms; liver fluke infection can involve the bile ducts and gallbladder; symptoms depend on worm burden. |
| Trematodes, blood | Schistosomes are pathogenic for humans; however, the loading dose of cercariae from infected water sources determines the outcome of disease; very light infections may not produce symptoms; heavy infections can lead to death. | Symptoms may range from asymptomatic in light infections to severe organ failure resulting from deposition of eggs and subsequent granuloma formation in the tissues; “pipe-stem” fibrosis is seen in blood vessels; collateral circulation may develop; severe disease can cause death. |
Particularly relevant for areas of the United States where Giardia sp. is the most common organism found.
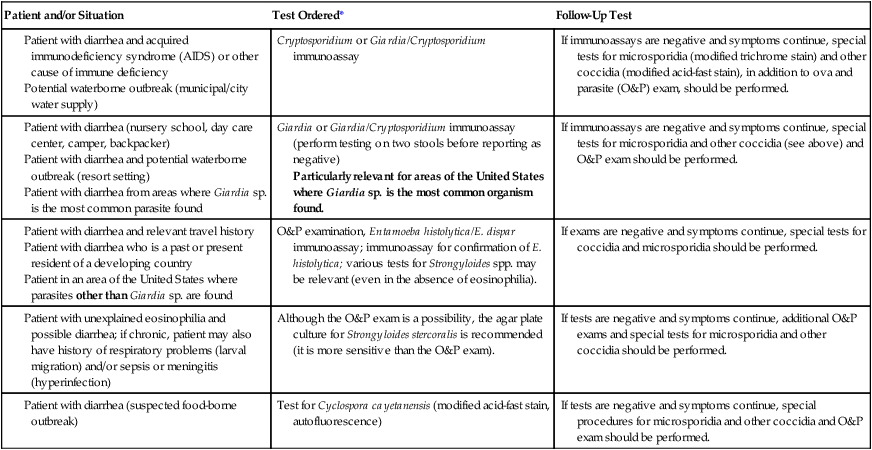
*Depending on the particular immunoassay kit used, various single or multiple organisms may be included. Selection of a particular kit depends on many variables, such as clinical relevance, cost, ease of performance, training, personnel availability, number of test orders, training of physician clients, sensitivity, specificity, equipment, and time to result. Very few laboratories handle this type of testing in exactly the same way. Many options are clinically relevant and acceptable for good patient care. It is critical that the laboratory report indicate specifically which organisms could be identified using the kit; a negative report should list the organisms relevant to that particular kit.
Laboratory Diagnosis
The ability to detect and identify human parasites is directly linked to the quality of the clinical specimen, submission of the appropriate specimen or specimens, relevant diagnostic test orders, and the experience and training of laboratory personnel (Table 47-8). A summary of human parasites and applicable collection fixatives, clinical specimens, diagnostic tests, the elements of a positive finding, and comments are presented in Tables 47-9 and 47-10.
TABLE 47-8
Stool Specimen Collection and Testing Options
| Option | Pros | Cons |
| Rejection of stools from inpatients who have been in-house for longer than 3 days | Data suggest that patients who begin to have diarrhea after they have been inpatients for a few days are not symptomatic from parasitic infections, but generally from other causes. | The chance always exists that the problem is related to a health care–associated (nosocomial) parasitic infection (rare); Cryptosporidium spp. and microsporidia may be possible considerations. |
| Examination of a single stool (ova and parasite [O&P]) Data suggest that 40% to 50% of organisms present are found with only a single stool exam. Two O&P exams (concentration, permanent stained smear) are acceptable but not always as good as three specimens (may be relatively cost-effective approach); any patient remaining symptomatic requires additional testing. |
Some think that most intestinal parasitic infections can be diagnosed from examination of a single stool. If the patient becomes asymptomatic after collection of the first stool, subsequent specimens may not be necessary. | Diagnosis from a single stool examination depends on the experience of the microscopist, proper collection, and the parasite load in the specimen. In a series of three stool specimens, frequently not all three specimens are positive and/or may be positive for different organisms. |
| Examine a second stool only after the first is negative and the patient is still symptomatic. | With additional examinations, yield of protozoa increases (Entamoeba histolytica, 22.7%; Giardia lamblia, 11.3%; and Dientamoeba fragilis, 31.1%). | Assumes the second (or third) stool is collected within the recommended 10-day time frame for a series of stools; protozoa are shed periodically. May be inconvenient for patient. |
| Examination of a single stool and an immunoassay (enzyme immunoassay [EIA], fluorescent antibody [FA], lateral or vertical flow cartridge) This approach is a mix: one immunoassay may be acceptable; however, immunoassay testing of two separate specimens may be required to confirm the presence of Giardia antigen. One O&P exam is not the best approach (review last option below). |
If the examinations are negative and the patient’s symptoms subside, probably no further testing is required. | Patients may show symptoms (off and on), so ruling out parasitic infections with only a single stool and one fecal immunoassay may be difficult. If the patient remains symptomatic, then even if two Giardia immunoassays are negative, other protozoa may be missed (Entamoeba histolytica/E. dispar group, E. histolytica, Dientamoeba fragilis, Cryptosporidium spp., microsporidia). Normally, there are specific situations in which fecal immunoassays OR O&P exams should be ordered. It is not recommended to perform both the O&P and fecal immunoassay automatically as a stool exam for parasites. |
| Pool three specimens for examination; perform one concentration and one permanent stain (the laboratory pools the specimens). | Three specimens are collected by the patient (three separate collection vials) over 7-10 days; pooling by the laboratory may save time and expense. | Organisms present in low numbers may be missed because of the dilution factor once the specimens have been pooled. |
TABLE 47-9
Fecal Fixatives Used in Diagnostic Parasitology (Intestinal Tract Specimens)
| Fixative | Concentration | Permanent Stained Smear Trichrome, Iron-Hematoxylin, Special Stains/Coccidia and Microsporidia | Immunoassays: Giardia lamblia Cryptosporidium spp. | Comments |
| 5% or 10% Formalin | Yes | No | Yes | Concentrations and IAs (EIA, FA, Rapids) |
| 5% or 10% Buffered formalin | Yes | No | Yes | Concentrations and IAs (EIA, FA, Rapids) |
| MIF | Yes | Polychrome IV stain | ND | No published data |
| SAF | Yes | Iron-hematoxylin best | Yes | Concentrations, permanent stains, and IAs (EIA, FA, Rapids) |
| Schaudinn’s (Hg base) no PVA* |
Rare | Yes | No | Permanent stains; Hg interferes with IAs; primarily used with fresh stool specimens (no fixative collection vials) |
| Schaudinn’s (Hg base) with PVA* |
Rare | Yes | No | Permanent stains; Hg and PVA interfere with IAs; considered gold standard fixative for permanent stains |
| Schaudinn’s (Cu base) with PVA† |
Rare | Yes | No | Permanent stains; PVA interferes with IAs; stains not as good as with Schaudinn’s fixative using Hg or Zn |
| Schaudinn’s (Zn base) with PVA‡ |
Rare | Yes | No | Permanent stains; PVA interferes with IAs; the same fixative as TOTAL-FIX without PVA (see below) |
| Ecofriendly ECOFIX (PVA)§ |
Rare | Yes | No | Permanent stains; PVA interferes with IAs; works best with ECOSTAIN; Wheatley’s trichrome second best |
Universal fixative, ecofriendly ecofriendlyTOTAL-FIX |
Yes | Yes | Yes | No formalin, no mercury, no PVA; concentrations, permanent stains, special stains, fecal IAs |
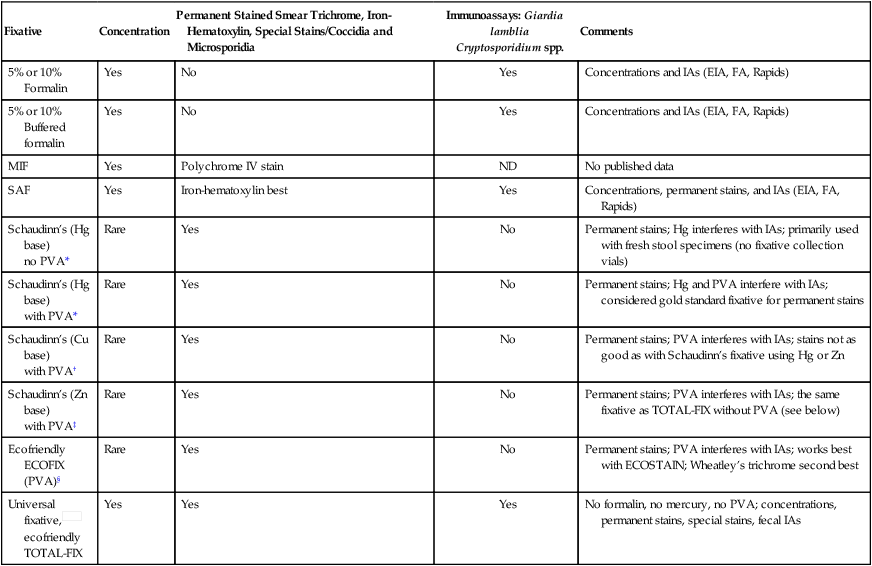
Cu, Copper; EIA, enzyme immunoassay; FA, fluorescent antibody; Hg, mercury; IA, immunoassay; MIF, merthiolate-iodine-formalin fixative; ND, no data; PVA, polyvinyl alcohol; Rapids, cartridge-format, membrane-flow IAs; SAF, sodium acetate–acetic acid–formalin; Zn, zinc.
Commentary
The most common collection option (original public health approach) is a two-vial system: one vial of 5% or 10% formalin or buffered formalin and one vial of fixative containing the plastic adhesive polyvinyl alcohol (PVA). The formalin vial is used for concentration and fecal immunoassays, and the PVA vial is used for the permanent stained smear. Regulations for formalin (see below) originally were developed for industry, not the clinical laboratory, where amounts of formalin tend to be quite low. However, a laboratory using any amount of formalin must be monitored (see below).
SEMIUNIVERSAL FIXATIVES
Examples of a semiuniversal fixative include sodium acetate–acetic acid–formalin (SAF) (no mercury or PVA; contains formalin) and ECOFIX (no mercury or formalin; contains PVA).
UNIVERSAL FIXATIVE
Currently, TOTAL-FIX is the only fixative that contains NO formalin, NO PVA, and NO mercury. TOTAL-FIX can be used without adding PVA to the fixative; adequate drying time for smears before staining is the most important step (minimum of 1 hr in 37°C incubator; more time required for thicker fecal smears). This fixative can be used for concentration, permanent stained smear, special stains for coccidia or microsporidia, and fecal immunoassays for Giardia and Cryptosporidium spp.
FORMALIN FIXATIVE
Formalin has been used for many years as an all-purpose fixative that is appropriate for helminth eggs and larvae and for protozoan cysts, oocysts, and spores. Two concentrations are commonly used: 5%, which is recommended for preservation of protozoan cysts, and 10%, which is recommended for helminth eggs and larvae. Although 5% is often recommended for all-purpose use, most commercial manufacturers provide 10%, which is more likely to kill all helminth eggs. To help maintain organism morphology, the formalin can be buffered with sodium phosphate buffers (i.e., neutral formalin). Selection of specific formalin formulations is at the user’s discretion. Aqueous formalin permits examination of the specimen as a wet mount only, a much less accurate technique than a permanent stained smear for identifying intestinal protozoa. However, the fecal immunoassays for Giardia lamblia and Cryptosporidium spp. can be performed from the aqueous formalin vial. Fecal immunoassays for the Entamoeba histolytica/E. dispar group and E. histolytica are limited to fresh or frozen fecal specimens or Cary-Blair transport medium. After centrifugation, special stains for the coccidia (modified acid-fast stains) and microsporidia (modified trichrome stains) can be performed from the concentrate sediment obtained from formalin-preserved stool material. Use of the sediment provides a more sensitive test.
OSHA REGULATIONS ON THE USE OF FORMALDEHYDE
Formaldehyde has been in use for more than a century as a disinfectant and preservative, and it is found in a number of industrial products. Disagreement exists about the carcinogenic potential of lower levels of exposure, and epidemiologic studies of the effects of formaldehyde exposure among humans have given inconsistent results. Studies of industry workers with known exposure to formaldehyde report little evidence of increased cancer risk. In addition, people with asthma appear to respond no differently than healthy individuals after exposure to concentrations of formaldehyde up to 3 ppm. The federal Occupational Safety and Health Administration (OSHA) requires all workers to be protected from dangerous levels of vapors and dust. Formaldehyde vapor is the most likely air contaminant to exceed the regulatory threshold in a laboratory, particularly in anatomic pathology. Current OSHA regulations require vapor levels not to exceed 0.75 ppm (measured as a time-weighted average [TWA]) and 2 ppm (measured as a 15-minute short-term exposure). OSHA requires monitoring for formaldehyde vapor wherever formaldehyde is used in the work place. The laboratory must have evidence at the time of inspection that formaldehyde vapor levels have been measured, and both 8-hour and 15-minute exposures must have been determined.
If each measurement is below the permissible exposure limit and the 8-hour measurement is below 0.5 ppm, no further monitoring is required as long as laboratory procedures remain constant. If the 0.5-ppm, 8-hour TWA or the 2-ppm, 15-minute level is exceeded, monitoring must be repeated semiannually. If either the 0.75-ppm, 8-hour TWA or the 2-ppm, 15-minute level is exceeded (very unlikely in a routine microbiology laboratory setting), employees must be required to wear respirators. Accidental skin contact with aqueous formaldehyde must be prevented with the use of proper clothing and equipment (gloves, laboratory coats).
The amendments of 1992 add medical removal protection provisions to supplement the existing medical surveillance requirements for employees suffering significant eye, nose, or throat irritation and for those experiencing dermal irritation or sensitization from occupational exposure to formaldehyde. In addition, these amendments establish specific hazard-labeling requirements for all forms of formaldehyde, including mixtures and solutions composed of at least 0.1% formaldehyde in excess of 0.1 ppm. Additional hazard labeling, including a warning label that formaldehyde presents a potential cancer hazard, is required where formaldehyde levels, under reasonably foreseeable conditions of use, may potentially exceed 0.5 ppm. The final amendments also provide for annual training of all employees exposed to formaldehyde at levels of 0.1 ppm or higher.
Note: The use of monitoring badges may not be a sensitive enough method to correctly measure the 15-minute exposure level. Contact the OSHA office in your institution for monitoring options. Usually, the accepted method involves monitoring airflow in the specific area or areas in the laboratory where formaldehyde vapors are found.
POLYVINYL ALCOHOL (PVA) ADHESIVE (NOT A FIXATIVE)
Polyvinyl alcohol (PVA) is a water-soluble, synthetic polymer used as a viscosity-increasing agent in pharmaceuticals, as an adhesive in parasitology fecal fixatives, and as a lubricant and protectant in ophthalmic preparations. PVA is also defined as a water-soluble polymer made by hydrolysis of a polyvinyl ester (e.g., polyvinyl acetate); it is used in adhesives, a textile and paper sizer, and for emulsifying, suspending, and thickening solutions. PVA is not a fixative, but rather an adhesive to help glue stool material onto the slide; this is the only purpose of PVA as an additive to parasitology fecal fixative formulations.
PVA is a plastic resin that is normally incorporated into Schaudinn’s fixative. Although some laboratories may perform a fecal concentration from a PVA-preserved specimen, some parasites do not concentrate well, and some do not exhibit the typical morphology that would be seen in concentration sediment from a formalin-based fixative. PVA fixative solution is highly recommended as a means of preserving cysts and trophozoites for later examination. Use of PVA fixative also allows specimens to be shipped (by regular mail service) from any location in the world to a laboratory for examination. PVA fixative is particularly useful for liquid specimens and should be used in the ratio of 3 parts PVA to 1 part fecal specimen.
Note: Very detailed information on all fixative options can be found in Garcia LS: Diagnostic medical parasitology, ed 5, Washington, DC, 2007, ASM Press,.
*These two fixatives use the mercuric chloride base in the Schaudinn’s fixative; this formulation is still considered the gold standard against which all other fixatives are evaluated (organism morphology after permanent staining).
†This modification uses a copper sulfate base rather than mercuric chloride; the morphology of stained organisms is not as good as with Hg or Zn.
‡This modification (proprietary formula) uses a zinc base rather than mercuric chloride and works well with both trichrome and iron-hematoxylin.
§This fixative uses a combination of ingredients but is prepared from a proprietary formula (contains PVA).
 This modification uses a combination of ingredients, including zinc, but is prepared from a proprietary formula. The aim is to provide a universal fixative that can be used for the fecal concentration, permanent stained smear, and available immunoassays for Giardia lamblia, Cryptosporidium spp., and Entamoeba histolytica (or the Entamoeba histolytica/E. dispar group). However, currently, fecal immunoassays for the Entamoeba histolytica/E. dispar group and Entamoeba histolytica (true pathogen) require fresh or frozen specimens; testing can also be performed from stool submitted in Cary-Blair transport medium.
This modification uses a combination of ingredients, including zinc, but is prepared from a proprietary formula. The aim is to provide a universal fixative that can be used for the fecal concentration, permanent stained smear, and available immunoassays for Giardia lamblia, Cryptosporidium spp., and Entamoeba histolytica (or the Entamoeba histolytica/E. dispar group). However, currently, fecal immunoassays for the Entamoeba histolytica/E. dispar group and Entamoeba histolytica (true pathogen) require fresh or frozen specimens; testing can also be performed from stool submitted in Cary-Blair transport medium.
TABLE 47-10
Common Human Parasites: Diagnostic Specimens, Tests, and Positive Findings
| Organism | Infection Acquired | Location in Host | Diagnostic Specimen | Diagnostic Test* | Positive Specimen | Comments |
| Intestinal Amebae | Ingestion of food or water contaminated with infective cysts; fecal-oral transmission | Intestinal tract; E. histolytica infection may disseminate to the liver (extraintestinal amebiasis); Blastocystis strains are pathogenic or nonpathogenic; cannot differentiate on morphology | Stool; sigmoidoscopy specimens | O&P exam; stained sigmoidoscopy slides; stool immunoassays | Trophozoites and/or cysts | Many of the protozoa can look very much alike; see diagnostic tables for details; immunoassays for E. histolytica/E. dispar group and E. histolytica (fresh, frozen stool, Cary Blair required) |
| Entamoeba histolytica Entamoeba dispar Entamoeba hartmanni Entamoeba coli Endolimax nana Iodamoeba bütschlii Blastocystis hominis |
||||||
| Free-Living Amebae | Contaminated water or soil; dust, contaminated eye solutions; organisms may enter through nasal mucosa, travel to brain via olfactory nerve | CNS (PAM, GAE); eye | CSF, corneal scrapings, biopsy, eye care solutions; CSF exam STAT REQUEST |
Stains, culture, FA, biopsy; B. mandrillaris cannot be grown on agar culture, whereas N. fowleri and Acanthamoeba spp. can | Trophozoites or cysts | CNS disease life-threatening with N. fowleri; other CNS infections more chronic; keratitis can lead to blindness |
| Naegleria fowleri Acanthamoeba spp. Balamuthia mandrillaris Sappinia spp. |
||||||
| Intestinal Flagellates | Ingestion of food or water contaminated with infective cysts or trophozoites (D. fragilis, T. hominis); fecal-oral transmission | Intestinal tract | Stool; duodenal specimens or Entero-Test capsule (string test) for Giardia lamblia | O&P exam; wet preparations or stains of duodenal material; stool immunoassays (can use fresh or formalin fixed specimens; no PVA); need two specimens for IA for G. lamblia | Trophozoites or cysts | G. lamblia is very difficult to recover; stool immunoassays are more sensitive than routine O&P exams; D. fragilis requires permanent stain for identification |
| Giardia lamblia Dientamoeba fragilis Chilomastix mesnili Pentatrichomonas hominis |
||||||
| Urogenital Flagellates | Sexually transmitted; wet towels less likely but possible | Urinary tract; genital system; males may be asymptomatic | Vaginal secretions, prostatic fluid, often recovered in urine sediment | Wet preparations, culture, immunoassays; molecular testing | Trophozoites | Often diagnosed by motility in urine sediment or wet preparations |
| Trichomonas vaginalis | ||||||
| Intestinal Ciliate | Ingestion of food or water contaminated with infective cysts; fecal-oral transmission | Intestinal tract | Stool | O&P exam; wet preparations better than permanent stained smear | Trophozoites and/or cysts | Not common in the United States; associated with pigs; seen in proficiency testing specimens |
| Balantidium coli | ||||||
| Intestinal Coccidia | Ingestion of food or water contaminated with infective oocysts; fecal-oral transmission | Intestinal tract; Cryptosporidium spp. can disseminate to other tissues in compromised host (lung, gallbladder) | Stool; biopsy; duodenal specimen; sputum | Modified acid-fast stains; stool immunoassays; concentration wet prep for I. belli | Oocysts in stool or scrapings; other developmental stages in tissues | Cryptosporidium spp. cause severe diarrhea in compromised patient; nosocomial transmission |
| Cryptosporidium spp. Cyclospora cayetanensis Isospora belli |
||||||
| Intestinal Microsporidia† | Ingestion of food or water contaminated with infective spores; fecal-oral transmission | Intestinal tract; organisms can disseminate to other body sites (kidney) | Stool; biopsy | Modified trichrome stains; optical brightening agents; experimental immunoassays; biopsy and histology (tissue Gram stains) | Spores in stool; other developmental stages in tissues | Can cause serious diarrhea in compromised host; less known about infections in normal host |
| Enterocytozoon bieneusi Encephalitozoon (Septata) intestinalis |
||||||
| Microsporidia, Other Body Sites | Ingestion of food or water contaminated with infective spores; fecal-oral transmission; inhalation; direct environmental contact to eyes; probably hands to eyes | All tissues | All body fluids and/or tissues relevant, depending on body site | Modified trichrome stains; optical brightening agents; experimental immunoassays; biopsy and histology (tissue Gram stains) | Spores in stool, urine, other body fluids; developmental stages in tissues | Can cause serious diarrhea in compromised host; less known about infections in normal host; a number of eye infections documented in immunocompetent patients |
| Encephalitozoon spp. Brachiola vesicularum Microsporidium Anncaliia Pleistophora Trachipleistophora Vittaforma Tubulinosema |
||||||
| Tissue Protozoa | T. gondii: ingestion of raw meat; oocysts from cat feces | Eye, CNS in compromised patient (T. gondii) | Biopsies (any tissue), CSF | Serology, tissue culture; recovery from CSF | Positive serology; recovery of trophozoites in CSF | Many people have positive serologies for T. gondii; infections are serious in immunocompromised patients |
| Toxoplasma gondii | ||||||
| Intestinal Nematodes | Ingestion of food or water contaminated with infective eggs; penetration of skin by infective larvae in soil | Intestine; S. stercoralis may disseminate (hyperinfection), primarily in immunocompromised patients | Stool; duodenal contents (S. stercoralis); Scotch tape preps or paddles for E. vermicularis | O&P exam; special concentrates and cultures; examination of tapes for E. vermicularis | Adult worms, eggs and/or larvae, depending on the roundworm involved | Review direct and indirect life cycles (migration through heart, lung, trachea to intestine), 4 to 6 consecutive tapes required to rule out Enterobius infection |
| Enterobius vermicularis Trichuris trichiura Ascaris lumbricoides Hookworm Strongyloides stercoralis |
||||||
| Tissue Nematodes | ||||||
| VLM, OLM (Toxocara spp.) | Ingestion of infective eggs | Migration through tissues | Serum | Serology | Positive serology | Human is accidental host for VLM, OLM, and CLM |
| CLM (dog/cat hookworm) | Skin penetration of larvae | Skin tracks, migration | Visual inspection | Presence of tracks/skin | Eosinophilia, visual tracks | |
| Trichinella spp. | Ingestion of raw pork | Muscle | Serum, muscle biopsy | Serology, squash prep | Positive serology, larvae | Outbreaks still occur |
| Anisakis, others | Ingestion of raw marine fish | Intestine | Submission of larvae | ID of larvae | Positive larval ID | Sometimes identified only after surgical removal |
| Intestinal Cestodes | Ingestion of: | Intestine | Eggs for the two Taenia spp. look alike; gravid proglottid or scolex is needed to make identification | |||
| Taenia saginata (beef) | Raw beef | Stool and/or proglottids | O&P, India ink proglottids | Eggs, proglottid branches | ||
| Taenia solium (pork) | Raw pork | Stool and/or proglottids | O&P, India ink proglottids | Eggs, proglottid branches | ||
| Diphyllobothrium latum | Raw freshwater fish | Stool and/or proglottids | O&P | Eggs, proglottid shape | ||
| Hymenolepis nana | Tapeworm eggs | Stool | O&P | Eggs | ||
| Hymenolepis diminuta | Grain beetles | Stool | O&P | Eggs | ||
| Dipylidium caninum | Fleas from dogs/cats | Stool and/or proglottids | O&P | Eggs, proglottid shape | ||
| Tissue Cestodes | Ingestion of: | |||||
| Echinococcus granulosus | Eggs from dog tapeworm | Liver, lung, and so on | Serum, hydatid cyst aspirate; biopsy | Serology, centrifugation of fluid; histology | Positive serology; hydatid sand, tapeworm tissue | E. granulosus (enclosed cyst) |
| E. multilocularis | Eggs from fox tapeworm | E. multilocularis (cyst wanders through tissue) | ||||
| Taenia solium (pork) | Eggs from human tapeworm | CNS, subcutaneous tissues | Serum, scans, biopsy | Serology, films, histology | Positive serology, positive scans, tapeworm tissue | Small, enclosed cysticerci (cysticercosis) |
| Intestinal Trematodes | Ingestion of metacercariae: | |||||
| Fasciolopsis buski | On water chestnuts | Intestine | Stool | O&P exam; M. yokogawai, H. heterophyes eggs very small; use high dry power | Eggs in stool | Eggs of F. buski look identical to those of the liver fluke, Fasciola hepatica |
| Metagonimus yokogawai | In raw fish | |||||
| Heterophyes heterophyes | ||||||
| Liver and Lung Trematodes | Ingestion of metacercariae: | |||||
| Fasciola hepatica | On watercress | Liver | Stool | O&P | Eggs in stool | Eggs of F. hepatica look almost identical to those of F. buski; lung fluke eggs in sputum resemble brown metal filings |
| Clonorchis sinensis | In raw fish | Liver, bile ducts | Stool, duodenal drainage | O&P | Eggs in stool, and so on | |
| Paragonimus spp. | In raw crabs | Lung | Stool, sputum | O&P | Eggs in stool and/or sputum | |
| Blood Trematodes | Veins over the: | |||||
| Schistosoma mansoni | Skin penetration of cercariae released from the freshwater snail intermediate host | Large intestine | Because the adult worms may become located in the “incorrect” veins, both urine and stool (unpreserved) should be examined | O&P; hatching test for egg viability (all specimens collected with no preservatives); concentrates performed with saline, not water | Eggs in stool and/or urine | When schistosomiasis is suspected, stool, spot urines, and 24-hr urine specimen (collected with no preservatives) |
| S. haematobium | Bladder | |||||
| S. japonicum | Small intestine | |||||
| Malaria | Preerythrocytic: | |||||
| Plasmodium vivax | Infection through mosquito bite, blood transfusion, shared drug needles; transplacental | Liver | Drawn immediately: STAT REQUEST Blood; draw every 6 hr until confirmed as positive or negative |
Thick, thin blood films; rapid immunoassay methods (not yet FDA approved in United States); concentration methods | Parasites present | P. falciparum and P. knowlesi infections are medical emergencies; complete patient history mandatory (travel, prophylaxis, prior history); Giemsa or other blood stain recommended |
| P. ovale | Blood | |||||
| P. malariae | Blood | |||||
| P. knowlesi | Blood | |||||
| P. falciparum | Blood Blood plus capillaries of deep tissues (spleen, liver, bone marrow) |
|||||
| Babesiosis Babesia spp. |
Tick borne; transfusion; organ transplants | Blood | Blood | Thick, thin blood films | Parasites present | Can mimic ring forms of P. falciparum; patient will have no travel history outside of United States |
| Trypanosomes | ||||||
| Trypanosoma brucei gambiense | Bite of tsetse fly | Blood, lymph nodes, CNS | Blood, node aspirate, CSF | Thick, thin films | Trypomastigotes | African sleeping sickness more common with T. b. gambiense Chagas disease (American trypanosomiasis) (xenodiagnosis an option) |
| T. b. rhodesiense | Bite of tsetse fly | Blood, lymph nodes, CNS | Thick, thin films | Trypomastigotes | ||
| Trypanosoma cruzi | Feces of triatomid bug (kissing bug) (bug feces scratched into bite site) | Blood, striated muscle (e.g., heart, GI tract) | Blood, cardiac changes, muscle biopsy | Thick, thin films, histology; culture | Trypomastigotes in blood, amastigotes in tissue | |
| Leishmaniae | Macrophages of: | |||||
| Leishmania tropica complex (cutaneous) | Bite of sand fly | Skin | Skin biopsy | Stained smears, cultures | Amastigotes in clinical specimens indicated | Animal inoculation rarely used; some research labs now using PCR |
| L. braziliensis complex (mucocutaneous) | Skin, mucous membranes | Skin, membrane biopsy | Stained smears, cultures | |||
| L. donovani complex (visceral) | Spleen, liver, bone marrow (RE system) | Blood, bone marrow, liver/spleen biopsy | Thick, thin blood films; stained smears, cultures | |||
| Filarial Nematodes | Bite of: | |||||
| Wuchereria bancrofti (S) | Mosquito | Lymphatics (adults), blood (microfilariae) Nodules (adults), skin, eye (microfilariae) |
Blood Skin snips, blood; biopsy nodule |
Thick, thin films; various concentrations Biopsy, tease skin apart in water; thick, thin films |
Microfilariae | Elephantiasis possible; periodicity a factor in finding microfilariae; some microfilariae sheathed (S), some not (NS) “African eye worm” |
| Onchocerca volvulus (NS) | Black fly | Microfilariae; adult in tissue nodules | ||||
| Less Common | ||||||
| Loa loa (S) | Black gnat | Eye (adults) Lymphatics (adults) blood (microfilariae) for all three |
Blood | Thick, thin films; various concentrations | Microfilariae, adult worm Microfilariae |
|
| Brugia malayi (S) | Mosquito | |||||
| Mansonella spp. (NS) | Mosquito |
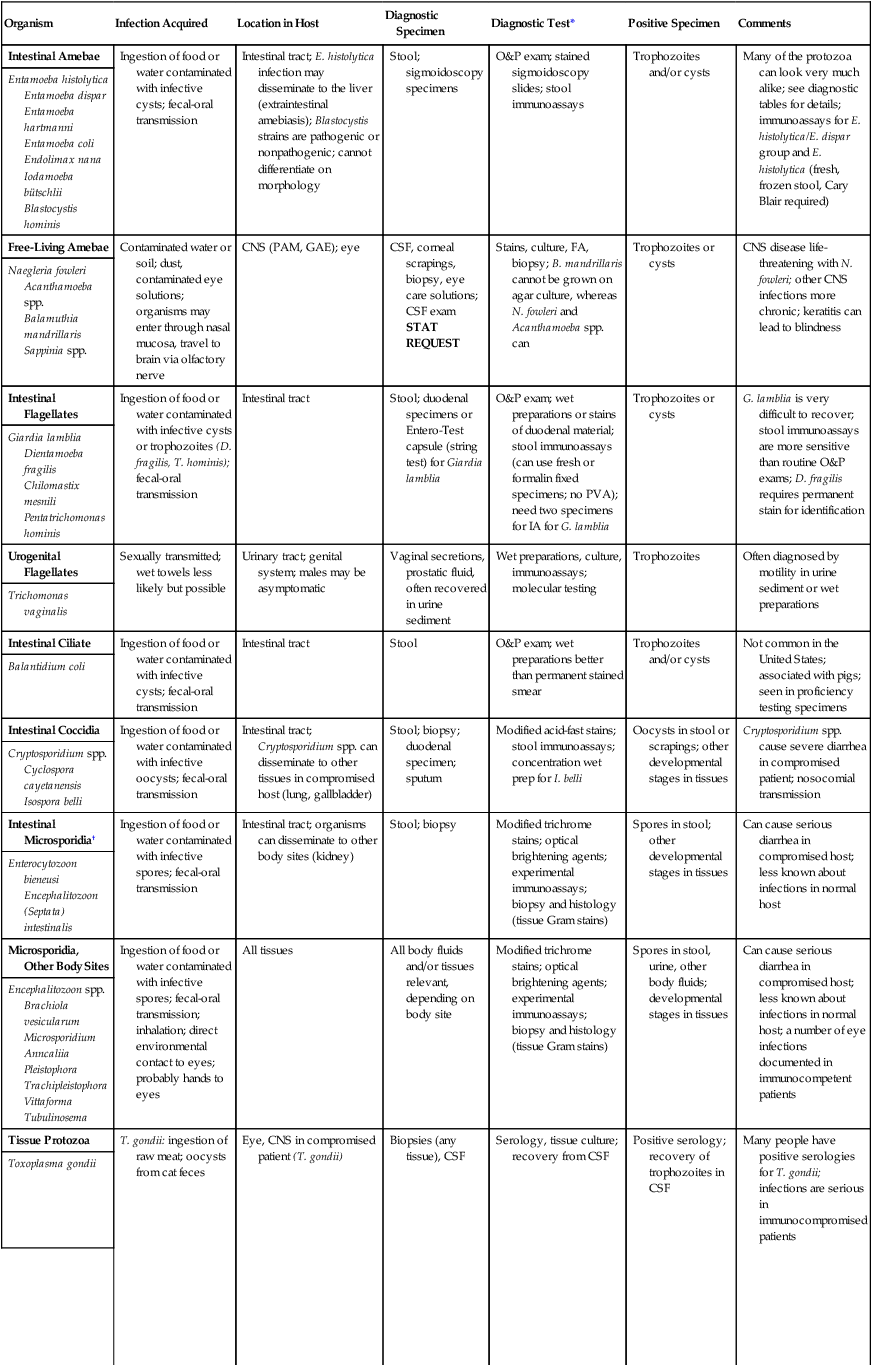
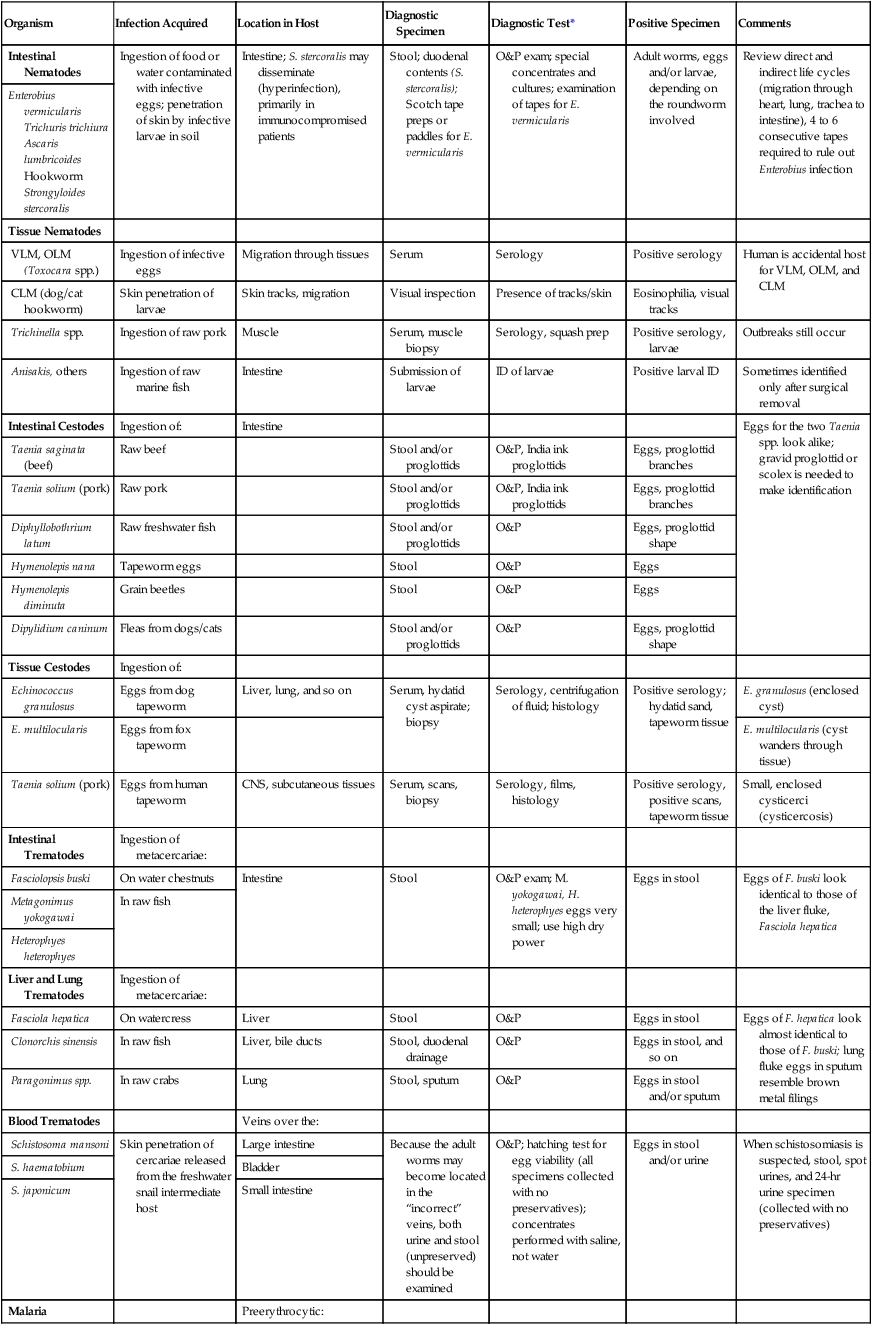
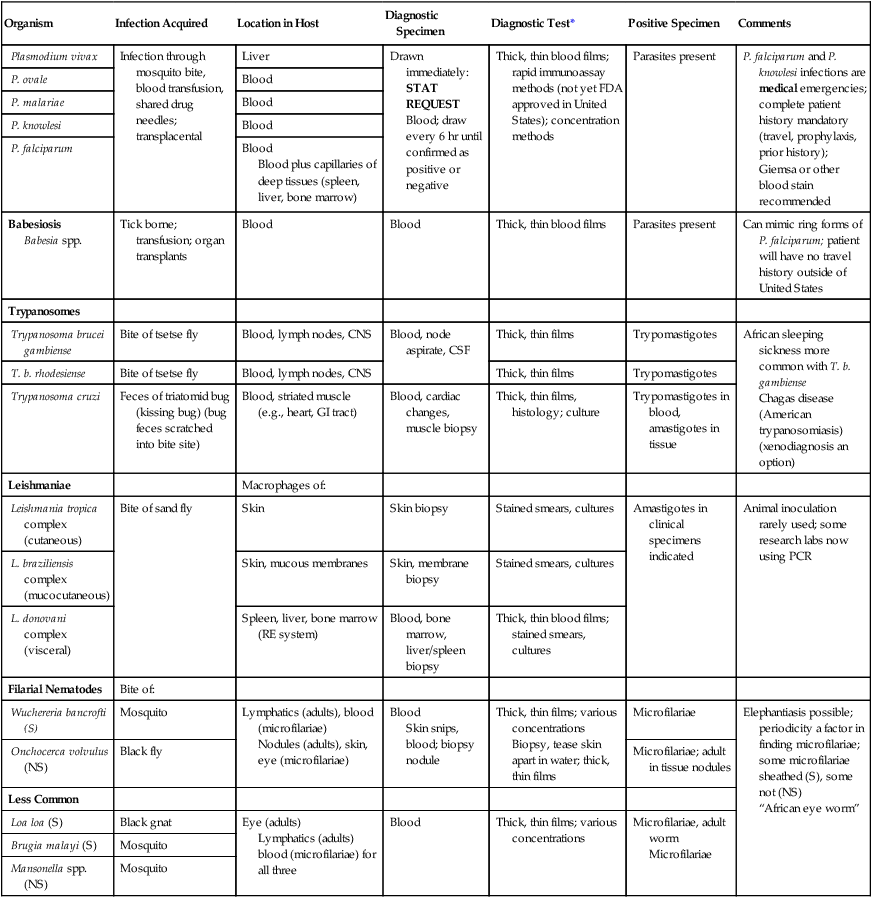
CLM, Cutaneous larva migrans; CSF, cerebrospinal fluid; CNS, central nervous system; FA, fluorescent antibody; GAE, granulomatous amebic encephalitis; IA, immunoassay; ID, identification; NS, not sheathed; OLM, ocular larva migrans; O&P, ova and parasite; PAM, primary amebic meningoencephalitis; PCR, polymerase chain reaction; RE, reticuloendothelium system; S, sheathed; VLM, visceral larva migrans.
*Although serologic tests are not always mentioned, they are available for a number of parasitic infections. Unfortunately, most are not routinely available. Contact your state Public Health Laboratory or the Centers for Disease Control and Prevention (CDC) in Atlanta, Georgia.







