Chapter 54 Kidney and Ureteral Carcinoma
Renal cell carcinoma, historically referred to as hypernephroma or Grawitz’s tumor, was first reported by Konig in 1826. In 1883, Grawitz3 noted that the fatty content of RCC was similar to that of adrenal cells and postulated that these tumors arose from adrenal rests within the kidney. In 1894, Birch-Hirschfel used the term hypernephroid to describe these tumors.
Primary cancers of the renal pelvis and ureter are uncommon. About 5% of urothelial carcinomas occur in the kidneys and ureters.4 Radical nephroureterectomy, with removal of the bladder cuff, is the main means of treatment. In recent years, laparoscopic and robot-assisted surgeries have become available as alternatives to an open approach. The use of more conservative surgery should be limited to well-selected patients who have low-grade and low-stage disease, small unifocal lesions, solitary kidney, or significant medical comorbidity. Patients with high-grade tumors or advanced-stage disease are at significant risk of local as well as distant relapse. Adjuvant chemotherapy and/or radiation therapy may improve the treatment outcome in patients with locally advanced disease.
Etiology and Epidemiology
Kidney
Epidemiology
Kidney cancer comprises approximately 3% of new cancer cases and 3% of cancer deaths in the United States each year.1,5 In 2010 there were approximately 58,240 new cases of kidney and renal pelvis cancer diagnosed in the United States and approximately 13,040 deaths attributed to kidney and renal pelvis cancer. Over 90% of kidney cancers among adults are classified as RCC.5
RCC is typically diagnosed in the seventh decade of life, with a median age at diagnosis of 65 years; however, it has been observed in children as young as 6 months old. According to data from the Surveillance, Epidemiology and End Results (SEER) program, the overall incidence of RCC in the United States has increased at a rate of 2% to 3% per year since the early 1970s.6,7 Although the greatest increase in RCC incidence has been among localized tumors, early detection of subclinical tumors cannot fully explain the rising incidence of RCC. There is also an upward trend for more advanced tumors as well as an overall increase in mortality rates.6,8–10
There is a strong gender preponderance, with incidence rates in men approximately twice that of women.1 Men account for roughly two thirds of RCC diagnoses and deaths in the United States.11 There is also a notable variability in RCC incidence across racial and ethnic groups. The highest rates are reported for whites and African Americans, whereas the lowest rates are reported for Asian Americans. Between 1975 and 1998, incidence rates among African Americans increased by 4.5% compared with only 2.9% in whites. Globally, the incidence rate of RCC is the highest in North America and Scandinavia and the lowest is in Asia and South America.12
Etiology
Cigarette smoking and obesity are two well-established risk factors for RCC, and each may increase the risk of RCC roughly twofold. These factors may account for 40% of all RCCs diagnosed in the United States.13–20 Although hypertension is often included as a risk factor, this association is considered less certain, given its correlation with both smoking and obesity.13,16–18 Some reports suggest that hypertension may increase the risk of RCC approximately twofold.21,22 There may be an inverse association (i.e., protective effect) of RCC development with moderate alcohol consumption and physical activity, as well as a positive association with a history of urinary tract infections.23–30 There are few consistent associations of RCC and dietary factors.18,31 Consumption of vegetables may be associated with reduced risk, whereas red meat consumption may slightly increase the risk of RCC32–34; however, a recent meta-analysis did not support an independent association of red meat consumption and RCC.35 Finally, occupational exposures to asbestos, gasoline, lead, and cadmium, use of Thorotrast, and treatment with phenacetin, as well as a history of end-stage renal disease or acquired cystic kidney disease, have been associated with increased risk of RCC.36–41
A positive family history has been reported to increase the risk of RCC as much as twofold.42 Although genes that are associated with inherited forms of RCC have been identified, there are currently very few published data regarding the role of germline genetic susceptibility and sporadic RCC.17,43,44 Small case-control studies have demonstrated that individuals carrying polymorphisms in specific genes (particularly phase I enzymes) may be at increased risk of RCC.45–48 The results of these studies have yet to be validated. Large genome-wide association studies of RCC risk are not available at this time.
Renal Pelvis and Ureter
Epidemiology
Approximately 5% of urothelial neoplasms occur in the kidneys and ureters.4 Data from the SEER program suggest that the age-adjusted annual incidence of renal pelvic and ureteral cancers is 0.73/100,000 person-years.49 Similar to RCC, there is a strong male predominance. The incidence increases with age, with the peak age at diagnosis in the sixth and seventh decades of life. Primary tumors of the ureter occur only half as frequently as those of the renal pelvis. The incidence of UUT urothelial cancers has been increasing.49,50 Disease-specific annual mortality is greater in black than in white individuals and in women than in men.
Etiology
Risk factors and environmental agents that cause bladder cancer appear to play similar roles in the development of upper tract urothelial cancers.51–55 Patients with carcinomas of the renal pelvis have more than a 30% to 80% risk of developing metachronous or synchronous bladder cancer. The risk of developing UUT malignancy after treatment of superficial bladder cancer is up to 9.8%.54 The median time to the development of secondary UUT cancer after bladder cancer diagnosis is 33 months. Contralateral metachronous UUT cancer is relatively uncommon, occurring in 3% to 6% of patients.53,56
Cigarette smokers have a 2.6- to 7.2-fold increased risk of UUT cancer.57–59 Chronic use of analgesics that contain phenacetin has been implicated as a risk factor.59–61 Capillarosclerosis is a pathognomonic change seen in patients with long-standing abuse of compound analgesics.62 Ingestion of Chinese herbs containing Aristolochia fangchi, which is used for weight reduction, results in a nephropathy characterized by progressive renal fibrosis and increased risk of urothelial cancer.63,64 There is a 57- to 100-fold increase in the incidence of UUT cancer in Balkan countries affected by an endemic nephropathy.65,66 The renal pathology is an interstitial nephritis. Patients with tumors from the endemic region present with higher-grade disease and more solid growth pattern compared with those from nonendemic regions.67 The cause of the endemic nephropathy is postulated to be high concentrations of radon and minerals in the drinking water in the area. Occupational exposure to organic chemicals is associated with a higher risk of UUT cancers for workers in the chemical, petrochemical, or plastic industries.57 Patients with a history of kidney or ureteral stones have a 2.5-fold increase in risk of renal and ureteral cancers, suggesting that chronic irritation and infection may play a role.68 There are reports of kindred with familial urothelial cancers of the UUT.69–71
Prevention and Early Detection
Kidney
Cessation of cigarette smoking is currently the most effective method of preventing RCC. A large case-control study in Iowa reported that the risk of RCC development decreases steadily from the point of smoking cessation.72 After 15 years of cessation the risk of developing RCC returns to that of nonsmokers. It is unclear whether reduction in weight would decrease the risk for RCC. Some data suggest that daily moderate alcohol consumption and physical activity reduce the risk of RCC; however, more investigations are needed to confirm their benefits.24,25
Early diagnosis of RCC is challenging. Twenty-five percent to 40% of patients are asymptomatic at the time of diagnosis.73 Over the past 3 decades the percentage of patients presenting with the classic triad of flank pain, hematuria, and a palpable mass has dropped to less than 10%. The increased use of imaging modalities including CT, MRI, and ultrasonography helps to detect disease at earlier stages, which may translate to greater probability of resectability.74
Renal Pelvis and Ureter
Lifestyle modification targeting known risk factors of UUT cancers may help to prevent these diseases. Smoking cessation is extremely important, with the reduction in risk correlating with the time of quitting cigarette smoking.58 Diet with frequent intake of both green and yellow vegetables may also reduce the risk of urothelial cancers.75 However, there is no known intervention that can prevent future development of metachronous lesions of the urothelial tract after a primary tumor has been diagnosed.
The use of urine cytology as a screening test is not clearly defined, even in populations at increased risk of developing these diseases (e.g., heavy smokers, workers in petrochemical industries). For patients with a prior history of bladder cancer the use of screening intravenous pyelography as a routine follow-up examination is controversial.76
Pathology and Pathways Of Spread
Kidney
Pathology
In 1997, an international consensus conference on RCC sponsored by the Union Internationale Contre le Cancer (UICC) and the American Joint Committee on Cancer (AJCC) outlined recommendations for the classification of RCC.77 The classification system originally proposed at the Heidelberg conference in 1996 was adopted.78 RCC is categorized as clear cell, papillary, chromophobe, or collecting duct subtypes. RCC that does not fall into one of these four groups is classified as “renal cell carcinoma, not otherwise specified.” Granular cell RCC was excluded from the classification because it encompassed oncocytoma (benign kidney tumor) as well as chromophobe and clear cell RCC and was not specific to a single subtype. This classification system recognizes that RCC consists of several histologic subtypes with distinct morphologic and genetic characteristics. Previous reports have demonstrated significant differences in patient outcome based on histologic subtypes.79–81 Patients with clear cell RCC have a worse prognosis compared with patients with papillary and chromophobe RCC. There is no statistically significant difference in outcome between patients with papillary RCC and those with chromophobe RCC.82
Furthermore, features predictive of a poorer prognosis, including histologic tumor necrosis and sarcomatoid differentiation, have been shown to differ by subtype.80–83 Histologic tumor necrosis is noted in 20% to 45% of RCC patients, whereas sarcomatoid differentiation occurs in 5% to 15% of patients.80–82
Fuhrman and colleagues84 developed a nuclear grading system based on the size and appearance of nuclei and nucleoli. The Fuhrman nuclear grade is predictive of outcome in patients with RCC.
Algorithms have been developed to predict outcomes for patients with RCC. Using retrospective data from 1801 patients with clear cell RCC, Frank and associates85 from the Mayo Clinic developed a predictive model using an SSIGN scoring system that incorporated stage, tumor size, nuclear grade, and histologic tumor necrosis. Ten-year cancer-specific survival can be estimated based on a patient’s SSIGN score. Kattan and coauthors86 developed a nomogram to predict 5-year probability of treatment failure using presenting symptoms, histology, tumor size, and stage. Zisman and colleagues87 developed a clinical outcome algorithm based on 814 patients treated at the University of California, Los Angeles, using stage, Fuhrman grade, and Eastern Cooperative Oncology Group (ECOG) performance status. This algorithm has been validated by two international multicenter studies.88,89
Pathways of Spread
RCC may spread by local extension through the renal capsule into the perinephric fat or the adrenal gland or by direct extension through the renal vein to the inferior vena cava (occasionally reaching the right atrium). Regional lymphatic drainage includes renal hilar, paracaval, aortic, and retroperitoneal lymph nodes. RCC may also spread by blood-borne metastases to the lung, soft tissue, bone, and brain or by retrograde venous drainage to the ovary or testis.90
Renal Pelvis and Ureter
Pathology
Transitional cell carcinoma (TCC) accounts for the majority of renal pelvis and ureter neoplasms.91 Squamous cell carcinoma (SCC) accounts for about 4% of all cases.92 Although patients with SCC tend to present with more advanced-stage diseases and worse prognosis, stage for stage, there is no significant difference in prognosis between TCC and SCC. Other cell types such as adenocarcinoma and sarcoma are very rare.
Both stage and tumor grade are important prognostic factors for survival.93–98 Lymph node metastasis and lymphovascular space invasion are associated with stage and predict for worse outcome.99,100
Gross tumor architecture (sessile vs. papillary growth pattern) is an independent predictor of tumor relapse and cancer-specific mortality.101 Sessile growth pattern is associated with higher tumor grade, more advanced stage, and nodal metastasis. A history of prior bladder cancer has also been associated with worse disease-specific survival (DSS).102
Pathway of Spread
Involvement of multiple areas of the urothelial epithelium by TCC, either synchronously or metachronously, is common in patients with renal pelvis and ureteral cancers. As the tumor progresses, it invades the surrounding tissues with direct extension through the ureteral wall into periureteral tissues or to adjacent renal parenchyma as well as extrarenal tissues. Lymph node metastasis is the most common site of metastasis in UUT cancers. In a multi-institutional series of 1363 patients,103 lymph node metastasis was shown to increase with advancing pathologic stage: less than 1% for T0/Ta/Tis, 2% for T1, 8% for T2, 17% for T3, and 46% for T4.
Kondo and coauthors104 outline the distribution of nodal metastatic sites. For tumors of the right renal pelvis, the primary metastatic sites are the right renal hilar, paracaval, and retrocaval nodes. Tumors of the upper two thirds of the right ureter primarily metastasize to the retrocaval and interaortocaval nodes. Tumors of the left renal pelvis metastasize to the left renal hilar and para-aortic nodes. Tumors of the upper two thirds of the left ureter primarily metastasize to the para-aortic nodes. Tumors of the lower ureter primarily metastasize inferiorly to the aortic bifurcation. The finding of lymph vessel invasion in the tumor is predictive of regional lymph node metastasis.105 Nodal metastasis is a predictor for distant metastasis and is associated with worse survival.99,103
Patients with high-grade or advanced-stage disease are at significant risk of developing distant metastasis.93,106 Common sites of distant failure include lung, liver, and bone.96
Biologic Characteristics and Molecular Biology
Kidney
RCC occurs in both familial as well as sporadic forms. There are several hereditary syndromes associated with the development of RCC, including the von Hippel-Lindau syndrome, tuberous sclerosis, hereditary papillary renal carcinoma, Birt-Hogg-Dubé (BHD) syndrome, and hereditary renal carcinoma.107 The von Hippel-Lindau syndrome, an autosomal dominant disorder affecting 1 in 40,000 individuals, is caused by a mutation of the VHL tumor suppressor gene located on chromosome 3p. Silencing of the VHL gene by either somatic mutation or hypermethylation is believed to play a role in 50% to 60% of sporadic cases of clear cell RCC.108 Tuberous sclerosis is an autosomal dominant disorder affecting 1 in 10,000 individuals and results from mutations of either the TSC1 gene on chromosome 9q or the TSC2 gene on chromosome 6p.109 Hereditary papillary renal carcinoma is also an autosomal dominant disorder in which patients develop bilateral, multifocal lesions with associated germline mutations of the MET proto-oncogene located on chromosome 7q.110 Birt-Hogg-Dubé syndrome is a dominantly inherited predisposition to benign fibrofolliculomas and other skin and soft tissue tumors, including RCC. The gene for Birt-Hogg-Dubé syndrome has been mapped to chromosome 17p12-q11.2.111
Gene expression profiling has provided important information on the genetic basis of RCC.112 Genes that have been shown to have a role in renal carcinogenesis include the FHIT and RASSF1A tumor suppressor genes, transforming growth factor-beta receptor, hypoxia-inducible factors, PTEN, vascular endothelial growth factor, and carbonic anhydrases.113–119 Other genes that have been investigated but for which the data suggest little involvement in RCC include E-cadherin, TP53, BCL2, RB, c-MYC, and c-ERBB2.120–123
Renal Pelvis and Ureter
The karyotypic profile of UUT urothelial cancer has been shown to be similar to that of bladder cancers.124 Loss of the entire chromosome or partial loss of the short arm of chromosome 9 is a common finding, suggesting that it may be an early important event in tumorigenesis. Gains of DNA sequences are frequently observed in chromosome regions 1q21, 2p23, and 8q21.125 Overexpression of TP53, cyclin A, and cyclin E is seen in 24% to 30% of these tumors and is associated with worse prognosis.126–128 The expression of TP53 and caspase correlates with the pathologic stage and grade.129 The expression of survivin and the apoptotic index are associated with shorter disease-specific survival. Low level of CDKN1B is associated with tumor invasion and unfavorable prognosis.130
Clinical Manifestations, Patient Evaluation, and Staging
Kidney
Clinical Manifestations
RCC may present as an incidental finding on imaging or may demonstrate multiple presenting signs and symptoms caused by either local extension or metastatic disease. Because of these multiple symptoms, RCC has been called the “internist’s tumor.” Symptoms resulting from local tumor extension include hematuria, abdominal pain, and a flank mass. However, this “classic triad” of symptoms occurs in only about 10% of cases.90 Symptoms caused by metastatic disease include fever, weight loss, and night sweats. Two percent of male patients present with a varicocele, usually left sided, as a result of impaired drainage of the testicular vein.131
Paraneoplastic syndromes occur in about 30% of patients with RCC. Symptoms include hypertension, hypercalcemia, pyrexia, and hepatic dysfunction.132,133
Patient Evaluation
The widespread use of CT, MRI, and ultrasonography to evaluate the upper abdomen has significantly increased the incidental finding of RCC. Currently, 25% to 40% of diagnoses are made after the incidental detection of a renal mass.134,135 These tumors usually are smaller and, therefore, are more likely to be surgically resectable.136,137
The diagnosis of RCC is usually based on clinical and radiologic findings and pathologically confirmed at the time of nephrectomy. The staging evaluation includes a history and physical examination, complete blood cell count, serum chemistries, determination of lactate dehydrogenase level, urinalysis, chest radiography, and CT or MRI of the abdomen and pelvis (Table 54-1). If clinically indicated, bone scintigraphy and brain MRI may be considered. CT can predict tumor extent preoperatively in 90% of cases.138 MRI has been demonstrated to be superior to CT in determining inferior vena cava tumor extent.139 Magnetic resonance angiography can be used to evaluate vasculature preoperatively, especially when nephron-sparing surgery is being considered for patients with bilateral cancers.
TABLE 54-1 Diagnostic Evaluation/Algorithm for Kidney Carcinoma
Staging
Two staging systems are used to define disease extent in RCC. Historically, Robson’s modification of the Flocks and Kadesky system was used.140 More recently, the AJCC Staging and End Results Reporting Classification has been used (Table 54-2).141 This system appears to be superior to the Robson system because it delineates more clearly local tumor extent and quantifies lymph node involvement.74
TABLE 54-2 American Joint Committee on Cancer Staging Classification System for Kidney and Ureteral Carcinoma, 7th Edition (2010)
| Primary Tumor (T) | |
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| Kidney | |
| T1 | Tumor 7 cm or less in greatest dimension, limited to the kidney |
| T1a | Tumor 4 cm or less in greatest dimension, limited to the kidney |
| T1b | Tumor more than 4 cm but not more than 7 cm in greatest dimension, limited to the kidney |
| T2 | Tumor more than 7 cm in greatest dimension, limited to the kidney |
| T2a | Tumor more than 7 cm but less than or equal to 10 cm in greatest dimension, limited to the kidney |
| T2b | Tumor more than 10 cm, limited to the kidney |
| T3 | Tumor extends into major veins or perinephric tissues but not into the ipsilateral adrenal gland and not beyond Gerota’s fascia |
| T3a | Tumor grossly extends into the renal vein or its segmental (muscle containing) branches, or tumor invades perirenal and/or renal sinus fat but not beyond Gerota’s fascia |
| T3b | Tumor grossly extends into the vena cava below the diaphragm |
| T3c | Tumor grossly extends into the vena cava above the diaphragm or invades the wall of the vena cava |
| T4 | Tumor invades beyond Gerota’s fascia (including contiguous extension into the ipsilateral adrenal gland) |
| Ureteral/Renal Pelvis | |
| Ta | Papillary noninvasive carcinoma |
| Tis | Carcinoma in situ |
| T1 | Tumor invades subepithelial connective tissue |
| T2 | Tumor invades muscularis |
| T3 | (Renal pelvis only) Tumor invades beyond muscularis into peripelvic fat or renal parenchyma |
| T3 | (Ureter only) Tumor invades beyond muscularis into periureteric fat |
| T4 | Tumor invades adjacent organs or through kidney into perinephric fat |
| Regional Lymph Node (N)* | |
| Kidney | |
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis in regional lymph node(s) |
| Ureteral/Renal Pelvis | |
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis in a single regional lymph node, 2 cm or less in greatest dimension |
| N2 | Metastasis in a single lymph node, more than 2 cm but not more than 5 cm in greatest dimension; or multiple lymph nodes, none more than 5 cm in greatest dimension |
| N3 | Metastasis in a lymph node more than 5 cm in greatest dimension |
| Distant Metastasis (M) | |
| MX | Distant metastasis cannot be assessed |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
| Stage Groupings | |
| Kidney | |
| I | T1N0M0 |
| II | T2N0M0 |
| III | T1-2N1M0 T3N0-1M0 |
| IV | T4, Any N0, M0 Any T, any N, M1 |
| Renal Pelvis and Ureteral Carcinoma | |
| 0a | TaN0M0 |
| 0is | TisN0M0 |
| I | T1N0M0 |
| II | T2N0M0 |
| III | T3N0M0 |
| IV | T4N0M0 Any T, N1-3, M0 Any T, any N, M1 |
* Laterality does not affect the N classification.
From Edge S, Byrd D, Compton C: AJCC Cancer Staging Manual. New York, Springer-Verlag, 2010.
Renal Pelvis and Ureter
Clinical Manifestations
The most common presenting symptom is gross or microscopic hematuria, which occurs in about 75% to 80% of patients. Flank pain is present in 27% to 35%.94,142 The pain may mimic that of ureteral calculus. Urinary symptoms such as frequency and dysuria are reported in 25% to 50% of patients. A palpable mass may be found in up to 10% of patients and is usually a hydronephrotic kidney. Constitutional symptoms such as malaise and weight loss are frequently seen in patients with extensive disease or metastases.
Patient Evaluation
Patients are evaluated with urine cytology, CT urography, and ureteroscopy. Cytology of voided urine detects 35% to 59% of UUT urothelial carcinomas and is more useful in higher-grade cancers.94 Urine specimens obtained by ureteral catheterization, or by washings of the upper tract, improve the positive yield of the study. Upper tract urine cytology is positive in up to 70% of patients.143 The positive predictive value of renal pelvis washing for high-grade cancer is 93% but is 43% for low-grade cancer.144 Radiologic findings of these tumors include a radiolucent filling defect or obstructive hydronephrosis. Ureteroscopy or percutaneous nephroscopy is performed if exfoliative cytology or radiologic studies show suspicious findings. Ureteroscopic biopsy provides the tissue diagnosis in up to 89% of cases.145
Additional studies include complete blood cell count and chemistries and chest radiography or chest CT. CT of the abdomen and pelvis is useful to assess the local extent of disease and intra-abdominal metastasis, although it is not a sensitive or specific test for nodal staging.146 If there is any concern about the function of the contralateral kidney, a renal scan should be performed before nephroureterectomy.
Staging
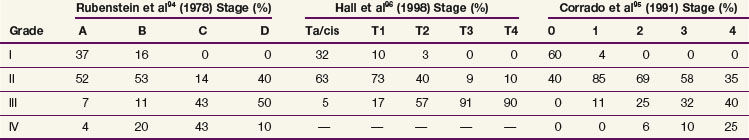
The current TNM staging system is listed in Table 54-2. Another staging system that is often used is the Jewett-Strong classification, which was originally used for bladder cancer: stage 0, limited to the mucosa; stage A, submucosal infiltration; stage B, invasion into but not through the muscle wall; stage C, invasion through the wall; stage D, lymphatic or distant metastasis.147 Tumor grade has a strong association with disease stage (Table 54-3). Both stage and tumor grade are important prognostic factors for survival. Table 54-4 illustrates the impact of stage and grade on the overall survival.
Primary Therapy
Kidney
Surgery
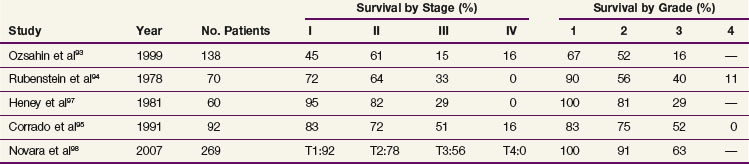
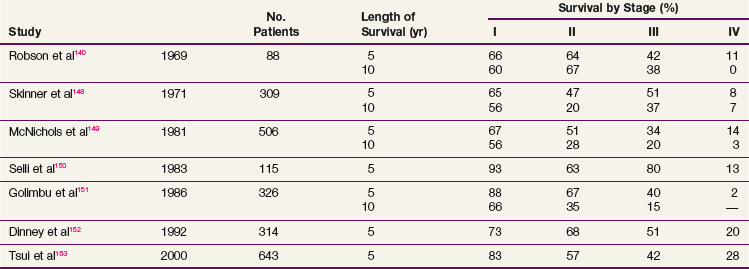
Complete surgical resection is the only possible curative treatment for RCC. A radical nephrectomy typically includes removal of the ipsilateral kidney with perinephric tissues including Gerota’s fascia and the ipsilateral adrenal gland. Survival results for treatment with standard open radical nephrectomy140,148–153 are delineated in Table 54-5. In a report of 643 patients,153 the 5-year DSS was 91% for those with stage I disease, 74% for those with stage II disease, 67% for those with stage III disease, and 32% for those with stage IV disease based on the 1997 TMN staging criteria. In a Mayo Clinic series of 1547 patients,154 the 10-year cause-specific survival (CSS) was 91% for T1, 70% for T2, 53% for T3a, 42% for T3b, and 43% for T3c tumors.
Removal of the adrenal gland is not required for RCC involving the middle or lower poles. However, upper pole cancers may necessitate adrenal gland removal with nephrectomy depending on the size, pathologic type, and extent of invasion of the renal tumor. Preoperative CT has demonstrated 99.6% specificity and a 94.4% negative predictive value in predicting adrenal gland involvement.155,156 In a review of 511 radical nephrectomies with adrenal gland removal,155 the incidence of renal cancer involvement with the adrenal gland was 5.7% and T1-2 tumors had an incidence of adrenal involvement of 0.6%.
A radical nephrectomy can include resection of hilar lymph nodes and occasionally includes regional lymph node dissection. Although regional lymph node dissection provides additional prognostic information, it has not been proven to prolong survival.74 The European Organization for Research and Treatment of Cancer (EORTC) conducted a phase III trial evaluating the role of lymph node dissection.157 Seven hundred seventy-two patients were randomized to a radical nephrectomy with or without a complete lymphadenectomy. All patients were clinically node negative based on preoperative CT. The incidence of positive lymph nodes was 4%. After a median follow-up of 12.6 years there were no differences in overall survival (OS), time to progression of disease, progression-free survival (PFS), or complications. One criticism of this trial is that 70% of patients were T1 or T2 category, suggesting a low risk of lymph node involvement. It is difficult to conclude whether patients with locally advanced tumors would benefit from a lymph node dissection. Researchers at the Mayo Clinic reported a review of 955 patients who underwent a lymph node dissection as part of a radical nephrectomy.158 High-grade tumor, presence of sarcomatoid features, tumor size greater than or equal to 10 cm, T3 category, and histologic tissue necrosis were significant predictors of lymph node-positive disease. The presence of these high-risk factors can help to determine which patients may benefit from an extensive lymph node dissection. Pantuck and colleagues159 retrospectively analyzed 900 patients with RCC and concluded that a lymph node dissection offers no survival advantage in clinically node negative patients but is associated with improved survival and improved response to immunotherapy in node-positive patients.
Partial nephrectomy, or nephron-sparing surgery, has been performed for small tumors. The initial indications for this surgery were presence of bilateral tumors or a cancer involving an anatomic or functional solitary kidney. Local recurrence rate was low (<6%), and this procedure has subsequently been performed in patients with small tumors (<4 cm).160 The incidence of local recurrence in these “electively” treated patients is 3% or less. Recent data from several institutions161,162 suggest larger tumors (>7 cm) can also be considered for nephron-sparing surgery. Patients undergoing nephron-sparing surgery have a decreased cumulative incidence of chronic renal insufficiency compared with patients who undergo radical nephrectomy.163,164
Laparoscopic radical nephrectomy and laparoscopic partial nephrectomy have become a commonly used surgical option for patients with RCC. Both pure and hand-assisted laparoscopic techniques for radical nephrectomy and nephron-sparing surgery achieve oncologic outcomes comparable to the standard open approach.165–169 Laparoscopic approaches have demonstrated advantages regarding blood loss, length of hospital stay, pain medication usage, cosmetics, and patient recovery compared with the standard open approach of radical nephrectomy.165,166
Ablation through radiofrequency heating and cryosurgical freezing are two frequently utilized techniques to obliterate suspicious small renal lesions.170,171 Employment of these techniques through an open, laparoscopic, or percutaneous approach has been demonstrated.170–172 A review of radiofrequency ablation and cryosurgical freezing with a laparoscopic or percutaneous approach173 revealed that laparoscopic ablation using either technique had a 0% to 15% relapse rate with a follow-up of 5 years. The percutaneous approach for ablation also demonstrated similar recurrence rates, but follow-up was only 2 years. The overall complication rate was 5%.
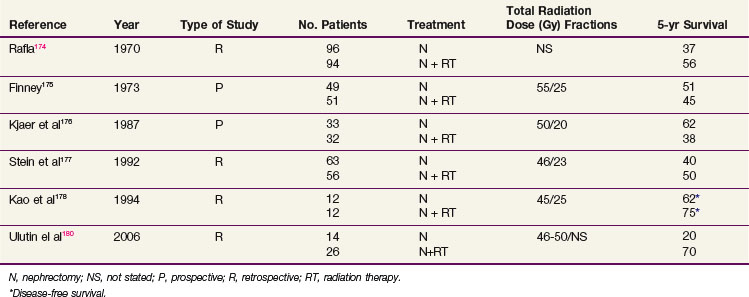
Adjuvant Irradiation
Although the use of postoperative irradiation has been evaluated both retrospectively and prospectively, its role remains controversial174,175–178 (Table 54-6). In a nonrandomized comparison of surgery with or without postoperative irradiation, Rafla174 found a 5-year OS advantage for 94 patients undergoing postoperative irradiation versus 96 patients treated by nephrectomy alone (56% vs. 37%). These data were confirmed in an expanded updated analysis. The 5-year OS was 38% (40/105) for patients who received postoperative irradiation versus 18% (24/135) for those undergoing nephrectomy alone.179
In a pseudo-randomized trial of 100 patients (randomization by odd vs. even date of birth), Finney175 found that incidence of distant metastases, local recurrence, and survival were not affected by the addition of postoperative radiation therapy (55 Gy in 25 fractions in 5 weeks). These results are difficult to interpret because randomization was not blinded or stratified by risk factors.
A small prospective, randomized study of postoperative radiation therapy was conducted by the Copenhagen Renal Cancer Study Group.176 Patients were randomized to nephrectomy alone (33 patients) versus nephrectomy and postoperative radiation therapy (32 patients). Postoperative irradiation consisted of 50 Gy in 20 fractions. The 5-year OS was 62% for nephrectomy alone versus 38% for nephrectomy and postoperative radiotherapy. Forty-four percent of the patients treated with radiation therapy had significant complications involving the stomach, duodenum, or liver that contributed to the death of 19% of these patients.
Four more recent retrospective studies evaluated the role of postoperative irradiation in patients at high risk for local recurrence after surgery for RCC, using multiple treatment fields and CT-based planning. Stein and associates177 evaluated 147 patients with localized RCC who underwent a nephrectomy. Fifty-six patients received postoperative irradiation with a median dose of 46 Gy. With a median follow-up of 19 months, there was no statistical difference in OS. However, there were more local recurrences in nonirradiated T3 patients (37% in nonirradiated patients vs. 11% in irradiated patients). In a follow-up descriptive study of patients treated with radiation after a nephrectomy, Gez and coauthors180 reported no benefit of postoperative radiation, even for T3 patients.
Kao and colleagues178 described 12 patients treated with nephrectomy alone and 12 patients treated with nephrectomy and postoperative irradiation, with a median dose of 45 Gy. After a median follow-up of 5 years, no difference in DFS was noted. However, the local failure rate was 30% for the nephrectomy-alone patients and 0% for those who had postoperative radiation therapy. In another retrospective study, Ulutin and colleagues181 found no difference in OS but a significant difference in DFS in favor of radiation therapy for 26 patients treated with postoperative irradiation compared with 14 patients treated with nephrectomy alone.
Rabinovitch and associates182 performed a patterns-of-failure analysis by CT on patients with localized RCC who underwent a radical or partial nephrectomy without adjuvant therapy. The risk of local failure after a gross resection was approximately 5%. Positive surgical margins and positive lymph nodes had a significant impact on local failure, increasing the rate of local failure to 21%. The risk of distant metastasis after gross resection was 26%. Lymph node involvement and renal vein involvement were significant prognosticators of distant metastases. Approximately half of patients who experienced a local relapse also experienced distant metastases concurrently or as the first site of relapse. Therefore the risk of micrometastatic disease at the time of surgery must be weighed against the potential benefit of local irradiation.
Adjuvant Chemotherapy, Immunotherapy, and Molecularly Targeted Treatment
Adjuvant therapy is given to patients with high risk of systemic disease relapse after curative surgery. Three separate clinical trials evaluating adjuvant interferon-alfa after nephrectomy183–185 failed to demonstrate a survival benefit. The Cytokine Working Group had conducted a high-dose intravenous bolus interleukin-2 (IL-2) trial versus observation in postnephrectomy patients,186 and it did not show any survival benefit. A different approach was studied by Jocham and colleagues187 using autologous tumor cell vaccine. Five hundred and fifty-eight patients with resected pT2-3b, pN0-3, M0 RCC were randomly assigned to vaccine compared with observation. At 4.5 years, PFS was 77% with the vaccine and 68% with observation. Its effect on OS would require longer follow-up. The role of targeted agents in the adjuvant setting is actively being investigated. One study in progress is the ECOG phase III study ECOG 2805, or the ASSURE study (Adjuvant Sorafenib or Sunitinib for Unfavorable risk REnal carcinoma).
Stereotactic Body Radiotherapy
The relative radioresistance of RCC and the previous experience with improvement in local control for patients treated with stereotactic radiosurgery for renal cell brain metastases have led to growing interests in the treatment of primary RCC with high-dose per fraction stereotactic body radiotherapy (SBRT). Beitler and colleagues188 reported on nine patients with nonmetastatic primary RCC who refused a nephrectomy. The majority of patients received 40 Gy in five fractions over 15 days. With a median follow-up of 26.7 months, four of nine patients survived. Only one patient had a local failure, which consisted of a new primary tumor in the unirradiated portion of the kidney. All surviving patients had an initial tumor size less than 3.4 cm and no clinical evidence of nodal disease, renal vein, or inferior vena caval extension or penetration of Gerota’s fascia. Wersall and associates189 treated eight patients with an inoperable primary or local inoperable recurrence. The median follow-up surpassed 58 months and only one of the patients experienced a local failure.
Svedman and colleagues190 conducted a single-institution phase II trial to evaluate the efficacy and safety of SBRT for RCC. Thirty patients with inoperable primary or metastatic RCC received treatment to 82 lesions. The most common dose fractionations were 32 to 40 Gy in four or five fractions of 8 Gy, 30 to 40 Gy in three or four fractions of 10 Gy, and 30 to 45 Gy in two or three fractions of 15 Gy. With a median follow-up time of 52 months for living patients and 22 months for deceased patients, local control was 98%. Sixteen of 28 patients experienced side effects, 96% of which were grade I or II.
Renal Pelvis and Ureter
Surgery
Nephroureterectomy with removal of the bladder cuff is the standard treatment for patients with UUT cancer. Locoregional relapses occur in 9% to 15% of patients with low-grade, low-stage disease and in 30% to 50% of those with high-grade, high-stage disease. The removal of the bladder cuff is recommended because ureteral stump recurrence is noted in 11% to 55% of patients if the stump is left in place.147,191,192 Patients with T2-4 disease should have lymphadenectomy. Nodal status is a significant prognostic factor and may be used to guide the decision for adjuvant treatment. For selected patients with distal ureteral cancers, distal ureterectomy and lymphadenectomy with reimplantation of ureter may be an option.
Laparoscopic nephroureterectomy has been shown to achieve similar disease control in many patients, with decrease in hospital stay and shorter recovery time, as compared with open nephroureterectomy.193–197 In a report of 115 patients,195 port-site metastasis occurred in one patient (0.9%). Minimally invasive surgery with robot-assisted ureterectomy and ureteral reconstruction appears to be safe and feasible in some patients, but the oncologic effectiveness of this procedure needs to be confirmed.198
There are conflicting reports96,97,199,200,201 about the efficacy of more conservative nephron-sparing surgery or partial ureterectomy, which often can be done endoscopically. One study reported up to 62% recurrence, and careful follow-up is imperative for these patients. The selection criteria usually include low-grade and low-stage disease, superficial disease, small lesion size (<1.0 to 1.5 cm), absence of multifocal disease, solitary kidney, bilateral disease, or major medical comorbidities. These patients would need to be followed very closely so that nephroureterectomy can be offered if recurrence is detected.
Primary Irradiation
Primary irradiation is rarely used as initial treatment. The few published reports using primary irradiation are mostly case reports or small series. Some patients with gross residual disease after nephroureterectomy or who have recurrent disease are treated with external beam irradiation. In a series with 19 patients with unresectable disease,191 the median survival was 11 months for the 11 patients treated with radiation therapy versus 4 months for those who did not receive radiation. Two patients were disease free after receiving 45 Gy and 50.4 Gy, respectively, for gross residual disease after surgery. Batata and coauthors147 reported one of eight patients had long-term disease control after EBRT for gross residual disease after surgery, using doses of 10 Gy in 5 fractions to 60 Gy in 35 fractions.
Adjuvant Irradiation
The risk of local relapse correlates with the stage of disease and tumor grade and can be up to 45% despite radical surgery. In a study by Cozad and colleagues,191 the 5-year local control rate was 90% for grade 1 and 2 tumors and 41% for grade 3 and 4 tumors, respectively. For stage I and II disease, the 5-year local control rate was 83%, compared with 52% for stage III disease. About half of the local relapses presented initially as isolated site of relapse. Distant metastasis occurred in 19% of patients with stage I and II disease and in 53% with stage III disease. Distant metastasis was the predominant site of initial relapse for stage III disease. The risk of lymph node metastasis also increases with disease stage and tumor grade.103 The pattern of failure demonstrates that patients with high-stage or high-grade disease have significant risks of both distant and local relapse, and many of them may have locoregional failure as the initial site of relapse.
Few studies have been published to evaluate the role of adjuvant irradiation in the management of UUT cancers.147,202,203 In a study by Cozad and colleagues,202 26 patients with T3 or T4 N0/+ disease had radical surgery, 9 of whom received adjuvant irradiation to a median dose of 50 Gy. Local failure occurred in 9 of 17 without and 1 of 9 with adjuvant irradiation (p = .07). The effect of adjuvant irradiation was seen only in high-grade tumors, because no local relapse developed in patients with low-grade disease regardless of whether adjuvant therapy was given. Metastasis developed in 4 of 9 and 8 of 17 patients with and without radiation therapy, respectively. Five-year OS was 44% with, and 24% without, adjuvant irradiation, respectively (p = .23). In a study by Brookland and Richter,203 adjuvant irradiation was given to 11 of 23 patients who had tumor grade 3 or 4, or pathologic stage C or D. The median dose was 50 Gy to the ureteropelvic bed, with or without inclusion of the para-aortic nodes. For the nonirradiated group, the median survival was 26 months and the local relapse rate was 45%, compared with 35 months and 11%, respectively, for those who received adjuvant irradiation. Other studies have questioned the benefits of adjuvant irradiation. In a series of 138 patients,93 45 received postoperative irradiation with a median dose of 50 Gy. The 5-year OS was 21% for those who received postoperative irradiation, compared with 33% for those who did not. However, patients who received postoperative irradiation had significantly higher T category and tumor grade. Maulard-Durdux and colleagues106 reported the use of 45 Gy of adjuvant irradiation in 26 patients and concluded that local control and OS were similar to those of other surgical series, with distant metastasis as the predominant site of relapse.
Czito and colleagues204 reported a series of 31 patients with T3/4 or N+ disease that was treated with adjuvant irradiation to a median dose of 46.9 Gy. Adjuvant MVAC chemotherapy (methotrexate, vinblastine, doxorubicin [Adriamycin], cisplatin) with concurrent cisplatin was given to 9 patients. Five-year OS, DSS, locoregional control, and metastasis-free survival rates were 39%, 52%, 67%, and 48%, respectively. Compared with irradiation alone, the use of concurrent cisplatin improved the OS (67% vs. 27%) and DSS (76% vs. 41%). This study suggests that the addition of concurrent cisplatin to adjuvant radiotherapy improves the survival of patients with locally advanced disease.
Adjuvant Chemotherapy
Urothelial cancer of the bladder has been shown to be sensitive to chemotherapy, with improvement in OS when preoperative chemotherapy is given. However, there are very few studies evaluating the use of adjuvant chemotherapy for UUT cancers. Kwak and colleagues205 reported a series in which 32 patients received four or more cycles of cisplatin-based adjuvant chemotherapy while 11 did not. Most of these patients had T3 cancer. There was a significant difference in OS and DFS. Disease relapses occurred in 38% of those who had adjuvant chemotherapy, compared with 64% for those who did not. The 3-year OS was 81% versus 36%. In another small series of 46 patients, most of whom had pT3N0 disease, 24 were given adjuvant MVAC chemotherapy while 22 were observed.206 Owing to side effects, only 9 patients were able to complete the three planned cycles of treatment. There was a significant improvement in 5-year intravesical recurrence-free survival in the chemotherapy group compared with the nonchemotherapy group (84% vs. 39%). However, there was no difference in OS.
In an international collaborative database that included 542 high-risk patients,207 among which 121 received adjuvant chemotherapy, there was no difference in median OS and DSS between the chemotherapy and nonchemotherapy group. However, the percentage of patients with high-grade and high-stage disease in the chemotherapy group was significantly more than in the nonchemotherapy group. Large prospective studies are needed to define the role of adjuvant chemotherapy for UUT cancer.
locally advanced disease
Kidney
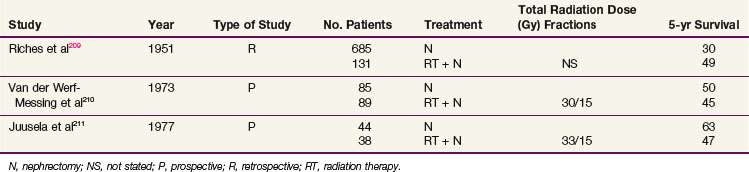
RCCs rarely invade adjacent organs at presentation. Large tumors tend to displace and compress adjacent tissue. However, if tumor directly invades contiguous tissues such as the liver, duodenum, large intestine, and perinephric muscle, surgical resection may not be successful. Preoperative irradiation can reduce the size of renal tumors and cause fibrosis, with thickening of the tumor capsule and sclerosis of small blood vessels, increasing the likelihood of an adequate surgical resection.208 In a retrospective review, Riches and colleagues209 reported apparent survival advantages of patients treated with preoperative irradiation versus surgery alone (49% vs. 30% 5-year OS).
Two prospective clinical trials evaluating the role of neoadjuvant radiation210,211 have been conducted (Table 54-7). Van der Werf-Messing210 reported a series of 126 patients from 1965 to 1972 randomized to nephrectomy alone versus low-dose preoperative radiation therapy (30 Gy in 15 fractions for 3 weeks) followed by immediate nephrectomy. Complete resection was more frequent for patients with tumors infiltrating intrarenal or extrarenal veins or lymph vessels, and survival was considerably better at 18 months, when radiation therapy was given preoperatively compared with surgery only. However, there was no difference in 5-year OS.
Juusela and associates211 also conducted a prospective, randomized study of preoperative irradiation followed by nephrectomy (38 patients) versus nephrectomy alone (44 patients). Patients treated with preoperative radiation received 2.2 Gy per day to a total dose of 33 Gy and had a 47% 5-year OS, compared with 63% for patients treated with nephrectomy alone.
Intraoperative Irradiation Containing Approaches for Locally Advanced Disease
Mayo Clinic Results
Frydenberg and colleagues reported on two patients with locally advanced RCC and six patients with locally recurrent RCC treated with preoperative EBRT (45 to 50 Gy) followed by resection and IOERT at the Mayo Clinic.212 The two patients with primary locally advanced disease were alive without evidence of disease at 15 and 50 months from initiation of EBRT. Of the six patients with locally recurrent disease, two were alive with no evidence of disease at 29 and 42 months. The locoregional control rate was 87.5%.
The Mayo Clinic IOERT series was updated to include 14 patients with locally advanced primary (2 patients) or recurrent (12 patients) renal cancers (see Table 54-8 on Expert Consult website).213 Preoperative EBRT was given in 11 of the 14 patients (dose = 44 Gy in 8 patients). The IOERT dose was 10 to 20 Gy in 12 of 14 patients (dose of 25 Gy given to 2 patients who received high-dose EBRT before referral to the Mayo Clinic with disease progression). In the renal cell cancer group of patients, 5 of 10 patients were alive.213 Three of 10 (30%) were free of disease at 37, 55, and 56 months from initiation of treatments, and 2 others died free of disease at 10.5 and 19 months (5 of 10, or 50%, relapse free). Two patients were alive with disease at 22 and 44 months, and 3 died of disease at 16, 19, and 31 months. Of the 4 patients with other cell types, 1 died free of disease at 28.5 months and 3 died of disease at 5, 8, and 12 months. Local or central relapse occurred in only 1 patient in each category. The distant metastasis rate was high at 57% (8 of 14 patients).
WEB-ONLY TABLE 54-8 Locally Advanced and Recurrent Renal Cell Carcinoma (RCC), Mayo Clinic: External Beam plus IOERT Survival, Disease Status, and Patterns of Failure
In the most recent update of the Mayo Clinic series,214 IOERT had been used as a supplement to EBRT and resection in 49 patients with genitourinary malignancies, including 28 patients with renal cancer. Other disease sites were bladder, 8; prostate, 7; ureter, 2; and miscellaneous, 4. Survival was best for patients with renal cancer (primary or recurrent) (5-year, 37% vs. 16%, p = .05), for patients with gross total resection preceding IOERT (5-year, 41% vs. 0%, p <.0001) and for primary vs. recurrent disease (5-year, 48% vs. 25%, p = .019). For the 28 patients with renal primary tumors, disease control was as follows: local, 90% (5-year); central, 88% (5-year, within IOERT field); and distant, 28% (3-year).
Pamplona Series
The University Clinic of Navarra in Pamplona used IOERT as a component of treatment for 11 patients with locally advanced primary (8 patients) or locally recurrent (3 patients) lesions.213,215 Patients received IOERT after maximal surgical resection, and 7 of the 11 received postoperative EBRT (30 to 45 Gy). Gross residual disease remained in 4 patients; the remaining 7 had close or micropositive margins. Histology was RCC in 10 patients and TCC in 1 patient. The IOERT dose ranged from 10 to 20 Gy (10 Gy, 2 patients; 15 Gy, 8 patients; 20 Gy, 1 patient).
In an early analysis (follow-up time of 2 to 33 months; median, 8 months), distant metastases occurred in 3 patients (lung, 3; liver, 2) and local relapse in 1 of the 3 (microresidual, IOERT dose 20 Gy and no EBRT).215 In an analysis update, 3 of 11 patients were free of disease at 3 or more years, similar to results of the Mayo Clinic series.
Heidelberg Series
Elbe and colleagues216 reported a University of Heidelberg series of 11 patients with renal cancer in whom IOERT was given as a component of treatment (primary, 3 patients; locally recurrent, 8 patients). Patients had maximal resection, IOERT (12 to 20 Gy), and postoperative EBRT (40 Gy in 2-Gy fractions, 5 days per week). With mean follow-up of 24 months, no local relapse occurred. Distant metastases, however, occurred in 5 of 11 patients, or 45% (lung, 3; bone, 2). At 3 years, OS and DFS were 47% and 34%, respectively.
University of California, San Francisco, Series
At UCSF,217 10 of 14 patients who underwent resection of recurrent renal cancer received IOERT. Nine patients died of progressive metastatic disease at a mean of 17 months. Five patients were alive (mean 66 months; range 14 to 86 months) and OS was 40% at 2 years and 30% at 5 years. Local re-recurrence was observed in 2 patients.
Renal Pelvis and Ureter
Patients with locally advanced or unresectable disease have poor prognosis. The performance status and medical comorbidities of these patients are major considerations in determining the aggressiveness of any therapeutic intervention. Radiation therapy, with or without chemotherapy, has been shown to achieve long-term disease control in some patients who have gross residual or locally recurrent disease.147,191 In a report by Cozad and colleagues,191 postoperative irradiation was given to 11 of 19 patients with gross residual disease after surgery. The median survival was 11 months for the irradiated group versus 4 months for those who did not receive radiation. Two patients who were treated with 45 Gy and 50.4 Gy of irradiation were disease free at 21 and 28 months. One of these 2 also had concurrent MVAC chemotherapy. In selected patients with locally advanced UUT cancers, preoperative irradiation with or without chemotherapy may downstage the disease to allow surgical resection.
IOERT is an appealing adjunct in the management of locally advanced UUT. After preoperative EBRT ± chemotherapy to downsize the tumor, patients may proceed to surgery. A boost may be given to areas of close or positive margins with IOERT while sparing the surrounding normal organs. Zhang and associates218 treated 17 patients with locally advanced ureteral cancer with nephroureterectomy and IOERT, followed by adjuvant EBRT. Eleven patients had T3 and 5 had T4 primary tumors. Six patients also had nodal involvement. Five patients had microscopic or grossly positive margins. The median IOERT dose was 14 Gy, and the median EBRT dose was 42 Gy. Adjuvant MVAC was given to 10 patients. The 5-year OS and local control rates were 46% and 51%, respectively. On multivariate analysis, disease grade and incomplete surgical resection were significant predictors of survival. Two patients developed late grade 3 gastrointestinal toxicities. Dose escalation using IOERT appears to be an option for patients with locally advanced UUT cancer and should be further evaluated.
Palliation and Metastatic Disease
Kidney
In patients with demonstrated widespread metastatic disease at initial diagnosis, nephrectomy is not justified if the intent is to induce a spontaneous regression; the incidence of such a regression is less than 1% and would not justify the high morbidity rates associated with nephrectomy. A palliative nephrectomy can be considered in patients suffering from repeated hemorrhages, tumor pain, or significant paraneoplastic syndromes.74
The role of nephrectomy has been evaluated in patients who present with solitary metastatic disease. Of 158 patients who presented to the Mayo Clinic between 1970 and 1980 with documented metastatic disease and no prior treatment at diagnosis,219 56 patients (35%) were noted to have a solitary metastasis. Nephrectomy was performed on 67.6% of patients who presented with multiple metastases and 83.9% of patients with solitary metastasis. The OS at 2 and 3 years for patients with solitary metastasis was 29.1% and 19%, respectively, versus 6.8% and 4.3% for patients with multiple lesions.219 Nephrectomy had a significant impact on survival only in patients with solitary metastasis, low-grade primary tumor, and weight loss of less than 10%.
Two clinical trials have shown that debulking nephrectomy may enhance the effect of immunotherapy. The Southwest Oncology Group (SWOG) randomized 241 patients with an ECOG performance status of 0 to 1 to initial nephrectomy followed by interferon versus interferon alone.220 The median survival of the nephrectomy group was significantly improved by 3 months (11.1 vs. 8.1 months). The EORTC also conducted a trial similar to the SWOG trial in 83 patients with metastatic RCC and an ECOG performance status of 0 to 1.221 Patients undergoing nephrectomy experienced a 10-month median survival benefit (17 vs. 7 months) and improved time to progression (5 vs. 3 months). These debulking nephrectomy trials show that patients with a good initial performance status who subsequently receive interferon immunotherapy experience an improved survival. There are no prospective trials evaluating debulking nephrectomy followed by IL-2 or molecularly targeted therapy.
Motzer and associates222 evaluated prognostic factors for 670 patients with metastatic or recurrent RCC to develop a predictive modal for survival. The absence of a nephrectomy predicted a lower survival in these patients. Other predictive factors included performance status and hemoglobin, calcium, and lactate dehydrogenase values.
Solitary Metastases
Treatment of the solitary metastatic site has been evaluated in patients with both synchronous and metachronous solitary metastasis. Of 18 patients who presented with a solitary metastasis at diagnosis at the Mayo Clinic,223 4 patients (22%) who underwent surgical treatment of the primary and metastatic sites survived longer than 2 years. Patients with metachronous solitary metastasis have a more favorable outlook. Of 26 patients treated with surgery or radiation therapy, 18 (69%) lived more than 2 years, and 5-year OS from the time of nephrectomy was 50%. Six of the 26 patients (23%) lived more than 5 years after removal of the solitary metastatic lesion.
Kjaer224 evaluated 25 patients with RCC and solitary metastasis. Twelve patients had a focus in the bone, and the remaining 13 patients had metastasis in soft tissue, including the lung, thyroid, flank, and epididymis. Radiation therapy was used primarily to treat the bone lesions. For the 13 patients with soft tissue metastasis, surgery was done in 7 patients and radiation therapy in 6. The median survival of this select group of patients was 4.3 years. The 5- and 10-year OS were 36% and 16%, respectively. Females had 5- and 10-year OS of 76% and 38%, respectively, versus 18% and 12% for males (p = .05).
Kavolius and coauthors225 retrospectively evaluated 278 patients initially treated with a curative nephrectomy who developed recurrent and/or metastatic RCC. The 5-year OS for 141 patients treated with a curative metastectomy for their first relapse was 44% as compared with 14% for patients who underwent noncurative surgery and 11% for patients treated nonsurgically. On multivariate analysis, factors associated with improved OS included a solitary site of first relapse, curative resection of first metastasis, and a long disease-free interval.
Bone and Soft Tissue Metastases
Metastases to the bone (solitary or multiple) may occur in 25% to 50% of all patients with RCC. Althausen and associates226 evaluated 38 patients with osseous metastases secondary to RCC. Patients were treated with resection with or without allograft implantation, radiation therapy alone, or a combination of treatments. The survival for the entire group was 90% at 6 months, 84% at 1 year, 55% at 5 years, and 39% at 10 years. Presentation without metastasis, long disease-free interval between nephrectomy and first metastases, appendicular skeletal location, and solitary metastasis were associated with longer survival. The authors concluded that patients who have an appendicular (preferably solitary) metastasis and who have a long duration from diagnosis of disease to metastasis should be treated with complex orthopedic surgical procedures if necessary. Postoperative irradiation should be considered in selected cases.
The results of irradiation alone in the treatment of bone metastases have been evaluated. Halperin and Harisiadis227 evaluated the results of 36 sites irradiated for bone pain. The pain responded in 77% of the treated sites, and the majority of the sites received a time-dose fractionation (TDF) equivalent dose ranging from 45 to 85. No relation was observed between the TDF equivalent dose and the probability of response. Twenty-four of the 28 sites (86%) were partially or completely free of pain for the remainder of the patients’ lives.
Wilson and colleagues228 reported partial and symptomatic response rates to palliative irradiation, with pain reduction of 67% and 73%, respectively. A dose response based on the biologic effective dose could not be established.
In a prospective phase II trial, Lee and associates229 evaluated the effect of radiation therapy on symptoms in 31 patients with symptomatic bone or soft tissue RCC metastases. With a median follow-up of 4.3 months, 83% of patients experienced site-specific pain relief with a median duration of site-specific pain response of 3 months. Global assessment of quality of life was limited owing to progression of disease outside the fields of treatment.
Stereotactic body radiotherapy (SBRT) or stereotactic radiosurgery (SRS) has been used for treatment of metastatic RCC to the spine. Gerszten and colleagues described 48 patients who underwent SRS to 60 spinal lesions.230 A single fraction with a median dose of 20 Gy was given. Eighty-nine percent of patients experienced improvement in pain. Six of 48 patients eventually required surgical intervention because of progression of disease. Nguyen and co-workers231 reviewed 48 patients who underwent SBRT to 55 spinal lesions. Doses included 24 Gy in one fraction, 27 Gy in three fractions, and 30 Gy in five fractions. Forty-four percent and 52% of patients were pain free at 1 month and 12 months, respectively, compared with 23% before treatment. No grade 3 or grade 4 neurologic toxicity occurred.
Brain Metastases
Brain metastases are diagnosed in approximately 10% of patients with metastatic RCC.232 Wronski and associates232 evaluated the results of whole-brain radiation therapy in a retrospective study of 119 patients treated at M.D. Anderson Cancer Center. The median radiation dose was 30 Gy (range, 18 to 56 Gy). The median OS from the time of diagnosis of the brain metastases was 4.4 months. Patients with multiple brain tumors had a median survival of 3.0 months, compared with 4.4 months for those with a single brain metastasis (p = .043). The cause of death was neurologic in 90 of the 119 patients (76%). Patients with brain metastases diagnosed synchronously with the renal primary tumor (24 patients) had a median survival of 3.4 months compared with 3.2 months for the 95 patients diagnosed metachronously. Favorable prognostic factors included solitary brain metastasis, lack of other distant metastases at diagnosis, and tumor diameter less than 2 cm.
In view of the poor outcome of patients after whole-brain irradiation, more aggressive treatments have been evaluated in selected patients with favorable prognostic features. Patients who could undergo surgical resection of brain metastases from RCC had a 12-month median survival.233 Several retrospective studies234,235,236–239 have reported local tumor control rates of 88% to 96% in patients treated with radiosurgery alone, or in combination with whole-brain irradiation. Brown and colleagues235 reported that the addition of whole-brain irradiation to SRS improved local control and decreased distant brain failure. The Radiation Therapy Oncology Group recursive partitioning analysis (RPA) class strongly influenced the survival rate.234,240
Chemotherapy, Immunotherapy, and Molecularly Targeted Treatment
Treatment options for patients with metastatic RCC include chemotherapy, immunotherapy, hormonal therapy, and, more recently, molecularly targeted treatment. Generally, RCC is not sensitive to chemotherapy, with a response rate of about 5.6%.241 One regimen worth mentioning is the combination of gemcitabine and continuous 5-fluorouracil, which achieved a response rate of 17% as the second-line treatment in a phase II study.242 In non–clear cell RCC, the response rates to chemotherapy appear to be better, and response rates of more than 10% to 15% have been reported using carboplatin/paclitaxel, cisplatin/gemcitabine, and doxorubicin/gemcitabine (for collecting duct or sarcomatoid histology).243 Current focus of systemic treatment is primarily on three different pathways: immunotherapy, vascular endothelial growth factor (VEGF) inhibition, and mammalian target of rapamycin (mTOR) inhibition. (A more extensive version of this section on immunotherapy of RCC can be found on the Expert Consult website.) ![]()
Immunotherapy of Renal Cell Carcinoma
Modified doses of IL-2 have been given by subcutaneous or inhalation routes instead of intravenously. Most of the regimens produce responses of 10% to 19%. In a study comparing different dosing schedules,44 higher response rate was achieved by high-dose intravenous bolus IL-2 (21%) compared with low-dose intravenous bolus IL-2 (13%) and subcutaneous IL-2 (10%). High-dose IL-2a also led to significant improvements in complete response durability and survival.
Molecularly Targeted Treatment
Sunitinib and sorafenib are tyrosine kinase (TK) inhibitors that are administered orally. Sunitinib inhibits the VEGF receptor TK, as well as other TKs associated with the platelet-derived growth factor (PDGF) receptor and c-KIT oncogene. For patients who had previously received cytokine treatment, response rates to sunitinib were 34% to 40%, with a median time to progression of 8 months. In a phase III trial of sunitinib versus interferon-alfa as first-line therapy for metastatic clear cell RCC, sunitinib treatment resulted in significantly better response rate (39% vs. 8%), median PFS (11 vs. 5 months), and median survival (26.4 vs. 21.8 months).45 Sorafenib is a small-molecule inhibitor of multiple TKs, as well as c-RAF and both mutant and wild-type b-RAF kinase. In the phase III TARGET trial, the median PFS was significantly longer in the sorafenib group compared with placebo (5.5 vs. 2.8 months). In a randomized phase II trial, good- and intermediate-risk patients with previously untreated advanced RCC were randomly assigned to sorafenib (400 mg twice a day) or interferon-alfa (9 million units three times per week).46 Sorafenib did not increase PFS (5.7 months with sorafenib vs. 5.6 months with interferon-alfa).
Another class of molecularly targeted drugs inhibits mTOR. Temsirolimus is a competitive inhibitor of mTOR kinase and is given intravenously, whereas everolimus is an orally administered drug. Both drugs have been shown to be beneficial to patients who have failed prior systemic treatments. In a phase III trial that included poor-prognosis patients with metastatic or recurrent RCC,47 temsirolimus significantly prolonged median survival (10.9 vs. 7.3 months) and median PFS (5.5 vs. 3.1 months), as compared with interferon-alfa alone. In another phase III trial for patients with metastatic clear cell RCC whose disease had progressed on a VEGF-targeted therapy,48 the median PFS with everolimus was significantly prolonged compared with placebo (4.0 vs. 1.9 months), although there was no significant difference in OS.
Bevacizumab is a monoclonal antibody to VEGF and is given intravenously. A randomized trial of bevacizumab in patients with metastatic RCC after cytokine failure showed a significant prolongation of PFS of 2.3 months.49 In the AVOREN trial,50 649 previously untreated patients were randomized to interferon-alfa plus either bevacizumab or placebo. The response rate and PFS were significantly improved with the combination of bevacizumab plus interferon-alfa (31% vs. 13% and 10.2 vs. 5.4 months). Similar results were reported in the Cancer and Leukemia Group B trial 90206.
Immunotherapy of Renal Cell Carcinoma
RCC is an immunologically responsive disease and spontaneous remissions have been reported in less than 1% of patients. In an attempt to reproduce this accentuated response, the efficacy of interleukin-2 (IL-2) or interferon has been studied. The overall response rate of interferon-alfa is 15%, with a delay in time to progression of 4 months. There are no long-term remissions.244 For metastatic RCC, IL-2 has achieved objective response rates from 14% to 19%, with 7% long-term remission, including prolonged survival of 10 years or more.245 Intravenous bolus IL-2 treatment is associated with significant side effects, including renal failure, congestive heart failure, respiratory failure secondary to capillary leak syndrome, coma, liver failure, and arrhythmias.246 Delivery of the drug requires admission of the patient to a monitored inpatient unit.
Modified doses of IL-2 have been given by subcutaneous or inhalation routes instead of intravenously. Most of the regimens produce responses of 10% to 19%. Yang and colleagues247 compared high-dose intravenous bolus, low-dose intravenous bolus, and low-dose daily subcutaneous IL-2. Higher response rate was achieved by high-dose intravenous bolus IL-2 (21%) compared with low-dose intravenous bolus IL-2 (13%) and subcutaneous IL-2 (10%). High-dose IL-2a also led to significant improvements in complete response durability and survival. Atzpodien and associates248 showed that subcutaneous IL-2, interferon, and 5-fluorouracil improved 3-year OS in patients with metastatic RCC compared with a non–IL-2 regimen (25 vs. 16 months). IL-2 is the only drug that may achieve a durable long-term response for patients with metastatic RCC.
Molecularly Targeted Treatment
Sunitinib
Sunitinib is an oral drug that inhibits the VEGF receptor tyrosine kinase (TK), as well as other TKs associated with the PDGF receptor and c-KIT oncogene. Sunitinib has been studied in patients who previously received cytokine treatment. In one study249 there were 25 partial responders (40%) among the 63 enrolled patients, 8 of whom remained progression free for 21+ to 24+ months. The median time to tumor progression and the survival were 8.7 and 16.4 months, respectively. Similar results were demonstrated in another study,250 with an overall response rate of 34% and a median time to progression of 8.3 months. In a phase III trial of sunitinib as first-line therapy,251 750 patients with primarily metastatic clear cell RCC were randomized to 6-week cycles of sunitinib (50 mg daily for 4 weeks, followed by 2 weeks off) or interferon-alfa (9 million units, three times per week). Sunitinib resulted in a significantly better response rate (39% vs. 8%) and median PFS (11 vs. 5 months). This benefit was noted in patients at good, intermediate, and poor risk (PFS 14.5 vs. 7.9, 10.6 vs. 3.8, and 3.7 vs. 1.2 months, respectively). The final analysis showed that median OS was prolonged with sunitinib (26.4 vs. 21.8 months, p = .051).252
Sorafenib
Sorafenib is a small-molecule inhibitor of multiple TKs, including VEGFR2, FLT3, PDGF receptor, and fibroblast growth factor receptor-1 (FGFR1). It also inhibits C-raf and both mutant and wild-type B-raf kinase.116 In the phase III TARGET trial,253 903 patients with advanced RCC who had failed prior standard therapy were randomized to sorafenib (400 mg twice daily) or placebo. The median PFS was significantly longer in the sorafenib group (5.5 vs. 2.8 months). In a secondary analysis,253,254 sorafenib significantly improved survival when patients who had received sorafenib after progressing on placebo were censored (median 17.8 vs. 14.3 months). In a randomized phase II trial,255 good- and intermediate-risk patients with previously untreated advanced RCC were randomly assigned to sorafenib (400 mg twice a day) or interferon-alfa (9 million units three times per week). Sorafenib did not increase progression-free survival (5.7 with sorafenib vs. 5.6 months with interferon-alfa).
Toxicity of Sunitinib and Sorafenib
In a meta-analysis,256 the overall incidence of hypertension with sunitinib is 22%, with severe hypertension in 7% of patients. A significant increase in the incidence of renal dysfunction is also observed (relative risk 1.36). For sorafenib, the incidence of hypertension is 23%, and it is severe or life threatening in 6% of patients.257 Sunitinib also causes an asymptomatic decline in cardiac ejection fraction. Patients treated with sunitinib may develop thyroid dysfunction that usually manifests as hypothyroidism, although transient thyrotoxicosis has been reported.258 Monitoring of the thyroid-stimulating hormone level is warranted during sunitinib therapy. Hand-foot syndrome is a common toxicity with both sunitinib and sorafenib.259
Temsirolimus
Temsirolimus is a competitive inhibitor of mammalian target of rapamycin (mTOR kinase) and is given intravenously. In a phase II trial,260 111 patients with advanced RCC who either had received previous interferon or interleukin therapy or were not considered candidates for such therapy were randomly assigned to one of three different dose levels of temsirolimus (25, 75, or 250 mg weekly). The response rate was 7%, including one complete response. Despite the low objective response rate, significant antitumor activity was suggested by the substantial number of patients with minor responses (26%) and stable disease for more than 6 months (17%), as well as the relatively long time to progression (5.8 months) and median survival (15 months). In a phase III trial, 626 previously untreated, poor-prognosis patients with metastatic or recurrent RCC were randomized to temsirolimus (25 mg IV/wk), the combination of temsirolimus (15 mg IV/wk) plus interferon-alfa (escalated up to 6 million units three times per week as tolerated), or interferon-alfa alone (escalated up to 18 million units three times per week as tolerated).261 Compared with interferon-alfa alone, temsirolimus significantly prolonged the median OS (10.9 vs. 7.3 months), as well as the median PFS (5.5 vs. 3.1 months). The combination of temsirolimus plus interferon-alfa was not significantly better than that of interferon-alfa alone.
Toxicity of Temsirolimus
The most frequent side effects are maculopapular rash, mucositis, asthenia, and nausea (76%, 70%, 50%, and 43%, respectively). Severe adverse events are uncommon. The most frequent grade 3 or 4 adverse events include hyperglycemia, hypophosphatemia, anemia, and hypertriglyceridemia (17%, 13%, 9%, and 6%, respectively).261 Hypersensitivity reactions and dyspnea have also been reported and may be life threatening.
Bevacizumab
Bevacizumab is a monoclonal antibody to VEGF and is given intravenously. A randomized trial of bevacizumab in patients with metastatic RCC after cytokine failure showed a significant prolongation of PFS of 2.3 months.262 In the AVOREN trial,263 649 previously untreated patients were randomized to interferon-alfa (9 million units three times per week for 1 year) plus either bevacizumab (10 mg/kg every 2 weeks) or placebo. The response rate and PFS were significantly improved with the combination of bevacizumab plus interferon-alfa (31% vs. 13% and 10.2 vs. 5.4 months). There were more grade 3 or 4 adverse events in patients treated with bevacizumab, including thromboembolic events and gastrointestinal perforation (3% vs. 1% and 1% vs. 0%, respectively). In the Cancer and Leukemia Group B trial 90206,264 732 previously untreated patients with metastatic RCC were randomized to treatment with interferon-alfa plus bevacizumab or with interferon-alfa plus placebo. Interim analysis found that progression-free survival was significantly increased in patients treated with the bevacizumab plus interferon-alfa regimen (median 8.5 vs. 5.2 months), and the objective response rate was also improved (25.5% vs. 13.1%).
Everolimus
Everolimus is an orally administered inhibitor of mTOR. In a phase III trial,265 410 patients with metastatic clear cell RCC whose disease had progressed on a VEGF-targeted therapy were randomized in a 2 : 1 ratio to everolimus (10 mg/day) or placebo. The median PFS with everolimus was significantly prolonged compared with placebo (4.0 vs. 1.9 months). Objective responses were rare (1% and 0% with everolimus and placebo, respectively), although stable disease was more common with everolimus (63% and 32%). There was no significant difference in OS. The most frequent severe laboratory abnormalities associated with everolimus were lymphopenia and hyperglycemia (15% and 12%, respectively). Grade 3 or 4 adverse events included stomatitis and pneumonitis (3% and 3%, respectively).
Pazopanib
Pazopanib is an oral VEGF1, VEGF2, VEGF3, PDGF receptor, and c-KIT inhibitor. Pazopanib was compared with placebo in a phase III randomized trial in patients with advanced renal cancer.266 There was an improvement in progression-free survival in treatment-naive patients (9.2 vs. 4.2 months, p <.0000001) and cytokine-pretreated patients (11.1 vs. 2.8 months, p <.001.) An overall survival benefit (21.1 vs. 8.7 months, p <.02) and an improved response rate (30% vs. 3%) were also demonstrated. Side effects included fatigue, mucositis, and hand-foot syndrome.
Renal Pelvis and Ureter
For patients with metastatic disease and good performance status, cisplatin-based chemotherapy can be considered. The response rates range from 54% to 70%.267–269 At present, no molecularly targeted treatment is available for advanced UUT cancer. EBRT can be used palliatively for local symptoms such as pain from bony metastasis.
Irradiation Techniques and Tolerance
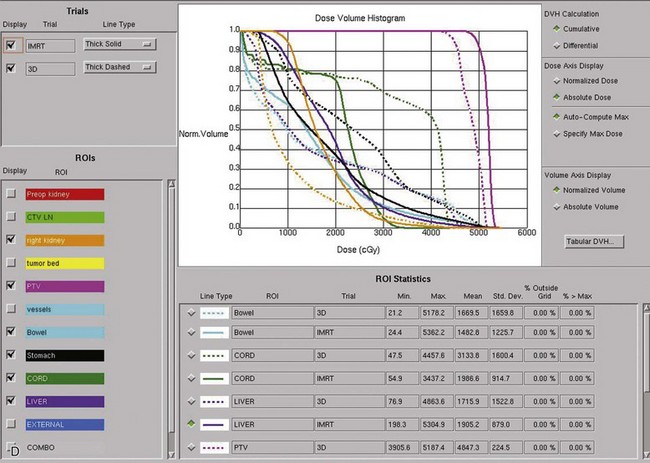
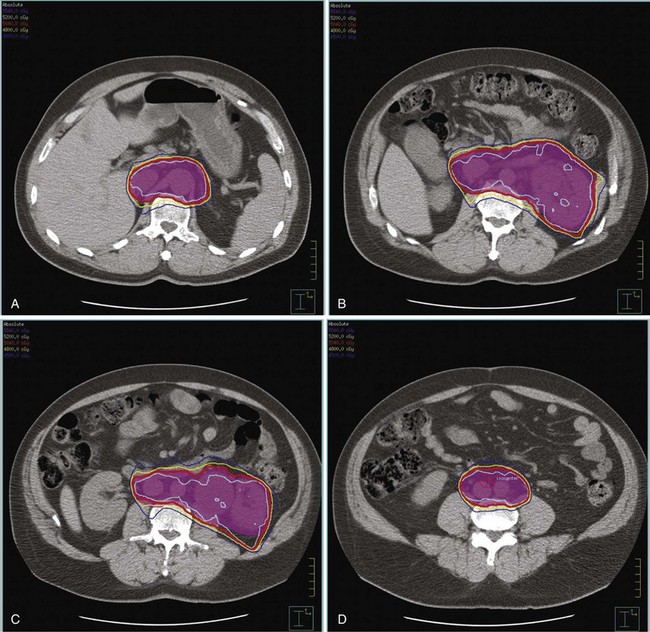
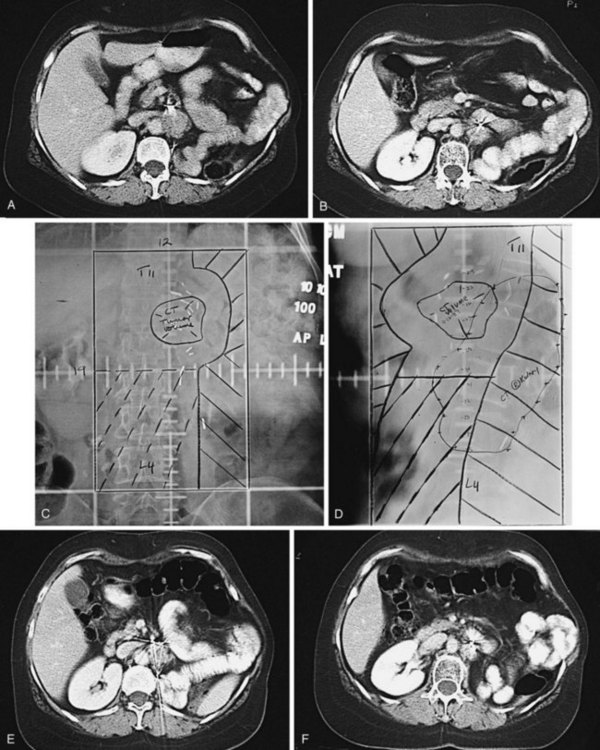
Kidney
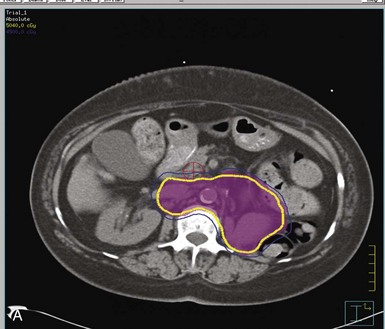
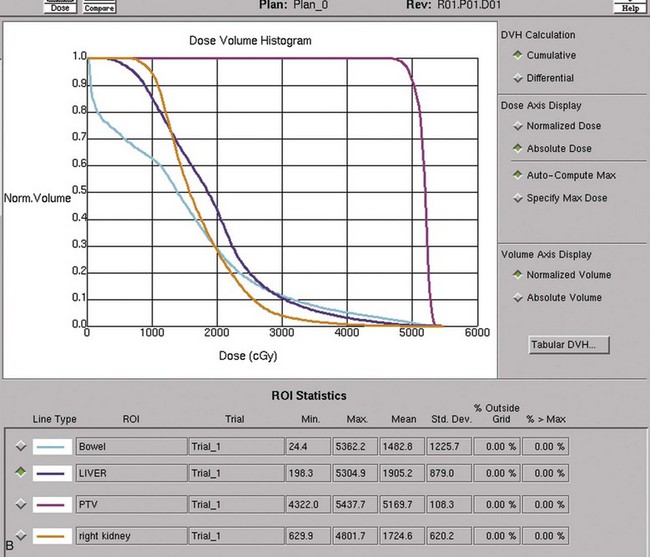
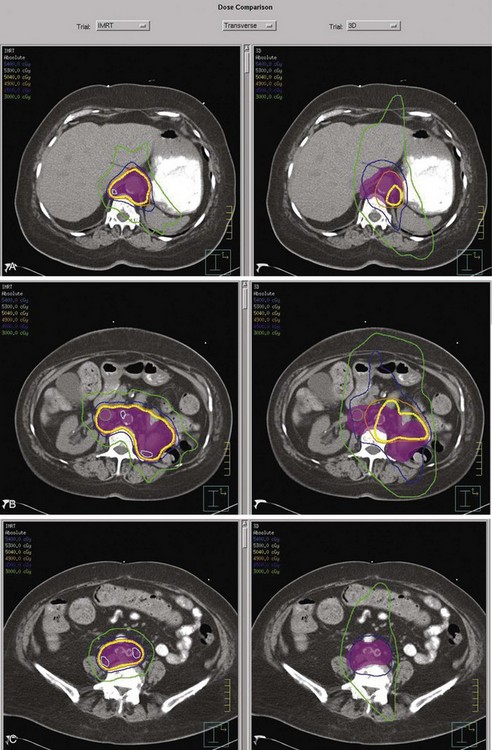
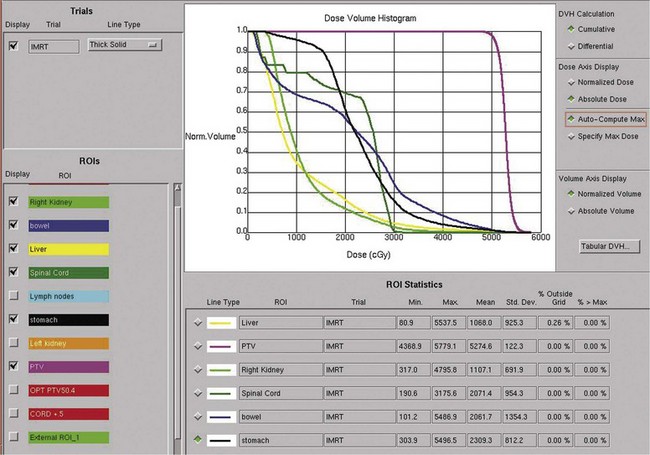
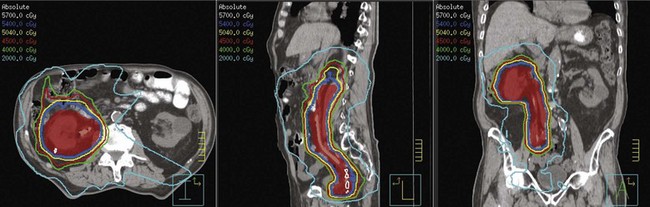
Postoperative radiation should be considered for patients at high risk for local recurrence after surgery. Pathologic features predictive of local recurrence include positive lymph nodes and positive surgical margins. The clinical target volume should include the tumor bed, surgical clips, regional lymph nodes (hilar and para-aortic lymph nodes), and vascular extent of tumor (Fig. 54-1). If feasible, the scar should be included in the target volume because scar recurrences have occasionally happened.177 Total radiation doses of 45 to 50.4 Gy should be delivered with 1.8- to 2-Gy fractions using multiple-field techniques. In addition, small-volume fields can be used to boost areas of residual disease to a total of 55 to 60 Gy. Delivery of the boost can be facilitated by surgical reconstruction to displace small intestine or stomach, placement of surgical clips at the site of residual disease, prone positioning, and false tabletop techniques. Intensity modulated radiation therapy (IMRT) can be used to treat the target volume and minimize the dose to the normal tissues (liver, small bowel, stomach, opposite kidney, and spinal cord) (Figs. 54-2 and 54-3; see also Fig. 54-1).
Preoperative irradiation treatment volumes for patients with locally advanced disease should encompass the primary tumor, renal hilar lymph nodes, para-aortic lymph nodes, and vascular extent of tumor. To spare dose-limiting organs, three-dimensional conformal field combinations of anterior-posterior/posterior-anterior (AP/PA), laterals, or obliques are recommended. IMRT can also be used to minimize the dose to normal tissues. Total doses of 45 to 50.4 Gy in 1.8- to 2.0-Gy fractions should be given preoperatively, followed by surgery in 4 to 6 weeks. If possible, an IORT supplement should be considered if residual disease or narrow margins exist after maximal surgical resection. At the Mayo Clinic, doses of 10 to 20 Gy are usually delivered with IOERT; the dose is dependent on margin status and amount of EBRT delivered preoperatively or planned postoperatively.212
Patients in whom a local recurrence has developed in the tumor bed or regional nodes after radical nephrectomy should be considered for aggressive salvage treatment.212 This treatment includes preoperative three-dimensional conformal EBRT or IMRT to the recurrent tumor volume, postoperative tumor bed, and renal hilar and para-aortic lymph nodes. A traditional field is shown in web-only Figure 54-1 on the Expert Consult website![]() . A total dose of 45 to 50.4 Gy in 1.8- to 2.0-Gy fractions should be delivered using multiple treatment fields. Maximal surgical debulking with consideration of IORT should occur within 4 to 6 weeks of completion of EBRT.
. A total dose of 45 to 50.4 Gy in 1.8- to 2.0-Gy fractions should be delivered using multiple treatment fields. Maximal surgical debulking with consideration of IORT should occur within 4 to 6 weeks of completion of EBRT.
Treatment-Related Toxicity
CT-aided radiation therapy treatment planning (three-dimensional conformal or IMRT) is essential to reduce the risk of treatment-related toxicity. The Copenhagen Renal Study Group randomized 32 patients to receive postoperative irradiation after surgery.176 Twenty-seven of the 32 patients randomized completed the course of postoperative radiation therapy to a dose of 50 Gy in 20 fractions. Significant complications to the stomach, duodenum, or liver occurred in 12 of the 27 patients and contributed to the death of 5 patients (19%). The increased fraction size of 2.5 Gy may have partially contributed to the high complication rate. In studies utilizing modern CT-based planning, the risk of late complications was less than 5%.177,178
Renal Pelvis and Ureter
The radiation treatment field should include the primary tumor bed down to the bladder wall at the ipsilateral trigone with an adequate margin, plus the regional lymph nodes adjacent to the primary tumor bed. The pattern of lymphatic metastasis based on the location of the primary lesion was summarized earlier in the section on pathway of spread.104 For renal pelvis and upper ureteral lesions, nodal metastasis most commonly occurs near and/or superior to the primary tumor site, involving the periaortic/pericaval lymph nodes and the renal hilar nodes. The initial treatment field, encompassing the renal pelvis and ureter and the regional nodes, should be treated to 45 to 50.4 Gy with 1.8-Gy per fraction. Areas of gross disease may be boosted to 54 to 60 Gy. CT-based three-dimensional conformal therapy is used to reduce the volume of normal organs such as small bowel, liver, and contralateral kidneys in the high-dose region. The dose to the contralateral functioning kidney should not exceed 18 Gy. The volume of irradiated liver (for patients with left-sided lesions) and stomach (for patients with right-sided lesion) can be reduced with AP/PA fields. Lateral fields may be used to deliver part of the treatment to keep the dose to the spinal cord within tolerance. Representative fields for a local recurrence of renal pelvis cancer are shown in web-only Figure 54-2 on the Expert Consult website![]() . For lower ureteral lesions, pelvic lymph nodes should be included in the treatment field. The posterior location of the ureter allows the use of a four-field or three-field (PA and opposed laterals) technique. The use of a prone treatment position and small bowel contrast is helpful to minimize the amount of small bowel in the treatment field. A representative radiation treatment field for a lower ureter cancer is shown in Figure 54-4.
. For lower ureteral lesions, pelvic lymph nodes should be included in the treatment field. The posterior location of the ureter allows the use of a four-field or three-field (PA and opposed laterals) technique. The use of a prone treatment position and small bowel contrast is helpful to minimize the amount of small bowel in the treatment field. A representative radiation treatment field for a lower ureter cancer is shown in Figure 54-4.
The use of IMRT may further spare the volume of normal organs in the high-dose region.270 Figure 54-5 shows the dose distribution of an IMRT treatment plan for an 88-year-old patient who had TCC in the midureter and positive cytology from the renal pelvis. He was medically unfit for surgery because of heart disease.
Treatment Algorithm, Conclusions, and Future Possibilities
Kidney
Figure 54-6 presents an algorithm for the current Mayo Clinic philosophy on diagnosis and treatment of RCC. Patients with a tumor 4 cm or smaller, a solitary kidney, bilateral RCCs, or impaired renal function may be candidates for a partial nephrectomy. Patients with tumors larger than 4 cm and a normal-functioning opposite kidney should be treated with radical nephrectomy. If gross total resection with negative margins is accomplished, the patient should be observed. If positive surgical margins or lymph node involvement is present at radical nephrectomy, consideration should be given to postoperative EBRT.177,178
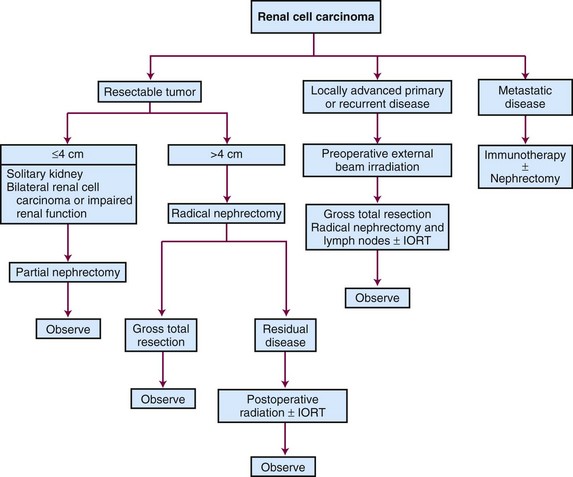
Figure 54-6 Diagnostic and treatment algorithms for renal cell carcinoma. IORT, intraoperative irradiation.
84 Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol. 1982;6:655-663.
85 Frank I, Blute ML, Cheville JC, et al. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis. The SSIGN score. J Urol. 2002;168:2395-2400.
87 Zisman A, Pantuck AJ, Wieder J, et al. Risk group assessment and clinical outcome algorithm to predict the natural history of patients with surgically resected renal cell carcinoma. J Clin Oncol. 2002;20:4559-4566.
93 Ozsahin M, Zouhair A, Villa S, et al. Prognostic factors in urothelial renal pelvis and ureter tumours. A multicentre Rare Cancer Network study. Eur J Cancer. 1999;35:738-743.
94 Rubenstein MA, Walz BJ, Bucy JG. Transitional cell carcinoma of the kidney 25-year experience. J Urol. 1978;119:594-597.
95 Corrado F, Ferri C, Mannini D, et al. Transitional cell carcinoma of the upper urinary tract. Evaluation of prognostic factors by histopathology and flow cytometric analysis. J Urol. 1991;145:1159-1163.
96 Hall MC, Womack S, Sagalowsky AI, et al. Prognostic factors, recurrence, and survival in transitional cell carcinoma of the upper urinary tract. A 30-year experience in 252 patients. Urology. 1998;52:594-601.
97 Heney NM, Nocks BN, Daly JJ, et al. Prognostic factors in carcinoma of the ureter. J Urol. 1981;125:632-636.
98 Novara G, De Marco V, Gottardo F, et al. Independent predictors of cancer-specific survival in transitional cell carcinoma of the upper urinary tract. Multi-institutional dataset from 3 European centers. Cancer. 2007;110:1715-1722.
103 Margulis V, Shariat SF, Matin SF, et al. Outcomes of radical nephroureterectomy. A series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer. 2009;115:1224-1233.
104 Kondo T, Nakazawa H, Ito F, et al. Primary site and incidence of lymph node metastases in urothelial carcinoma of upper urinary tract. Urology. 2007;69:265-269.
106 Maulard-Durdux C, Dufour B, Hennequin C, et al. Postoperative radiation therapy in 26 patients with invasive transitional cell carcinoma of the upper urinary tract. No impact on survival? J Urol. 1996;155:115-117.
154 Gettman MT, Blute ML, Spotts B, et al. Pathologic staging of renal cell carcinoma. Significance of tumor classification with the 1997 TNM staging system. Cancer. 2001;91:354-361.
155 Tsui KH, Shvarts O, Barbaric Z, et al. Is adrenalectomy a necessary component of radical nephrectomy? UCLA experience with 511 radical nephrectomies. J Urol. 2000;163:437-441.
157 Blom JH, van Poppel H, Marechal JM, et al. Radical nephrectomy with and without lymph-node dissection. Final results of European Organization for Research and Treatment of Cancer (EORTC) randomized phase 3 trial 30881. Eur Urol. 2009;55:28-34.
175 Finney R. The value of radiotherapy in the treatment of hypernephroma—a clinical trial. Br J Urol. 1973;45:258-269.
176 Kjaer M, Iversen P, Hvidt V, et al. A randomized trial of postoperative radiotherapy versus observation in stage II and III renal adenocarcinoma. A study by the Copenhagen Renal Cancer Study Group. Scand J Urol Nephrol. 1987;21:285-289.
177 Stein M, Kuten A, Halpern J, et al. The value of postoperative irradiation in renal cell cancer. Radiother Oncol. 1992;24:41-44.
178 Kao GD, Malkowicz SB, Whittington R, et al. Locally advanced renal cell carcinoma. Low complication rate and efficacy of postnephrectomy radiation therapy planned with CT. Radiology. 1994;193:725-730.
181 Ulutin HC, Aksu G, Fayda M, et al. The value of postoperative radiotherapy in renal cell carcinoma. A single-institution experience. Tumori. 2006;92:202-206.
182 Rabinovitch RA, Zelefsky MJ, Gaynor JJ, Fuks Z. Patterns of failure following surgical resection of renal cell carcinoma. Implications for adjuvant local and systemic therapy. J Clin Oncol. 1994;12:206-212.
190 Svedman C, Sandstrom P, Pisa P, et al. A prospective phase II trial of using extracranial stereotactic radiotherapy in primary and metastatic renal cell carcinoma. Acta Oncol. 2006;45:870-875.
191 Cozad SC, Smalley SR, Austenfeld M, et al. Transitional cell carcinoma of the renal pelvis or ureter. Patterns of failure. Urology. 1995;46:796-800.
199 Krambeck AE, Thompson RH, Lohse CM, et al. Imperative indications for conservative management of upper tract transitional cell carcinoma. J Urol. 2007;178:792-796. discussion 796-797
202 Cozad SC, Smalley SR, Austenfeld M, et al. Adjuvant radiotherapy in high stage transitional cell carcinoma of the renal pelvis and ureter. Int J Radiat Oncol Biol Phys. 1992;24:743-745.
203 Brookland RK, Richter MP. The postoperative irradiation of transitional cell carcinoma of the renal pelvis and ureter. J Urol. 1985;133:952-955.
204 Czito B, Zietman A, Kaufman D, et al. Adjuvant radiotherapy with and without concurrent chemotherapy for locally advanced transitional cell carcinoma of the renal pelvis and ureter. J Urol. 2004;172:1271-1275.
205 Kwak C, Lee SE, Jeong IG, Ku JH. Adjuvant systemic chemotherapy in the treatment of patients with invasive transitional cell carcinoma of the upper urinary tract. Urology. 2006;68:53-57.
206 Soga N, Arima K, Sugimura Y. Adjuvant methotrexate, vinblastine, adriamycin, and cisplatin chemotherapy has potential to prevent recurrence of bladder tumors after surgical removal of upper urinary tract transitional cell carcinoma. Int J Urol. 2008;15:800-803.
207 Hellenthal NJ, Shariat SF, Margulis V, et al. Adjuvant chemotherapy for high risk upper tract urothelial carcinoma. Results from the Upper Tract Urothelial Carcinoma Collaboration. J Urol. 2009;182:900-906.
210 van der Werf-Messing B. Proceedings. Carcinoma of the kidney. Cancer. 1973;32:1056-1061.
211 Juusela H, Malmio K, Alfthan O, Oravisto KJ. Preoperative irradiation in the treatment of renal adenocarcinoma. Scand J Urol Nephrol. 1977;11:277-281.
212 Frydenberg M, Gunderson L, Hahn G, et al. Preoperative external beam radiotherapy followed by cytoreductive surgery and intraoperative radiotherapy for locally advanced primary or recurrent renal malignancies. J Urol. 1994;152:15-21.
218 Zhang Q, Fu S, Liu T, et al. Adjuvant intraoperative electron radiotherapy and external beam radiotherapy for locally advanced transitional cell carcinoma of the ureter. Urol Oncol. 2009;27:14-20.
220 Flanigan RC, Salmon SE, Blumenstein BA, et al. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med. 2001;345:1655-1659.
221 Mickisch GH, Garin A, van Poppel H, et al. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma. A randomised trial. Lancet. 2001;358:966-970.
225 Kavolius JP, Mastorakos DP, Pavlovich C, et al. Resection of metastatic renal cell carcinoma. J Clin Oncol. 1998;16:2261-2266.
229 Lee J, Hodgson D, Chow E, et al. A phase II trial of palliative radiotherapy for metastatic renal cell carcinoma. Cancer. 2005;104:1894-1900.
230 Gerszten PC, Burton SA, Ozhasoglu C, et al. Stereotactic radiosurgery for spinal metastases from renal cell carcinoma. J Neurosurg Spine. 2005;3:288-295.
231 Nguyen QN, Shiu AS, Rhines LD, et al. Management of spinal metastases from renal cell carcinoma using stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 2010;76:1185-1192.
232 Wronski M, Maor MH, Davis BJ, et al. External radiation of brain metastases from renal carcinoma. A retrospective study of 119 patients from the M.D. Anderson Cancer Center. Int J Radiat Oncol Biol Phys. 1997;37:753-759.
233 Wronski M, Arbit E, Russo P, Galicich JH. Surgical resection of brain metastases from renal cell carcinoma in 50 patients. Urology. 1996;47:187-193.
235 Brown PD, Brown CA, Pollock BE, et al. Stereotactic radiosurgery for patients with “radioresistant” brain metastases. Neurosurgery. 2002;51:656-665. discussion 665-667
247 Yang JC, Sherry RM, Steinberg SM, et al. Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal cancer. J Clin Oncol. 2003;21:3127-3132.
251 Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115-124.
255 Escudier B, Szczylik C, Hutson TE, et al. Randomized phase II trial of first-line treatment with sorafenib versus interferon alfa-2a in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:1280-1289.
261 Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271-2281.
262 Yang JC, Haworth L, Sherry RM, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427-434.
263 Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma. A randomised, double-blind phase III trial. Lancet. 2007;370:2103-2111.
265 Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma. A double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449-456.
1 Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277-300.
2 Cheville JC, Lohse CM, Zincke H, et al. Sarcomatoid renal cell carcinoma. An examination of underlying histologic subtype and an analysis of associations with patient outcome. Am J Surg Pathol. 2004;28:435-441.
3 Growitz VP. Die sogenannten Lipome der Niere. Pathol Anat. 1883;93:39.
4 Genega EM, Porter CR. Urothelial neoplasms of the kidney and ureter. An epidemiologic, pathologic, and clinical review. Am J Clin Pathol. 2002;117(Suppl):S36-S48.
5 Eble JNSG, Epstein JI, et al. World Health Organization Classification of Tumors. Pathology and Genetics of Tumors of the Urinary System and Male Genital Organs. IARC Press, Lyon, France, 2004..
6 Chow WH, Devesa SS, Warren JL, Fraumeni JFJr. Rising incidence of renal cell cancer in the United States. JAMA. 1999;281:1628-1631.
7 Murai M, Oya M. Renal cell carcinoma. Etiology, incidence and epidemiology. Curr Opin Urol. 2004;14:229-233.
8 Pantuck AJ, Zisman A, Belldegrun AS. The changing natural history of renal cell carcinoma. J Urol. 2001;166:1611-1623.
9 Jayson M, Sanders H. Increased incidence of serendipitously discovered renal cell carcinoma. Urology. 1998;51:203-205.
10 Hock LM, Lynch J, Balaji KC. Increasing incidence of all stages of kidney cancer in the last 2 decades in the United States. An analysis of surveillance, epidemiology and end results program data. J Urol. 2002;167:57-60.
11 Aron M, Nguyen MM, Stein RJ, Gill IS. Impact of gender in renal cell carcinoma. An analysis of the SEER database. Eur Urol. 2008;54:133-140.
12 Parkin DM, Pisani P, Ferlay J. Estimates of the worldwide incidence of eighteen major cancers in 1985. Int J Cancer. 1993;54:594-606.
13 Mellemgaard A. Human renal-cell carcinoma—epidemiological and mechanistic aspects. IARC Sci Publ. 1999;147:68-80.
14 McLaughlin JK, Lipworth L. Epidemiologic aspects of renal cell cancer. Semin Oncol. 2000;27:115-123.
15 Hunt JD, van der Hel OL, McMillan GP, et al. Renal cell carcinoma in relation to cigarette smoking. Meta-analysis of 24 studies. Int J Cancer. 2005;114:101-108.
16 Dhote R, Thiounn N, Debre B, Vidal-Trecan G. Risk factors for adult renal cell carcinoma. Urol Clin North Am. 2004;31:237-247.
17 Moore LE, Wilson RT, Campleman SL. Lifestyle factors, exposures, genetic susceptibility, and renal cell cancer risk. A review. Cancer Invest. 2005;23:240-255.
18 Lipworth L, Tarone RE, McLaughlin JK. The epidemiology of renal cell carcinoma. J Urol. 2006;176:2353-2358.
19 Benichou J, Chow WH, McLaughlin JK, et al. Population attributable risk of renal cell cancer in Minnesota. Am J Epidemiol. 1998;148:424-430.
20 McCann J. Obesity, cancer links prompt new recommendations. J Natl Cancer Inst. 2001;93:901-902.
21 Chow WH, Gridley G, Fraumeni JFJr, Jarvholm B. Obesity, hypertension, and the risk of kidney cancer in men. N Engl J Med. 2000;343:1305-1311.
22 Weikert S, Boeing H, Pischon T, et al. Blood pressure and risk of renal cell carcinoma in the European prospective investigation into cancer and nutrition. Am J Epidemiol. 2008;167:438-446.
23 Parker AS, Cerhan JR, Lynch CF, et al. History of urinary tract infection and risk of renal cell carcinoma. Am J Epidemiol. 2004;159:42-48.
24 Nicodemus KK, Sweeney C, Folsom AR. Evaluation of dietary, medical and lifestyle risk factors for incident kidney cancer in postmenopausal women. Int J Cancer. 2004;108:115-121.
25 Parker AS, Cerhan JR, Lynch CF, et al. Gender, alcohol consumption, and renal cell carcinoma. Am J Epidemiol. 2002;155:455-462.
26 Lee JE, Hunter DJ, Spiegelman D, et al. Alcohol intake and renal cell cancer in a pooled analysis of 12 prospective studies. J Natl Cancer Inst. 2007;99:801-810.
27 Setiawan VW, Stram DO, Nomura AM, et al. Risk factors for renal cell cancer. The multiethnic cohort. Am J Epidemiol. 2007;166:932-940.
28 Parker AS. Re: The epidemiology of renal cell carcinoma. J Urol. 2007;177:1953. author reply 1953-1954
29 Chiu BC, Gapstur SM, Chow WH, et al. Body mass index, physical activity, and risk of renal cell carcinoma. Int J Obes (Lond). 2006;30:940-947.
30 Pan SY, DesMeules M, Morrison H, Wen SW. Obesity, high energy intake, lack of physical activity, and the risk of kidney cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:2453-2460.
31 Rubagotti A, Martorana G, Boccardo F. Epidemiology of kidney cancer. Eur Urol Suppl. 2006;5:558-565.
32 Wolk A, Gridley G, Niwa S, et al. International renal cell cancer study. VII. Role of diet. Int J Cancer. 1996;65:67-73.
33 Hu C, Chow WH, Boffetta P, et al. Canadian Cancer Registries Epidemiology Research Group. Diet and vitamin or mineral supplements and risk of renal cell carcinoma in Canada. Cancer Causes Control. 2003;14:705-714.
34 Faramawi MF, Johnson E, Fry MW, et al. Consumption of different types of meat and the risk of renal cancer. Meta-analysis of case-control studies. Cancer Causes Control. 2007;18:125-133.
35 Alexander DD, Cushing CA. Quantitative assessment of red meat or processed meat consumption and kidney cancer. Cancer Detect Prev. 2009;32:340-351.
36 McCredie M, Stewart JH, Day NE. Different roles for phenacetin and paracetamol in cancer of the kidney and renal pelvis. Int J Cancer. 1993;53:245-249.
37 Farivar-Mohseni H, Perlmutter AE, Wilson S, et al: Renal cell carcinoma and end stage renal disease, J Urol 175:2018-2020, 2021, discussion.
38 Schwarz A, Vatandaslar S, Merkel S, Haller H. Renal cell carcinoma in transplant recipients with acquired cystic kidney disease. Clin J Am Soc Nephrol. 2007;2:750-756.
39 Doublet JD, Peraldi MN, Gattegno B, et al. Renal cell carcinoma of native kidneys. Prospective study of 129 renal transplant patients. J Urol. 1997;158:42-44.
40 Kauzlaric D, Barmeir E, Luscieti P, et al. Renal carcinoma after retrograde pyelography with Thorotrast. AJR Am J Roentgenol. 1987;148:897-898.
41 Mandel JS, McLaughlin JK, Schlehofer B, et al. International renal-cell cancer study. IV. Occupation. Int J Cancer. 1995;61:601-605.
42 Mellemgaard A, Engholm G, McLaughlin JK, Olsen JH. Risk factors for renal cell carcinoma in Denmark. I. Role of socioeconomic status, tobacco use, beverages, and family history. Cancer Causes Control. 1994;5:105-113.
43 Linehan WM, Vasselli J, Srinivasan R, et al. Genetic basis of cancer of the kidney. Disease-specific approaches to therapy. Clin Cancer Res. 2004;10:6282S-6289S.
44 Phillips JL, Pavlovich CP, Walther M, et al. The genetic basis of renal epithelial tumors. Advances in research and its impact on prognosis and therapy. Curr Opin Urol. 2001;11:463-469.
45 Bruning T, Lammert M, Kempkes M, et al. Influence of polymorphisms of GSTM1 and GSTT1 for risk of renal cell cancer in workers with long-term high occupational exposure to trichloroethene. Arch Toxicol. 1997;71:596-599.
46 Schulz WA, Krummeck A, Rosinger I, et al. Increased frequency of a null-allele for NAD(P)H: quinone oxidoreductase in patients with urological malignancies. Pharmacogenetics. 1997;7:235-239.
47 Semenza JC, Ziogas A, Largent J, et al. Gene-environment interactions in renal cell carcinoma. Am J Epidemiol. 2001;153:851-859.
48 Sweeney C, Farrow DC, Schwartz SM, et al. Glutathione S-transferase M1, T1, and P1 polymorphisms as risk factors for renal cell carcinoma. A case-control study. Cancer Epidemiol Biomarkers Prev. 2000;9:449-454.
49 Munoz JJ, Ellison LM. Upper tract urothelial neoplasms. Incidence and survival during the last 2 decades. J Urol. 2000;164:1523-1525.
50 Mellemgaard A, Carstensen B, Norgaard N, et al. Trends in the incidence of cancer of the kidney, pelvis, ureter and bladder in Denmark 1943-88. Scand J Urol Nephrol. 1993;27:327-332.
51 Hisataki T, Miyao N, Masumori N, et al. Risk factors for the development of bladder cancer after upper tract urothelial cancer. Urology. 2000;55:663-667.
52 Kang CH, Yu TJ, Hsieh HH, et al. The development of bladder tumors and contralateral upper urinary tract tumors after primary transitional cell carcinoma of the upper urinary tract. Cancer. 2003;98:1620-1626.
53 Holmang S, Johansson SL. Bilateral metachronous ureteral and renal pelvic carcinomas. Incidence, clinical presentation, histopathology, treatment and outcome. J Urol. 2006;175:69-72. discussion 72-73
54 Hurle R, Losa A, Manzetti A, Lembo A. Upper urinary tract tumors developing after treatment of superficial bladder cancer. 7-Year follow-up of 591 consecutive patients. Urology. 1999;53:1144-1148.
55 Wright JL, Hotaling J, Porter MP. Predictors of upper tract urothelial cell carcinoma after primary bladder cancer. A population based analysis. J Urol. 2009;181:1035-1039. discussion 1039
56 Novara G, De Marco V, Dalpiaz O, et al. Independent predictors of contralateral metachronous upper urinary tract transitional cell carcinoma after nephroureterectomy. Multi-institutional dataset from three European centers. Int J Urol. 2009;16:187-191.
57 Jensen OM, Knudsen JB, McLaughlin JK, Sorensen BL. The Copenhagen case-control study of renal pelvis and ureter cancer. Role of smoking and occupational exposures. Int J Cancer. 1988;41:557-561.
58 McLaughlin JK, Silverman DT, Hsing AW, et al. Cigarette smoking and cancers of the renal pelvis and ureter. Cancer Res. 1992;52:254-257.
59 Ross RK, Paganini-Hill A, Landolph J, et al. Analgesics, cigarette smoking, and other risk factors for cancer of the renal pelvis and ureter. Cancer Res. 1989;49:1045-1048.
60 Pommer W, Bronder E, Klimpel A, et al. Urothelial cancer at different tumour sites. Role of smoking and habitual intake of analgesics and laxatives. Results of the Berlin Urothelial Cancer Study. Nephrol Dial Transplant. 1999;14:2892-2897.
61 McCredie M, Stewart JH, Carter JJ, et al. Phenacetin and papillary necrosis. Independent risk factors for renal pelvic cancer. Kidney Int. 1986;30:81-84.
62 Palvio DH, Andersen JC, Falk E. Transitional cell tumors of the renal pelvis and ureter associated with capillarosclerosis indicating analgesic abuse. Cancer. 1987;59:972-976.
63 Nortier JL, Martinez MC, Schmeiser HH, et al. Urothelial carcinoma associated with the use of a Chinese herb (Aristolochia fangchi). N Engl J Med. 2000;342:1686-1692.
64 Lord GM, Cook T, Arlt VM, et al. Urothelial malignant disease and Chinese herbal nephropathy. Lancet. 2001;358:1515-1516.
65 Petkovic SD. Epidemiology and treatment of renal pelvic and ureteral tumors. J Urol. 1975;114:858-865.
66 Cukuranovic R, Ignjatovic M, Stefanovic V. Urinary tract tumors and Balkan nephropathy in the South Morava River basin. Kidney Int Suppl. 1991;34:S80-S84.
67 Jankovic Velickovic L, Hattori T, Dolicanin Z, et al. Upper urothelial carcinoma in Balkan endemic nephropathy and non-endemic regions. A comparative study of pathological features. Pathol Res Pract. 2009;205:89-96.
68 Chow WH, Lindblad P, Gridley G, et al. Risk of urinary tract cancers following kidney or ureter stones. J Natl Cancer Inst. 1997;89:1453-1457.
69 Frischer Z, Waltzer WC, Gonder MJ. Bilateral transitional cell carcinoma of the renal pelvis in the cancer family syndrome. J Urol. 1985;134:1197-1198.
70 Orphali SL, Shols GW, Hagewood J, et al. Familial transitional cell carcinoma of renal pelvis and upper ureter. Urology. 1986;27:394-396.
71 Lynch HT, Ens JA, Lynch JF. The Lynch syndrome II and urological malignancies. J Urol. 1990;143:24-28.
72 Parker AS, Cerhan JR, Janney CA, et al. Smoking cessation and renal cell carcinoma. Ann Epidemiol. 2003;13:245-251.
73 Curti BD. Renal cell carcinoma. JAMA. 2004;292:97-100.
74 Sokoloff MH, deKernion JB, Figlin RA, Belldegrun A. Current management of renal cell carcinoma. CA Cancer J Clin. 1996;46:284-302.
75 Wakai K, Hirose K, Takezaki T, et al. Foods and beverages in relation to urothelial cancer. Case-control study in Japan. Int J Urol. 2004;11:11-19.
76 Hastie KJ, Hamdy FC, Collins MC, Williams JL. Upper tract tumours following cystectomy for bladder cancer. Is routine intravenous urography worthwhile? Br J Urol. 1991;67:29-31.
77 Storkel S, Eble JN, Adlakha K, et al. Classification of renal cell carcinoma. Workgroup No. 1. Union Internationale Contre le Cancer (UICC) and the American Joint Committee on Cancer (AJCC). Cancer. 1997;80:987-989.
78 Kovacs G, Akhtar M, Beckwith BJ, et al. The Heidelberg classification of renal cell tumours. J Pathol. 1997;183:131-133.
79 Ljungberg B, Alamdari FI, Stenling R, Roos G. Prognostic significance of the Heidelberg classification of renal cell carcinoma. Eur Urol. 1999;36:565-569.
80 Moch H, Gasser T, Amin MB, et al. Prognostic utility of the recently recommended histologic classification and revised TNM staging system of renal cell carcinoma. A Swiss experience with 588 tumors. Cancer. 2000;89:604-614.
81 Amin MB, Tamboli P, Javidan J, et al. Prognostic impact of histologic subtyping of adult renal epithelial neoplasms. An experience of 405 cases. Am J Surg Pathol. 2002;26:281-291.
82 Cheville JC, Lohse CM, Zincke H, et al. Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinoma. Am J Surg Pathol. 2003;27:612-624.
83 Delahunt B, Eble JN. Papillary renal cell carcinoma. A clinicopathologic and immunohistochemical study of 105 tumors. Mod Pathol. 1997;10:537-544.
84 Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol. 1982;6:655-663.
85 Frank I, Blute ML, Cheville JC, et al. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis. The SSIGN score. J Urol. 2002;168:2395-2400.
86 Kattan MW, Reuter V, Motzer RJ, et al. A postoperative prognostic nomogram for renal cell carcinoma. J Urol. 2001;166:63-67.
87 Zisman A, Pantuck AJ, Wieder J, et al. Risk group assessment and clinical outcome algorithm to predict the natural history of patients with surgically resected renal cell carcinoma. J Clin Oncol. 2002;20:4559-4566.
88 Patard JJ, Kim HL, Lam JS, et al. Use of the University of California Los Angeles integrated staging system to predict survival in renal cell carcinoma. An international multicenter study. J Clin Oncol. 2004;22:3316-3322.
89 Han KR, Bleumer I, Pantuck AJ, et al. Validation of an integrated staging system toward improved prognostication of patients with localized renal cell carcinoma in an international population. J Urol. 2003;170:2221-2224.
90 Motzer RJ, Bander NH, Nanus DM. Renal-cell carcinoma. N Engl J Med. 1996;335:865-875.
91 Johansson S, Angervall L, Bengtsson U, Wahlqvist L. A clinicopathologic and prognostic study of epithelial tumors of the renal pelvis. Cancer. 1976;37:1376-1383.
92 Holmang S, Lele SM, Johansson SL. Squamous cell carcinoma of the renal pelvis and ureter. Incidence, symptoms, treatment and outcome. J Urol. 2007;178:51-56.
93 Ozsahin M, Zouhair A, Villa S, et al. Prognostic factors in urothelial renal pelvis and ureter tumours. A multicentre Rare Cancer Network study. Eur J Cancer. 1999;35:738-743.
94 Rubenstein MA, Walz BJ, Bucy JG. Transitional cell carcinoma of the kidney. 25-Year experience. J Urol. 1978;119:594-597.
95 Corrado F, Ferri C, Mannini D, et al. Transitional cell carcinoma of the upper urinary tract. Evaluation of prognostic factors by histopathology and flow cytometric analysis. J Urol. 1991;145:1159-1163.
96 Hall MC, Womack S, Sagalowsky AI, et al. Prognostic factors, recurrence, and survival in transitional cell carcinoma of the upper urinary tract. A 30-year experience in 252 patients. Urology. 1998;52:594-601.
97 Heney NM, Nocks BN, Daly JJ, et al. Prognostic factors in carcinoma of the ureter. J Urol. 1981;125:632-636.
98 Novara G, De Marco V, Gottardo F, et al. Independent predictors of cancer-specific survival in transitional cell carcinoma of the upper urinary tract. Multi-institutional dataset from 3 European centers. Cancer. 2007;110:1715-1722.
99 Roscigno M, Shariat SF, Margulis V, et al. Impact of lymph node dissection on cancer specific survival in patients with upper tract urothelial carcinoma treated with radical nephroureterectomy. J Urol. 2009;181:2482-2489.
100 Akao J, Matsuyama H, Yamamoto Y, et al. Clinical significance of lymphovascular invasion in upper urinary tract urothelial cancer. BJU Int. 2008;102:572-575.
101 Remzi M, Haitel A, Margulis V, et al. Tumour architecture is an independent predictor of outcomes after nephroureterectomy. A multi-institutional analysis of 1363 patients. BJU Int. 2009;103:307-311.
102 Mullerad M, Russo P, Golijanin D, et al. Bladder cancer as a prognostic factor for upper tract transitional cell carcinoma. J Urol. 2004;172:2177-2181.
103 Margulis V, Shariat SF, Matin SF, et al. Outcomes of radical nephroureterectomy. A series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer. 2009;115:1224-1233.
104 Kondo T, Nakazawa H, Ito F, et al. Primary site and incidence of lymph node metastases in urothelial carcinoma of upper urinary tract. Urology. 2007;69:265-269.
105 Miyake H, Hara I, Gohji K, et al. The significance of lymphadenectomy in transitional cell carcinoma of the upper urinary tract. Br J Urol. 1998;82:494-498.
106 Maulard-Durdux C, Dufour B, Hennequin C, et al. Postoperative radiation therapy in 26 patients with invasive transitional cell carcinoma of the upper urinary tract. No impact on survival? J Urol. 1996;155:115-117.
107 Linehan WM, Walther MM, Zbar B. The genetic basis of cancer of the kidney. J Urol. 2003;170:2163-2172.
108 Gnarra JR, Tory K, Weng Y, et al. Mutations of the VHL tumour suppressor gene in renal carcinoma. Nat Genet. 1994;7:85-90.
109 van Slegtenhorst M, de Hoogt R, Hermans C, et al. Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science. 1997;277:805-808.
110 Iliopoulos O, Eng C. Genetic and clinical aspects of familial renal neoplasms. Semin Oncol. 2000;27:138-149.
111 Khoo SK, Bradley M, Wong FK, et al. Birt-Hogg-Dubé syndrome. Mapping of a novel hereditary neoplasia gene to chromosome 17p12-q11.2. Oncogene. 2001;20:5239-5242.
112 Takahashi M, Rhodes DR, Furge KA, et al. Gene expression profiling of clear cell renal cell carcinoma. Gene identification and prognostic classification. Proc Natl Acad Sci U S A. 2001;98:9754-9759.
113 Martinez A, Fullwood P, Kondo K, et al. Role of chromosome 3p12-p21 tumour suppressor genes in clear cell renal cell carcinoma. Analysis of VHL dependent and VHL independent pathways of tumorigenesis. Mol Pathol. 2000;53:137-144.
114 Morrissey C, Martinez A, Zatyka M, et al. Epigenetic inactivation of the RASSF1A 3p21.3 tumor suppressor gene in both clear cell and papillary renal cell carcinoma. Cancer Res. 2001;61:7277-7281.
115 Copland JA, Luxon BA, Ajani L, et al. Genomic profiling identifies alterations in TGFbeta signaling through loss of TGFbeta receptor expression in human renal cell carcinogenesis and progression. Oncogene. 2003;22:8053-8062.
116 Turner KJ, Moore JW, Jones A, et al. Expression of hypoxia-inducible factors in human renal cancer. Relationship to angiogenesis and to the von Hippel-Lindau gene mutation. Cancer Res. 2002;62:2957-2961.
117 Shin Lee J, Seok Kim H, Bok Kim Y, et al. Expression of PTEN in renal cell carcinoma and its relation to tumor behavior and growth. J Surg Oncol. 2003;84:166-172.
118 Igarashi H, Esumi M, Ishida H, Okada K. Vascular endothelial growth factor overexpression is correlated with von Hippel-Lindau tumor suppressor gene inactivation in patients with sporadic renal cell carcinoma. Cancer. 2002;95:47-53.
119 Ashida S, Nishimori I, Tanimura M, et al. Effects of von Hippel-Lindau gene mutation and methylation status on expression of transmembrane carbonic anhydrases in renal cell carcinoma. J Cancer Res Clin Oncol. 2002;128:561-568.
120 Kuczyk MA, Serth J, Bokemeyer C, et al. Detection of p53 gene alteration in renal-cell cancer by micropreparation techniques of tumor specimens. Int J Cancer. 1995;64:399-406.
121 Papadopoulos I, Rudolph P, Weichert-Jacobsen K. Value of p53 expression, cellular proliferation, and DNA content as prognostic indicators in renal cell carcinoma. Eur Urol. 1997;32:110-117.
122 Shimazui T, Oosterwijk E, Debruyne FM, Schalken JA. Molecular prognostic factor in renal cell carcinoma. Semin Urol Oncol. 1996;14:250-255.
123 Rasmuson T, Grankvist K, Ljungberg B. Soluble ectodomain of c-erbB-2 oncoprotein in relation to tumour stage and grade in human renal cell carcinoma. Br J Cancer. 1997;75:1674-1677.
124 Fadl-Elmula I, Gorunova L, Mandahl N, et al. Cytogenetic analysis of upper urinary tract transitional cell carcinomas. Cancer Genet Cytogenet. 1999;115:123-127.
125 Rigola MA, Fuster C, Casadevall C, et al. Comparative genomic hybridization analysis of transitional cell carcinomas of the renal pelvis. Cancer Genet Cytogenet. 2001;127:59-63.
126 Rey A, Lara PC, Redondo E, et al. Overexpression of p53 in transitional cell carcinoma of the renal pelvis and ureter. Relation to tumor proliferation and survival. Cancer. 1997;79:2178-2185.
127 Furihata M, Ohtsuki Y, Sonobe H, et al. An overexpression in carcinoma of the renal pelvis and ureter including dysplasia. Immunohistochemical findings in relation to prognosis. Clin Cancer Res. 1997;3:1399-1404.
128 Furihata M, Ohtsuki Y, Sonobe H, et al. Prognostic significance of cyclin E and p53 protein overexpression in carcinoma of the renal pelvis and ureter. Br J Cancer. 1998;77:783-788.
129 Jeong IG, Kim SH, Jeon HG, et al. Prognostic value of apoptosis-related markers in urothelial cancer of the upper urinary tract. Hum Pathol. 2009;40:668-677.
130 Kamai T, Takagi K, Asami H, et al. Prognostic significance of p27Kip1 and Ki-67 expression in carcinoma of the renal pelvis and ureter. BJU Int. 2000;86:14-19.
131 Ritchie AW, Chisholm GD. The natural history of renal carcinoma. Semin Oncol. 1983;10:390-400.
132 Sufrin G, Chasan S, Golio A, Murphy GP. Paraneoplastic and serologic syndromes of renal adenocarcinoma. Semin Urol. 1989;7:158-171.
133 Chisholm GD. Nephrogenic ridge tumors and their syndromes. Ann N Y Acad Sci. 1974;230:403-423.
134 Porena M, Vespasiani G, Rosi P, et al. Incidentally detected renal cell carcinoma. Role of ultrasonography. J Clin Ultrasound. 1992;20:395-400.
135 Konnak JW, Grossman HB. Renal cell carcinoma as an incidental finding. J Urol. 1985;134:1094-1096.
136 Thompson IM, Peek M. Improvement in survival of patients with renal cell carcinoma—the role of the serendipitously detected tumor. J Urol. 1988;140:487-490.
137 Smith SJ, Bosniak MA, Megibow AJ, et al. Renal cell carcinoma. Earlier discovery and increased detection. Radiology. 1989;170:699-703.
138 Johnson CD, Dunnick NR, Cohan RH, Illescas FF. Renal adenocarcinoma. CT staging of 100 tumors. AJR Am J Roentgenol. 1987;148:59-63.
139 Semelka RC, Shoenut JP, Magro CM, et al. Renal cancer staging. Comparison of contrast-enhanced CT and gadolinium-enhanced fat-suppressed spin-echo and gradient-echo MR imaging. J Magn Reson Imaging. 1993;3:597-602.
140 Robson CJ, Churchill BM, Anderson W. The results of radical nephrectomy for renal cell carcinoma. J Urol. 1969;101:297-301.
141 Edge S, Byrd D, Compton C, et al. AJCC Cancer Staging Manual. New York: Springer-Verlag; 2010.
142 Charbit L, Gendreau MC, Mee S, Cukier J. Tumors of the upper urinary tract. 10 Years of experience. J Urol. 1991;146:1243-1246.
143 Chow NH, Tzai TS, Cheng HL, et al. Urinary cytodiagnosis. Can it have a different prognostic implication than a diagnostic test? Urol Int. 1994;53:18-23.
144 Williams SK, Denton KJ, Minervini A, et al. Correlation of upper-tract cytology, retrograde pyelography, ureteroscopic appearance, and ureteroscopic biopsy with histologic examination of upper-tract transitional cell carcinoma. J Endourol. 2008;22:71-76.
145 Witte D, Truong LD, Ramzy I. Transitional cell carcinoma of the renal pelvis. The diagnostic role of pelvic washings. Am J Clin Pathol. 2002;117:444-450.
146 Badalament RA, Bennett WF, Bova JG, et al. Computed tomography of primary transitional cell carcinoma of upper urinary tracts. Urology. 1992;40:71-75.
147 Batata MA, Whitmore WF, Hilaris BS, et al. Primary carcinoma of the ureter. A prognostic study. Cancer. 1975;35:1626-1632.
148 Skinner DG, Colvin RB, Vermillion CD, et al. Diagnosis and management of renal cell carcinoma. A clinical and pathologic study of 309 cases. Cancer. 1971;28:1165-1177.
149 McNichols DW, Segura JW, DeWeerd JH. Renal cell carcinoma. Long-term survival and late recurrence. J Urol. 1981;126:17-23.
150 Selli C, Hinshaw WM, Woodard BH, Paulson DF. Stratification of risk factors in renal cell carcinoma. Cancer. 1983;52:899-903.
151 Golimbu M, Joshi P, Sperber A, et al. Renal cell carcinoma. Survival and prognostic factors. Urology. 1986;27:291-301.
152 Dinney CP, Awad SA, Gajewski JB, et al. Analysis of imaging modalities, staging systems, and prognostic indicators for renal cell carcinoma. Urology. 1992;39:122-129.
153 Tsui KH, Shvarts O, Smith RB, et al. Prognostic indicators for renal cell carcinoma. A multivariate analysis of 643 patients using the revised 1997 TNM staging criteria. J Urol. 2000;163:1090-1095. quiz 1295
154 Gettman MT, Blute ML, Spotts B, et al. Pathologic staging of renal cell carcinoma. Significance of tumor classification with the 1997 TNM staging system. Cancer. 2001;91:354-361.
155 Tsui KH, Shvarts O, Barbaric Z, et al. Is adrenalectomy a necessary component of radical nephrectomy? UCLA experience with 511 radical nephrectomies. J Urol. 2000;163:437-441.
156 Paul R, Mordhorst J, Busch R, et al. Adrenal sparing surgery during radical nephrectomy in patients with renal cell cancer. A new algorithm. J Urol. 2001;166:59-62.
157 Blom JH, van Poppel H, Marechal JM, et al. Radical nephrectomy with and without lymph-node dissection. Final results of European Organization for Research and Treatment of Cancer (EORTC) randomized phase 3 trial 30881. Eur Urol. 2009;55:28-34.
158 Blute ML, Leibovich BC, Cheville JC, et al. A protocol for performing extended lymph node dissection using primary tumor pathological features for patients treated with radical nephrectomy for clear cell renal cell carcinoma. J Urol. 2004;172:465-469.
159 Pantuck AJ, Zisman A, Dorey F, et al. Renal cell carcinoma with retroperitoneal lymph nodes: role of lymph node dissection. J Urol. 2003;169:2076-2083.
160 Lerner SE, Hawkins CA, Blute ML, et al. Disease outcome in patients with low stage renal cell carcinoma treated with nephron sparing or radical surgery. J Urol. 1996;155:1868-1873.
161 Patard JJ, Shvarts O, Lam JS, et al. Safety and efficacy of partial nephrectomy for all T1 tumors based on an international multicenter experience. J Urol. 2004;171:2181-2185. quiz 2435
162 Lam JS, Shvarts O, Alemozaffard M. Nephro-sparing surgery as the new gold standard for T1 renal cell carcinoma. Results of a contemporary UCLA series. J Urol. 2004;171(4 Suppl):469. [abstract 1774]
163 Lau WK, Blute ML, Weaver AL, et al. Matched comparison of radical nephrectomy vs nephron-sparing surgery in patients with unilateral renal cell carcinoma and a normal contralateral kidney. Mayo Clin Proc. 2000;75:1236-1242.
164 McKiernan J, Simmons R, Katz J, Russo P. Natural history of chronic renal insufficiency after partial and radical nephrectomy. Urology. 2002;59:816-820.
165 Ono Y, Kinukawa T, Hattori R, et al. Laparoscopic radical nephrectomy for renal cell carcinoma. A five-year experience. Urology. 1999;53:280-286.
166 Portis AJ, Yan Y, Landman J, et al. Long-term followup after laparoscopic radical nephrectomy. J Urol. 2002;167:1257-1262.
167 Stifelman M, Taneja C, Cohen M. Hand assisted laparoscopic nephrectomy. A multi-institutional study evaluation oncological control [abstract]. J Urol. 2002;167:668.
168 Berger A, Brandina R, Atalla MA, et al. Laparoscopic radical nephrectomy for renal cell carcinoma. Oncological outcomes at 10 years or more. J Urol. 2009;182:2172-2176.
169 Lane BR, Gill IS. 5-Year outcomes of laparoscopic partial nephrectomy. J Urol. 2007;177:70-74. discussion 74
170 Harmon J, Parulkar B, Doble A. Critical assessment of cancer recurrence following renal cryoablation. A multi-center review. J Urol. 2004;4 Suppl:469. [abstract 1775]
171 Shingleton B, Sewell P. Percutaneous renal tumor cryoablation. Results in the first 90 patients. J Urol. 2004;4 Suppl:463. [abstract 1751]
172 Savage SJ, Gill IS. Renal tumor ablation. Energy-based technologies. World J Urol. 2000;18:283-288.
173 Gontero P, Joniau S, Zitella A, et al. Ablative therapies in the treatment of small renal tumors. How far from standard of care? Urol Oncol. 2010;28:251-259.
174 Rafla S. Renal cell carcinoma. Natural history and results of treatment. Cancer. 1970;25:26-40.
175 Finney R. The value of radiotherapy in the treatment of hypernephroma—a clinical trial. Br J Urol. 1973;45:258-269.
176 Kjaer M, Iversen P, Hvidt V, et al. A randomized trial of postoperative radiotherapy versus observation in stage II and III renal adenocarcinoma. A study by the Copenhagen Renal Cancer Study Group. Scand J Urol Nephrol. 1987;21:285-289.
177 Stein M, Kuten A, Halpern J, et al. The value of postoperative irradiation in renal cell cancer. Radiother Oncol. 1992;24:41-44.
178 Kao GD, Malkowicz SB, Whittington R, et al. Locally advanced renal cell carcinoma. Low complication rate and efficacy of postnephrectomy radiation therapy planned with CT. Radiology. 1994;193:725-730.
179 Rafla S. The Role of Adjuvant Radiotherapy in the Management of Renal Cell Carcinoma. New York: Thieme-Stratton; 1984. pp 93-107
180 Gez E, Libes M, Bar-Deroma R, et al. Postoperative irradiation in localized renal cell carcinoma. The Rambam Medical Center experience. Tumori. 2002;88:500-502.
181 Ulutin HC, Aksu G, Fayda M, et al. The value of postoperative radiotherapy in renal cell carcinoma. A single-institution experience. Tumori. 2006;92:202-206.
182 Rabinovitch RA, Zelefsky MJ, Gaynor JJ, Fuks Z. Patterns of failure following surgical resection of renal cell carcinoma. Implications for adjuvant local and systemic therapy. J Clin Oncol. 1994;12:206-212.
183 Messing EM, Manola J, Wilding G, et al. Phase III study of interferon alfa-NL as adjuvant treatment for resectable renal cell carcinoma. An Eastern Cooperative Oncology Group/Intergroup trial. J Clin Oncol. 2003;21:1214-1222.
184 Pizzocaro G, Piva L, Colavita M, et al. Interferon adjuvant to radical nephrectomy in Robson stages II and III renal cell carcinoma. A multicentric randomized study. J Clin Oncol. 2001;19:425-431.
185 Porzsolt F. Adjuvant therapy of renal cell cancer with interferon alpha-2a [abstract]. Proc Am Soc Clin Oncol. 1992;11:202a.
186 Clark JI, Atkins MB, Urba WJ, et al. Adjuvant high-dose bolus interleukin-2 for patients with high-risk renal cell carcinoma. A cytokine working group randomized trial. J Clin Oncol. 2003;21:3133-3140.
187 Jocham D, Richter A, Hoffmann L, et al. Adjuvant autologous renal tumour cell vaccine and risk of tumour progression in patients with renal-cell carcinoma after radical nephrectomy. Phase III, randomised controlled trial. Lancet. 2004;363:594-599.
188 Beitler JJ, Makara D, Silverman P, Lederman G. Definitive, high-dose-per-fraction, conformal, stereotactic external radiation for renal cell carcinoma. Am J Clin Oncol. 2004;27:646-648.
189 Wersall PJ, Blomgren H, Lax I, et al. Extracranial stereotactic radiotherapy for primary and metastatic renal cell carcinoma. Radiother Oncol. 2005;77:88-95.
190 Svedman C, Sandstrom P, Pisa P, et al. A prospective phase II trial of using extracranial stereotactic radiotherapy in primary and metastatic renal cell carcinoma. Acta Oncol. 2006;45:870-875.
191 Cozad SC, Smalley SR, Austenfeld M, et al. Transitional cell carcinoma of the renal pelvis or ureter. Patterns of failure. Urology. 1995;46:796-800.
192 Zincke H, Neves RJ. Feasibility of conservative surgery for transitional cell cancer of the upper urinary tract. Urol Clin North Am. 1984;11:717-724.
193 McDougall EM, Clayman RV, Elashry O. Laparoscopic nephroureterectomy for upper tract transitional cell cancer. The Washington University experience. J Urol. 1995;154:975-979. discussion 979-980
194 Manabe D, Saika T, Ebara S, et al. Comparative study of oncologic outcome of laparoscopic nephroureterectomy and standard nephroureterectomy for upper urinary tract transitional cell carcinoma. Urology. 2007;69:457-461.
195 Muntener M, Schaeffer EM, Romero FR, et al. Incidence of local recurrence and port site metastasis after laparoscopic radical nephroureterectomy. Urology. 2007;70:864-868.
196 Capitanio U, Shariat SF, Isbarn H, et al. Comparison of oncologic outcomes for open and laparoscopic nephroureterectomy. A multi-institutional analysis of 1249 cases. Eur Urol. 2009;56:1-9.
197 Seifman BD, Montie JE, Wolf JSJr. Prospective comparison between hand-assisted laparoscopic and open surgical nephroureterectomy for urothelial cell carcinoma. Urology. 2001;57:133-137.
198 Glinianski M, Guru KA, Zimmerman G, et al. Robot-assisted ureterectomy and ureteral reconstruction for urothelial carcinoma. J Endourol. 2009;23:97-100.
199 Krambeck AE, Thompson RH, Lohse CM, et al. Imperative indications for conservative management of upper tract transitional cell carcinoma. J Urol. 2007;178:792-796. discussion 796-797
200 Leitenberger A, Beyer A, Altwein JE. Organ-sparing treatment for ureteral carcinoma? Eur Urol. 1996;29:272-278.
201 Wallace DM, Wallace DM, Whitfield HN, et al. The late results of conservative surgery for upper tract urothelial carcinomas. Br J Urol. 1981;53:537-541.
202 Cozad SC, Smalley SR, Austenfeld M, et al. Adjuvant radiotherapy in high stage transitional cell carcinoma of the renal pelvis and ureter. Int J Radiat Oncol Biol Phys. 1992;24:743-745.
203 Brookland RK, Richter MP. The postoperative irradiation of transitional cell carcinoma of the renal pelvis and ureter. J Urol. 1985;133:952-955.
204 Czito B, Zietman A, Kaufman D, et al. Adjuvant radiotherapy with and without concurrent chemotherapy for locally advanced transitional cell carcinoma of the renal pelvis and ureter. J Urol. 2004;172:1271-1275.
205 Kwak C, Lee SE, Jeong IG, Ku JH. Adjuvant systemic chemotherapy in the treatment of patients with invasive transitional cell carcinoma of the upper urinary tract. Urology. 2006;68:53-57.
206 Soga N, Arima K, Sugimura Y. Adjuvant methotrexate, vinblastine, adriamycin, and cisplatin chemotherapy has potential to prevent recurrence of bladder tumors after surgical removal of upper urinary tract transitional cell carcinoma. Int J Urol. 2008;15:800-803.
207 Hellenthal NJ, Shariat SF, Margulis V, et al. Adjuvant chemotherapy for high risk upper tract urothelial carcinoma. Results from the Upper Tract Urothelial Carcinoma Collaboration. J Urol. 2009;182:900-906.
208 Malkin RB. Regression of renal carcinoma following radiation therapy. J Urol. 1975;114:782-783.
209 Riches EW, Griffiths IH, Thackray AC. New growths of the kidney and ureter. Br J Urol. 1951;23:297-356.
210 van der Werf-Messing B. Proceedings: Carcinoma of the kidney. Cancer. 1973;32:1056-1061.
211 Juusela H, Malmio K, Alfthan O, Oravisto KJ. Preoperative irradiation in the treatment of renal adenocarcinoma. Scand J Urol Nephrol. 1977;11:277-281.
212 Frydenberg M, Gunderson L, Hahn G, et al. Preoperative external beam radiotherapy followed by cytoreductive surgery and intraoperative radiotherapy for locally advanced primary or recurrent renal malignancies. J Urol. 1994;152:15-21.
213 Calvo F, Zincke H, Gunderson LL. Genitourinary IORT. In: Gunderson LL, Willet CG, Harrison JB, Calvo F, editors. Intraoperative irradiation—techniques and results. Totowa, NJ: Humana Press; 1999:421-436.
214 Haddock M, Miller RC, Zincke H: Intraoperative electron irradiation (IOERT) for locally advanced genitourinary malignancies, ISIORT Abstracts, #5.1, 2002.
215 Santos M, Ucar A, Ramos H, et al. [Intraoperative radiotherapy in locally advanced carcinoma of the kidney. Initial experience]. Actas Urol Esp. 1989;13:36-40. In Spanish
216 Eble MJ, Stahler G, Wannenmacher M. IORT for locally advanced or recurrent renal cell carcinoma. Front Radiat Ther Oncol. 1997;31:253-255.
217 Master VA, Gottschalk AR, Kane C, Carroll PR. Management of isolated renal fossa recurrence following radical nephrectomy. J Urol. 2005;174:473-477. discussion 477
218 Zhang Q, Fu S, Liu T, et al. Adjuvant intraoperative electron radiotherapy and external beam radiotherapy for locally advanced transitional cell carcinoma of the ureter. Urol Oncol. 2009;27:14-20.
219 Neves RJ, Zincke H, Taylor WF. Metastatic renal cell cancer and radical nephrectomy. Identification of prognostic factors and patient survival. J Urol. 1988;139:1173-1176.
220 Flanigan RC, Salmon SE, Blumenstein BA, et al. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med. 2001;345:1655-1659.
221 Mickisch GH, Garin A, van Poppel H, et al. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma. A randomised trial. Lancet. 2001;358:966-970.
222 Motzer RJ, Mazumdar M, Bacik J, et al. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol. 1999;17:2530-2540.
223 O’Dea MJ, Zincke H, Utz DC, Bernatz PE. The treatment of renal cell carcinoma with solitary metastasis. J Urol. 1978;120:540-542.
224 Kjaer M. The treatment and prognosis of patients with renal adenocarcinoma with solitary metastasis. Ten-year survival results. Int J Radiat Oncol Biol Phys. 1987;13:619-621.
225 Kavolius JP, Mastorakos DP, Pavlovich C, et al. Resection of metastatic renal cell carcinoma. J Clin Oncol. 1998;16:2261-2266.
226 Althausen P, Althausen A, Jennings LC, Mankin HJ. Prognostic factors and surgical treatment of osseous metastases secondary to renal cell carcinoma. Cancer. 1997;80:1103-1109.
227 Halperin EC, Harisiadis L. The role of radiation therapy in the management of metastatic renal cell carcinoma. Cancer. 1983;51:614-617.
228 Wilson D, Hiller L, Gray L, et al. The effect of biological effective dose on time to symptom progression in metastatic renal cell carcinoma. Clin Oncol (R Coll Radiol). 2003;15:400-407.
229 Lee J, Hodgson D, Chow E, et al. A phase II trial of palliative radiotherapy for metastatic renal cell carcinoma. Cancer. 2005;104:1894-1900.
230 Gerszten PC, Burton SA, Ozhasoglu C, et al. Stereotactic radiosurgery for spinal metastases from renal cell carcinoma. J Neurosurg Spine. 2005;3:288-295.
231 Nguyen QN, Shiu AS, Rhines LD, et al. Management of spinal metastases from renal cell carcinoma using stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 2010;76:185-1192.
232 Wronski M, Maor MH, Davis BJ, et al. External radiation of brain metastases from renal carcinoma. A retrospective study of 119 patients from the M.D. Anderson Cancer Center. Int J Radiat Oncol Biol Phys. 1997;37:753-759.
233 Wronski M, Arbit E, Russo P, Galicich JH. Surgical resection of brain metastases from renal cell carcinoma in 50 patients. Urology. 1996;47:187-193.
234 Sheehan JP, Sun MH, Kondziolka D, et al. Radiosurgery in patients with renal cell carcinoma metastasis to the brain. Long-term outcomes and prognostic factors influencing survival and local tumor control. J Neurosurg. 2003;98:342-349.
235 Brown PD, Brown CA, Pollock BE, et al. Stereotactic radiosurgery for patients with “radioresistant” brain metastases. Neurosurgery. 2002;51:656-665. discussion 665-667
236 Amendola BE, Wolf AL, Coy SR, et al. Brain metastases in renal cell carcinoma. Management with gamma knife radiosurgery. Cancer J. 2000;6:372-376.
237 Payne BR, Prasad D, Szeifert G, et al. Gamma surgery for intracranial metastases from renal cell carcinoma. J Neurosurg. 2000;92:760-765.
238 Marko NF, Angelov L, Toms SA, et al. Stereotactic radiosurgery as single-modality treatment of incidentally identified renal cell carcinoma brain metastases. Surg Neurol. 2009. Jul 14. [Epub ahead of print]
239 Samlowski WE, Majer M, Boucher KM, et al. Multidisciplinary treatment of brain metastases derived from clear cell renal cancer incorporating stereotactic radiosurgery. Cancer. 2008;113:2539-2548.
240 Hernandez L, Zamorano L, Sloan A, et al. Gamma knife radiosurgery for renal cell carcinoma brain metastases. J Neurosurg. 2002;97:489-493.
241 Yagoda A, Petrylak D, Thompson S. Cytotoxic chemotherapy for advanced renal cell carcinoma. Urol Clin North Am. 1993;20:303-321.
242 Rini BI, Vogelzang NJ, Dumas MC, et al. Phase II trial of weekly intravenous gemcitabine with continuous infusion fluorouracil in patients with metastatic renal cell cancer. J Clin Oncol. 2000;18:2419-2426.
243 Milowsky MI, Rosmarin A, Tickoo SK, et al. Active chemotherapy for collecting duct carcinoma of the kidney. A case report and review of the literature. Cancer. 2002;94:111-116.
244 Motzer RJ, Bacik J, Murphy BA, et al. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol. 2002;20:289-296.
245 Fyfe G, Fisher RI, Rosenberg SA, et al. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol. 1995;13:688-696.
246 Fisher RI, Rosenberg SA, Fyfe G. Long-term survival update for high-dose recombinant interleukin-2 in patients with renal cell carcinoma. Cancer J Sci Am. 2000;6(Suppl 1):S55-S57.
247 Yang JC, Sherry RM, Steinberg SM, et al. Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal cancer. J Clin Oncol. 2003;21:3127-3132.
248 Atzpodien J, Kirchner H, Jonas U, et al. Interleukin-2- and interferon alfa-2a-based immunochemotherapy in advanced renal cell carcinoma. A prospectively randomized trial of the German Cooperative Renal Carcinoma Chemoimmunotherapy Group (DGCIN). J Clin Oncol. 2004;22:1188-1194.
249 Motzer RJ, Michaelson MD, Redman BG, et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24:16-24.
250 Motzer RJ, Rini BI, Bukowski RM, et al. Sunitinib in patients with metastatic renal cell carcinoma. JAMA. 2006;295:2516-2524.
251 Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115-124.
252 Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:3584-3590.
253 Ratain MJ, Eisen T, Stadler WM, et al. Phase II placebo-controlled randomized discontinuation trial of sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24:2505-2512.
254 Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125-134.
255 Escudier B, Szczylik C, Hutson TE, et al. Randomized phase II trial of first-line treatment with sorafenib versus interferon alfa-2a in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:1280-1289.
256 Zhu X, Stergiopoulos K, Wu S. Risk of hypertension and renal dysfunction with an angiogenesis inhibitor sunitinib. Systematic review and meta-analysis. Acta Oncol. 2009;48:9-17.
257 Wu S, Chen JJ, Kudelka A, et al. Incidence and risk of hypertension with sorafenib in patients with cancer. A systematic review and meta-analysis. Lancet Oncol. 2008;9:117-123.
258 Rini BI, Tamaskar I, Shaheen P, et al. Hypothyroidism in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst. 2007;99:81-83.
259 Rosenbaum SE, Wu S, Newman MA, et al. Dermatological reactions to the multitargeted tyrosine kinase inhibitor sunitinib. Support Care Cancer. 2008;16:557-566.
260 Atkins MB, Hidalgo M, Stadler WM, et al. Randomized phase II study of multiple dose levels of CCI-779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. J Clin Oncol. 2004;22:909-918.
261 Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271-2281.
262 Yang JC, Haworth L, Sherry RM, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427-434.
263 Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma. A randomised, double-blind phase III trial. Lancet. 2007;370:2103-2111.
264 Rini BI, Halabi S, Rosenberg JE, et al. Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma. CALGB 90206. J Clin Oncol. 2008;26:5422-5428.
265 Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma. A double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449-456.
266 Sternberg C, Szczylik C, Lee E, et al. A randomized, double blind phase III study of pazopanib in treatment naïve and cytokine-pretreated patients with advanced renal cell carcinoma (RCC). J Clin Oncol. 27, 2009. [abstract 5021]
267 Burch PA, Richardson RL, Cha SS, et al. Phase II study of paclitaxel and cisplatin for advanced urothelial cancer. J Urol. 2000;164:1538-1542.
268 Lerner SE, Blute ML, Richardson RL, Zincke H. Platinum-based chemotherapy for advanced transitional cell carcinoma of the upper urinary tract. Mayo Clin Proc. 1996;71:945-950.
269 Moore MJ, Winquist EW, Murray N, et al. Gemcitabine plus cisplatin, an active regimen in advanced urothelial cancer. A phase II trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 1999;17:2876-2881.
270 Heron DE, Gerszten K, Selvaraj RN, et al. Conventional 3D conformal versus intensity-modulated radiotherapy for the adjuvant treatment of gynecologic malignancies. A comparative dosimetric study of dose-volume histograms small star, filled. Gynecol Oncol. 2003;91:39-45.