Chapter 417 Laboratory Evaluation
417.1 Radiologic Assessment
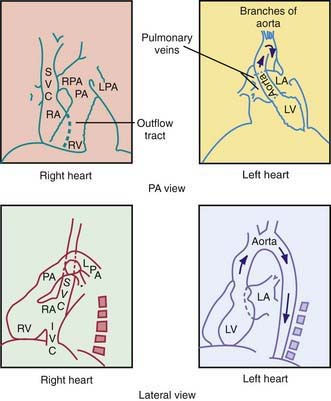
In the posteroanterior view, the left border of the cardiac shadow consists of three convex shadows produced, from above downward, by the aortic knob, the main and left pulmonary arteries, and the left ventricle (Fig. 417-1). In cases of moderate to marked left atrial enlargement, the atrium may project between the pulmonary artery and the left ventricle. The outflow tract of the right ventricle does not contribute to the shadows formed by the left border of the heart. The aortic knob is not as easily seen in infants and children as in adults. The side of the aortic arch (left or right) can often be inferred as being opposite the side of the midline from which the air-filled trachea is visualized. This observation is important because a right-sided aortic arch is often present in cyanotic congenital heart disease, particularly in tetralogy of Fallot. Three structures contribute to the right border of the cardiac silhouette. In the view from above, they are the superior vena cava, the ascending aorta, and the right atrium.
417.2 Electrocardiography
Developmental Changes
The marked changes that occur in cardiac physiology and chamber dominance during the perinatal transition (Chapter 415) are reflected in the evolution of the ECG during the neonatal period. Because vascular resistance in the pulmonary and systemic circulations is nearly equal in a term fetus, the intrauterine work of the heart results in an equal mass of both the right and left ventricles. After birth, systemic vascular resistance rises when the placental circulation is eliminated, and pulmonary vascular resistance falls when the lungs expand. These changes are reflected in the ECG as the right ventricular wall begins to thin.
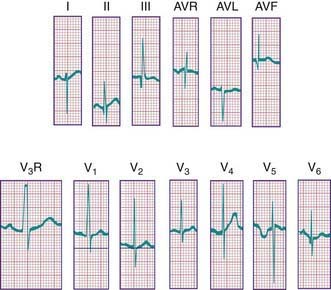
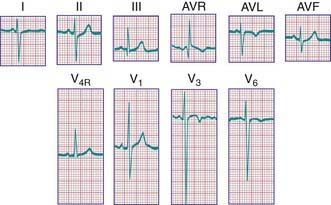
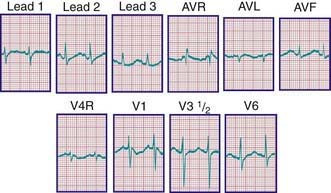
During the 1st days of life, right axis deviation, large R waves, and upright T waves in the right precordial leads (V3R or V4R and V1) are the norm (Fig. 417-2). As pulmonary vascular resistance decreases in the 1st few days after birth, the right precordial T waves become negative. In the great majority of instances, this change occurs within the 1st 48 hr of life. Upright T waves that persist in leads V3R, V4R, or V1 beyond 1 wk of life are an abnormal finding indicating right ventricular hypertrophy or strain, even in the absence of QRS voltage criteria. The T wave in V1 should never be positive before 6 yr of age and may remain negative into adolescence. This finding represents one of the most important, yet subtle differences between pediatric and adult ECGs and is a common source of error when adult cardiologists interpret pediatric ECGs.
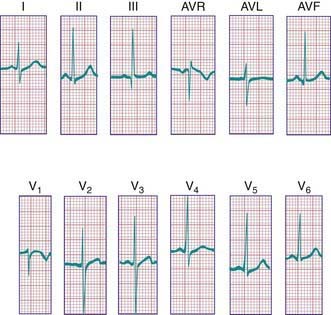
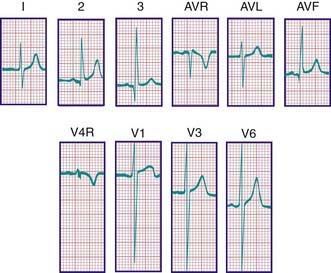
In a newborn, the mean QRS frontal-plane axis normally lies in the range of +110 to +180 degrees. The right-sided chest leads reveal a larger positive (R) than negative (S) wave and may do so for months because the right ventricle remains relatively thick throughout infancy. Left-sided leads (V5 and V6) also reflect right-sided dominance in the early neonatal period, when the R : S ratio in these leads may be <1. A dominant R wave in V5 and V6 reflecting left ventricular forces quickly becomes evident within the 1st few days of life (Fig. 417-3). Over the years, the QRS axis gradually shifts leftward, and the right ventricular forces slowly regress. Leads V1, V3R, and V4R display a prominent R wave until 6 mo to 8 yr of age. Most children have an R : S ratio >1 in lead V4R until they are 4 yr of age. The T waves are inverted in leads V4R, V1, V2, and V3 during infancy and may remain so into the middle of the 2nd decade of life and beyond. The processes of right ventricular thinning and left ventricular growth are best reflected in the QRS-T pattern over the right precordial leads. The diagnosis of right or left ventricular hypertrophy in a pediatric patient can be made only with an understanding of the normal developmental physiology of these chambers at various ages until adulthood is reached. As the left ventricle becomes dominant, the ECG evolves to the characteristic pattern of older children (Fig. 417-4) and adults (Fig. 417-5).
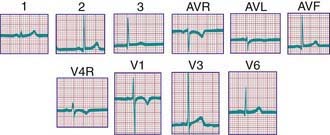
Figure 417-4 Electrocardiogram of a normal child. Note the relatively tall R waves and inversion of the T waves in V4R and V1.
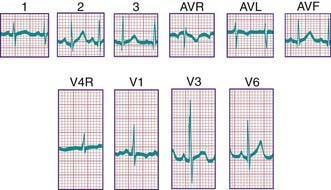
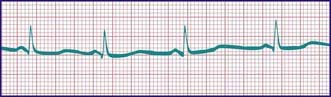
The diagnosis of pathologic right ventricular hypertrophy is difficult in the 1st wk of life because physiologic right ventricular hypertrophy is a normal finding. Serial tracings are often necessary to determine whether marked right axis deviation and potentially abnormal right precordial forces or T waves, or both, will persist beyond the neonatal period (Fig. 417-6). In contrast, an adult electrocardiographic pattern (see Fig. 417-5) seen in a neonate suggests left ventricular hypertrophy. The exception is a premature infant, who may display a more “mature” ECG than a full-term infant (Fig. 417-7) as a result of lower pulmonary vascular resistance secondary to underdevelopment of the medial muscular layer of the pulmonary arterioles. Some premature infants display a pattern of generalized low voltage across the precordium.
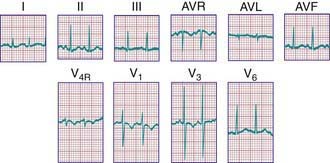
Figure 417-7 Electrocardiogram of a premature infant (weight 2 kg and age 5 wk at the time of the tracing). The cardiovascular system was clinically normal. Left ventricular dominance is manifested by R-wave progression across the chest, similar to tracings obtained from older children. Compare with the tracing from a normal full-term infant (see Fig. 417-3).
P Waves
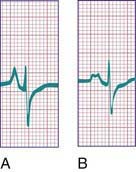
Tall (>2.5 mm), narrow, and spiked P waves are indicative of right atrial enlargement and are seen in congenital pulmonary stenosis, Ebstein anomaly of the tricuspid valve, tricuspid atresia, and sometimes cor pulmonale. These abnormal waves are most obvious in leads II, V3R, and V1 (Fig. 417-8A). Similar waves are sometimes seen in thyrotoxicosis. Broad P waves, commonly bifid and sometimes biphasic, are indicative of left atrial enlargement (Fig. 417-8B). They are seen in some patients with large left-to-right shunts (ventricular septal defect [VSD], patent ductus arteriosus [PDA]) and with severe mitral stenosis or regurgitation. Flat P waves may be encountered in hyperkalemia.
QRS Complex
Right Ventricular Hypertrophy
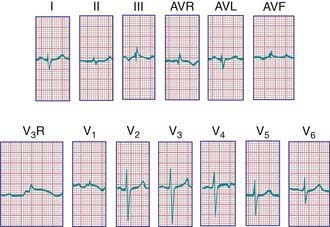
For the most accurate assessment of ventricular hypertrophy, pediatric ECGs should include the right precordial lead V3R or V4R, or both. The diagnosis of right ventricular hypertrophy depends on demonstration of the following changes (see Fig. 417-6): (1) a qR pattern in the right ventricular surface leads; (2) a positive T wave in leads V3R-V4R and V1-V3 between the ages of 6 days and 6 yr; (3) a monophasic R wave in V3R, V4R, or V1; (4) an rsR′ pattern in the right precordial leads with the 2nd R wave taller than the initial one; (5) age-corrected increased voltage of the R wave in leads V3R-V4R or the S wave in leads V6-V7, or both; (6) marked right axis deviation (>120 degrees in patients beyond the newborn period); and (7) complete reversal of the normal adult precordial RS pattern. At least 2 of these changes should be present to support a diagnosis of right ventricular hypertrophy.
Abnormal ventricular loading can be characterized as either systolic (as a result of obstruction of the right ventricular outflow tract, as in pulmonic stenosis) or diastolic (as a result of increased volume load, as in atrial septal defects [ASDs]). These two types of abnormal loads result in distinct electrocardiographic patterns. The systolic overload pattern is characterized by tall, pure R waves in the right precordial leads. In older children, the T waves in these leads are initially upright and later become inverted. In infants and children <6 yr, the T waves in V3R-V4R and V1 are abnormally upright. The diastolic overload pattern (typically seen in patients with ASDs) is characterized by an rsR′ pattern (Fig. 417-9) and a slightly increased QRS duration (minor right ventricular conduction delay). Patients with mild to moderate pulmonary stenosis may also exhibit an rsR′ pattern in the right precordial leads.
Left Ventricular Hypertrophy
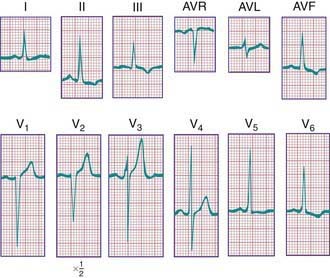
The following features indicate the presence of left ventricular hypertrophy (Fig. 417-10): (1) depression of the ST segments and inversion of the T waves in the left precordial leads (V5, V6, and V7), known as a left ventricular strain pattern—these findings suggest the presence of a severe lesion; (2) a deep Q wave in the left precordial leads; and (3) increased voltage of the S wave in V3R and V1 or the R wave in V6-V7, or both. It is important to emphasize that evaluation of left ventricular hypertrophy should not be based on voltage criteria alone. The concepts of systolic and diastolic overload, though not always consistent, are also useful in evaluating left ventricular enlargement. Severe systolic overload of the left ventricle is suggested by straightening of the ST segments and inverted T waves over the left precordial leads; diastolic overload may result in tall R waves, a large Q wave, and normal T waves over the left precordium. Finally, an infant with an ECG that would be considered “normal” for an older child may, in fact, have left ventricular hypertrophy.
Bundle Branch Block
A complete right bundle branch block may be congenital or may be acquired after surgery for congenital heart disease, especially when a right ventriculotomy has been performed, as in repair of the tetralogy of Fallot. Congenital left bundle branch block is rare; this pattern is occasionally seen with cardiomyopathy. A bundle branch block pattern may be indicative of a bypass tract associated with one of the pre-excitation syndromes (Chapter 429).
P-R and Q-T Intervals
The duration of the Q-T interval varies with the cardiac rate; a corrected Q-T interval (Q-Tc) can be calculated by dividing the measured Q-T interval by the square root of the preceding R-R interval. A normal Q-Tc should be <0.45. It is often lengthened with hypokalemia and hypocalcemia; in the former instance, a U wave may be noted at the end of the T wave (Fig. 417-11). There are a number of medications that can also lengthen the Q-T interval. A congenitally prolonged Q-T interval (Fig. 417-12) may also be seen in children with one of the long Q-T syndromes. These patients are at high risk for ventricular arrhythmias, including a form of ventricular tachycardia known as torsades de pointes, and sudden death (Chapter 429.5).
ST Segment and T-wave Abnormalities
T-wave inversion may occur in myocarditis and pericarditis, or it may be a sign of either right or left ventricular hypertrophy and strain. Hypothyroidism may produce flat or inverted T waves in association with generalized low voltage. In hyperkalemia, the T waves are commonly of high voltage and are tent-shaped (Fig. 417-13).
Garson A. The electrocardiogram in infants and children: a systematic approach. Philadelphia: Lea & Febiger; 1983.
Lawless CE, Best TM. Electrocardiograms in athletes: interpretation and diagnostic accuracy. Med Sci Sports Exerc. 2008;40:787-798.
O’Connor M, McDaniel N, Brady WJ. The pediatric electrocardiogram: part I: age-related interpretation. Am J Emerg Med. 2008;26:506-512.
O’Connor M, McDaniel N, Brady WJ. The pediatric electrocardiogram: part III: congenital heart disease and other cardiac syndromes. Am J Emerg Med. 2008;26:497-503.
417.4 Echocardiography
Transthoracic echocardiography has replaced invasive studies such as cardiac catheterization for the initial diagnosis of most forms of congenital heart disease. The echocardiographic examination can be used to evaluate cardiac structure in congenital heart lesions, estimate intracardiac pressures and gradients across stenotic valves and vessels, quantitate cardiac contractile function (both systolic and diastolic), determine the direction of flow across a defect, examine the integrity of the coronary arteries, and detect the presence of vegetations from endocarditis, as well as the presence of pericardial fluid, cardiac tumors, and chamber thrombi. Echocardiography may also be used to assist in the performance of interventional procedures, including pericardiocentesis, balloon atrial septostomy (Chapter 425.2), atrial or ventricular septal defect closure and endocardial biopsy, and in the placement of flow-directed pulmonary artery (Swan-Ganz) monitoring catheters. Transesophageal echocardiography is used routinely to monitor ventricular function in patients during difficult surgical procedures and can provide an immediate assessment of the results of surgical repair of congenital heart lesions. A complete transthoracic echocardiographic examination usually entails a combination of M-mode and two-dimensional (2-D) imaging, as well as pulsed, continuous, and color Doppler flow studies. Doppler tissue imaging and other new technologies provide more quantitative assessments of ventricular function. Three-dimensional (3-D) echocardiography provides valuable information regarding cardiac morphology.
M-mode Echocardiography
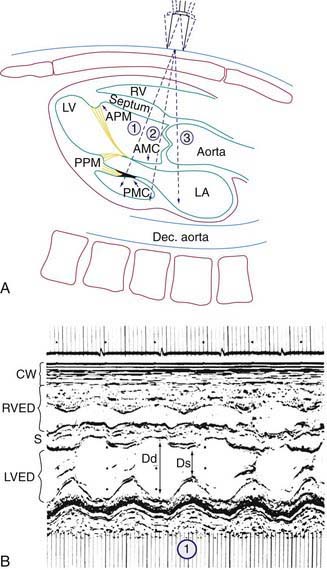
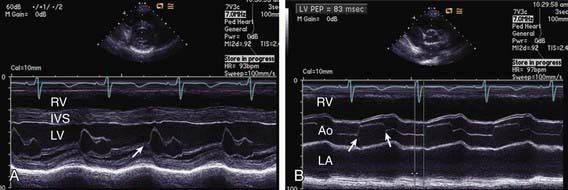
M-mode echocardiography displays a one-dimensional slice of cardiac structure varying over time (Fig. 417-14). It is used mostly for the measurement of cardiac dimensions (wall thickness and chamber size) and cardiac function (fractional shortening, wall thickening). M-mode echocardiography is also useful for assessing the motion of intracardiac structures (opening and closing of valves, movement of free walls and septa) and the anatomy of valves (Fig. 417-15). The most frequently used index of cardiac function in children is percent fractional shortening (%FS), which is calculated as (LVED − LVES)/LVED, where LVED is left ventricular (LV) dimension at end-diastole and LVES is LV dimension at end-systole. Normal fractional shortening is approximately 28-40%. Other M-mode indices of cardiac function include the mean velocity of fiber shortening (mean VCF), systolic time intervals (LVPEP = LV pre-ejection period, LVET = LV ejection time), and isovolemic contraction time. More sophisticated indices of cardiac function can be derived noninvasively with the assistance of echocardiography (pressure-volume relationship, end-systolic wall stress-strain relationship).
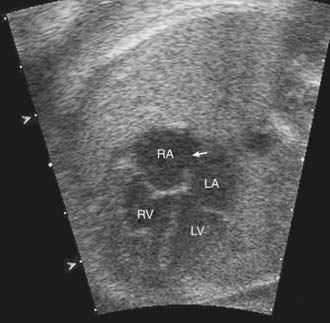
Two-Dimensional Echocardiography
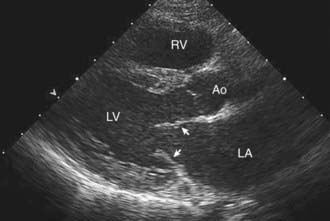
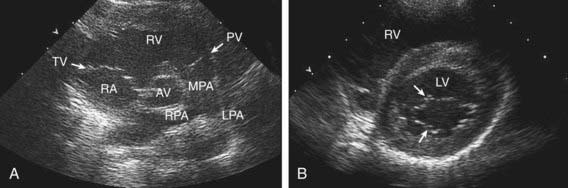
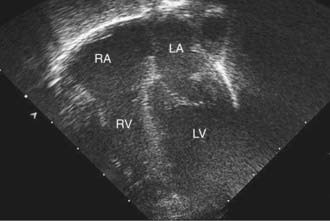
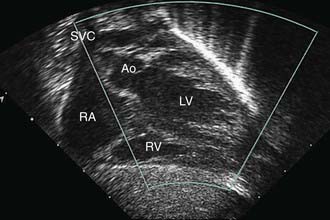
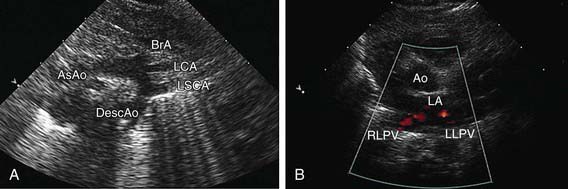
2-D echocardiography provides a real-time image of cardiac structures. With 2-D echocardiography, the contracting heart is imaged in real time using several standard views, including parasternal long axis (Fig 417-16), parasternal short axis (Fig. 417-17), apical four chamber (Fig. 417-18), subcostal (Fig. 417-19), and suprasternal (Fig. 417-20) windows, each of which emphasizes specific structures. 2-D echocardiography has replaced cardiac angiography for the preoperative diagnosis of most, but not all congenital heart lesions; it exceeds angiography in imaging the atrioventricular valves and their chordal attachments. When information from the cardiac examination or other studies is not consistent with the echocardiogram (e.g., the size of a left-to-right shunt), cardiac catheterization remains an important tool to confirm the anatomic diagnosis and evaluate the degree of physiologic derangement.
Doppler Echocardiography
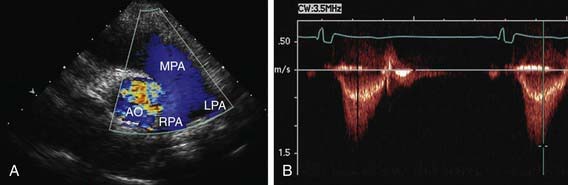
Doppler echocardiography displays blood flow in cardiac chambers and vascular channels based on the change in frequency imparted to a sound wave by the movement of erythrocytes. In pulsed Doppler and continuous wave Doppler, the speed and direction of blood flow in the line of the echo beam change the transducer’s reference frequency. This frequency change can be translated into volumetric flow (L/min) data for estimating systemic or pulmonary blood flow and into pressure (mm Hg) data for estimating gradients across the semilunar or atrioventricular valves or across septal defects or vascular communications such as shunts. Color Doppler permits highly accurate assessment of the presence and direction of intracardiac shunts and allows identification of small or multiple left-to-right or right-to-left shunts (Fig. 417-21). The severity of valvular insufficiency can be evaluated with both pulsed and color Doppler (Fig. 417-22). Alterations in venous Doppler flow patterns can be used to detect abnormalities of systemic and pulmonary veins and alterations of atrioventricular valve Doppler flow patterns can be used to assess ventricular diastolic functional abnormalities.
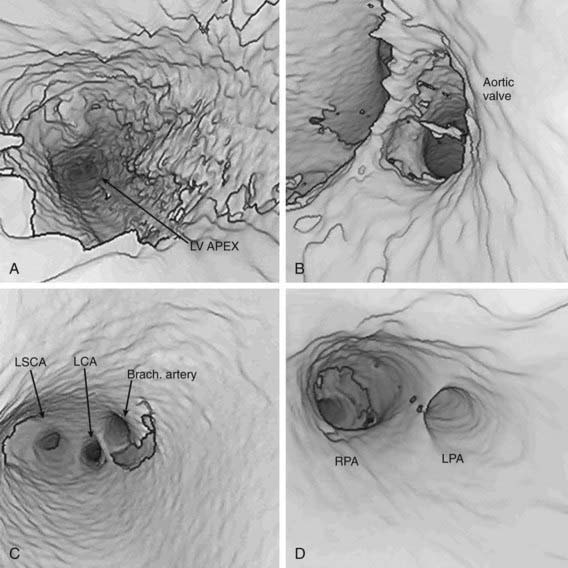
Three-Dimensional Echocardiography
Real-time 3-D echocardiographic reconstruction is valuable for the assessment of cardiac morphology (Fig. 417-23). Details of valve structure, the size and location of septal defects, abnormalities of the ventricular myocardium, and details of the great vessels, which may not be as readily apparent using 2-D imaging, can often be appreciated on 3-D echo. Reconstruction of the view that the surgeon will encounter in the operating room makes this technique a valuable adjunct for preoperative imaging.
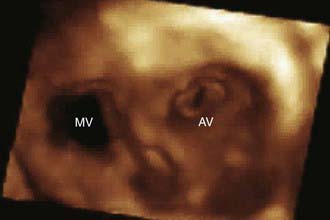
Fetal Echocardiography
Fetal echocardiography can be used to evaluate cardiac structures or disturbances in cardiac rhythm (Fig. 417-24). Perinatologists often detect gross abnormalities in cardiac structure on routine obstetric ultrasonography or may refer the patient because of unexplained hydrops fetalis, a family history of congenital heart disease, or a maternal condition associated with fetal cardiac pathology such as gestational diabetes. Fetal echocardiography is capable of diagnosing most significant congenital heart lesions as early as 17-19 wk of gestation; however, accuracy at this early stage is still limited and families should understand that these studies cannot totally eliminate the possibility of congenital heart disease. Serial fetal echocardiograms have also demonstrated the importance of flow disturbance in the pathogenesis of congenital heart disease; such studies can show the intrauterine progression of a moderate lesion, such as aortic stenosis, into a more severe lesion, such as hypoplastic left heart syndrome. M-mode echocardiography can diagnose rhythm disturbances in the fetus and can determine the success of antiarrhythmic therapy administered to the mother. A screening fetal echocardiogram is recommended for women with a previous child or 1st-degree relative with congenital heart disease, for those who are at higher risk of having a child with cardiac disease (insulin-dependent diabetics, patients with exposure to teratogenic drugs during early pregnancy), and in any fetus in which a chromosomal abnormality is suspected or confirmed.
Alagarsamy S, Chhabra M, Gudavalli M, et al. Comparison of clinical criteria with echocardiographic findings in diagnosing PDA in preterm infants. J Perinat Med. 2005;33:161-164.
Baker GH, Shirali G, Ringewald JM, et al. Usefulness of live three-dimensional transesophageal echocardiography in a congenital heart disease center. Am J Cardiol. 2009;103:1025-1028.
Benavidez OJ, Gauvreau K, Jenkins KJ, et al. Diagnostic errors in pediatric echocardiography: development of taxonomy and identification of risk factors. Circulation. 2008;117:2995-3001.
DeGroff CG. Doppler echocardiography. Pediatr Cardiol. 2002;23:307-333.
Friedberg MK, Silverman NH, Dubin AM, et al. Mechanical dyssynchrony in children with systolic dysfunction secondary to cardiomyopathy: a Doppler tissue and vector velocity imaging study. J Am Soc Echocardiogr. 2007;20:756-763.
Friedberg MK, Silverman NH, Moon-Grady AJ, et al. Prenatal detection of congenital heart disease. J Pediatr. 2009;15:26-31.
Frommelt PC. Update on pediatric echocardiography. Curr Opin Pediatr. 2005;17:579-585.
Hijazi ZM, Shivkumar K, Sahn DJ. Intracardiac echocardiography during interventional and electrophysiological cardiac catheterization. Circulation. 2009;119:587-596.
Kluckow M, Seri I, Evans N. Echocardiography and the neonatologist. Pediatr Cardiol. 2008;29:1043-1047.
417.5 Exercise Testing
Braden DS, Strong WF. Cardiovascular responses to exercise in childhood. Am J Dis Child. 1990;144:1255-1260.
Cava JR, Danduran MJ, Fedderly RT, et al. Exercise recommendations and risk factors for sudden cardiac death. Pediatr Clin North Am. 2004;51:1401-1420.
Friedberg MK, Silverman NH, Moon-Grady AJ, et al. Prenatal detection of congenital heart disease. J Pediatr. 2009;15:26-31.
James FW, Blomqvist CG, Freed MD, et al. Standards for exercise testing in the pediatric age group: American Heart Association Council on Cardiovascular Disease in the Young. Ad hoc committee on exercise testing. Circulation. 1982;66:1377A-1397A.
Stephens PJr, Paridon SM. Exercise testing in pediatrics. Pediatr Clin North Am. 2004;51:1569-1587.
Washington RL, van Gundy JC, Cohen C, et al. Normal aerobic and anaerobic exercise data for North American school-age children. J Pediatr. 1988;112:223-233.
417.6 MRI, MRA, CT, and Radionuclide Studies
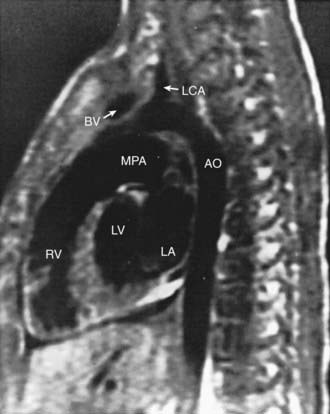
Magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA) are extremely helpful in the diagnosis and management of patients with congenital heart disease. These techniques produce tomographic images of the heart in any projection (Fig. 417-25), with excellent contrast resolution of fat, myocardium, and lung, as well as moving blood from blood vessel walls. MRI has been useful in evaluating areas that are less well visualized by echocardiography, such as distal branch pulmonary artery anatomy and anomalies in systemic and pulmonary venous return.
Computer processing of MRA images allows the noninvasive visualization of the cardiovascular system from inside of the heart or vessels, a technique known as fly-through imaging. These images allow the cardiologist to image the interiors of various cardiovascular structures (Fig. 417-26). These imaging techniques are especially helpful in imaging complex peripheral arterial stenoses, especially after balloon angioplasty.
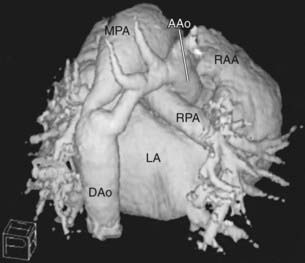
CT scanning can now be used to perform rapid, respiration-gated cardiac imaging in children with resolutions down to 0.5 mm. 3-D reconstruction of CT images (Fig. 417-27) are especially useful in evaluating branch pulmonary arteries, anomalies in systemic and pulmonary venous return, and great vessel anomalies such as coarctation of the aorta.
Chan FP. MR and CT imaging of the pediatric patient with structural heart disease. Semin Thorac Cardiovasc Surg. 2008;20:393-399.
Feinstein JA, Gatzoulis MA. Use of magnetic resonance imaging and computed tomography. Cardiol Young. 2009;19(Suppl 1):16-22.
Mertens L, Ganame J, Eyskens B. What is new in pediatric cardiac imaging? Eur J Pediatr. 2008;167:1-8.
Prakash A, Powell AJ, Krishnamurthy R, et al. Magnetic resonance imaging evaluation of myocardial perfusion and viability in congenital and acquired pediatric heart disease. Am J Cardiol. 2004;93:657-661.
Wald RM, Haber I, Wald R, et al. Effects of regional dysfunction and late gadolinium enhancement on global right ventricular function and exercise capacity in patients with repaired tetralogy of Fallot. Circulation. 2009;119:1370-1377.
Wolfson BJ. Radiologic interpretation of congenital heart disease. Clin Perinatol. 2001;28:71-89.
417.7 Diagnostic and Interventional Cardiac Catheterization
Diagnostic Cardiac Catheterization
Diagnostic catheterization is still performed: (1) to assist in the initial diagnosis of some complex congenital heart lesions (e.g., tetralogy of Fallot with pulmonary atresia and major aortopulmonary collateral arteries [MAPCAs], pulmonary atresia with intact ventricular septum and coronary sinusoids, hypoplastic left heart syndrome with mitral stenosis); (2) in cases in which other imaging studies are equivocal; (3) in patients for whom hemodynamic assessment is critical (to determine the size of a left-to-right shunt in borderline cases, or to determine the presence or absence of pulmonary vascular disease in an older patient with a left-to-right shunt); (4) between stages of repair of complex congenital heart disease (e.g., hypoplastic left or right heart syndromes); (5) for myocardial biopsy in the diagnosis of cardiomyopathy or in screening for cardiac rejection after cardiac transplantation; and (6) for electrophysiologic study in the evaluation of cardiac arrhythmias (Chapter 429).
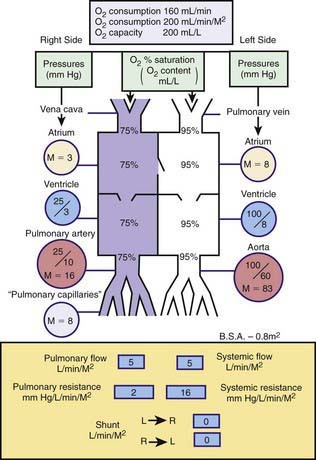
Catheterization may be limited to the right-sided cardiac structures, the left-sided structures, or both the right and left sides of the heart. The catheter is passed into the heart under fluoroscopic guidance through a percutaneous entry point in a femoral or jugular vein. In infants and in a number of older children, the left side of the heart can be accessed by passing the catheter across a patent foramen ovale to the left atrium and left ventricle. If the foramen is closed, the left side of the heart can be catheterized by passing the catheter retrograde via a percutaneous entry site in the femoral artery, or if necessary, via a trans-atrial septal puncture. The catheter can be manipulated through abnormal intracardiac defects (ASDs, VSDs). Blood samples are obtained for measuring oxygen saturation and calculating shunt volumes, pressures are measured for calculating gradients and valve areas, and radiopaque contrast is injected to delineate cardiac and vascular structures. A catheter with a thermosensor tip can be utilized for measurement of cardiac output by thermodilution. Specialized catheters can be utilized to measure more sophisticated indices of cardiac function: Those with pressure-transducer tips can be utilized to measure the first derivative of left ventricular pressure (dP/dt); and conductance catheters can be used to generate pressure-volume loops, from which indices of both contractility (end-systolic elastance) and relaxation can be derived. Complete hemodynamics can be calculated, including cardiac output, intracardiac left-to-right and right-to-left shunts, and systemic and pulmonary vascular resistances. Normal circulatory dynamics are depicted in Figure 417-28.
Interventional Cardiac Catheterization
The miniaturization of catheter delivery systems has allowed for the safe application of many of these interventional catheterization techniques, even in neonates and premature infants. Catheter treatment is now the standard of practice for most cases of isolated pulmonary or aortic valve stenosis (see Fig. 421-4) as well as for re-coarctation of the aorta. A special catheter with a sausage-shaped balloon at the distal end is passed through the obstructed valve. Rapid filling of the balloon with a mixture of contrast material and saline solution results in tearing of the stenotic valve tissue, usually at the site of inappropriately fused raphe. Valvular pulmonary stenosis can be treated successfully by balloon angioplasty; in most patients, angioplasty has replaced surgical repair as the initial procedure of choice. The clinical results of this procedure are similar to those obtained by open heart surgery, but without the need for sternotomy or prolonged hospitalization. Balloon valvuloplasty for aortic stenosis has also yielded excellent results, although, as with surgery, aortic stenosis often recurs as the child grows and multiple procedures may thus be required. One complication of both valvuloplasty and surgery is the creation of valvular insufficiency. This complication has more serious implications when it occurs on the aortic vs the pulmonary side of the circulation because regurgitation is less well tolerated at systemic arterial pressures. Balloon angioplasty is the procedure of choice for patients with re-stenosis of coarctation of the aorta after earlier surgery. It is controversial whether angioplasty is the best procedure for native (unoperated) coarctation of the aorta because of reports of later aneurysm formation and most centers still refer primary coarctation in infants and young children for surgical repair. However, in older patients with previously undiagnosed coarctation, especially those with decreased left ventricular function, primary angioplasty with possible stent placement, may be considered. Other applications of the balloon angioplasty technique include amelioration of mitral stenosis, dilatation of surgical conduits (Mustard or Senning atrial baffles), relief of branch pulmonary artery narrowing, dilatation of systemic or pulmonary venous obstructions, and the long-used balloon atrial septostomy (Rashkind procedure) for transposition of the great arteries (Chapter 425.2).
Interventional catheterization techniques are being adapted for use in the fetus with lesions such as aortic stenosis in an attempt to prevent their progression to more complex lesions such as hypoplastic left heart syndrome. In these procedures, after administration of appropriate anesthesia, a needle is passed through the maternal abdominal wall, the uterine wall, and the fetal chest wall and directly into the fetal left ventricle (see Fig. 425-13). A coronary angioplasty balloon catheter is passed through the needle and across the stenotic aortic valve, which is then dilated. With the restoration of normal left ventricular blood flow, it is to be hoped that normal left ventricular growth potential is restored. Mid-term results with this technique in a growing number of patients show mixed results with good ventricular growth leading to a two-ventricle circulation in approximately 25% of patients.
In patients with branch pulmonary artery stenoses, the previously mixed results with balloon angioplasty alone have been enhanced with the use of intravascular stents (Fig. 417-29) delivered over a balloon catheter and expanded within the vessel lumen. Once placed, they can often be dilated to successively greater sizes as the patient grows, although their use in younger infants and children is limited by the extent to which they can be further expanded. Research into biodissolvable stents may solve this problem in the future. Stents are also being utilized in adolescents and young adults with coarctation of the aorta.
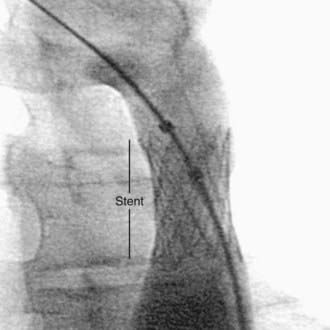
Figure 417-29 Intravascular stent placed in the descending aorta for treatment of recurrent coarctation of the aorta.
Closure of a small PDA is now routinely achieved with catheter-delivered coils (see Fig. 420-11), whereas a larger PDA can be closed with a variety of sandwich-type devices. Closure of anomalous vascular connections (coronary fistulas, veno-venous collaterals in cyanotic heart lesions) can also be achieved using coils. Secundum ASDs are now routinely closed with a double disc occluder device (see Fig. 420-3). Versions of these devices are currently in clinical trials for closure of surgically hard-to-reach muscular VSDs and even for the more common perimembranous VSD. Catheter-delivered devices may also be used as an adjunct to complex surgical repairs (dilation or stenting of branch pulmonary artery or pulmonary vein stenosis or closure of a difficult to reach muscular VSD). High-risk patients undergoing the Fontan operation (Chapter 430.4) often have a small fenestration created between the right and left sides of the circulation to serve as a “popoff valve” for high right-sided pressure in the early surgical period. Patients with these “fenestrated Fontans” are usually candidates for subsequent closure of the fenestration with a catheter-delivered device.
Andrews RE, Tulloh RMR. Interventional cardiac catheterization in congenital heart disease. Arch Dis Child. 2004;89:1168-1173.
Bacha EA, Cao QL, Galantowicz ME, et al. Multicenter experience with periventricular device closure of muscular ventricular septal defects. Pediatr Cardiol. 2005;26:169-175.
Feinstein JA, Kim N, Reddy VM, et al. Percutaneous pulmonary valve placement in a 10-month-old patient using a hand crafted stent-mounted porcine valve. Catheter Cardiovasc Interv. 2006;67:644-649.
Holzer R, Hijazi ZM. Interventional approach to congenital heart disease. Curr Opin Cardiol. 2004;19:84-90.
Knauth AL, Lock JE, Perry SB, et al. Transcatheter device closure of congenital and postoperative residual ventricular septal defects. Circulation. 2004;110:501-507.
Kutty S, Zahn EM. Interventional therapy for neonates with critical congenital heart disease. Catheter Cardiovasc Interv. 2008;72:663-674.
Masura J, Gavora P, Podnar T. Long-term outcome of transcatheter secundum-type atrial septal defect closure using Amplatzer septal occluders. J Am Coll Cardiol. 2005;45:505-507.
Michelfelder E, Polzin W, Hirsch R. Hypoplastic left heart syndrome with intact atrial septum: Utilization of a hybrid catheterization facility for cesarean section delivery and prompt neonatal intervention. Catheter Cardiovasc Interv. 2008;72:983-987.
Tworetzky W, Marshall AC. Fetal interventions for cardiac defects. Pediatr Clin North Am. 2004;51:1503-1513.