Chapter 31 Laboratory Investigations in Diagnosis and Management of Neurological Disease
The history and examination are key to making the diagnosis in a patient with neurological disease (see Chapter 1). Laboratory investigations are becoming increasingly important in diagnosis and management, however, and are discussed in some detail in later chapters on the specific disorders. A test may be diagnostic (e.g., the finding of cryptococci in the cerebrospinal fluid [CSF] of a patient with a subacute meningitis, a low vitamin E level in a patient with ataxia and tremor, a low serum vitamin B12 level in a patient with a combined myelopathy and neuropathy).
It is important to use laboratory tests judiciously and to understand their sensitivity, specificity, risks, and costs. The physician must understand how to interpret the hematological, biochemical, and bacteriological studies and the specific neurodiagnostic investigations. The latter studies include clinical neurophysiology, neuroimaging, and the pathological study of biopsy tissue. Knowledge of the various DNA tests available and their interpretation is critical before they are ordered; their results may have far-reaching implications not only for the patient but for all other family members. The neurologist also must have a working knowledge of several related disciplines that provide specific investigations to aid in neurological diagnosis. These include neuropsychology, neuro-ophthalmology, neuro-otology, uroneurology, neuroepidemiology, clinical neurogenetics, neuroimmunology and neurovirology, and neuroendocrinology. Chapter 34, Chapter 35, Chapter 36, Chapter 37, Chapter 38, Chapter 39, Chapter 40, Chapter 41, Chapter 42 describe these disciplines and the investigations they offer.
The investigations used to diagnose neurological disease change rapidly. Genetic studies of DNA mutations in the blood now allow the diagnosis of Huntington disease (HD), a growing number of spinocerebellar ataxias and parkinsonian disorders, a form of autosomal dominant dystonia (DYT1), Duchenne and other muscular dystrophies, many forms of Charcot-Marie-Tooth disease, Rett syndrome, fragile X premutation, and a variety of other neurogenetic disorders (see http://www.ncbi.nlm.nih.gov/sites/GeneTests/?db=GeneTests; http://www.genetests.org; http://www.geneclinics.org; http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=OMIM). Blood tests for human immunodeficiency virus infection (HIV), Lyme disease, and other infections and for various paraneoplastic syndromes affecting the nervous system also can be diagnostic. For example, three types of anti–Purkinje cell antibodies are recognized: anti-Yo (PCA-1), seen with tumors of breast, ovary, and adnexa; atypical anti–cytoplasmic antibody (anti-Tr or PCA-Tr), seen with Hodgkin disease and tumors of the lung and colon; and PCA-2, identified mostly with lung tumors. In addition, three antineuronal antibodies can be detected: anti-Hu (ANNA-1), seen in conjunction with encephalomyelitis, small cell lung tumor, and tumors of breast, prostate, and neuroblastoma; anti-Ri (ANNA-2), found with tumors of breast and ovary; and atypical anti-Hu, seen with tumors of lung, colon, adenocarcinoma, and lymphoma. Anti-CV2 (CRMP) antibody, expressed by oligodendrocytes, is associated with a syndrome of ataxia and optic neuritis and has been seen with small cell lung carcinoma. The role of antineuronal antibodies, such as those presumably directed to components of the basal ganglia, is not established and may be of doubtful pathogenic relevance.
Risk and Cost of Investigations
Risk-to-Benefit Analysis
Lumbar Puncture
LP carries significant risks, the most disastrous being cerebral or cerebellar herniation. The LP may suddenly release elevated CSF pressure produced by an expanding supratentorial lesion and may force the medial temporal lobe through the tentorium cerebelli to compress the midbrain. In the case of an expanding infratentorial lesion, it may cause the cerebellar tonsils to herniate through the foramen magnum and compress the cervicomedullary junction (see Chapter 50B). These herniations can be fatal, so never perform an LP in a patient with a possible space-occupying lesion without first examining the optic fundi for evidence of papilledema or consulting a recent head CT or MRI.
Cerebral Arteriography
The question of whether to request percutaneous cerebral arteriography (see Chapter 33B) entails analysis of the risks and benefits for each patient. In a patient with cerebrovascular disease, the study may show thrombotic or embolic occlusion of arteries and abnormalities of the arterial wall, including arteriosclerotic plaques, fibromuscular hyperplasia, medial dissection, and arteritis. It also may demonstrate an intracranial aneurysm or arteriovenous malformation (AVM). Any of these findings can clarify the diagnosis, treatment, and prognosis. Many of these diagnostic outcomes can be identified with advanced MRI or a combination of MRI, CT, and ultrasound strategies. Thus, it is critical that the attending neurologist have a specific hypothesis in mind before ordering such examinations, as well as understand the relative ability of noninvasive versus invasive tests to demonstrate specific abnormalities in the blood vessels. If treatment (e.g., aneurysm occlusion) can be combined with a diagnostic procedure (e.g., catheter angiography), this may also alter the decision-making outcome.
Brain Biopsy
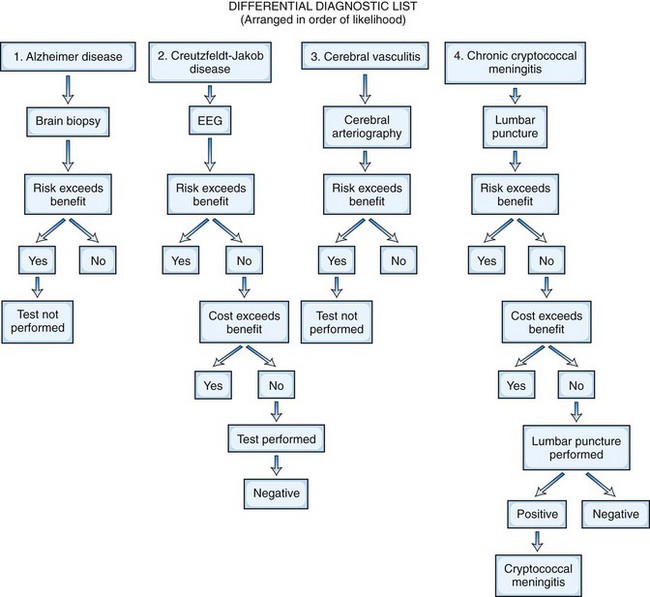
The treatability of the possible cause of the disease is the crucial benefit to consider in the risk-to-benefit analysis. If the neuroimaging study suggests a malignant glioma, for which treatment is likely to be ineffective, biopsy alone may not be considered justified, although resection and tissue diagnosis would be. If it suggests a primary lymphoma of the brain, which is likely to respond to radiotherapy, then confirmatory biopsy may be recommended. If the differential diagnosis in a patient with acquired immunodeficiency syndrome includes toxoplasmosis or lymphoma, it may be reasonable to give anti-Toxoplasma therapy rather than perform a brain biopsy; biopsy would be needed only if the lesions do not respond to 2 to 3 weeks of treatment. Figure 31.1 presents a risk-to-benefit analysis and prioritization of investigations for an 80-year-old man with possible cerebral degeneration.
Patient Confidentiality
Some diagnostic tests, such as the DNA genetic test for HD and the test for HIV 1, necessitate prior counseling about the implications of these tests for possibly affected persons and their families. Results of such tests should be kept separate from the rest of the chart to maintain strict confidentiality for the patient. Physicians and their staff in the United States need to comply with the Health Insurance Portability and Accountability Act of 1996 (http://www.hhs.gov/ocr/hipaa).