Labeling Techniques in Immunoassay
At the conclusion of this chapter, the reader should be able to:
• Compare heterogeneous and homogeneous immunoassays.
• Name and cite applications of at least three types of labels that can be used in an immunoassay.
• Describe and compare chemiluminescence, enzyme immunoassay (EIA), and immunofluorescence techniques.
• Briefly compare direct immunofluorescent, inhibition immunofluorescent, and indirect immunofluorescent assays.
• Describe the advantages, disadvantages, and application of Q dots, SQUID technology, luminescent oxygen-channeling immunoassay, fluorescent in situ hybridization, signal amplification technology, and magnetic labeling technology.
• Analyze a case study related to immunoassay.
• Correctly answer case study related multiple choice questions.
• Be prepared to participate in a discussion of critical thinking questions.
• Describe the principle of the solid-phase immunosorbent assay for pregnancy testing.
• Describe the direct fluorescent antibody test for N. gonorrhea.
competitive enzyme immunoassay
direct fluorescent antibody (DFA)
fluorescence in situ hybridization (FISH)
fluorescence polarization immunoassay
indirect fluorescent assay (IFA)
inhibition immunofluorescent assay
luminescent oxygen-channeling immunoassay (LOCI)
noncompetitive enzyme immunoassay
superconducting quantum interference device (SQUID)
Types of Labels
The principles and applications of enzyme immunoassays, chemiluminescence, and fluorescent substances as labels are presented in this chapter (Table 12-1).
Table 12-1
| Type | Antibody | Comments |
| Enzyme immunoassay (EIA; enzyme-linked immunosorbent assay, ELISA) | Enzyme-labeled antibody (e.g., horseradish peroxidase) | Competitive ELISA Noncompetitive (e.g., direct ELISA, indirect ELISA) |
| Chemiluminescence | Chemiluminescent molecule–labeled antibody (e.g., isoluminol or acridinium ester labels) | Competitive or sandwich immunoassay |
| Electrochemiluminescence | Electrochemiluminescent molecule–labeled antibody (e.g., ruthenium label) | — |
| Fluoroimmunoassay | Fluorescent molecule–labeled antigen (e.g., europium or fluorescein label) | Heterogeneous (e.g., time-resolved immunofluoroassay) Homogeneous (e.g., fluorescence polarization immunoassay) |
Enzyme Immunoassay
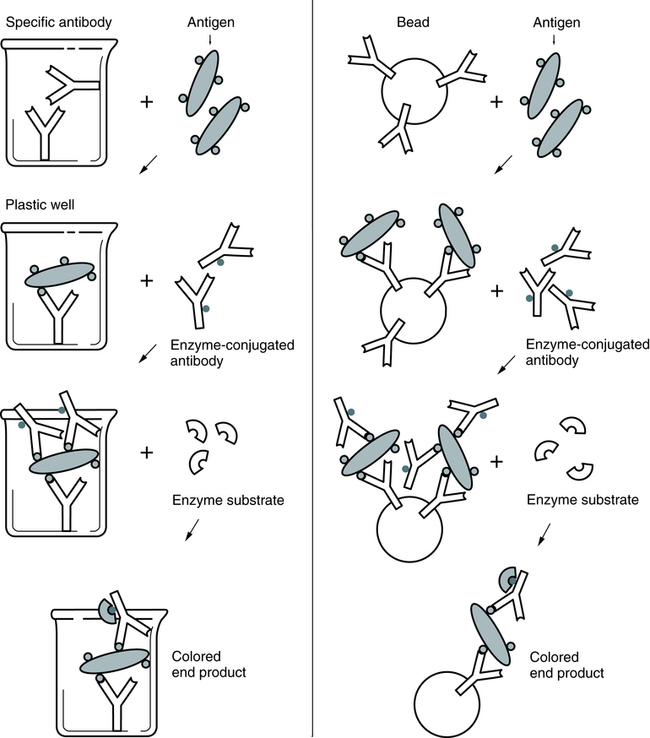
The EIA method uses the catalytic properties of enzymes to detect and quantitate immunologic reactions. An enzyme-labeled antibody or enzyme-labeled antigen conjugate is used in immunologic assays (Box 12-1). The enzyme, with its substrate, detects the presence and quantity of antigen or antibody in a patient specimen. In some tissues, an enzyme-labeled antibody can identify antigenic locations.
Various enzymes are used in enzyme immunoassay (Table 12-2). Common enzyme labels are horseradish peroxidase, alkaline phosphatase, glucose-6-phosphate dehydrogenase, and beta-galactosidase. To be used in an EIA, an enzyme must fulfill the following criteria:
Table 12-2
Enzymes Used in Enzyme Immunoassays
| Enzyme | Source |
| Acetylcholinesterase | Electrophorous electicus |
| Alkaline phosphatase | Escherichia coli |
| β-Galactosidase | Escherichia coli |
| Glucose oxidase | Aspergillus niger |
| Glucose-6-phosphate dehydrogenase (G6PD) | Leuconostoc mesenteroides |
| Lysozyme | Egg white |
| Malate dehydrogenase | Pig heart |
| Peroxidase | Horseradish |
In a representative EIA test, a plastic bead or plastic plate is coated with antigen (e.g., virus; Fig. 12-1). The antigen reacts with antibody in the patient’s serum. The bead or plate is then incubated with an enzyme-labeled antibody conjugate. If antibody is present, the conjugate reacts with the antigen-antibody complex on the bead or plate. The enzyme activity is measured spectrophotometrically after the addition of the specific chromogenic substrate. For example, peroxidase cleaves its substrate, o-dianisidine, causing a color change. In some cases, the test can be read subjectively.
Chemiluminescence
Chemiluminescence refers to light emission produced during a chemical reaction; it is used extensively in automated immunoassays (see Chapter 13). This methodology has excellent sensitivity and dynamic range. It does not require sample radiation and nonselective excitation and source instability are eliminated. Most chemiluminescent reagents and conjugates are stable and relatively nontoxic.
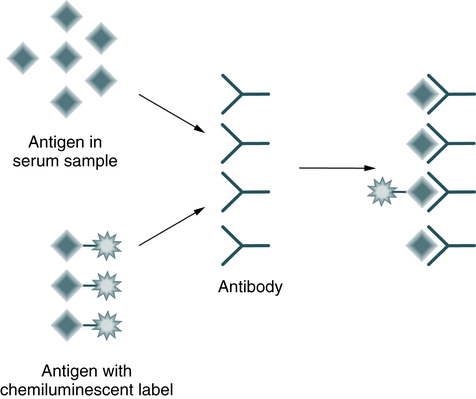
In a competitive immunoassay, a fixed amount of labeled antigen competes with unlabeled antigen from a patient specimen for a limited number of antibody-binding sites (Fig. 12-2). The amount of light emitted is inversely proportional to the amount of analyte (antigen) measured.
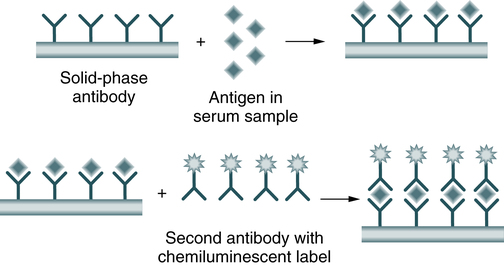
In a sandwich immunoassay, the sample antigen binds to an antibody fixed onto a solid phase; a second antibody, labeled with a chemiluminescent label, binds to the antigen-antibody complex on the solid phase (Fig. 12-3). In the sandwich assay, the emitted light is directly proportional to the analyte concentration. The detection device for analyses is a simple photomultiplier tube used to detect the emitted light.
Immunofluorescence
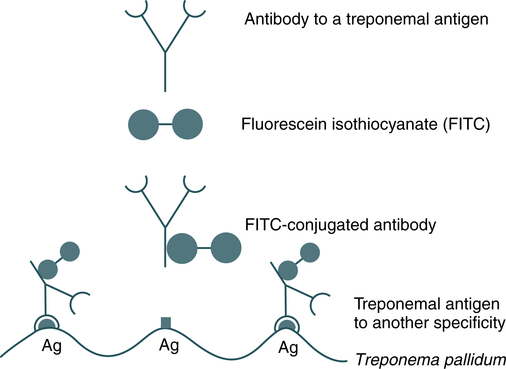
Fluorescent labeling is another method used to demonstrate the complexing of antigens and antibodies (Fig. 12-4). Fluorescent molecules are used as substitutes for radioisotope or enzyme labels. The fluorescent antibody technique consists of labeling antibody with fluorescein isothiocyanate (FITC), a fluorescent compound with an affinity for proteins, to form a complex (conjugate). This conjugate is able to react with antibody-specific antigen.
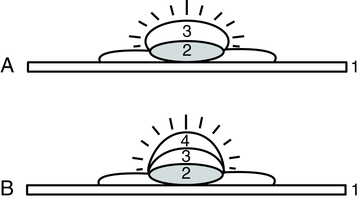
A, Direct fluorescence. B, Indirect fluorescence. 1, Microscopic slide; 2, cell (cytoplasm and nucleus); 3, antiserum (conjugate in A, unconjugate in B); 4, conjugated antiglobulin serum.
Fluorescent conjugates are used in the following basic methods, which are widely used:
Direct Immunofluorescent Assay
In the direct fluorescent antibody (DFA) technique, a conjugated antibody is used to detect antigen-antibody reactions at a microscopic level (Fig. 12-5). DFA can be applied to tissue sections or in smears for microorganisms (see Color Plate 2).
Indirect Immunofluorescent Assay
The basis for indirect fluorescent assay (IFA) is that antibodies (immunoglobulins) not only react with homologous antigens, but also can act as antigens and react with antiimmunoglobulins (Box 12-2). IFA is the serologic method most widely used for the detection of diverse antibodies. Immunofluorescence is used extensively in the detection of autoantibodies and antibodies to tissue and cellular antigens. For example, antinuclear antibodies (ANAs)—a heterogeneous group of circulating immunoglobulins that react with the whole nucleus or nuclear components (e.g., nuclear proteins, DNA, histones) in host tissues—are frequently assayed by indirect fluorescence. By using tissue sections that contain a large number of antigens, it is possible to identify antibodies to several different antigens in a single test. The antigens are differentiated according to their different staining patterns.
Immunofluorescence can also be used to identify specific antigens on live cells in suspension (flow cytometry). When a live stained cell suspension is put through a fluorescence-activated cell sorter (FACS), which measures its fluorescent intensity, the cells are separated according to their particular fluorescent brightness. This technique permits the isolation of different cell populations with different surface antigens (e.g., CD4+ and CD8+ lymphocytes; see Chapter 4).
Emerging Labeling Technologies
Quantum Dots (Q dots)
Magnetic Labeling Technology
Magnetic labeling technology is an application of the high-resolution magnetic recording technology developed for the computer disk drive industry. Increased density of microscopic, magnetically labeled biological samples (e.g., nucleic acid on a biochip) translates directly into reduced sample-processing times. Magnetic labeling can be applied to automated DNA sequences, DNA probe technology, and gel electrophoresis (Fig. 12-6). Compared with other nonradioactive labeling systems, magnetic labels are inherently safe, instrumentation is less expensive, signals are almost permanent, and spatial resolution is increased.
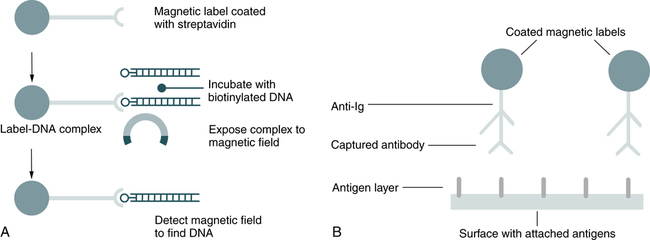
A, Detection of deoxyribonucleic acid. B, Detection of antibodies. (Adapted from Adelman L: Laboratory technology: magnetic labeling technology, Adv Med Lab Admin 11:131, 1999.)
In a magnetic label–based gel electrophoresis application sphere, DNA is analyzed. DNA is separated into bands using electrophoresis and magnetic labels are bound to the DNA in each band. By applying and then removing a magnetic field, the magnetic domains in each label are oriented in the same direction, resulting in a net magnetic field near the bands in the direction of the applied field (Fig. 12-7).
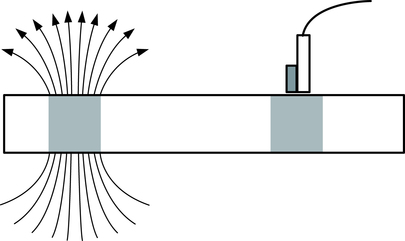
Left, Arrows on band represent the magnetic field resulting from the magnetized labels. Right, Band has a sensor near the surface. (Adapted from Adelman L: Laboratory technology: magnetic labeling technology, Adv Med Lab Admin 11:131, 1999.)
Chapter Highlights
• Heterogeneous immunoassays have a solid phase (microwell, bead) and require washing steps to remove unbound antigens or antibodies. Faster and easier to automate, homogeneous immunoassays have only a liquid phase and do not require washing steps.
• The ideal label should be measurable by several methods, including visual inspection.
• Enzyme immunoassay (EIA) uses a nonisotopic label and is safer than but shares the specificity, sensitivity, and rapidity of radioimmunoassay (RIA).
• In EIA antibody detection, the antigen in question is firmly fixed to a solid matrix (microplate well, outside of bead); this is called solid-phase immunosorbent assay.
• Chemiluminescence is the technology of choice of most immunodiagnostics manufacturers. In competitive and sandwich immunoassays, chemiluminescent labels can be attached to an antigen or antibody.
• Fluorescent labeling (direct and indirect) also demonstrates the complexing of antigens and antibodies. Fluorescent antibodies are used as substitutes for radioisotope or enzyme labels.
• Fluorescent conjugates are used in the basic methods of direct, inhibition, and indirect immunofluorescent assay. In direct immunofluorescence, a conjugated antibody is used to detect antigen-antibody reactions. In the indirect method, antibodies react with homologous antigens but also can act as antigens.
• Emerging labeling technologies include Q dots, SQUID, LOCI, signal amplification, and magnetic labeling.
• Fluorescence in situ hybridization (FISH) is often applied in immunology, hematopathology, and oncology.