Chapter 27 Kidney and genitourinary tract
• Diuretic drugs: their sites and modes of action, classification, adverse effects and uses in cardiac, hepatic, renal and other conditions.
• Carbonic anhydrase inhibitors.
• Cation-exchange resins and their uses.
• Drug-induced renal disease: by direct and indirect biochemical effects and by immunological effects.
• Prescribing for renal disease: adjusting the dose according to the characteristics of the drug and to the degree of renal impairment.
• Nephrolithiasis and its management.
• Pharmacological aspects of micturition.
Diuretic drugs
Definition
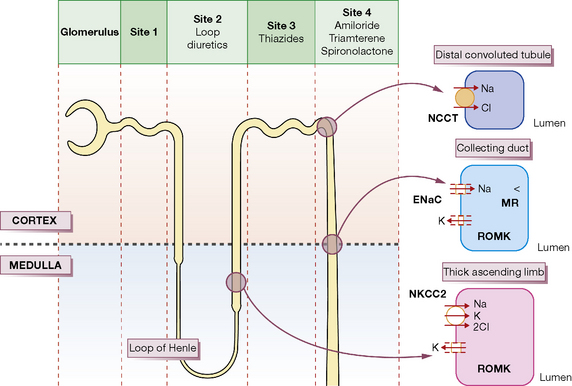
Each day the body produces 180 L of glomerular filtrate which is modified in its passage down the renal tubules to appear as 1.5 L of urine. Thus, if reabsorption of tubular fluid falls by 1%, urine output doubles. Most clinically useful diuretics are organic anions, which are transported directly from the blood into tubular fluid. The following brief account of tubular function with particular reference to sodium transport will help to explain where and how diuretic drugs act; it should be read with reference to Figure 27.1.
Sites and modes of action
Proximal convoluted tubule
Osmotic diuretics such as mannitol are non-resorbable solutes which retain water in the tubular fluid (Site 1, Fig. 27.1). Their effect is to increase water rather than sodium loss, and this is reflected in their special use acutely to reduce intracranial or intraocular pressure and not states associated with sodium overload.
Loop of Henle
The tubular fluid now passes into the loop of Henle where 25% of the filtered sodium is reabsorbed. There are two populations of nephron: those with short loops that are confined to the cortex, and the juxtamedullary nephrons whose long loops penetrate deep into the medulla and are concerned principally with water conservation;1 the following discussion refers to the latter.
The physiological changes are best understood by considering first the ascending limb. In the thick segment (Site 2, Fig. 27.1), sodium and chloride ions are transported from the tubular fluid into the interstitial fluid by the three-ion co-transporter system (i.e. Na+/K+/2Cl− called NKCC2) driven by the sodium pump. The co-transport of these ions is dependent on potassium returning to the lumen through the rectifying outer medullary potassium (ROMK) channel; otherwise potassium would be rate limiting. As the tubule epithelium is ‘tight’ here, i.e. impermeable to water, the tubular fluid becomes dilute, the interstitium becomes hypertonic, and fluid in the adjacent descending limb, which is permeable to water, becomes more concentrated as it approaches the tip of the loop, because the hypertonic interstitial fluid sucks water out of this limb of the tubule. The ‘hairpin’ structure of the loop thus confers on it the property of a countercurrent multiplier, i.e. by active transport of ions a small change in osmolality laterally across the tubular epithelium is converted into a steep vertical osmotic gradient.
The high osmotic pressure in the medullary interstitium is sustained by the descending and ascending vasa recta, long blood vessels of capillary thickness that lie close to the loops of Henle and act as countercurrent exchangers, for the incoming blood receives sodium from the outgoing blood.2Furosemide, bumetanide, piretanide, torasemide and ethacrynic acid act principally at Site 2 by inhibiting the three-ion transporter, thus preventing sodium ion reabsorption and lowering the osmotic gradient between cortex and medulla; this results in the formation of large volumes of dilute urine. Hence, these drugs are called ‘loop’ diuretics.
Distal convoluted tubule
The ascending limb of the loop then re-enters the renal cortex where its morphology changes into the thin-walled distal convoluted tubule (Site 3, Fig. 27.1). Here uptake is still driven by the sodium pump but sodium and chloride are taken up through a different transporter, the Na–Cl co-transporter, called NCC (formerly NCCT). Both ions are rapidly removed from the interstitium because cortical blood flow is high and there are no vasa recta present; the epithelium is also tight at Site 3 and consequently the urine becomes more dilute. Thiazides act principally at this region of the cortical diluting segment by blocking the NCC transporter.
Collecting duct
In the collecting duct (Site 4), sodium ions are exchanged for potassium and hydrogen ions. The sodium ions enter through the epithelial Na channel (called ENaC), which is stimulated by aldosterone. The aldosterone (mineralocorticoid) receptor is inhibited by the competitive receptor antagonist spironolactone, whereas the sodium channel is inhibited by amiloride and triamterene. All three of these diuretics are potassium sparing because potassium is normally secreted through the potassium channel, ROMK (see Fig. 27.1), down the potential gradient created by sodium reabsorption.
Individual diuretics
High-efficacy (loop) diuretics
Furosemide
Furosemide acts on the thick portion of the ascending limb of the loop of Henle (Site 2) to produce the effects described above. Because more sodium is delivered to Site 4, exchange with potassium leads to urinary potassium loss and hypokalaemia. Magnesium and calcium loss are increased by furosemide to about the same extent as sodium; the effect on calcium is utilised in the emergency management of hypercalcaemia (see p. 458).
Moderate-efficacy diuretics
(See also Hypertension, Ch. 24.)
Thiazides
Adverse effects
in general are discussed below. Rashes (sometimes photosensitive), thrombocytopenia and agranulocytosis occur. Thiazide-type drugs increase total plasma cholesterol concentration, but in long-term use this is less than 5%, even at high doses. The questions about the appropriateness of thiazides for mild hypertension, of which ischaemic heart disease is a common complication, are laid to rest by their proven success in randomised outcome comparisons (see Ch. 24).
Bendroflumethiazide
is a satisfactory member for routine use. For a diuretic effect the oral dose is 5–10 mg, which usually lasts less than 12 h, so that it should be given in the morning. As an antihypertensive, 2.5 mg is commonly prescribed. However, this dose does not achieve maximal blood pressure reduction in patients with salt-dependent hypertension (which includes most patients receiving a blocker of the renin–angiotensin system), and has not been shown to prevent strokes or heart attacks. New guidance is likely to recommend some return to higher doses – and/or greater use of the thiazide-like diuretics (below), with or without co-prescribed K+-sparing diuretic. Important potassium depletion is uncommon, but plasma potassium concentration should be checked in potentially vulnerable groups such as the elderly (see Ch. 25). If marked hypokalaemia occurs hyperaldosteronism should be excluded.
Low-efficacy diuretics
Spironolactone
(Aldactone) is structurally similar to aldosterone and competitively inhibits its action in the distal tubule (Site 4; exchange of potassium for sodium); excessive secretion of aldosterone contributes to fluid retention in hepatic cirrhosis, nephrotic syndrome, congestive heart failure (see specific use in Ch. 25) and primary hypersecretion (Conn’s syndrome). Spironolactone is also useful in the treatment of resistant hypertension, where increased aldosterone sensitivity is increasingly recognised as a contributory factor.
Adverse effects. Oestrogenic effects are the major limitation to its long-term use. They are dose dependent, but in the Randomized Aldactone Evaluation Study (RALES)3 (see Ch. 25) even 25 mg/day caused breast tenderness or enlargement in 10% of men. Women may also report breast discomfort or menstrual irregularities, including amenorrhoea. Minor gastrointestinal upset also occurs and there is increased risk of gastroduodenal ulcer and bleeding. These are reversible on stopping the drug. Spironolactone is reported to be carcinogenic in rodents, but many years of clinical experience suggest that it is safe in humans. Nevertheless, the UK licence for its use in essential hypertension was withdrawn (i.e. possible use long term in a patient group that includes the relatively young), but is retained for other indications.
Indications for diuretics
• Oedema states associated with sodium overload, e.g. cardiac, renal or hepatic disease, and also without sodium overload, e.g. acute pulmonary oedema following myocardial infarction. Note that oedema may also be localised, e.g. angioedema over the face and neck or around the ankles with some calcium channel blockers, or due to low plasma albumin, or immobility in the elderly; in none of these circumstances is a diuretic indicated.
• Hypertension, by reducing intravascular volume and probably by other mechanisms too, e.g. reduction of sensitivity to noradrenergic vasoconstriction.
• Hypercalcaemia. Furosemide reduces calcium reabsorption in the ascending limb of the loop of Henle, which action may be utilised in the emergency reduction of raised plasma calcium levels in addition to rehydration and other measures.
• Idiopathic hypercalciuria, a common cause of renal stone disease, may be reduced by thiazide diuretics.
• The syndrome of inappropriate secretion of antidiuretic hormone secretion (SIADH) may be treated with furosemide if there is a dangerous degree of volume overload (see also p. 423).
• Nephrogenic diabetes insipidus, paradoxically, may respond to diuretics which, by contracting vascular volume, increase salt and water reabsorption in the proximal tubule, and thus reduce urine volume.
Therapy
Congestive cardiac failure
The main account appears in Chapter 25, where the emphasis is now on early use of angiotensin-converting enzyme (ACE) inhibitors and β-adrenoceptor antagonists that are specifically diuretic sparing. But oral diuretics are easily given repeatedly, and lack of supervision can result in insidious over-treatment. Relief at disappearance of the congestive features can mask exacerbation of the low-output symptoms of heart failure, such as tiredness and postural dizziness due to reduced blood volume. A rising blood urea level is usually evidence of reduced glomerular blood flow consequent on a fall in cardiac output, but does not distinguish whether the cause of the reduced output is over-diuresis or worsening of the heart failure itself. The simplest guide to the success or failure of diuretic regimens is to monitor body-weight, which the patient can do equipped with just bathroom scales. Fluid intake and output charts are more demanding of nursing time, and often less accurate.
Adverse effects characteristic of diuretics
Potassium depletion
Diuretics that act at Sites 1, 2 and 3 (see Fig. 27.1) cause more sodium to reach the sodium–potassium exchange site in the distal tubule (Site 4) and so increase potassium excretion. This subject warrants discussion because hypokalaemia may cause cardiac arrhythmia in patients at risk (e.g. receiving digoxin). The safe lower limit for plasma potassium concentration is normally quoted as 3.5 mmol/L. Whether or not diuretic therapy causes significant lowering of serum potassium levels depends both on the drug and on the circumstances in which it is used:
• The loop diuretics produce a smaller fall in serum potassium concentration than do the thiazides, for equivalent diuretic effect, but have a greater capacity for diuresis, i.e. higher efficacy especially in large dose, and so are associated with greater decline in potassium levels. If diuresis is brisk and continuous, clinically important potassium depletion is likely to occur.
• Low dietary intake of potassium predisposes to hypokalaemia; the risk is particularly notable in the elderly, many of whom ingest less than 50 mmol per day (the dietary normal is 80 mmol).
• Hypokalaemia may be aggravated by other drugs, e.g. β2-adrenoceptor agonists, theophylline, corticosteroids, amphotericin.
• Hypokalaemia during diuretic therapy is also more likely in hyperaldosteronism, whether primary or more commonly secondary to severe liver disease, congestive cardiac failure or nephrotic syndrome.
• Potassium loss occurs with diarrhoea, vomiting or small bowel fistula, and may be aggravated by diuretic therapy.
• When a thiazide diuretic is used for hypertension, there is probably no case for routine prescription of a potassium supplement if no predisposing factors are present (see Ch. 25).
Potassium depletion can be minimised or corrected by:
• Maintaining a good dietary potassium intake (fruits, fruit juices, vegetables).
• Combining a potassium-depleting with a potassium-sparing drug.
• Intermittent use of potassium-losing drugs, i.e. drug holidays.
• Potassium supplements: KCl is preferred because chloride is the principal anion excreted along with sodium when high-efficacy diuretics are used. Potassium-sparing diuretics generally defend plasma potassium more effectively than potassium supplements. All forms of potassium are irritant to the gastrointestinal tract, and in the oesophagus may even cause ulceration. The elderly, in particular, should be warned never to take such tablets dry but always with a large cupful of liquid and sitting upright or standing.
Hyperkalaemia
may occur, especially if a potassium-sparing diuretic is given to a patient with impaired renal function. ACE inhibitors and angiotensin II receptor antagonists can also cause a modest increase in plasma potassium levels. They may cause dangerous hyperkalaemia if combined with KCl supplements or other potassium-sparing drugs, in the presence of impaired renal function. These may not be obvious, for example, to the patient who is using an unprescribed ‘low sodium’ salt substitute to reduce their salt (NaCl) intake.4 However, with suitable monitoring the combination can be used safely, as was well illustrated by the RALES trial.5 Ciclosporin, tacrolimus, indometacin and possibly other NSAIDs may cause hyperkalaemia with the potassium-sparing diuretics.
Depends on the severity and the following measures are appropriate:
• Any potassium-sparing diuretic should be discontinued.
• A cation-exchange resin, e.g. polystyrene sulphonate resin (Resonium A, Calcium Resonium, see below) can be used orally (more effective than rectally), to remove body potassium by the gut.
• Potassium may be moved rapidly from plasma into cells by giving:
 sodium bicarbonate, 50 mL 8.4% solution through a central line, and repeated in a few minutes if characteristic ECG changes persist
sodium bicarbonate, 50 mL 8.4% solution through a central line, and repeated in a few minutes if characteristic ECG changes persist nebulised β2-agonist, salbutamol 5–10 mg, is effective in stimulating the pumping of potassium into skeletal muscle.
nebulised β2-agonist, salbutamol 5–10 mg, is effective in stimulating the pumping of potassium into skeletal muscle.• In the presence of ECG changes, calcium gluconate, 10 mL of 10% solution, should be given i.v. and repeated if necessary in a few minutes; it has no effect on the serum potassium but opposes the myocardial effect of a raised serum potassium level. Calcium may potentiate digoxin and should be used cautiously, if at all, in a patient taking this drug. Sodium bicarbonate and calcium salt must not be mixed in a syringe or reservoir because calcium precipitates.
• Dialysis may be needed in refractory cases and is highly effective.
Methylxanthines
The general properties of the methylxanthines (theophylline, caffeine) are discussed elsewhere (see p. 155). Their mild diuretic action probably depends in part on smooth muscle relaxation in the afferent arteriolar bed increasing renal blood flow, and in part on a direct inhibitory effect on salt reabsorption in the proximal tubule. Their uses in medicine depend on other properties.
Carbonic anhydrase inhibitors
Alteration of urine pH
Alteration of urine pH by drugs is sometimes desirable. The most common reason is in the treatment of poisoning (a fuller account is given on p. 126). A summary of the main indications appears below.
Alkalinisation of urine:
• increases the elimination of salicylate, phenobarbital and chlorophenoxy herbicides, e.g. 2,4-D, MCPA
• treats crystal nephropathy by increasing drug solubility, e.g. of methotrexate, sulphonamides and triamterene. NB indinavir requires acidification
• reduces irritation of an inflamed urinary tract
• discourages the growth of certain organisms, e.g. Escherichia coli.
The urine can be made alkaline by sodium bicarbonate i.v., or by potassium citrate by mouth. Sodium overload may exacerbate cardiac failure, and sodium or potassium excess are dangerous when renal function is impaired.
Drugs and the kidney
Drug-induced renal disease
Drugs and other chemicals damage the kidney by:
1. Direct biochemical effect. Substances that cause such toxicity include:
2. Indirect biochemical effect:
3. Immunological effect. A wide range of drugs produces a wide range of injuries:
A drug may cause damage by more than one of the above mechanisms, e.g. gold. The sites and pathological types of injury are as follows:
Tubule damage
The counter-current multiplier and exchange systems of urine concentration (see p. 453) cause some drugs to accumulate in the renal medulla. Analgesic nephropathy is often first evident at this site, partly because of high tissue concentration and partly, it is believed, because of ischaemia through inhibition of locally produced vasodilator prostaglandins by NSAIDs. The distal tubule is the site of lithium-induced nephrotoxicity; damage to the medulla and distal nephron is manifested by failure to concentrate the urine after fluid deprivation and by failure to acidify urine after ingestion of ammonium chloride.
Other drug-induced lesions
• Vasculitis, caused by allopurinol, isoniazid, sulphonamides.
• Allergic interstitial nephritis, caused by penicillins (especially), thiazides, allopurinol, phenytoin, sulphonamides.
• Drug-induced lupus erythematosus, caused by hydralazine, procainamide, sulfasalazine.
Drugs may thus induce any of the common clinical syndromes of renal injury, namely:
• Acute renal failure, e.g. aminoglycosides, cisplatin.
• Nephrotic syndrome, e.g. penicillamine, gold, captopril (only at higher doses than now recommended).
• Chronic renal failure, e.g. NSAIDs.
• Functional impairment, i.e. reduced ability to dilute and concentrate urine (lithium), potassium loss in urine (loop diuretics), acid–base imbalance (acetazolamide).
Prescribing in renal disease
• exacerbate renal disease (see above)
• be ineffective, e.g. thiazide diuretics in moderate or severe renal failure; uricosurics
• be potentiated by accumulation due to failure of renal excretion.
The profound influence of impaired renal function on the elimination of some drugs is illustrated in Table 27.1.
Table 27.1 Drug t½ (h) in normal and severely impaired renal function
| Normal | Severe renal impairmenta | |
|---|---|---|
| Captopril | 2 | 25 |
| Amoxicillin | 2 | 14 |
| Gentamicin | 2.5 | > 50 |
| Atenolol | 6 | 100 |
| Digoxin | 36 | 90 |
a Glomerular filtration rate < 5 mL/min (normal value is 120 mL/min). These values illustrate the major effect of impaired renal function on the elimination of certain drugs. Depending on the circumstances, alternative drugs must be found or special care exercised when prescribing drugs that depend significantly on the kidney for elimination.
Administering the correct dose to a patient with renal disease must therefore take into account both the extent to which the drug normally relies on renal elimination and the degree of renal impairment; the best guide to the latter is the creatinine clearance and not the serum creatinine level itself,6 which can be notoriously misleading in the elderly and at extremes of body mass.
Dose adjustment for patients with renal impairment
• Adjustment of the initial dose (or where necessary the priming or loading dose, see p. 96) is generally unnecessary, as the volume into which the drug has to distribute should be the same in the uraemic as in the healthy subject. There are exceptions to this rule of thumb; for example, the volume of distribution of digoxin is contracted in uraemic patients due to altered tissue binding of the drug.
• Adjustment of the maintenance dose involves either reducing each dose given or lengthening the time between doses.
• Special caution is needed when the patient is hypoproteinaemic and the drug is usually extensively plasma protein bound, or in advanced renal disease when accumulated metabolic products may compete for protein binding sites. Careful observation is required in the early stages of dosing until response to the drug can be gauged.
General rules
1. Drugs that are completely or largely excreted by the kidney, or drugs that produce active, renally eliminated metabolites: give a normal or, if there is special cause for caution (see above), a slightly reduced initial dose, and lower the maintenance dose or lengthen the dose interval in proportion to the reduction in creatinine clearance.
2. Drugs that are completely or largely metabolised to inactive products: give normal doses. When the note of special caution (see above) applies, a modest reduction of initial dose and the maintenance dose rate are justified while drug effects are assessed.
3. Drugs that are partly eliminated by the kidney and partly metabolised: give a normal initial dose and modify the maintenance dose or dose interval in the light of what is known about the patient’s renal function and the drug, its dependence on renal elimination and its inherent toxicity.
Recall that the time to reach steady-state blood concentration (see p. 83) is dependent only on drug t½, and a drug reaches 97% of its ultimate steady-state concentration in 5 × t½. Thus, if t½ is prolonged by renal impairment, so also will be the time to reach steady state.
Nephrolithiasis
Management
• Thiazide diuretics reduce the excretion of calcium and oxalate in the urine, and reduce the rate of stone formation.
• Sodium cellulose phosphate (Calcisorb) binds calcium in the gut, reduces urinary calcium excretion and may benefit calcium stone-formers.
• Allopurinol is effective in those who have high excretion of uric acid in the urine.
• Potassium citrate, which alkalinises the urine, should be given to prevent formation of pure uric acid stones.
Pharmacological aspects of micturition
Functional abnormalities
The main abnormalities that require treatment are:
• Unstable bladder or detrusor instability, characterised by uninhibited, unstable contractions of the detrusor which may be of unknown aetiology or secondary to an upper motor neurone lesion or bladder neck obstruction.
• Decreased bladder activity or hypotonicity due to a lower motor neurone lesion or over-distension of the bladder, or both.
• Urethral sphincter dysfunction which is due to various causes including weakness of the muscles and ligaments around the bladder neck, descent of the urethrovesical junction and periurethral fibrosis; the result is stress incontinence.
Benign prostatic hyperplasia (BPH)
α-Adrenoceptor antagonists
These adverse events are avoided by using tamsulosin, which selectively blocks the α1A subclass7 of adrenoceptors and is therefore less likely to affect blood pressure, provided the single 400-microgram daily dose of tamsulosin is not exceeded.
Finasteride
Finasteride (t½ 6 h) is taken as a single 5-mg tablet each day. The improvement in urine flow appears over 6 months (as the prostate shrinks in size), and in 5–10% of patients may be at the cost of some loss of libido. The serum concentration of prostate-specific antigen is approximately halved. Although this may reflect a real reduction in risk of prostatic cancer, in patients receiving finasteride it is safer to regard values of the antigen in the upper half of the usual range as abnormal. Lower doses of finasteride have also been used successfully to halt the development of baldness.8Dutasteride is an alternative 5α-reductase inhibitor.
Erectile dysfunction
Erectile dysfunction (ED), the inability to achieve or maintain a penile erection sufficient to permit satisfactory sexual intercourse, is estimated to affect over 100 million men worldwide, with a prevalence of 39% in those aged 40 years.9
Sildenafil
Adverse effects are short lived, dose related, and comprise headache, flushing, nasal congestion and dyspepsia. High doses can inhibit PDE6, which is needed for phototransduction in the retina, and some patients report a transient blue coloration to their vision.10 Some patients experience non-arteritic anterior ischaemic optic neuropathy (NAION), consisting of blurred vision and/or visual field loss generally within 24 h of taking sildenafil. Priapism11 has been reported.
Alprostadil
is a stable form of prostaglandin E1, a powerful vasodilator (see p. 401), and is effective for psychogenic and neuropathic erectile dysfunction. Alprostadil increases arterial inflow and reduces venous outflow by contracting the corporal smooth muscle that occludes draining venules. It can be administered either as a urethral suppository (0.125–1 mg) or injected directly into the dorsolateral aspect of the proximal third of the penis (so-called intracavernosal injection). The duration and grade of erection are dose related. The patient package insert from the manufacturer provides some helpful drawings. The dose (5–20 micrograms) is titrated initially in the doctor’s surgery, aiming for an erection lasting for not more than 1 h. Painful erection is the commonest adverse effect.
Papaverine,
an alkaloid (originally extracted from opium but devoid of narcotic properties12), is also a non-specific phosphodiesterase inhibitor. It is effective (up to 80%) for psychogenic and neurogenic erectile dysfunction by intracavernosal self-injection shortly before intercourse (efficacy may be increased by also administering the α-adrenoceptor blocker phentolamine),13 although its use has waned with the availability of orally active selective PDE5 inhibitors such as sildenafil. Papaverine used in this way can cause priapism requiring aspiration of the corpora cavernosa and injection of an α-adrenoceptor agonist, e.g. metaraminol.
• The actions of drugs on the kidney are of an importance disproportionate to the low prevalence of kidney disorders.
• The kidney is the main site of loss, or potential loss, of all body substances. It is among the functions of drugs to help reduce losses of desirable substances and increase losses of undesired substances.
• The kidney is also at increased risk of toxicity from foreign substances because of the high concentrations these can achieve in the renal medulla.
• Diuretics are among the most commonly used drugs, perhaps because the evolutionary advantages of sodium retention have left an ageing population without salt-losing mechanisms of matching efficiency.
• Loop diuretics, acting on the ascending loop of Henle, are the most effective, and are used mainly to treat the oedema states. Potassium is lost as well as sodium.
• Thiazides, acting on the cortical diluting segment of the tubule, have lower natriuretic efficacy, but slightly greater antihypertensive efficacy than loop diuretics. Potassium loss is rarely a significant problem with thiazides, and thiazides reduce loss of calcium.
• Potassium retention with hyperkalaemia can occur with potassium-sparing diuretics, which block sodium transport in the last part of the distal tubule, either directly (e.g. amiloride) or by blocking aldosterone receptors (spironolactone).
• Drugs have little ability to alter the filtering function of the kidney when this is reduced by nephron loss.
• Prostatic enlargement is the main disease of the lower urinary tract; drugs can be used to postpone, or avoid, surgery. The symptoms of benign prostatic hyperplasia are partially relieved either by α1-adrenoceptor blockade or by inhibiting synthesis of dihydrotestosterone in the prostate.
• Drugs are effective for the relief of erectile dysfunction, notably sildenafil, a highly specific phosphodiesterase inhibitor.
Basnyat B., Murdoch D.R. High-altitude illness. Lancet. 2003;361:1967–1974.
Brown M.J. The choice of diuretic in hypertension: saving the baby from the bathwater. Heart. 2011;97:1547–1551.
Ernst M.E., Moser M. Use of diuretics in patients with hypertension. N. Engl. J. Med.. 2009;361:2153–2164.
Hood S.J., Taylor K.P., Ashby M.J., et al. The Spironolactone, Amiloride, Losartan and Thiazide (SALT) double-blind crossover trial in patients with low-renin hypertension and elevated aldosterone/renin ratio. Circulation. 2007;116:268–275.
Lameire N., Van Biesen W., Vanholder R., et al. Acute renal failure. Lancet. 2005;365:417–430.
McMahon C.N., Smith C.J., Shabsigh R., et al. Treating erectile dysfunction when PDE5 inhibitors fail. Br. Med. J.. 2006;332:589–592.
Moe O.W. Kidney stones: pathophysiology and medical management. Lancet. 2006;367:333–344.
Moynihan R. The marketing of a disease: female sexual dysfunction. Br. Med. J.. 2005;330:192–194.
Ouslander J.G. Management of overactive bladder. N. Engl. J. Med.. 2004;350(8):786–799.
Quaseem A., Snow V., Denberg T.D., et al. Hormonal testing and pharmacologic treatment of erectile dysfunction: a clinical practice guideline from the American College of Physicians. Available online at: http://www.annals.org/content/early/2009/10/19/0000605-200911030-00151.full, 2009. (accessed 18.11.11.)
Thorpe A., Neal D. Benign prostatic hyperplasia. Lancet. 2003;361:1359–1367.
Vidal L., Shavit M., Fraser A., et al. Systematic comparison of four sources of drug information regarding adjustment of dose for renal function. Br. Med. J. 2005;331:263–266.
1 Beavers and other freshwater-adapted mammals typically have nephrons with short loops, whereas desert-adapted mammals have long loops.
2 The most easily comprehended counter-current exchange mechanism (in this case for heat) is that in wading birds in cold climates whereby the veins carrying cold blood from the feet pass closely alongside the arteries carrying warm blood from the body and heat exchange takes place. The result is that the feet receive blood below body temperature (which does not matter) and the blood from the feet, which is often very cold, is warmed before it enters the body so that the internal temperature is maintained more easily. The principle is the same for maintaining renal medullary hypertonicity.
3 Pitt B, Zannad F, Remme W J et al 1999 The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. New England Journal of Medicine 341:709–717.
4 These typically contain equal proportions by weight of NaCl and KCl, so 1 g could contain 7 mmole of KCl and consuming the recommended 6 g/d of salt as the ‘low sodium’ form could provide > 40 mmol/d of KCl!
5 Pitt B, Zannad F, Remme W J et al 1999 The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. New England Journal of Medicine 341:709–717.
6 The creatinine clearance can be predicted from the serum creatinine concentration, sex, age and weight using formulae such as the Cockcroft–Gault or MDRD formulae. A number of free online calculators are available, e.g. http://www.medical-calculator.nl/calculator/GFR/. Free apps are also available for smartphones e.g. from www.qxmd.com.
7 There are three cloned subtypes for the α1-adrenoceptor: α1A, α1B and α1D. The α1A is the predominant subtype in the bladder base and prostatic urethra, whereas contraction of vascular smooth muscle is largely mediated by the α1B subtype. Hence, α1A selectivity would confer, at least in principle, ‘prostatic’ selectivity. But selectivity determined in vitro against cloned α1 receptors only poorly predicts in vivo ‘uroselectivity’, which also diminishes as dose is increased (compare the discussion of β-adrenoceptor selectivity with β-blocking drugs, Ch. 24, p. 404).
8 It has also been used as a treatment for hirsutism in women. Scalp follicles (of both sexes) contain type II 5α-reductase and the levels are increased in balding scalps (Tartagni M, Schonauer M, Cicinelli E et al 2004 Intermittent low-dose finasteride is as effective as daily administration for the treatment of hirsute women. Fertility and Sterility 82(3):752–755).
9 Feldman H A, Goldstein I, Hatzichristou D G et al 1994 Impotence and its medical and psychological correlates: results of Massachusetts male aging study. Journal of Urology 151:54–61.
10 The problem is reported much less frequently with the newer and more PDE5-specific taladafil and vardenafil. This very unusual drug effect is reminiscent of the disturbed colour perception caused by digoxin (in overdose), except here patients report yellowed vision (xanthopsia). This may not be an adverse effect in all cases, as it has been suggested that xanthopsia is the explanation for the predominance of yellow in Van Gogh’s art.
11 Persistent erection (> 4 h) of the penis, with pain and tenderness. In Greek mythology, Priapus was a god of fertility. He was also a patron of seafarers and shepherds.
12 Papaveretum, whose actions are principally those of its morphine content, has occasionally been supplied in error, to the surprise, distress and hazard of the subject.
13 Brindley G S 1986 Pilot experiments on the actions of drugs injected into the human corpus cavernosum penis. British Journal of Pharmacology 87:495 – an account of self-experimentation with 17 drugs.


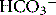 ) ions. This process is fundamental to the production of either acid or alkaline secretions, and high concentrations of carbonic anhydrase are present in the gastric mucosa, pancreas, eye and kidney. Because the number of H+ ions available to exchange with Na+ in the proximal tubule is reduced, sodium loss and diuresis occur. But
) ions. This process is fundamental to the production of either acid or alkaline secretions, and high concentrations of carbonic anhydrase are present in the gastric mucosa, pancreas, eye and kidney. Because the number of H+ ions available to exchange with Na+ in the proximal tubule is reduced, sodium loss and diuresis occur. But 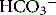 reabsorption from the tubule is also reduced, and its loss in the urine leads within days to metabolic acidosis, which attenuates the diuretic response to carbonic anhydrase inhibition. Consequently, inhibitors of carbonic anhydrase are obsolete as diuretics, but still have specific uses. Acetazolamide is the most widely used carbonic anhydrase inhibitor.
reabsorption from the tubule is also reduced, and its loss in the urine leads within days to metabolic acidosis, which attenuates the diuretic response to carbonic anhydrase inhibition. Consequently, inhibitors of carbonic anhydrase are obsolete as diuretics, but still have specific uses. Acetazolamide is the most widely used carbonic anhydrase inhibitor.