38 Kiva System in the Treatment of Vertebral Osteoporotic Compression Fractures
KEY POINTS
Introduction
Vertebral compression fractures (VCFs) have a high incidence in the elderly population and are the most common fractures in osteoporotic bones. The majority present without a history of major trauma.1 The incidence of VCF is increased in patients with a prior vertebral compression fracture, with studies indicating that nearly 20% of patients who have an osteoporotic VCF will develop a second fracture within a year of the first.2 Not only do these fractures cause significant morbidity in terms of pain, loss of mobility, and kyphosis, but the relative risk of death after a vertebral fracture is nearly nine times greater than in people without a vertebral fracture.2
Percutaneous vertebroplasty (PVP) was introduced in France in 1984 by Galibert and Deramond as a treatment for a malignant aggressive hemangioma.1 It is a therapeutic procedure performed to reduce pain and perhaps to stabilize vertebral lesions. Subsequently PVP was used to treat painful lesions such as hemangiomas, metastasis, multiple myeloma, and osteoporotic fractures. Today most patients undergoing PVP suffer from vertebral osteoporotic compression fractures. PVP is generally seen as a safe and efficient procedure for treatment of painful osteoporotic fractures.
Vertebral augmentation using balloon kyphoplasty and vertebroplasty3 is traditionally performed with polymethyl methacrylate (PMMA) cement. Clinical studies have demonstrated the efficacy of both methods in reducing fracture-related pain.2,4 However, there have also been reports of complications: extrusion of cement into surrounding tissue, vascular embolism in the corresponding vascular system, adverse systemic reactions to unpolymerized toxic monomers, and thermal damage to adjacent structures. The two latter complications are specific to PMMA. For this reason, and because PMMA does not become osseointegrated, attempts have been increasingly made in recent years to explore the possibilities of alternative cements by looking into biomaterials based on calcium phosphate (CaP). The properties of such bone cements, however, would have to fit a specific profile that takes into account the following parameters: setting behavior, mechanical fitness, and biological behavior.
Contraindications
Precautions
Operative Technique
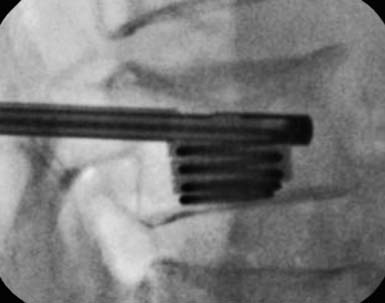
Deployment of the Distraction Sleeve
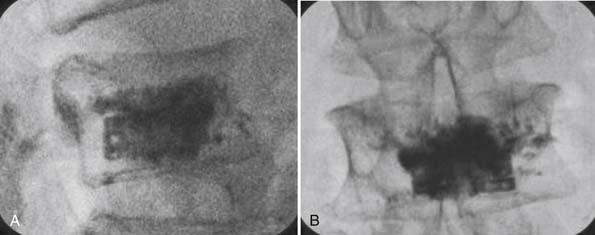
Injecting Pmma Bone Cement
Conclusions and Discussion
These findings, albeit short-term, suggest robust and consistent clinical improvement for pain and function outcomes, following this novel vertebral augmentation procedure in patients with painful VCFs. Clinically relevant gains were realized early postoperatively and maintained through follow-up. The device could be deployed and implanted without adverse events, with improvement in VAS pain scores (p = .0002) and ODI scores (p < .0001), with the following overall clinical success criteria: 2-point improvement in VAS and 15-point improvement in ODI. Two cases of cement extravasations occurred without clinical manifestations.
Although the PMMA cement has proved useful in these procedures, biodegradable vertebroplasty materials are being tested. Arecent report5 showed for the first time that there is no difference in clinical and morphologic outcomes after kyphoplasty using either CaP cement (Calcibon) or conventional PMMA material in patients with painful osteoporotic vertebral fractures for at least 3 years of follow-up. There was no significant difference with regard to postoperative pain reduction or the improvement of mobility between the CaP and PMMA groups. Furthermore, there was a comparable height restoration of the fractured vertebral bodies, and no significant difference in the number of vertebral follow-up fractures during the 3-year study period. In daily routine, PMMA is used for the internal stabilization of vertebral fractures by kyphoplasty. However, PMMA is not biodegradable and heals with a fibrous tissue layer around the implant. Therefore CaP cement materials have been developed, which are biodegradable by osteoclastic resorption and allow a direct osseous integration of the entire surface of the implant, whereby a slow replacement by normal bone tissue seems possible.
Vertebroplasty is widely accepted as an effective, minimally invasive procedure, and is becoming the standard of care for the management of painful osteoporotic VCFs. Significant pain relief has been reported in 78% to 95% of patients suffering from osteoporotic VCFs. However, very few articles in the literature have focused on those patients who failed to respond to the initial PV. Although one study reported that a repeat PVP performed on previously treated vertebral levels for recurrent pain might offer therapeutic benefits (these patients experienced pain relief for 8 to 167 days after the initial PV), we are not aware of any studies on the use of repeat PVs in patients whose pain does not resolve after the initial treatment.5
1. Rousing R., Andersen Mikkel O., Jespersen Stig M., Thomsen K., Lauritsen J. Percutaneous vertebroplasty compared to conservative treatment in patients with painful acute or subacute osteoporotic vertebral fractures: three-months follow-up in a clinical randomized study. Spine. 2009;34(13 June 1,):1349-1354.
2. Becky Benz K., John Gemery M., John McIntyre J., Clifford Eskey J. Value of immediate preprocedure magnetic resonance imaging in patients scheduled to undergo vertebroplasty or kyphoplasty. Spine. 2009;34(6 March 15):609-612.
3. Blattert Thomas R., Jestaedt L., Weckbach A. Suitability of a calcium phosphate cement in osteoporotic vertebral body fracture augmentation: a controlled, randomized, clinical trial of balloon kyphoplasty comparing calcium phosphate versus polymethylmethacrylate. Spine. 2009;34(2 January 15):108-114.
4. He Shi-Cheng, Gao-Jun Teng, Gang Deng, Wen Fang, Jin-He Guo, Guang-Yu Zhu, Guo-Zhao Li. Repeat vertebroplasty for unrelieved pain at previously treated vertebral levels with osteoporotic vertebral compression fractures. Spine. 2008;33(6 March 15):640-647.
5. Grafe Ingo A., Baier M., Nöldge G., Weiss C., Da Fonseca K., Hillmeier J., Libicher M., Rudofsky G., Metzner C., Nawroth P., Meeder P.-J., Kasperk C. Calcium-phosphate and polymethylmethacrylate cement in long-term outcome after kyphoplasty of painful osteoporotic vertebral fractures. Spine. 2008;33(11 May 15):1284-1290.