34 Kyphoplasty
KEY POINTS
Brief Description
Spinal osteoporosis alone is asymptomatic. If allowed to progress, however, it confers increasing risk of fragility fracture. The principal manifestation of osteoporotic vertebral compression fractures (VCFs) is back pain. Some minimally symptomatic patients do not present for medical evaluation.1 Others require hospital admission for unrelenting pain. Typically, over 3 months, the fracture heals and the back pain subsides.2 Although the nonunion rate is low, not all VCFs heal.
Back pain can persist after fracture healing. From 33% to 75% of fractures precipitate chronic back pain.3 The chronic pain has been attributed to hyperkyphosis, leading to excessive muscular strain. Excessive anterior vertebral body loading engendered by this malalignment may propagate stress fractures in the surrounding endplates.4 Late kyphosis is occasionally associated with myelopathy.5
Indications and Contraindications
Over time, kyphoplasty indications have gradually been expanded to include conditions such as multiple myeloma and osteolytic metastases. Moreover, kyphoplasty has been added to open decompression and internal fixation procedures. Hybrid procedures may be indicated for more complex fracture patterns, significant compression of the neural elements, and neoplastic lesions with cortical destruction.7 Another hybrid option combines radiosurgery and kyphoplasty. Conventional radiotherapy remains the index treatment in many patients with vertebral body metastasis.7 Used alone, radiation is associated with delayed pain relief and further vertebral collapse due to both the previous bone erosion and the radiation itself. Newer radiation therapy techniques allow more focused radiation to be applied via intense treatments over a shorter time course.
Absolute contraindications to kyphoplasty include the following:
While less common than VCF, osteoporotic burst fractures (senile burst fractures) are not rare. Any fracture precipitating more than 50% height loss will have associated posterior cortical compromise. In many cases, this compromise takes the form of cortical buckling. When the canal occlusion is less than 33%, kyphoplasty can be considered. On the other hand, in the face of cortical comminution, avoid percutaneous kyphoplasty because of the increased risk of cement extravasation. In patients with neurologic injury, open surgery may be required.
Background of Scientific Testing and Clinical Outcomes
Outcomes data include a number of retrospective studies. Very recently prospective data have been reported from the FREE trial.10 This trial included 21 sites in 8 countries that enrolled 300 patients with acute VCF and randomized them to either kyphoplasty (149) or nonoperative care (151). As of this writing, the complete paper has not been published, but early pain relief seems to be a clear advantage of kyphoplasty. Whether that advantage persists is more difficult. The primary outcome was the difference in the Short Form (SF-36) physical component summary at 1 month. Quality of life measurements and spine radiographs were assessed through 12 months. Kyphoplasty subjects reported greater improvement than controls in their SF-36 physical component (5.2 point difference; p < 0001) at one month). By 12 months, the difference declined to 1.5 points and was no longer significant (p = .2). Kyphoplasty improved quality of life by the 1-point EuroQol questionnaire at 1 (0.18 points; 95% CI, 0.08–0.28; p < .001) and 12 (0.12; 95% CI, 0.01–0.22; p = .025) months. Back function, as measured by the 24-point Roland-Morris scale, was improved by 4.0 points by kyphoplasty at 1 month (p < .001) and 2.6 points at 12 months (p = .001). Kyphoplasty patients reported fewer days with limited activity, less back pain, and less use of analgesics and walking aids.
Of note, the FREE study was funded by the manufacturer and many of its authors are Kyphon consultants. On the other hand, three other small studies comparing kyphoplasty with conventional medical treatment also found that kyphoplasty consistently improved pain and physical function, with results sustained at 6 months.11–13
In 2005, Hadjipavlou et al14 combined the available vertebroplasty and kyphoplasty outcome reports in an effort to compare the procedures. Using meta-regression techniques, the authors found that individual study design had a considerable impact on subsequent analysis. For prospective studies, the rates of success with vertebroplasty and kyphoplasty were not significantly different at 92% and 93% respectively. However, in retrospective studies, kyphoplasty was more successful (95% vs. 86%; p = .019)
Aside from pain relief, a major benefit of VBA lies in the restoration of mobility. In one series of 11 wheelchair-bound cancer patients, 73% were able to walk shortly after vertebroplasty.15 Other studies reported restoration of mobility after kyphoplasty in 84% to 100%.8,16 In terms of other types of physical functioning, a number of different outcome measures have been used. In a retrospective analysis of patients with painful osteoporotic VCF, 49 patients who were available for follow-up at a mean 9-month interval had an improvement in visual analogue pain scale score of seven points (p < .05), and an improvement in Roland-Morris Disability Survey of 11 points (p < .05).17
In a retrospective analysis of 52 patients with 82 painful osteoporotic VCFs, kyphoplasty restored 4.6 mm and 3.9 mm to the heights of the anterior and medial columns, respectively.17 The mean Cobb angle increased by 14%. In a meta-analysis, Hadjipavlou et al concluded that, although postural reduction can improve vertebral height following a compression fracture, better reductions are obtained with kyphoplasty than with vertebroplasty.14 Better reductions may be achieved with earlier treatment.
Clinical Presentation and Evaluation
Successful kyphoplasty hinges on distinction of compression fracture pain from other etiologies. Clinical assessment involves an evaluation of the patient’s spinal alignment and gait, followed by palpation of the spine, ilium, sacrum, and paravertebral tissues. The importance of local tenderness over the involved spinous process as a principal sign of a painful VCF has been analyzed in two studies. In the first, which comprised 10 patients, Gaughen et al18 noted that local tenderness was not present despite imaging findings suggestive of an acute fracture. Recently, Gaitanis and coworkers16 found that spinous process tenderness corresponded to the level of pathology in 100% of osteolytic tumors and in 96% of VCFs when correlated with magnetic resonance imaging (MRI) findings of an acute fracture.
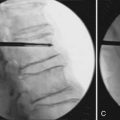
Several imaging techniques are employed in the evaluation of a painful VCF. Recently, flexion and extension or standing and supine lateral radiographs have been used to assess fracture mobility. A number of studies have examined the presence of intravertebral clefts. Although the exact cause of these intraosseous nitrogen pockets has been debated, the so-called Kummel sign may characterize pseudarthrosis. A cone-down lateral view directly perpendicular to the involved level is required in the assessment, because these clefts can easily be missed with standing lateral radiographs alone.19
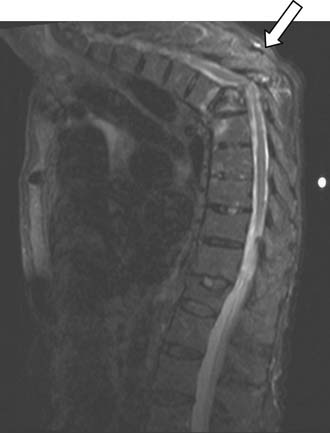
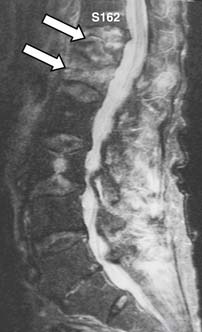
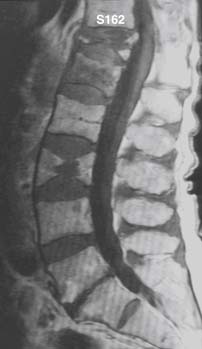
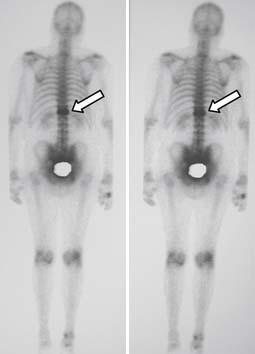
Magnetic resonance imaging is an important technique for detection of osteoporotic compression fractures (Figure 34-1). It is more sensitive than plain radiography, with a reported accuracy of 96%.19 Fracture acuity (or failure of healing) is also best observed as intense signal on sagittal MRI with short tau inversion recovery (STIR) sequences (Figures 34-2 and 34-3).20 For patients unable to undergo MRI, the combination of a technetium bone scan with computed tomography (CT) of the scintigraphically active levels can provide useful information on relatively fresh vertebral fractures (Figure 34-4).21
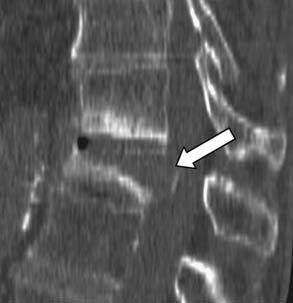
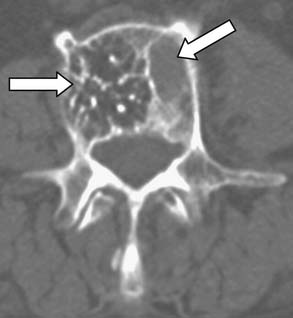
There are patients in whom both MRI and CT imaging is useful. For those with questionable endplate erosion, the greater bone–soft tissue contrast of the CT scan often demonstrates erosions more clearly (Figure 34-5). Similarly, a fine-cut (2 mm) CT scan with sagittal reconstructions may demonstrate small lytic lesions not otherwise seen in the fractured vertebral body on MRI (Figure 34-6). Most commonly, however, the CT is ordered as an adjunct to MRI in patients with canal compromise from their fracture.
Operative Technique
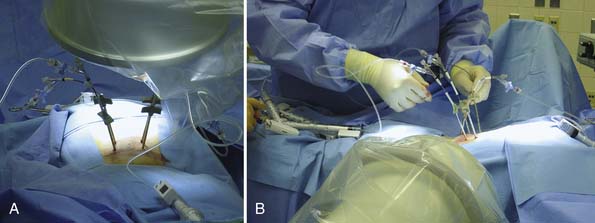
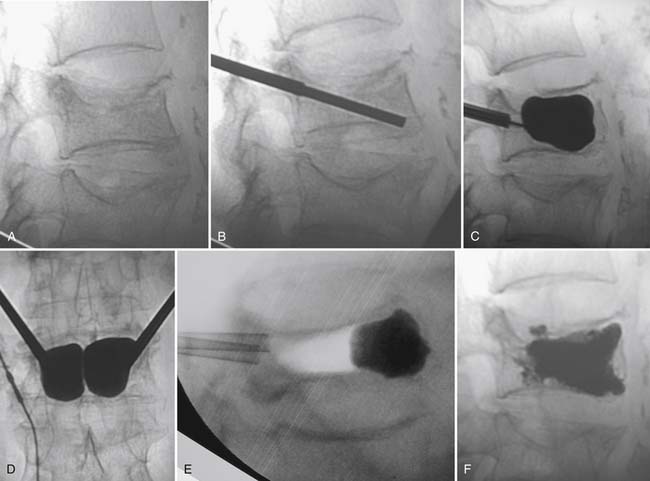
Kyphoplasty begins with true anteroposterior (AP) and lateral fluoroscopic images (Figures 34-7 and 34-8). Ensure a true AP with the spinous process in the midline between the pedicles. On the lateral view, the pedicles should line up and yield a clear view of the foramen and the posterior vertebral cortex. Both images should show the endplates of the level selected as a single line, not an oval.
Complications and Avoidance
Kyphoplasty complications can be categorized: medical, anesthesia related, instrument placement, and PMMA problems. In most cases, failure to improve is due to inappropriate patient selection. The more diffuse the patient’s pain, the less likely they are to benefit from VBA. Placement of PMMA into the spine may increase the risk of adjacent segment fracture.
The most devastating technical complication of kyphoplasty arises from PMMA extravasation. Leakage is clinically silent in the vast majority of cases, with symptomatic leaks representing only a small portion of the total.22 PMMA may extravasate into the vascular tree, disc space, anterior and lateral soft tissues, and spinal canal. Extravasation is most common in metastatic osteolytic tumors or myeloma.15
Interestingly, leakage into the central canal is better tolerated in most cases than intraforaminal leak; however, when symptomatic, central canal extravasation leads to more devastating neurological symptoms, such as paraplegia. In most cases, symptoms are transient and respond well to nerve root blocks or oral medication; rarely do they require surgical decompression.23
Along with the more viscous cement applied, void creation and bone compacting effects may decrease extravasation rates compared with vertebroplasty. A cadaveric study by Belkoff et al24 reported reduced rates of PMMA extravasation after kyphoplasty compared with vertebroplasty. In a series of patients with metastatic disease, Fourney et al25 reported a 9% extravasation rate after vertebroplasty, but no cases of extravasation following kyphoplasty.
Improper instrumentation placement most frequently stems from difficulty delineating the bony anatomy in patients in whom poor bone quality coexists with spinal deformity, such as degenerative scoliosis or marked spondylosis. Once the instruments are in place, care must be taken not to apply too much force, because leverage may lead to fractures. Pedicle and transverse process fractures may lead to postoperative pain, irritate local nerve roots, or destabilize the spine. Finally, these breaches create a path for inadvertent leakage of cement into the spinal canal. In one multicenter study, instrument placement problems led to postoperative hematoma in two patients, and a direct injury to the spinal cord when an extrapedicular approach was used on a vertebra with a fractured pedicle.26
Methacrylate monomer is toxic. Some recommend that more than 30 ml PMMA be injected per session.27 The more viscous the cement, the less likely it is that untoward blood pressure or blood gas effects will occur.14
Several VBA reports suggest an increased risk of secondary fractures adjacent to the augmented vertebra.28,29 Two small studies suggest that kyphoplasty decreases adjacent fracture risk. Kasperk and colleagues11,30 found that at 6-month follow-up, 30% (6 of 20) nonoperatively treated patients developed secondary fractures, whereas only 12.5% of 40 kyphoplasty patients had secondary fractures. Similarly, Komp et al12 reported that 65% of 17 nonoperatively treated patients had new fractures, whereas only 37% of 19 kyphoplasty patients had additional fractures. In the FREE study, on the other hand, at 12 months, new vertebral fractures were slightly higher but not statistically significantly different between the kyphoplasty (41.8%) and nonsurgical (37.8%) groups (p = .5).10 The exact effects of VBA on adjacent levels probably vary with steroid exposure, spinal level, local spondylosis, and muscular factors; these require further study.
1. Guermazi A., Mohr A., Grigorian M., et al. Identification of vertebral fractures in osteoporosis. Semin. Musculoskelet. Radiol.. 2002;6:241-252.
2. Lyritis G.P., Mayasis B., Tsakalakos N., et al. The natural history of osteoporotic vertebral fracture. Clin. Rheumatol.. 1989;8:66-69.
3. Pluijm S.M., Tromp A.M., Smit J.H., et al. C Consequences of vertebral deformities in older men and women. J. Bone Miner. Res.. 2000;15:1564-1572.
4. Kayanja M.M., Ferrara L.A., Lieberman I.H. Distribution of anterior cortical shear strain after a thoracic wedge compression fracture. Spine J.. 2004;4:76-87.
5. Hadjipavlou A.G., Katonis P.G., Tzermiadianos M.N., et al. Principles of management of osteometabolic disorders affecting the aging spine. Eur. Spine J.. 2003;12:S113-S131.
6. Truumees E., Hilibrand A., Vaccaro A.R. Percutaneous vertebral augmentation. Spine J.. 2004;4:218-229.
7. Lowe R., Phillips F. Percutaneous vertebral augmentation for malignant disease of the spine. Curr. Opin. Orthop.. 2005;16:489-493.
8. Ledlie J.T., Renfro M.B. Kyphoplasty treatment of vertebral fractures: 2-year outcomes show sustained benefits. Spine. 2006;31:57-64.
9. Lieberman I., Reinhardt M.K: Vertebroplasty and kyphoplasty for osteolytic vertebral collapse, Clin. Orthop. Relat. Res., Suppl. 415:2003, S176-S186.
10. Muller C., Wardlaw D., Bastien L., et al. A randomized trial of balloon kyphoplasty and nonsurgical care for patients with acute vertebral compression fractures: one year results, The Internet Journal of Minimally Invasive Spine Technology, Available at http://www.ispub.com/journal/the_internet_journal_of_minimally_invasive_spinal_technology/volume_2_number_3_1/article/a_randomized_trial_of_balloon_kyphoplasty_and_nonsurgical_care_for_patients_with_acute_vertebral_compression_fractures_one_year_results.html Supplement I – to IJMIST Vol. 1 No 2. 2008.
11. Kasperk C., Hillmeier J., Noldge G., et al. Treatment of painful vertebral fractures by kyphoplasty in patients with primary osteoporosis: a prospective nonrandomized controlled study. J. Bone Miner. Res.. 2005;20:604-612.
12. Komp M., Ruetten S., Godolias G. Minimally invasive therapy for functionally unstable osteoporotic vertebral fracture by means of kyphoplasty: a prospective comparative study of 19 surgically and 17 conservatively treated patients. J. Miner. Stoffwechs.. 2004;1:13-15.
13. Weisskopf M., Herlein S., Birnbaum K., et al. Kyphoplasty—a new minimally invasive treatment for repositioning and stabilizing vertebral bodies. Z. Orthop. Ihre. Grenzgeb.. 2003;141:406-411.
14. Hadjipavlou A.G., Tzermiadianos M.N., Katonis P.G., et al. Percutaneous vertebroplasty and balloon kyphoplasty for the treatment of osteoporotic vertebral compression fractures and osteolytic tumours. J. Bone Joint Surg. Br.. 2005;87:1595-1604.
15. Alvarez L., Perez-Higueras A., Quinones D., et al. Vertebroplasty in the treatment of vertebral tumors: postprocedural outcome and quality of life. Eur. Spine J.. 2003;12:356-360.
16. Gaitanis I.N., Hadjipavlou A.G., Katonis P.G., et al. Balloon kyphoplasty for the treatment of pathological vertebral compressive fractures. Eur. Spine J.. 2005;14:250-260.
17. Rhyne A.3rd, Banit D., Laxer E., et al. Kyphoplasty: report of eighty-two thoracolumbar osteoporotic vertebral fractures. J. Orthop. Trauma. 2004;18:294-299.
18. Gaughen J.R.Jr., Jensen M.E., Schweickert P.A., et al. Lack of preoperative spinous process tenderness does not affect clinical success of percutaneous vertebroplasty. J. Vasc. Interv. Radiol.. 2002;13:1135-1138.
19. McKiernan F., Faciszewski T. Intravertebral clefts in osteoporotic vertebral compression fractures. Arthritis Rheum.. 2003;48:1414-1419.
20. Qaiyum M., Tyrrell P.N., McCall I.W., et al. MRI detection of unsuspected vertebral injury in acute spinal trauma: incidence and significance. Skeletal Radiol.. 2001;30:299-304.
21. Maynard A.S., Jensen M.E., Schweickert P.A., et al. Value of bone scan imaging in predicting pain relief from percutaneous vertebroplasty in osteoporotic vertebral fractures. AJNR Am. J. Neuroradiol.. 2000;21:1807-1812.
22. Mathis J.M., Ortiz A.O., Zoarski G.H. Vertebroplasty versus kyphoplasty: a comparison and contrast. AJNR Am. J. Neuroradiol.. 2004;25:840-845.
23. Weill A., Chiras J., Simon J.M., et al. Spinal metastases: indications for and results of percutaneous injection of acrylic surgical cement. Radiology. 1996;199:241-247.
24. Belkoff S.M., Jasper L.E., Stevens S.S. An ex vivo evaluation of an inflatable bone tamp used to reduce fractures within vertebral bodies under load. Spine. 2002;27:1640-1643.
25. Fourney D.R., Schomer D.F., Nader R., et al. Percutaneous vertebroplasty and kyphoplasty for painful vertebral body fractures in cancer patients. J. Neurosurg.. 2003;98:21-30.
26. Garfin S.R., Yuan H.A., Reiley M.A. New technologies in spine: kyphoplasty and vertebroplasty for the treatment of painful osteoporotic compression fractures. Spine. 2001;26:1511-1515.
27. Coumans J.V., Reinhardt M.K., Lieberman I.H. Kyphoplasty for vertebral compression fractures: 1-year clinical outcomes from a prospective study. J. Neurosurg.. 2003;99:44-50.
28. Legroux-Gerot I., Lormeau C., Boutry N., et al. Long-term follow-up of vertebral osteoporotic fractures treated by percutaneous vertebroplasty. Clin. Rheumatol.. 2004;23:310-317.
29. Grados F., Depriester C., Cayrolle G., et al. Long-term observations of vertebral osteoporotic fractures treated by percutaneous vertebroplasty. Rheumatology (Oxford). 2000;39:1410-1414.
30. Kasperk C., Hillmeier J., Noldge G., et al. Prospective controlled study of the treatment of painful osteoporotic vertebral fractures by kyphoplasty. Osteoporos. Int.. 2004;15:S108.