Acquired Immunodeficiency Syndrome
At the conclusion of this chapter, the reader should be able to:
• Describe the etiology and viral characteristics of human immunodeficiency virus (HIV-1).
• Explain the epidemiology, including modes of transmission, and prevention of HIV-1.
• Discuss the signs and symptoms of various stages and the classification of HIV infection.
• Describe the immunologic manifestations and cellular abnormalities of HIV-1 infection.
• Explain the serologic markers and diagnostic evaluation of HIV.
• Compare the features of fourth generations HIV testing to other generations of testing.
• Analyze a representative HIV-1 case study.
• Correctly answer case study related multiple choice questions.
• Be prepared to participate in a discussion of critical thinking questions.
• Describe the principles of the Rapid HIV antibody test, GS HIV Combo Antigen/Antibody EIA, and simulation of HIV-1 Detection.
Etiology
Viral Characteristics
Viral Structure
The HIV-1 virus (Fig. 25-1) is composed of a lipid membrane, structural proteins, and glycoproteins that protrude. The viral genome consists of three important structural components—pol, gag, and env. These gene components code for various products (Table 25-1). Long terminal redundancies (LTRs) border these three components. HIV-2 has a different envelope and slightly different core proteins.
Table 25-1
| Component | Product |
| pol | Produces DNA polymerase |
| Produces endonuclease | |
| gag | Codes for p24 and for proteins such as p17, p9, and p7 |
| env | Codes for two glycoproteins, gp41 and gp120 |

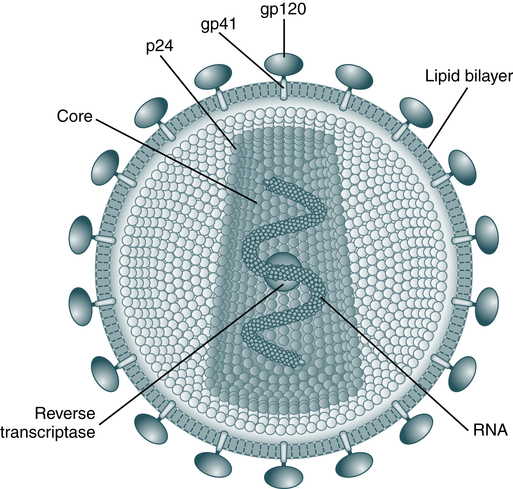
The envelope is made up of glycoproteins (gp) of 120 kDa and 41 kDa. The main core protein is p24. As an RNA virus, it relies on reverse transcriptase to produce complementary DNA for transcription and translation. (From Peakman M, Vergani D: Basic and clinical immunology, ed 2, London, 2009, Churchill Livingstone.)
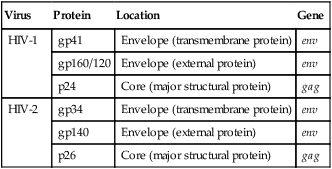
Cells infected with HIV can be examined with an electron microscope. The virus may appear as buds of the cell membrane particles. The virion has a double-membrane envelope and an electron-dense laminar crescent or semicircular cores. An intermediate, less electron-dense layer lies between the envelope and core. In a mature, free extracellular virion, the core appears as a bar-shaped nucleoid structure in cross section. This structure appears circular and is frequently located eccentrically. It is composed of structural proteins and glycoproteins that occupy the core and envelope regions of the particle. The virion consists of knoblike structures composed of a protein called glycoprotein (gp) 120, which is anchored to another protein called gp41. Each knob includes three sets of these protein molecules. The core of the virus includes a major structural protein called p25 or p24 encoded for by the gag gene. After human exposure, these and other viral components may induce an antibody response important in serodiagnosis (Table 25-2).
Table 25-2
HIV Proteins of Serodiagnostic Importance
| Virus | Protein | Location | Gene |
| HIV-1 | gp41 | Envelope (transmembrane protein) | env |
| gp160/120 | Envelope (external protein) | env | |
| p24 | Core (major structural protein) | gag | |
| HIV-2 | gp34 | Envelope (transmembrane protein) | env |
| gp140 | Envelope (external protein) | env | |
| p26 | Core (major structural protein) | gag |
The env gene encodes for a polyprotein that contains numerous glycosylation sites. The glycoprotein gp160 is found on infected cells but is deficient on viral particles; however, gp160 gives rise to two glycoproteins, gp120 and gp41, which are associated with the viral envelope. The encoding genes and gene products, or antigens, of the AIDS virus may induce an antibody response after human exposure (Table 25-3).
Table 25-3
Encoding Genes and Antigens of AIDS Virus
| Encoding Gene | Antigen |
| gag | p55 |
| gag | p24 |
| gag | p17 |
| pol | p66 |
| pol | p51 |
| sor | p24 |
| env | gp160 |
| env | gp120 |
| env | gp41 |
| 3′ orf | p27 |
Viral Replication
The replication of HIV is complicated and involves several steps (Fig. 25-2). The HIV life cycle is that of a retrovirus (Box 25-1). Retroviruses are so named because they reverse the normal flow of genetic information. In body cells, the genetic material is DNA. When genes are expressed, DNA is first transcribed into messenger RNA (mRNA), which then serves as the template for the production of proteins. The genes of a retrovirus are encoded in RNA; before they can be expressed, the RNA must be converted into DNA. Only then are the viral genes transcribed and translated into proteins in the usual sequence.
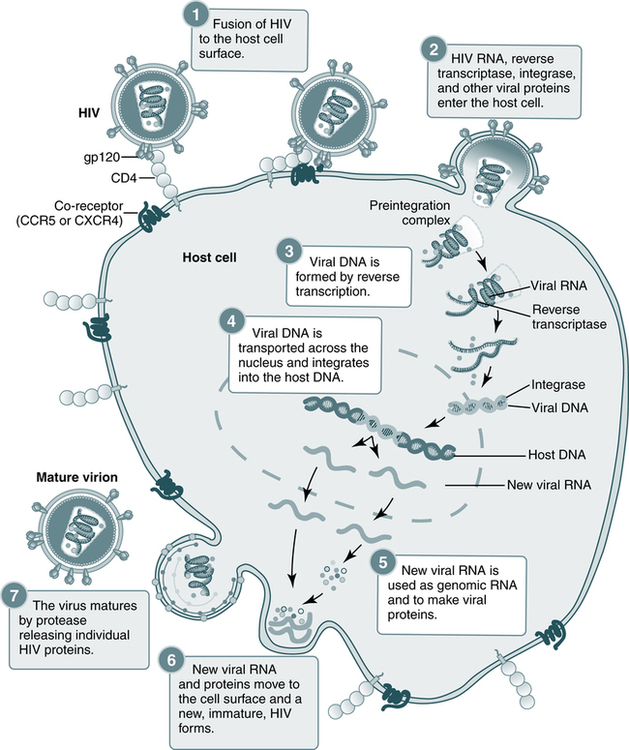
Steps in the HIV replication cycle
1. Fusion of the HIV cell to the host cell surface; 2. HIV RNA, reverse transcriptase, integrase, and other viral proteins enter the host cell; 3. viral DNA is formed by reverse transcription; 4. viral DNA is transported across the nucleus and integrates into the host DNA; 5. new viral RNA is used as genomic RNA and to make viral proteins; 6. new viral RNA and proteins move to the cell surface and a new, immature, HIV virus forms; 7. the virus matures by protease releasing individual HIV proteins. (Courtesy National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Md.)
Target Cells
The infectious process begins when the gp120 protein on the viral envelope binds to the protein receptor, called CD4, located on the surface of a target cell. HIV-1 has a marked preference for the CD4+ subset of T lymphocytes (Fig. 25-3). In addition to T lymphocytes, macrophages, peripheral blood monocytes, and cells in the lymph nodes, skin, and other organs also express measurable amounts of CD4 and can be infected by HIV-1. About 5% of the B lymphocytes may express CD4 and may be susceptible to HIV-1 infection. Macrophages may play an important role in spreading HIV infection in the body, both to other cells and to the target organs of HIV. Monocyte-macrophages enable HIV-1 to enter the immune-protected domain of the central nervous system (CNS), including the brain and spinal cord.
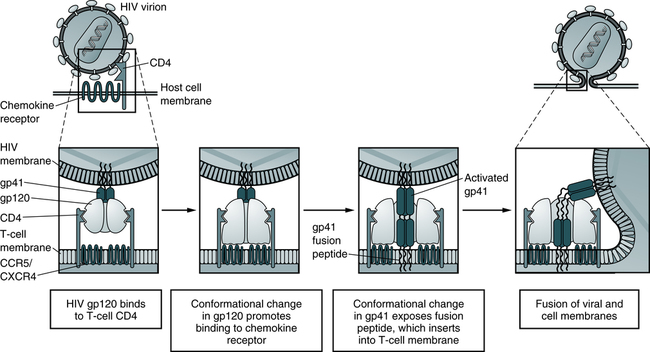
In the model, depicted sequential conformational changes in gp120 and gp41 promote fusion of the HIV-1 and host cell membranes. The fusion peptide of activated gp41 contains hydrophobic amino acid residues that promote insertion into the host cell plasma membrane lipid bilayer. (Adapted from Abbas AK, Lichtman AH, Pillai S: Cellular and molecular immunology, ed 6, Philadelphia, 2007, Saunders.)
Replication
Retroviruses carry a single, positive-stranded RNA and use reverse transcriptase to convert viral RNA into DNA. The life cycle of the HIV-1 virus consists of five phases (see Box 25-1):
1. The virus attaches and penetrates target cells (e.g., lymphocytes) that express the CD4 receptor. After penetration, the virus loses its protein coat, exposing the RNA core.
2. Reverse transcriptase converts viral RNA into proviral DNA.
3. The proviral DNA is integrated into the genome (genetic complement of the host cell).
4. New virus particles are produced as a result of normal cellular activities of transcription and translation.
Epidemiology
Classification System
The revised definition of HIV infection, which applies to both HIV-1 and HIV-2, incorporates the reporting criteria for HIV infection and AIDS into a single case definition (Box 25-2). The revised HIV criteria apply to AIDS-defining conditions for adults and children that require laboratory evidence of HIV.
Infectious Patterns
HIV-1
The progression of the natural history and immunopathogenesis of HIV-1 infection can be demonstrated in six discrete stages (Fig. 25-4). These stages are based on the sequential appearance in plasma of HIV-1 viral RNA, the gag p24 protein antigen, antibodies that bind to fixed viral proteins. No matter how HIV-1 was acquired, the timing of the appearance of viral and other markers of infection is generally uniform and follows an orderly pattern.
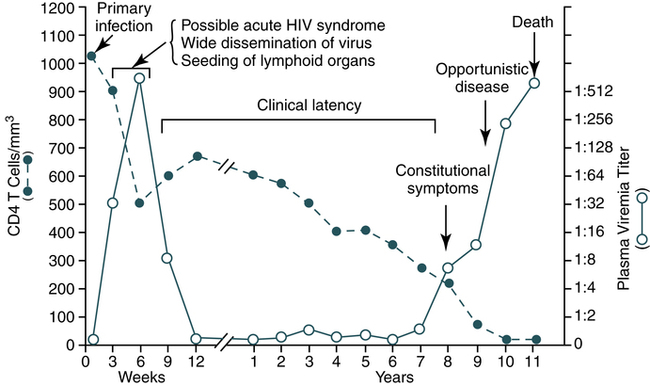
During the early period after primary infection, there is widespread dissemination of virus and a sharp decrease in the number of CD4 T cells in peripheral blood. An immune response to HIV ensues, with a decrease in detectable viremia followed by a prolonged period of clinical latency. The CD4 T cell count continues to decrease during the following years until it reaches a critical level below which there is a substantial risk of opportunistic diseases. (From Pantaleo G, Graziosi C, Fauci A: The immunopathogenesis of human immunodeficiency virus infection, N Engl J Med 328:327-335, 1993.)
Signs and Symptoms
The CDC has a classification used to define stages of HIV-related illness (Fig. 25-5). Infection with HIV produces a chronic infection with symptoms that range from asymptomatic to the end-stage complications of AIDS.
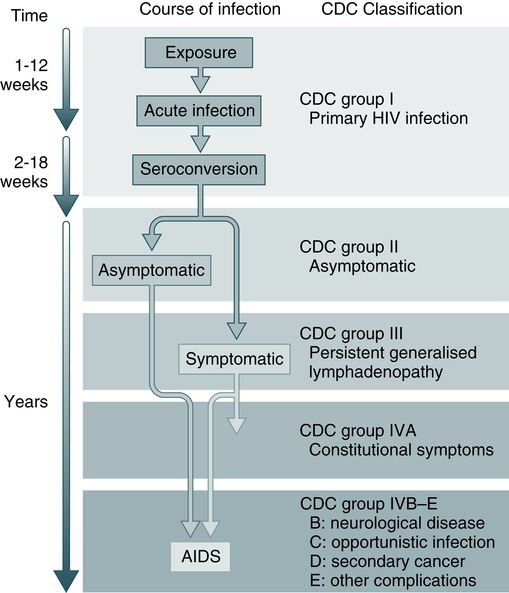
The CDC classification is used to define stages of HIV-related illness. (From Peakman M, Vergani D: Basic and clinical immunology, ed 2, London, 2009, Churchill Livingstone.)
Typically, patients in the early stages of HIV infection are completely asymptomatic or show mild chronic lymphadenopathy. The early phase may last from many months to many years after viral exposure. Although the course of HIV-1 infection may vary somewhat in individual patients, a common pattern of development has been recognized. The newly revised HIV classification system provides uniform and simple criteria for categorizing conditions (see Box 25-2).
Opportunistic Infections
The end stage of AIDS is characterized by the occurrence of neoplasms and opportunistic infections (Box 25-3). The most common opportunistic infections are P. jiroveci (P. carinii) (Fig. 25-6), cytomegalovirus (CMV), Mycobacterium avium-intracellulare, Cryptococcus, Toxoplasma, Mycobacterium tuberculosis, herpes simplex, and Legionella. Histoplasma capsulatum is being recognized with increasing frequency. The most frequent malignancy observed is an aggressive, invasive variant of Kaposi’s sarcoma, discovered in many cases on autopsy. Malignant B cell lymphomas are increasingly recognized in patients with or at high risk for AIDS.
Immunologic Manifestations
Cellular Abnormalities
Serologic Markers
Detection of Core Antigen
After initial infection, the body mounts a vigorous immune response against the viremia (see Color Plate 10). The first signal of an immune response to HIV-1 infection is the appearance of acute-phase reactants, including α1-antitrypsin and serum amyloid in plasma 3 to 5 days after transmission. This is followed by a steep rise in the HIV-1 viral load (ramp-up viremia) that coincides with a large burst of inflammatory cytokines led by interferon-α and interleukin-15 (IL 15) and by a burst of plasma microparticles derived from infected and activated CD4 T cells undergoing apoptosis.
Testing Methods
Testing assays for HIV (Table 25-4) are categorized into the following three main types:
Table 25-4
HIV Assays and Characteristics
| Assay | Format | Target Molecule | Comments |
| Lymphocyte CD4 absolute count | Flow cytometry | CD4+ | |
| HIV antigen assay for serum and plasma | EIA | HIV p24 antigen | |
| HIV types 1 and 2 (HIV-1, HIV-2) antibody detection in serum or plasma | EIA (first- and second-generation tests) | Recombinant HIV-1 env and gag and HIV-2 env proteins or Purified, inactivated HIV-1 virus propagated in T-lymphocyte culture |
If reactive, confirm with molecular testing (Western blot). |
| Detection of HIV-1 groups M and O | EIA (third generation) | Purified, inactivated HIV-1 viral lysate proteins, envelope proteins, and HIV-1 group O transmembrane protein or Purified gp160 and p24 recombinant proteins from HIV-1, HIV-2 transmembrane gp36, and synthetic epitope of HIV-1 group O |
|
| Enzyme-linked fluorescence p24 or EIA p24 |
EIA (fourth generation) | HIV-1 gp160, p24 antigen, and peptides representing regions of gp41 from HIV-1 group O and gp36 from HIV-2 or HIV-1 antigens p31 and gp41, HIV-2 p36 recombinant protein, HIV-1 group O gp41, and anti-p24 monoclonal antibodies |
|
| HIV antibody detection in serum or plasma | Western blot | Purified and inactivated HIV-1 strain LAV grown in CEM cell line or Purified and inactivated HIV-1 propagated in H9/HTLV-IIIB T lymphocyte cell line |
|
| HIV viral load assays | PCR | Reverse-transcriptase PCR or Nucleic acid sequence–based amplification or Signal amplification, branched-chain DNA |
|
| Rapid testing | Rapid immunoassay | Uses recombinant proteins representing regions of HIV-1 envelope proteins or Uses synthetic HIV structural proteins |
|
| Molecular Testing | |||
| HIV-1 RNA | Quantitative real-time PCR | Aids in assessing viral response to antiretroviral treatment | |
| HIV-1 RNA | Quantitative bDNA | Quantitative branched-chain DNA | Aids in assessing viral response to antiretroviral treatment |
| HIV-1 DNA | Qualitative PCR | Detects HIV-1 proviral DNA in infants <48 hr old; repeat testing at 1-2 mo and 3-6 mo of age | |
| HIV-2 antibody | Qualitative enzyme immunoassay, qualitative immunoblot | Screen for HIV-2 infection in a patient with an epidemiologic link to Africa. | |
| HIV-1 genotyping | Reverse transcription PCR/nucleic acid sequencing | Detect changes in the viral genome associated with drug resistance. Use in conjunction with CD4 measurement to monitor treatment efficacy. |
|
| HIV-2 antibody confirmation | Qualitative immunoblot | Confirm positive screening results. | |
| HIV-1 antibody | Qualitative chemiluminescent immunoassay | ||
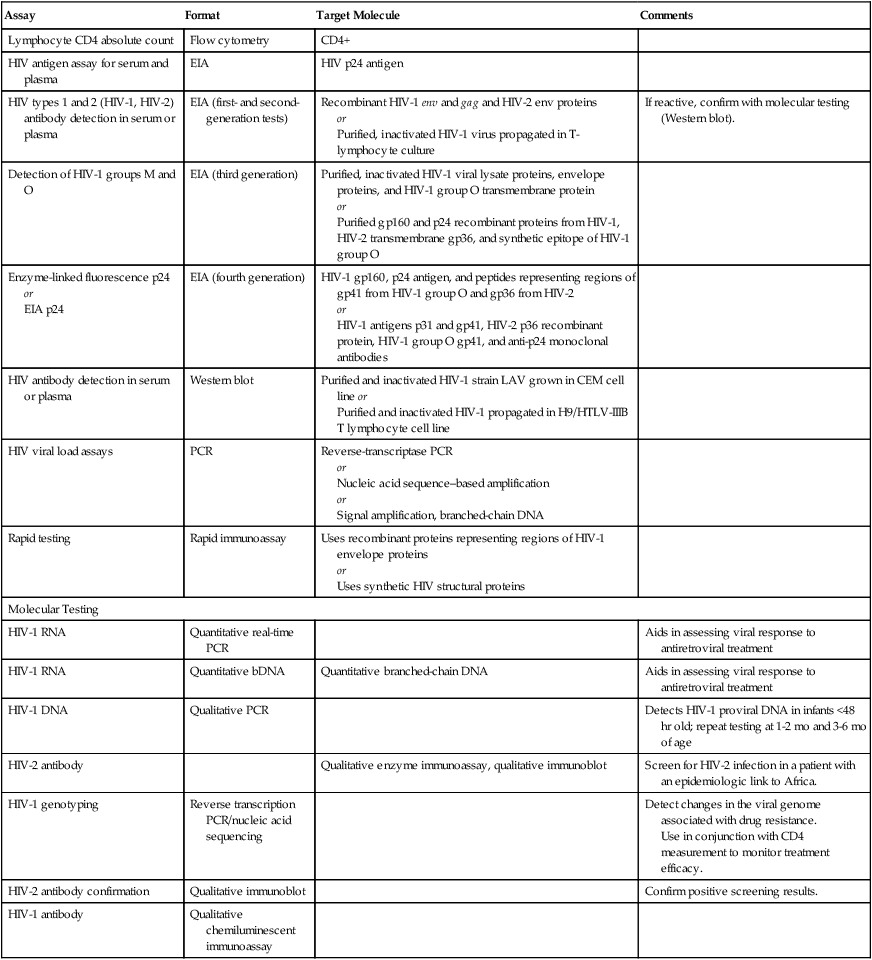
EIA, Enzyme immunoassay; PCR, polymerase chain reaction.
Adapted from Zetola N, Klausner JD: HIV testing: an update, MLO Med Lab Obs 38:58–62, 2006.
HIV-1 Antibodies
Antibodies to HIV can be detected by EIA (specificity 99%, sensitivity 98%; Table 25-5) and confirmed by the immunoblot technique. Antibody testing by EIA remains the standard method for screening potential blood donors. Simultaneous testing for p24 antigenemia is considered unnecessary. Third-generation serologic assays have demonstrated that seroconversion typically occurs 3 to 12 weeks after infection, but significant delays can occur in some individuals.
Table 25-5
Causes of False-Positive and False-Negative HIV Enzyme Immunoassay Results
| False-Positive Result | False-Negative Result |
| Positive RPR (syphilis serology) test | Laboratory glove starch |
| Hematologic malignant disorder | Window period before seroconversion |
| DNA viral infections | Immunosuppressive therapy |
| Autoimmune disorders | Malignancies |
| Alcoholic hepatitis | Bone marrow transplantation |
| Vaccinations (e.g., hepatitis B, influenza) | Kits that mainly detect antibodies to p24 |
| Chronic renal failure | |
| Renal transplantation |
Adapted from Specialty Laboratories, Santa Monica, Calif.
Confirmatory Testing
Western Blot
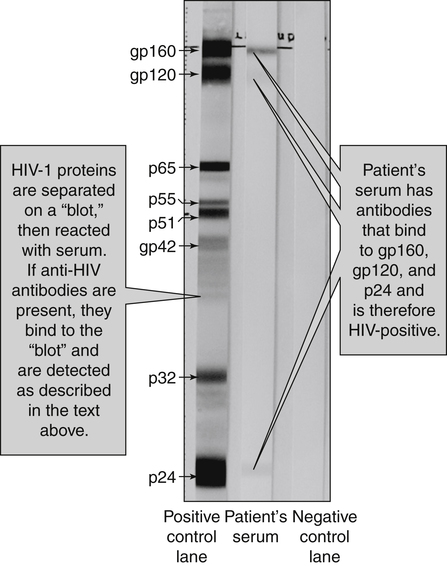
Before an HIV result is considered positive, the results should be reproducible and confirmable by at least one additional test. Western blot (WB) analysis is currently the standard method for confirming HIV-1 seropositivity (Fig. 25-7).
• The technical skill and experience of the technologist performing the procedure
• Characteristics of the technical methodology
• General sensitivity of the WB in detecting antibodies specific for various HIV-1 antigens (especially during the window period of seronegativity)
• Frequent lack of specificity because of contamination of the viral reference preparation by histocompatibility and other antigens that electrophoretically migrate with p24 and gp41
• Variation in band reactivity patterns in sera from an individual over the course of HIV-1 infection
• Serologic tests in the process of seroconversion; anti-p24 is usually the first antibody to appear.
• End-stage HIV infection, usually with loss of core antibody
• Cross-reacting nonspecific antibodies, as seen with collagen vascular disease, autoimmune diseases, lymphoma, liver disease, injection drug use, multiple sclerosis, parity, or recent immunization
• Infection with O strain or HIV-2
• Perinatally exposed infants who are seroconverting (losing maternal antibody)
False-positive WB results, especially those with a majority of bands, are extremely uncommon.
Tests for Therapeutic Monitoring
CD4 T Lymphocyte Testing
Monitoring of CD4 T lymphocytes measures immune function. This information guides the initiation of antiretroviral therapy (ART) and monitors a patient’s response to antiretroviral treatment. CD4 T lymphocyte counts are expressed as absolute counts or as a percentage of the total population of CD4/CD8 as a ratio. Flow cytometry, in conjunction with fluorescence-activated cell sorting (FACS; see Chapter 13) is considered to be the gold standard in CD4 T lymphocyte counting.
Prevention
Reducing Viral Transmission
Health care personnel should assume that the blood and other body fluids from all patients are potentially infectious (Standard Precautions; see Chapter 6).
Vaccines
Types of potential HIV vaccines include the following:
To date, the results of clinical trials have been disappointing (Box 25-4). Live attenuated vaccines are not currently being developed for use in human beings because of safety concerns. The first AIDS vaccine using the subunit concept, AIDSVAX gp120 vaccine, failed to protect against HIV infection in an efficacy trial. Many of the current AIDS vaccines in development are DNA vaccines. DNA vaccines will not cause HIV infection because they do not contain all the genes of the live pathogen. Another common strategy in AIDS vaccine development is recombinant vector vaccines. These will not cause HIV infection because they contain copies of only one or several HIV genes, not all of them. It is hoped that the addition of a vector will allow the vaccine to be more effective in creating an immune response than a DNA vaccine used alone.
Continued testing of vaccines is needed to determine whether they are more immunogenic in different doses, in different populations, and in combination with other candidate HIV vaccines (see Chapter 16).
More than 30 clinical trials have been in progress to develop a vaccine to prevent HIV (see www.iavi.org). The first generation of successful HIV vaccines likely will offer some protection but will not be entirely protective—no vaccine is 100% effective. Future generations of a preventive HIV vaccine will become increasingly more effective over time as scientific knowledge improves. However, even partially effective vaccines could make a difference in the following ways:
Treatment
Investigational Drugs
• Phase I takes about 1 year and includes 20 to 80 healthy volunteers who are tested for the safety of a new drug.
• Phase II lasts about 2 years and expands the number of volunteers to 100 to 300 persons with the disease to assess the effectiveness of a drug and observe for adverse side reactions.
• Phase III of a clinical trial lasts about 3 years and expands the number of patients with a specific disease to 1000 to 3000 to verify effectiveness further and identify any specific negative side effects of the drug.
Drug Resistance
• The number of mistakes per genome per replication cycle. This is extremely high in HIV because reverse transcriptase has no so-called proofreading ability.
• The number of viral replication cycles per unit of time. This is reflected in an infected patient’s viral load.
HIV Antiretroviral Drug Resistance: Genotypes, Phenotypes, Virtual Phenotypes, and Tropism Testing
A patient’s response to therapy depends on a number of factors, including patient compliance, percentage of resistant virus population, dosing, and drug pharmacology issues. Genotypic or phenotypic assays can be used to assess HIV drug resistance. The vircoTYPE HIV-1 assay (Janssen Diagnostics, Raritan, NJ) predicts HIV-1 drug resistance based on the nucleic acid sequence of the patient’s human immunodeficiency virus. This test analyzes sequences that encompass the entire protease gene and codons 1-335 of the reverse transcriptase gene. An analysis using the Virtual Phenotype Linear Modeling analysis (http://www. janssendiagnostics. com) can be used.
 Rapid HIV Antibody Test
Rapid HIV Antibody Test
Principle
Reporting Results
• Nonreactive: No reddish purple line next to the triangle labeled T; reddish purple line appears next to the triangle labeled C.
• Reactive: Reddish purple line next to the triangle labeled T; reddish purple line appears next to the triangle labeled C. One line may be darker than the other. A test is reactive if any color appears next to the T triangle and next to the C triangle, regardless of how faint.
• No precision pipetting, predilutions, or special instruments are required to perform the OraQuick ADVANCE Rapid HIV-1/2 Antibody Test.
• Standard Precautions must be practiced throughout the testing procedure.
• The test should be performed at temperatures in the range of 15° C to 37° C (59° F to 98.6° F). All refrigerated reagents must reach room temperature before testing.
• If the test kit is stored at temperatures outside ambient temperatures of 2° C to 27° C (35.6° F to 80.6° F) or used outside the operating temperature of 15° C to 37° C (59° F to 98.6° F), use the kit controls to ensure performance of the test.
 GS HIV Combo Ag/Ab EIA
GS HIV Combo Ag/Ab EIA
• Monoclonal antibodies against HIV-1 p24 antigen
• HIV antigens: HIV-1 gp160 recombinant protein, a synthetic peptide mimicking a totally artificial (i.e., encoded by no existing virus) HIV-1 group O–specific epitope and a peptide mimicking the immunodominant epitope of the HIV-2 envelope protein. The conjugates are based on the use of biotinylated polyclonal antibodies to HIV p24 Ag (conjugate 1) and peroxidase-conjugated streptavidin and peroxidase-conjugated HIV-1 antigens (gp41 and gp36 peptides mimicking the immunodominant epitopes of the HIV-1 and HIV-2 envelope glycoproteins, and the same synthetic peptide mimicking a totally artificial HIV-1 group O-specific epitope used for the solid phase; conjugate 2)
See the ![]() website for the procedural protocol.
website for the procedural protocol.
Chapter Highlights
• HIV-1 is the predominant virus responsible for AIDS. In addition to the original HIV-1, a second AIDS-causing virus, HIV-2, was identified in 1985.
• The HIV virus is composed of structural proteins and glycoproteins that occupy the core and envelope regions of the particle.
• Retroviruses contain a single, positive-stranded RNA with the genetic information of the virus and a special enzyme, reverse transcriptase, in their core. Reverse transcriptase enables the virus to convert viral RNA into DNA.
• HIV has a marked preference for the CD4+ subset of lymphocytes. Macrophages, as many as 40% of the peripheral blood monocytes, and cells in the lymph nodes, skin, and other organs also express measurable amounts of CD4 and can be infected by HIV. In addition, about 5% of B lymphocytes may express CD4 and be susceptible to HIV infection.
• Transmission of HIV is believed to be restricted to intimate contact with body fluids from an infected person; casual contact with infected persons has not been documented as a mode of transmission.
• The early phase of HIV-1 infection may last months to years after initial infection. Typically, patients in the early stages of HIV-1 infection are completely asymptomatic or show mild, chronic lymphadenopathy. HIV-1 causes a predictable progressive derangement of immune function; AIDS is one late manifestation of that process.
• Two to 10 years after HIV infection, replication of the virus flares again and the infection enters its final stage. An average of 8 or 9 years may pass before AIDS is fully developed. The virus behaves differently depending on the host cell and its level of mitotic activity. The end stage of AIDS is characterized by neoplasms and opportunistic infections.
• Immunologic activities associated with HIV-1 infection include the production of different types of antibodies against HIV-1. Some antibodies neutralize it, others prevent it from binding to cells, and others stimulate cytotoxic cells to attack HIV-infected cells.
• A window period of seronegativity exists from the time of initial infection to 6 or 12 weeks or longer. Using EIA methods based on defined HIV-1 proteins produced by recombinant DNA methods, antibodies specific for gp41 are detectable for weeks or months before assays specific for p24. The appearance of antibodies specific for p24 precedes that of anti-gp41 in Western blot serum specimens.
• Laboratory evaluation of HIV-infected patients consists of assessment of cellular and humoral components. Screening of blood donors and patients is usually by serologic methods. In patients with signs and symptoms of AIDS, both the assessment of cellular concentrations and function and the diagnosis and treatment of opportunistic infections become important.
• Antibodies to HIV-1 are usually detected by EIA and confirmed by Western blot, currently the standard for confirming HIV-1 seropositivity. If positive for band p41 or p24 with a positive EIA, the test is confirmatory.

 – HIV p24 antigen test, including neutralization assay, for a child aged >1 month– HIV isolation (viral culture)
– HIV p24 antigen test, including neutralization assay, for a child aged >1 month– HIV isolation (viral culture) HIV nucleic acid (DNA or RNA) detection tests are the virologic methods of choice for the diagnosis of exclusion of infection in children aged <18 months. Although HIV culture can be used, culture is less standardized and less sensitive than nucleic acid detection tests. The use of p24 antigen testing to exclude infection in children aged <18 months is not recommended because of poor sensitivity, especially in the presence of HIV antibody. Commercial tests for RNA and DNA detection have become widely available. Quantitative RNA tests have been approved by the Food and Drug Administration (FDA) for monitoring HIV infection, and qualitative RNA tests have been approved to aid diagnosis. The quantitative and qualitative RNA tests meet FDA standards for high analytic and clinical sensitivity and specificity (14-16). All available tests detect the subtypes of group M and strains of group O. HIV-2 can be diagnosed with HIV-2 DNA PCR. HIV RNA tests sometimes do not detect HIV-2 because the viral loads in some HIV-2–infected persons are below detectable levels. Because of the possibility of mutation or recombination involving the sequences detected by a particular test, occasionally, virus might not be detected in a specimen from an HIV-2 infected individual. If HIV-2 infection seems likely but results are negative, testing with a different assay might be advisable.
HIV nucleic acid (DNA or RNA) detection tests are the virologic methods of choice for the diagnosis of exclusion of infection in children aged <18 months. Although HIV culture can be used, culture is less standardized and less sensitive than nucleic acid detection tests. The use of p24 antigen testing to exclude infection in children aged <18 months is not recommended because of poor sensitivity, especially in the presence of HIV antibody. Commercial tests for RNA and DNA detection have become widely available. Quantitative RNA tests have been approved by the Food and Drug Administration (FDA) for monitoring HIV infection, and qualitative RNA tests have been approved to aid diagnosis. The quantitative and qualitative RNA tests meet FDA standards for high analytic and clinical sensitivity and specificity (14-16). All available tests detect the subtypes of group M and strains of group O. HIV-2 can be diagnosed with HIV-2 DNA PCR. HIV RNA tests sometimes do not detect HIV-2 because the viral loads in some HIV-2–infected persons are below detectable levels. Because of the possibility of mutation or recombination involving the sequences detected by a particular test, occasionally, virus might not be detected in a specimen from an HIV-2 infected individual. If HIV-2 infection seems likely but results are negative, testing with a different assay might be advisable.