Chapter 45 Kawasaki Disease
Kawasaki disease, initially described by Dr. Tomisaku Kawasaki in 1967,1 is an acute systemic vasculitis of uncertain etiology that predominantly affects infants and young children. The disease has been described worldwide and occurs in all populations. The classic presentation of the illness is marked by at least 4 days of fever, oral mucositis, nonexudative conjunctivitis, erythematous nonvesicular rash, changes in the hands and feet including edema or erythema, and cervical lymphadenopathy2 (Box 45-1). Coronary artery dilation or aneurysms affect up to 25% of those who are not treated with intravenous gamma globulin (IVIG) early in the course of the disease. In patients who develop aneurysms, angina, myocardial infarction (MI), and death may ensue during the acute phase3 or months to years later.4
![]() Box 45-1 Principal Clinical Findings in Kawasaki Disease*
Box 45-1 Principal Clinical Findings in Kawasaki Disease*
Fever persisting at least 4 days or more without other source in association with at least 4 principal features:
Adapted from American Heart Association Scientific Statement: Diagnosis, treatment, and long-term management of Kawasaki disease. Circulation 110:2751, 2004.
Epidemiology
Despite its description in diverse areas of the world, by far the greatest number of cases have been reported in Japan. Indeed, in the most recent Japanese nationwide survey performed in calendar years 2007 and 2008, the incidence rate was over 200 per 100,000 children younger than 5 years of age.5 In Japan, 1.4% of cases occur in children with a previously affected sibling. The disease can recur (≈︀3.5% in Japan), but person-to-person transmission is unusual. Lack of a mandatory national reporting system in the United States hinders epidemiological analysis, but administrative data from hospital discharge abstracts suggested that more than 4000 U.S. hospitalizations were associated with Kawasaki disease in the year 2000.6 Among children younger than age 5, occurrence was greatest in Asians (33.3/100,000), somewhat less in African Americans (23.4/100,000), and lowest in Caucasians (12.7/100,000).6 Outbreaks are more likely in the late winter and early spring, suggesting an infectious etiology, but a steady background activity of cases is noted throughout the remainder of the year. Males outnumber females, generally in a ratio of 1.4:1. Although Kawasaki disease is most common in children younger than age 5, the illness is being more commonly recognized in older children and adolescents.7
Etiology and Pathogenesis
The search for an etiological agent has been wide-ranging over the course of the past 3 decades, but to date the cause of Kawasaki disease is unknown. Although Kawasaki disease is not spread by person-to-person transmission, features suggesting an infectious etiology include its peak incidence in young children; clinical features including fever, rash, and conjunctivitis; the increase in incidence in winter and early spring; and the past occurrence of nationwide epidemics in Japan. Prior exposure of index cases to freshly cleaned carpets has been reported to be a risk factor by some investigators,8 although other studies have not confirmed this association,9 and no specific organism or toxin has been identified in the rugs. Some researchers hold that Kawasaki disease is caused by a single agent,10 but others believe the marked immune response typical in Kawasaki disease can be triggered by a variety of different agents.11 Reports of selective expansion of Vβ2 and Vβ8 T-cell receptor families have implicated specific superantigens, including TSST-1-secreting strains of Staphylococcus aureus and streptococcal pyrogenic exotoxin B- and C-producing streptococci.12 The only animal model of Kawasaki disease, which uses Lactobacillus casei to induce coronary arteritis in mice, implicates a toxin-mediated etiology.13 By contrast, Rowley has found support for a typical antigen-driven response by demonstrating oligoclonal immunoglobulin (Ig)A plasma cells and IgA heavy chain genes, as well as macrophages and CD8 T lymphocytes in inflamed arterial tissue of individuals with fatal Kawasaki disease.14 With a synthetic IgA antibody, these investigators have demonstrated intracytoplasmic inclusion bodies with aggregates of nucleic acids and viral proteins in the proximal bronchial epithelium and coronary arteries in most postmortem specimens from Kawasaki disease.15 These ribonucleic acid (RNA)-containing cytoplasmic inclusion bodies were demonstrated in 85% of postmortem specimens from Kawasaki disease patients but few young age-matched children. The finding that 25% of older children but adult controls have such inclusion bodies postmortem is consistent with the hypothesis that Kawasaki disease could be caused by a ubiquitous RNA virus that persists and causes the clinical features of Kawasaki disease in susceptible children.16,17
Kawasaki disease is accompanied by significant derangements in the immunoregulatory system that lead to coronary inflammation and coronary artery abnormalities (dilation, aneurysm formation, and giant aneurysms) in some patients. Profound immune activation is evidenced by release of proinflammatory cytokines and growth factors, endothelial cell (EC) activation, and infiltration of the coronary arterial wall first by neutrophils, and then by CD68+ monocyte/macrophages, CD8+, CD3+, and CD20+ lymphocytes, and IgA plasma cells.18–26
Release of matrix metalloproteinases (MMPs) may further disrupt arterial wall integrity, leading to aneurysms of the coronary arteries and occasionally of other extraparenchymal medium-sized muscular arteries.27 Kawasaki disease patients with coronary artery lesions have higher levels of MMPs and higher ratios of MMPs to tissue inhibitors of metalloproteinases (TIMPs) than those without coronary abnormalities,28 suggesting these circulating proteins may play an active role in coronary arterial remodeling. Data to suggest an important role for MMPs in the pathogenesis of aneurysms also comes from recent research on genetic risk factors, showing that MMP-3 rs3025058 (−/T) and haplotypes containing MMP-3 rs3025058 (−/T) and MMP-12 rs2276109 (A/G) were associated with a higher risk of aneurysm formation.29
Genetic factors have long been recognized to play an important role in susceptibility to Kawasaki disease. Children of Japanese ancestry have a relative risk 10 to 15 times higher than that of Caucasian children, whether they live in Japan or the United States. Furthermore, siblings have a relative risk 6 to 10 times greater than that of children without a family history. The parents of Japanese children with Kawasaki disease are twice as likely to have had the disease themselves in childhood than expected in the general population.30 In addition to MMP haplotypes, increased susceptibility to Kawasaki disease has been related to genetic variations in the transforming growth factor (TGF)-β pathway,31 CC chemokine receptor 5 (CCR5) and/or its ligand CCL3L1,32 and ITPKC (a negative regulator of T cell activation)33 among others. In a genomewide association study, investigators recently identified a single functional network containing LNX1, CAMK2D, ZFHX3, CSMD1, and TCP1, believed to be relevant to inflammation and apoptosis.34 Functional genomics may eventually allow development of new diagnostic tests and therapies for Kawasaki disease.
Pathology
Because mortality in this disease is uncommon (ranging from 0%-0.17% in the United States), published studies on the histopathology of early Kawasaki disease are limited.35,36 The original pathological description of Kawasaki disease by Fujiwara and Hamashima was based on autopsy findings of children dying in the acute and subacute phases of Kawasaki disease before available treatment with IVIG.35 In this early study, they established four categories of illness on the basis of time from onset of the disease: stage 1 (0-9 days), stage 2 (12-25 days), stage 3 (28-31 days), and stage 4 (40 days-4 years). Stage 1 was characterized by vasculitis of small vessels and microvessels, perivasculitis and endarteritis of the coronary vessels, and pancarditis. Pancarditis was seen also in stage 2, with coronary artery vasculitis, sometimes with aneurysm formation and coronary thrombosis. By stage 3, the acute inflammation had subsided, but myointimal proliferation of the coronary arteries was evident. In stage 4, coronary artery stenosis was noted for the first time. In stage 1, death resulted from arrhythmia and myocarditis, with evolution to ischemia and infarction with greater time from onset of disease to postmortem examination. Indeed, patients who die late after Kawasaki disease often have coronary artery stenoses resulting from neointimal proliferation and fibrosis.35,36 Within aneurysms, the internal elastic lamina is disrupted, and medial smooth muscle is replaced by fibroblasts and extracellular matrix (ECM).37 Finally, growth factors are expressed in the areas subjected to the greatest shear stress, particularly at the proximal and distal ends of an aneurysm.38 Peripheral artery aneurysms (e.g., occurring in axillary or iliac arteries) are found only among patients with giant coronary artery aneurysms.
Clinical Presentation
Because no laboratory test or pathognomonic feature is available for Kawasaki disease, the diagnosis must be made on clinical grounds. First described by Kawasaki on the basis of his observations in Japanese children,39 the classic criteria have continued to serve as the standard adopted by the American Heart Association (AHA; see Box 45-1) for arriving at the diagnosis.2 They include fever for 4 days or more and at least 4 of the 5 following findings: (1) a nonexudative bilateral conjunctivitis, (2) oral changes with erythematous or dry cracked lips, strawberry tongue, or pharyngitis, (3) a nonvesicular rash, often involving the trunk, perineum, and extremities, (4) erythema of the palmar and plantar surfaces, edema of the hands or feet, or periungual desquamation 2 weeks after illness onset, and (5) anterior cervical lymphadenopathy of 1.5 cm or greater, usually unilateral. Alternatively, the diagnosis can be made with fewer than 4 of 5 criteria in the presence of coronary artery abnormalities. Not all criteria have to be present simultaneously to make the diagnosis; indeed, it is common for some findings to resolve as others appear, making serial evaluation of the child essential.
The epidemiological case definition is not fulfilled in almost a third of children who develop coronary artery aneurysms.40 To capture incomplete cases, the 2004 AHA recommendations include an algorithm for evaluation and treatment of suspected Kawasaki disease in children with at least 5 days of fever and only two or three clinical criteria.2 Furthermore, infants younger than 6 months of age present a particular challenge because they often have incomplete criteria, yet are at greater risk for development of coronary artery abnormalities. The diagnosis should be considered and echocardiography performed in young infants who have fever for at least 7 days without documented source and whose laboratory tests indicate substantial systemic inflammation.2
Other supportive signs are present in many children with Kawasaki disease (Box 45-2). The rash, when perineal in location, often desquamates by the end of the first week of illness. Anterior uveitis can be identified by slit-lamp examination in 83% of patients early in the course.41 Arthralgia and arthritis of large and small joints may be severe enough that children refuse to walk or perform tasks with their hands, but the arthritis is virtually never chronic. Abdominal signs including vomiting, diarrhea, or hydrops of the gallbladder are common.
![]() Box 45-2 Other Significant Clinical and Laboratory Findings in Kawasaki Disease
Box 45-2 Other Significant Clinical and Laboratory Findings in Kawasaki Disease
Cardiovascular
On auscultation, gallop rhythm or distant heart sounds; ECG changes (arrhythmias, abnormal Q waves, prolonged PR or QT intervals or both; occasionally low-voltage or ST-T wave changes); chest x-ray abnormalities (cardiomegaly); echocardiographic changes (pericardial effusion, coronary aneurysms, or decreased contractility); mitral or aortic valvular insufficiency or both; and (rarely) aneurysms of peripheral arteries (e.g., axillary), angina pectoris, or MI
Adapted from American Heart Association Scientific Statement: Diagnosis, treatment, and long-term management of Kawasaki disease. Circulation 110:2751, 2004.
Laboratory values in the acute phase are consistent with systemic inflammation. Acute-phase reactants, including erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), are markedly increased. White blood cell (WBC) count is elevated with a leftward shift, and a normochromic, normocytic anemia is noted within the first week of illness. Thrombocytosis is usually present by the second week of the disease, often peaking at counts greater than 1,000,000 mm3 in association with hypercoagulability. These parameters often persist over the first month of the illness and gradually decline. Hepatocellular inflammation is accompanied by increases in γ-glutamyl transferase. Sterile pyuria and pleocytosis of cerebrospinal fluid,42 both with mononuclear cells, are found frequently. Laboratory measures of systemic inflammation usually return to normal by 6 to 8 weeks after illness onset.
Cardiac Manifestations
Coronary Artery Abnormalities
Kawasaki disease causes coronary artery abnormalities in 15% to 25% of patients who are not treated in the acute phase of the disease with high-dose IVIG. Lesions may be observed by echocardiography as early as 1 week from the onset of fever, with further progression of aneurysms ensuing in the next 3 to 4 weeks. Given the difficulty in reaching diagnostic confirmation of the illness using the classic criteria, identification of those at higher risk for coronary disease and for whom early treatment could reduce extent of involvement has led investigators to focus on predictive factors for coronary artery abnormalities. Many studies have explored predictors of coronary artery aneurysms. Late diagnosis and treatment with IVIG is the most important modifiable risk factor.43 Infants younger than age 6 months are at the highest risk for developing aneurysms, even when they are treated within the first 10 days of illness. The youngest infants frequently present with incomplete or atypical features, further increasing their risk of aneurysms by delaying diagnosis and IVIG treatment. Older children are also at higher risk for coronary aneurysms, in part because care providers do not consider Kawasaki disease as high in the differential diagnosis of the older child with fever.44 Many studies have highlighted the association of coronary aneurysms with persistent and recrudescent fever, as well as with IVIG resistance.45,46 Greater derangement of laboratory measures of the acute-phase response, reflecting more severe vasculitis, is also predictive and include lower hematocrit or hemoglobin (Hb), lower serum albumin, lower serum sodium, higher alanine aminotransferase, higher CRP and ESR, lower baseline serum IgG, and elevations in interleukin (IL)-6, IL-8, and other biomarkers.47–49 In addition, genetic polymorphisms (e.g., MMP haplotypes,29 polymorphisms of vascular endothelial growth factor [VEGF] and its receptors50) affect host susceptibility to aneurysms.
Myocarditis
In acute Kawasaki disease, myocarditis is a frequent finding at autopsy and by biopsy.51,52 Gallium-67 citrate scans53 and technetium-99 m-labeled WBC scans54 have also identified inflammatory myocardial changes in 50% to 70% of patients. Congestive heart failure (CHF) in the acute phase of the illness is generally the result of myocarditis and improves rapidly with IVIG treatment, in many cases within 24 hours from initiation of treatment.55 Later implications of these early changes is speculative, but several investigators have evaluated pathology and clinical function late after Kawasaki disease. Myocardial biopsies in patients with long-term follow-up have shown fibrosis, abnormal branching, and hypertrophy of myocytes unrelated to duration from illness onset.56 Although late noninvasive studies of myocardial function are encouraging, data on long-term myocardial function should accrue as the earliest Japanese cohorts reach middle age.57
Valve Regurgitation
Mitral and aortic regurgitation have been associated with Kawasaki disease in both early and chronic phases of the disease. In the acute stage, one in four children has mitral regurgitation of mild to moderate severity detected by two-dimensional (2D) echocardiography, which resolves in most children by the convalescent phase.58 The frequency of aortic regurgitation has been reported to be as high as 5% in Japanese children59 but only 1% in a recent North American population with Kawasaki disease,58 and there are rare reports of late-onset aortic regurgitation necessitating valve replacement.60,61 The cause for aortic insufficiency is not known; however, it has been reported that the aortic root enlarges from baseline and remains dilated during the first year after illness onset,58,62 raising the possibility that coaptation of the leaflets is disturbed.
Other Cardiac Findings
Rarely, patients in the acute or subacute phase of Kawasaki disease can develop tamponade due to a pericardial effusion, although effusions greater than 1 mm occur in fewer than 5% of patients.58,63 Rupture of a giant aneurysm into the pericardial space is an even rarer cause of pericardial tamponade.64–66
Cardiac Testing
Using 2D echocardiography, visualization of the proximal coronary arteries in young children is almost always possible, and measurements correlate closely with those identified by angiography. In addition to measurements of the proximal left main, anterior descending, circumflex, proximal, and middle right and posterior descending branches, assessment of ventricular function, pericardial fluid, and mitral and aortic regurgitation should be obtained. Coronary artery dilation may be present as early as the end of the first week of illness. In addition, findings on echocardiography are used to determine the need for IVIG treatment of children with suspected Kawasaki disease on the basis of fever and incomplete criteria.2 As a result, echocardiography should be undertaken when the diagnosis is entertained, particularly as an incomplete clinical picture, with coronary artery abnormalities sufficient to make the diagnosis and initiate therapy.2,67 Serial studies are obtained at 10 to 14 days from onset of illness to assess for the presence of aneurysm development and at the end of the subacute phase, 6 to 8 weeks from onset. Echocardiography should be performed more frequently in the child with coronary dilation at baseline, persistent fever, or other factors raising the likelihood of development of coronary aneurysms. Finally, children with giant aneurysms are at highest risk for development of coronary thrombosis in the first months after illness onset. For this reason, echocardiography may be performed once or twice a week until 8 weeks after illness onset or until coronary arteries stop enlarging and systemic inflammation has subsided.
The definition of normal coronary artery dimensions has been the subject of some controversy. Criteria established by the Japanese Ministry of Health in 1984 defined as abnormal those coronary arteries with lumen diameter greater than 3 mm in children younger than 5 years, or greater than 4 mm in those older than age 5; lumen diameter 1.5 times the size of an adjacent segment; or irregular lumen.68 Others have shown that coronary artery size in normal children correlates linearly with increasing body size. In patients with Kawasaki disease whose coronary arteries are classified as normal by Japanese Ministry of Health criteria, the dimensions are larger than expected when adjusted for body surface area (BSA) in all phases of the disease.69 Indeed, the median coronary z score at presentation in a multicenter trial was 1.43, significantly larger than the expected population norm in afebrile children of 0.49 Of note, normative data for febrile children are not available.
Echocardiography in the acute phase of Kawasaki disease is also useful for assessing left ventricular (LV) dysfunction.55 Among patients without coronary aneurysms, systolic function rapidly improves following IVIG administration.58 Diastolic function, specifically relaxation, is impaired in acute Kawasaki disease; patients with coronary aneurysms may continue to have long-term diastolic dysfunction, even when systolic function is preserved.70
Although echocardiography has high sensitivity and specificity for detection of dilation in the proximal coronary arteries, it is less useful for detection of coronary stenoses and aneurysms in the distal coronary vasculature. The quality of coronary imaging by echocardiography also diminishes as children grow larger, limiting its utility in long-term follow-up of the patient with Kawasaki disease. Therefore, imaging of the coronary arteries by ultrafast computed tomographic angiography (CTA) and magnetic resonance angiography (MRA) are used to obtain high-resolution images.71–75 Because the long-term effects of ionizing radiation are of special concern in children, CTA is used sparingly. Although the quality of coronary artery images may be superior with CTA, MRA does not require ionizing radiation and, in the child who is too young to exercise on a treadmill or bicycle, can be used with dobutamine or adenosine stress to delineate reversible ischemia.
Stress testing for inducible ischemia is an important component of periodic testing for patients with Kawasaki disease, but literature is limited to case series.76–79 As in so many other domains of care of the child with this disease, choice of stress test type is based upon literature related to adults with atherosclerotic coronary disease. As already noted, additional considerations include risks of repeated exposure to ionizing radiation and inability of the youngest children to exercise on a treadmill or bicycle, in whom pharmacological stress is required. To avoid false-positive test results, stress testing should only be performed in children with a history of coronary artery aneurysms.
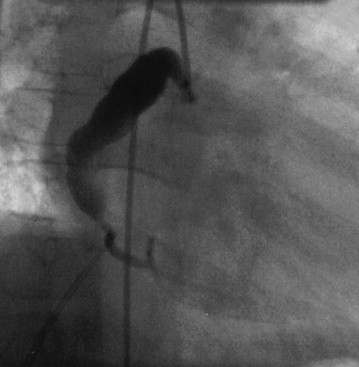
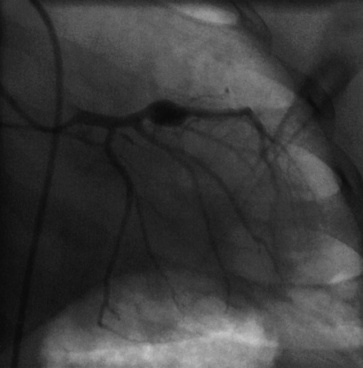
Coronary angiography has been used extensively for diagnostic assessment of coronary abnormalities. With rapid technical improvement in noninvasive imaging modalities and concerns about the use of ionizing radiation in children, invasive studies are generally reserved for children with aneurysms in whom noninvasive testing is insufficient to guide treatment or in whom revascularization is needed. Aneurysms are described as localized or extensive, the former being further subclassified as fusiform or saccular (Fig. 45-1). Extensive aneurysms, those that involve more than one segment, are ectatic (dilated uniformly; Fig. 45-2) or segmented (multiple dilated segments joined by normal or stenotic segments). Aneurysms may also involve other medium- to large-sized extraparenchymal arteries, particularly the subclavian, axillary, femoral, iliac, renal, and mesenteric arteries. Occasionally, aneurysms of the aorta may occur.
Clinical Course
Persistent Coronary Aneurysms
After expansion in diameter over the first 4 to 6 weeks after illness onset, coronary aneurysms in Kawasaki disease tend to regress over time. In a study of 594 patients, regression occurred over a 13.6-year period in 54% of those with aneurysms, with 90% regressing within 2 years from the onset of illness.80 Substantial regression in aneurysm diameter after 2 years is unlikely. The potential for aneurysm regression to normal internal lumen diameter is determined by the initial size of the aneurysm, with smaller abnormalities more likely to improve.81 Giant aneurysms (i.e., diameter ≥8 mm) are least likely to regress.82 In regressed aneurysms, intravascular ultrasound examination reveals myointimal thickening,83,84 worst in patients whose coronary diameters were initially the largest.83 Furthermore, coronary and peripheral vascular reactivity may be impaired in patients with either persistent or regressed aneurysms.84
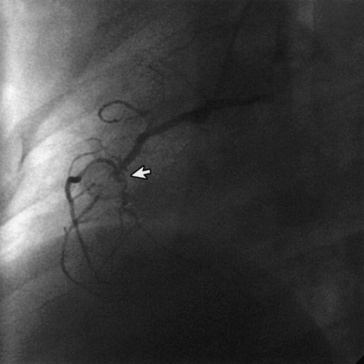
Among patients with persistent coronary aneurysms, prevalence of arterial stenosis increases linearly with time, owing to myointimal proliferation at the entrance or exit of the aneurysm. Giant aneurysms (diameter >8 mm) are at highest risk; stenoses commonly occur at the distal end of these lesions (Fig. 45-3) and, together with sluggish blood flow in the grossly dilated arterial segment, predispose to thrombosis and infarction. Relative to smaller aneurysms, giant aneurysms are more frequently associated with late sudden death from infarction. In a recent review, coronary artery stenoses had developed at 15-year follow-up in half of aneurysms with maximum diameter of at least 6 mm.85 In the largest reported series of patients with giant aneurysms (median observation 19 years), survival rates at 10, 20, and 30 years after disease onset were 95%, 88%, and 88%, and cumulative rates of coronary intervention at 5, 15, and 25 years were 28%, 43%, and 59%, respectively.82
Myocardial infarction due to Kawasaki disease, although infrequent, is a feared complication. In the largest series of cases from Japan, many patients were asymptomatic before their event.86 Most (73%) had infarction in the initial year following onset of illness, and approximately 50% of infarctions occurred within the first 3 months after illness onset. Symptoms were more common in those older than 4 years (83%) compared with younger children (17%) and included crying, chest pain, shock, abdominal pain, vomiting, dyspnea, and arrhythmia. Often, infarction occurred during rest or sleep rather than during exercise or play. First MI was associated with a mortality rate of 22%, most often the result of obstruction of the left main coronary artery or of both the right and left anterior descending (LAD) coronary arteries. Survivors tended to have involvement of a single artery other than the left main. Mortality rose to 62.5% after a second infarct and to nearly 100% in those with a third infarct. At later follow-up, a sizable proportion (41%) reported no symptoms. Not surprisingly, ejection fraction is a significant predictor of long-term survival after MI, with poor 30-year survival when ejection fraction is 45% or less.87
Regressed Coronary Aneurysms
Regression of aneurysms occurs as a result of myointimal proliferation, identified at postmortem examination with use of transluminal ultrasound.88 Histological examination of regressed aneurysms shows pathological findings similar to those seen in atherosclerosis,89 raising concern that individuals with Kawasaki disease might be predisposed to early onset of coronary disease. In a study of patients with Kawasaki disease receiving isosorbide dinitrate at catheterization, those segments with regressed aneurysms, as well as regions with persistent aneurysms, had diminished reactivity relative to coronary arteries that had never been dilated or to coronary arteries of control patients.84 Furthermore, the decreased reactivity in abnormal areas progressed as duration from illness increased.
No Detectable Lesions
In the absence of a history of coronary aneurysms, Kawasaki disease has not been associated with an increase in standardized mortality ratio in adulthood. Concerns that have been raised about the health of the coronary vasculature thus are based solely on studies searching for preclinical disease. Autopsy studies of incidental deaths are limited. In one series, five children who died of incidental causes following Kawasaki disease underwent postmortem examination.56 Microscopic examination of the coronary arteries revealed intimal thickening and fibrosis indistinguishable from that of atherosclerosis, but correlation with clinical status was not possible. On positron emission tomography (PET), Kawasaki patients who never had aneurysms, compared to controls, had lower myocardial reserve and higher total coronary resistance without regional perfusion abnormalities, suggesting an abnormal coronary microcirculation.90 Testing coronary endothelial function by infusing acetylcholine in the epicardial coronary arteries of Kawasaki patients and comparison patients has yielded conflicting results,91,92 as have studies on peripheral arterial stiffness and brachial reactivity.93–96 Finally, some investigators have speculated that long-term sequelae may result from acute myocarditis that occurs during the acute illness.57 Theoretical concerns about coronary and myocardial sequelae in Kawasaki disease patients without detectable coronary dilation in any phase of illness should be tempered by the adverse consequences of creating “cardiac non-disease.”97
Treatment
Antiinflammatory Therapy During Acute Kawasaki Disease
Aspirin
Aspirin has been a mainstay of therapy for Kawasaki disease, for both its antiinflammatory and antithrombotic effects. Most clinicians use high-dose aspirin, 80 to 100 mg/kg/day divided into four daily doses until defervescence, and then reduce the dosage to 3 to 5 mg/kg/day, administered once daily until the end of the subacute phase (6-8 weeks; Box 45-3) to reduce the likelihood for Reye’s syndrome or gastrointestinal bleeding. Meta-analysis has shown that low-dose or high-dose aspirin regimens in conjunction with IVIG have a similar incidence of coronary abnormalities at 30 and 60 days from onset of illness.98 In patients with aneurysms, low-dose aspirin therapy is continued, sometimes in combination with anticoagulants or other antiplatelet agents.
Intravenous gamma globulin
Intravenous Ig was first administered to children with Kawasaki disease in 198499 and shown to have beneficial effects in reducing the likelihood of coronary aneurysms and the inflammatory response in the acute phase. Many subsequent studies have confirmed the efficacy of high-dose IVIG; the lowest incidence of coronary artery lesions occurs in those patients treated with IVIG 2 g/kg, administered as a single dose over 8 to 12 hours98,100 (see Box 45-3). The mechanism of action of IVIG remains unknown. Children who are acutely ill with Kawasaki disease should be administered IVIG 2 g/kg as a single dose no later than the 10th day of illness and ideally within the first 7 days.101 Patients with fever beyond 10 days of illness should also receive IVIG,102 as should those with aneurysm formation and evidence for persistent inflammation. Approximately 15% of children have persistent or recrudescent fever after IVIG treatment (so-called IVIG resistance).103 Although not tested in a randomized trial, retreatment with IVIG 2 g/kg is generally administered if recurrent or recrudescent fever is present more than 36 hours after completion of an initial course of IVIG.2 If a second dose of IVIG is ineffective in diminishing fever, particularly in the presence of worsening coronary dilation, alternative antiinflammatory therapies are often administered (see later) discussion.
Corticosteroids
Corticosteroids are widely used in the treatment of other vasculitides, but data on the efficacy of primary and secondary (rescue) steroid therapy in Kawasaki disease are mixed. Although uncontrolled studies and one open multicenter trial previously suggested benefit for primary steroid therapy,104–109 a randomized multicenter placebo-blind study showed that primary therapy with pulsed-dose intravenous (IV) methylprednisolone did not improve coronary outcomes when added to conventional treatment with IVIG and aspirin.103 However, efficacy of other corticosteroid dosage regimens for primary treatment are currently being studied.
Corticosteroids are often used as rescue therapy in the patient with IVIG-resistant Kawasaki disease, with most reports comprising case series.110–112 Similar to findings in primary steroid treatment, rescue treatment with pulsed steroids was reported to shorten fever duration and length of hospital stay, but did not improve coronary outcomes in a randomized trial of pulsed steroid therapy.112 Based on the differing dosage regimens that have been used in studying the effect of steroids on coronary outcomes, and the limited power for randomized trials of rescue therapy, data are insufficient to conclude whether steroids have a place in the armamentarium of IVIG-resistant Kawasaki disease. Most experts consider use of steroids, either as a pulsed dose or chronic oral regimen in children who have persistent or recrudescent fever despite at least two courses of IVIG 2 g/kg.
Additional Therapies
Limited data are available concerning efficacy of other therapies that can be used in the IVIG-resistant patient. Plasmapheresis has been reported to lower the incidence of coronary disease,113 but it is technically complex, requiring placement of large-bore catheters and the commitment of the local blood bank to assist in the exchange. Abciximab, a platelet glycoprotein (GP) IIb/IIIa receptor inhibitor that prevents platelet aggregation, has been used as an antithrombotic agent in acute Kawasaki disease. A retrospective study reported that patients treated with abciximab, compared to those who were not, had smaller aneurysm size and a greater percentage decrease in aneurysm diameter.114 Infliximab, a chimeric monoclonal antibody to tumor necrosis factor (TNF)-α, has been used as rescue therapy in IVIG-resistant patients.115,116 A two-center retrospective study suggested that infliximab as rescue therapy shortens fever duration but does not reduce prevalence or size of aneurysms.117 Finally, in the most refractory cases of Kawasaki disease where coronary aneurysms are progressively enlarging despite all other medical therapies, treatment with cytotoxic agents has been reported.111,118
Antithrombotic therapy
The combination of active vasculitis with endothelial damage, thrombocytosis, and hypercoagulable state create the clinical setting for thrombosis of coronary arteries. Peak occurrence for such events is 15 to 45 days from onset of illness. The current recommendation for therapy is aspirin 3 to 5 mg/kg/day for 6 to 8 weeks. If the echocardiogram is normal at that time, aspirin is discontinued, but in the presence of dilation or aneurysms, aspirin is continued until regression to normal vessel lumen size. For patients with moderate to large aneurysms, some clinicians use combination antiplatelet therapy consisting of aspirin together with an inhibitor of adenosine diphosphate (ADP)-induced platelet aggregation (e.g., thienopyridine).2
Finally, patients with giant aneurysms, defined as those with an internal lumen diameter of 8 mm or greater or a z score of 10 or more,119 have the greatest risk for coronary thrombosis and are conventionally treated with a combination of anticoagulation and aspirin. One series has shown that patients with giant aneurysms have a lower risk of MI if treated with aspirin and warfarin than with aspirin alone.120 Low-molecular-weight heparin (LMWH) may be used in those for whom warfarin management is difficult. Newer oral direct thrombin inhibitors and factor Xa inhibitors have not yet been approved for use in the pediatric population but hold promise for future treatment of Kawasaki disease patients requiring anticoagulation. Women of childbearing age who have giant coronary aneurysms and are contemplating pregnancy should be counseled about the effects of warfarin and, once pregnant, should be treated in accordance with a protocol similar to that used for the pregnant woman with a mechanical prosthetic valve.121
Coronary Revascularization
The criteria for coronary revascularization in Kawasaki disease are based upon consensus of experts, experience in adult patients with atherosclerotic CAD, and small retrospective reviews of experience in Kawasaki disease.122–125
Coronary Artery Bypass Surgery
The decision to undertake coronary artery bypass graft (CABG) surgery is generally based on a combination of factors, including evidence of reversible ischemia on stress-imaging tests, viable myocardium in the region of distribution of the affected vessel, and absence of evidence for severe disease distal to the site of the planned graft.126 Initial reports of bypass grafting in Kawasaki disease involved use of saphenous veins, but early failures, particularly among younger children, led to introduction of internal mammary artery grafts. In the current era, systemic arterial grafts (e.g., internal mammary, radial) are preferred because they increase in length and diameter with somatic growth.122–124 Even young children have undergone successful surgical revascularization, but freedom from reintervention is longer when bypass is performed at an older age. Kitamura et al. recently reported his single-center experience; at 25 years, freedom from reintervention was only 60%, but patient survival was 95%.124
Percutaneous Coronary Intervention
Experience with percutaneous coronary intervention (PCI) in children affected by Kawasaki disease is even more limited than CABG, but techniques for interventional catheterization procedures used in these patients are similar to those in adults. Based on recommendations of the Research Committee of the Japanese Ministry of Health, Labor, and Welfare, patients with the following criteria should be considered for PCI: ischemic symptoms, presence of reversible ischemia on stress testing, or at least 75% stenosis of the LAD coronary artery.127 These recommendations suggest that CABG is superior to PCI in the setting of severe LV dysfunction or presence of multiple ostial or long-segment coronary artery stenoses. Although early success rates for percutaneous transluminal coronary angiography, rotational ablation, and stent placement are all high, restenosis is common.128 Furthermore, neoaneurysm formation is a risk of percutaneous dilation related to use of high inflation pressures necessary to dilate the heavily calcified arteries found in Kawasaki disease patients.128 For this reason, rotational ablation and stent placement are generally preferred to percutaneous transluminal coronary angioplasty, after a few years have passed since disease onset. Because pediatric cardiologists have limited experience with interventional coronary artery techniques, percutaneous intervention should be performed by adult invasive cardiologists, with support from pediatric teams when children are small.
Randomized clinical trials of CABG versus PCI have not been conducted; sample size and power would be limited by the infrequency with which revascularization is required, even in Japanese children. Using survey data comparing outcomes in patients whose first intervention was either PCI or CABG, rates of mortality and MI were similar. However, repeat revascularization therapies were administered more often to children whose first intervention was percutaneous.129
Preventive Cardiology
Patients who have regressed or persistent aneurysms are likely to be at increased risk for premature atherosclerotic cardiovascular disease. Histopathological findings have noted atherosclerosis on autopsies performed in children who die years after illness onset.130 Those with a history of aneurysms have increased myointimal thickness in the coronary arteries on intravascular ultrasound,83 and evidence of premature atherosclerosis is seen on carotid artery ultrasound 6 to 20 years after illness onset.131 Late after Kawasaki disease, peripheral arteries with coronary aneurysms have been noted to be stiffer with diminished vascular reactivity, compared to normal controls.84 High-sensitivity CRP levels are greater in Kawasaki disease patients with a history of aneurysms than among those who never developed aneurysms or in normal children.132,133 Data about future risk in children who never had coronary aneurysms continue to be controversial.
All patients with a history of Kawasaki disease should be counseled about risk factors for atherosclerotic cardiovascular disease, including the importance of a heart-healthy diet, exercise, avoidance of smoking, and for parents, the importance of maintaining a smoke-free home. In the AHA recommendations, the threshold for pharmacological treatment of hypertension and hyperlipidemia is lower for children with current and regressed coronary aneurysms.134 Exercise recommendations for participation in competitive sports depend upon severity of coronary disease, but all patients with a history of Kawasaki disease should avoid a sedentary life style.135
1 Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children (Japanese). Jpn J Allergy. 1967;116(3):178–222.
2 Newburger J.W., Takahashi M., Gerber M.A., et al. Diagnosis, treatment, and long-term management of Kawasaki disease. A statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004;110(17):2747–2771.
3 Hayasaka S., Nakamura Y., Yashiro M., et al. Analyses of fatal cases of Kawasaki disease in Japan using vital statistical data over 27 years. J Epidemiol. 2003;13(5):246–250.
4 Tsuda E., Matsuo M., Naito H., et al. Clinical features in adults with coronary arterial lesions caused by presumed Kawasaki disease. Cardiol Young. 2007;17(1):84–89.
5 Nakamura Y., Yashiro M., Uehara R., et al. Epidemiologic features of Kawasaki disease in Japan: results of the 2007-2008 nationwide survey. J Epidemiol. 2010;20(4):302–307.
6 Holman R.C., Curns A.T., Belay E.D., et al. Kawasaki syndrome hospitalizations in the United States, 1997 and 2000. Pediatrics. 2003;112(3 Pt 1):495–501.
7 Stockheim J.A., Innocentini N., Shulman S.T. Kawasaki disease in older children and adolescents. J Pediatr. 2000;137(2):250–252.
8 Rauch A.M., Glode M.P., Wiggins J.W.Jr, et al. Outbreak of Kawasaki syndrome in Denver, Colorado: association with rug and carpet cleaning. Pediatrics. 1991;87(5):663–669.
9 Burns J.C., Mason W.H., Glode M.P., et al. Clinical and epidemiologic characteristics of patients referred for evaluation of possible Kawasaki disease. J Pediatr. 1991;118(5):680–686.
10 Rowley A.H., Shulman S.T. Recent advances in the understanding and management of Kawasaki disease. Curr Infect Dis Rep. 2010;12(2):96–102.
11 Burns J.C., Glode M.P. Kawasaki syndrome. Lancet. 2004;364(9433):533–544.
12 Leung D.Y.M. Superantigens related to Kawasaki syndrome. Springer Semin Immunopathol. 1996;17(4):385–396.
13 Yeung R.S. Lessons learned from an animal model of Kawasaki disease. Clin Exp Rheumatol. 2007;25(1 Suppl 44):S69–S71.
14 Rowley A.H. Kawasaki disease: novel insights into etiology and genetic susceptibility. Annu Rev Med. 2011;62:69–77.
15 Rowley A.H., Baker S.C., Shulman S.T., et al. Cytoplasmic inclusion bodies are detected by synthetic antibody in ciliated bronchial epithelium during acute Kawasaki disease. J Infect Dis. 2005;192(10):1757–1766.
16 Rowley A.H., Baker S.C., Shulman S.T., et al. RNA-containing cytoplasmic inclusion bodies in ciliated bronchial epithelium months to years after acute Kawasaki disease. PLoS ONE. 2008;3(2):e1582.
17 Rowley A.H., Baker S.C., Shulman S.T., et al. Ultrastructural, immunofluorescence, and RNA evidence support the hypothesis of a “new” virus associated with Kawasaki disease. J Infect Dis. 2011;203(7):1021–1030.
18 Rowley A.H., Shulman S.T., Spike B.T., et al. Oligoclonal IgA response in the vascular wall in acute Kawasaki disease. J Immunol. 2001;166(2):1334–1343.
19 Brown T.J., Crawford S.E., Cornwall M.L., et al. CD8 T lymphocytes and macrophages infiltrate coronary artery aneurysms in acute Kawasaki disease. J Infect Dis. 2001;184(7):940–943.
20 Yasukawa K., Terai M., Shulman S.T., et al. Systemic production of vascular endothelial growth factor and FMS-like tyrosine kinase-1 receptor in acute Kawasaki disease. Circulation. 2002;105(6):766–769.
21 Asano T., Ogawa S. Expression of monocyte chemoattractant protein-1 in Kawasaki disease: the anti-inflammatory effect of gamma globulin therapy. Scand J Immunol. 2000;51(1):98–103.
22 Freeman A.F., Crawford S.E., Finn L.S., et al. Inflammatory pulmonary nodules in Kawasaki disease. Pediatr Pulmonol. 2003;36(2):102–106.
23 Ohno T., Yuge T., Kariyazono H., et al. Serum hepatocyte growth factor combined with vascular endothelial growth factor as a predictive indicator for the occurrence of coronary artery lesions in Kawasaki disease. Eur J Pediatr. 2002;161(2):105–111.
24 Nomura Y., Masuda K., Maeno N., et al. Serum levels of interleukin-18 are elevated in the subacute phase of Kawasaki syndrome. Int Arch Allergy Immunol. 2004;135(2):161–165.
25 Terai M., Honda T., Yasukawa K., et al. Prognostic impact of vascular leakage in acute Kawasaki disease. Circulation. 2003;108(3):325–330.
26 Takahashi K., Oharaseki T., Naoe S., et al. Neutrophilic involvement in the damage to coronary arteries in acute stage of Kawasaki disease. Pediatr Int. 2005;47(3):305–310.
27 Takeshita S., Tokutomi T., Kawase H., et al. Elevated serum levels of matrix metalloproteinase-9 (MMP-9) in Kawasaki disease. Clin Exp Immunol. 2001;125(2):340–344.
28 Gavin P.J., Crawford S.E., Shulman S.T., et al. Systemic arterial expression of matrix metalloproteinases 2 and 9 in acute Kawasaki disease. Arterioscler Thromb Vasc Biol. 2003;23(4):576–581.
29 Shimizu C., Matsubara T., Onouchi Y., et al. Matrix metalloproteinase haplotypes associated with coronary artery aneurysm formation in patients with Kawasaki disease. J Hum Genet. 2010;55(12):779–784.
30 Uehara R., Yashiro M., Nakamura Y., et al. Kawasaki disease in parents and children. Acta Paediatr. 2003;92(6):694–697.
31 Shimizu C., Jain S., Davila S., et al. Transforming growth factor-beta signaling pathway in patients with Kawasaki disease. Circ Cardiovasc Genet. 2011;4(1):16–25.
32 Mamtani M., Matsubara T., Shimizu C., et al. Association of CCR2-CCR5 haplotypes and CCL3L1 copy number with Kawasaki disease, coronary artery lesions, and IVIG responses in Japanese children. PLoS ONE. 2010;5(7):e11458.
33 Onouchi Y., Gunji T., Burns J.C., et al. ITPKC functional polymorphism associated with Kawasaki disease susceptibility and formation of coronary artery aneurysms. Nat Genet. 2008;40(1):35–42.
34 Burgner D., Davila S., Breunis W.B., et al. A genome-wide association study identifies novel and functionally related susceptibility Loci for Kawasaki disease. PLoS Genet. 2009;5(1):e1000319.
35 Fujiwara H., Hamashima Y. Pathology of the heart in Kawasaki disease. Pediatrics. 1978;61(1):100–107.
36 Naoe S., Takahasha M., Masuda H., et al. Kawasaki disease with particular emphasis on arterial lesions. Acta Pathol Jpn. 1991;41(1):785–797.
37 Burns J.C. Kawasaki disease update. Indian J Pediatr. 2009;76(1):71–76.
38 Suzuki A., Miyagawa-Tomita S., Komatsu K., et al. Active remodeling of the coronary arterial lesions in the late phase of Kawasaki disease: immunohistochemical study. Circulation. 2000;101(25):2935–2941.
39 Kawasaki T., Kosaki F., Okawa S., et al. A new infantile acute febrile mucocutaneous lymph node syndrome (MLNS) prevailing in Japan. Pediatrics. 1974;54(3):271–276.
40 Yellen E.S., Gauvreau K., Takahashi M., et al. Performance of 2004 American Heart Association recommendations for treatment of Kawasaki disease. Pediatrics. 2010;125(2):e234–e241.
41 Burns J.C., Joffe L., Sargent R.A., et al. Anterior uveitis associated with Kawasaki syndrome. Pediatr Infect Dis. 1985;4(3):258–261.
42 Dengler L.D., Capparelli E.V., Bastian J.F., et al. Cerebrospinal fluid profile in patients with acute Kawasaki disease. J Ped Inf Dis. 1998;17(6):478–481.
43 Minich L.L., Sleeper L.A., Atz A.M., et al. Delayed diagnosis of Kawasaki disease: what are the risk factors? Pediatrics. 2007;120(6):e1434–e1440.
44 Muta H., Ishii M., Sakaue T., et al. Older age is a risk factor for the development of cardiovascular sequelae in Kawasaki disease. Pediatrics. 2004;114(3):751–754.
45 Song D., Yeo Y., Ha K., et al. Risk factors for Kawasaki disease-associated coronary abnormalities differ depending on age. Eur J Pediatr. 2009;168(11):1315–1321.
46 Kim T., Choi W., Woo C.W., et al. Predictive risk factors for coronary artery abnormalities in Kawasaki disease. Eur J Pediatr. 2007;166(5):421–425.
47 Beiser A.S., Takahashi M., Baker A.L., et al. A predictive instrument for coronary artery aneurysms in Kawasaki disease. Am J Cardiol. 1998;81(9):1116–1120.
48 Sabharwal T., Manlhiot C., Benseler S.M., et al. Comparison of factors associated with coronary artery dilation only versus coronary artery aneurysms in patients with Kawasaki disease. Am J Cardiol. 2009;104(12):1743–1747.
49 McCrindle B.W., Li J.S., Minich L.L., et al. Coronary artery involvement in children with Kawasaki disease: risk factors from analysis of serial normalized measurements. Circulation. 2007;116(2):174–179.
50 Kariyazono H., Ohno T., Khajoee V., et al. Association of vascular endothelial growth factor (VEGF) and VEGF receptor gene polymorphisms with coronary artery lesions of Kawasaki disease. Pediatr Res. 2004;56(6):953–959.
51 Fujiwara H., Hamashima Y. Pathology of the heart in Kawasaki disease. Pediatrics. 1978;61(1):100–107.
52 Yutani C., Okano K., Kamiya T., et al. Histopathological study on right endomyocardial biopsy of Kawasaki disease. Br Heart J. 1980;43(5):589–592.
53 Matsuura H., Ishikita T., Yamamoto S., et al. Gallium-67 myocardial imaging for the detection of myocarditis. Br Heart J. 1987;58(4):385–392.
54 Kao C.H., Hsieh K.S., Wang Y.L., et al. Tc-99m HMPAO labeled WBC scan for the detection of myocarditis in different phases of Kawasaki disease. Clin Nucl Med. 1992;17(3):185–190.
55 Moran A.M., Newburger J.W., Sanders S.P., et al. Abnormal myocardial mechanics in Kawasaki disease: rapid response to gamma-globulin. Am Heart J. 2000;139(2 Pt 1):217–223.
56 Tanaka N., Naoe S., Masuda H., et al. Pathological study of sequelae of Kawasaki disease (MCLS). With special reference to the heart and coronary arterial lesions. Acta Pathol Jpn. 1986;36(10):1513–1527.
57 Gordon J.B., Kahn A.M., Burns J.C. When children with Kawasaki disease grow up: myocardial and vascular complications in adulthood. J Am Coll Cardiol. 2009;54(21):1911–1920.
58 Printz B.F., Sleeper L.A., Newburger J.W., et al. Noncoronary cardiac abnormalities are associated with coronary artery dilation and with laboratory inflammatory markers in acute Kawasaki disease. J Am Coll Cardiol. 2010;57(1):86–92.
59 Nakano H., Nojima K., Saito A., et al. High incidence of aortic regurgitation following Kawasaki disease. J Pediatr. 1985;107(1):59–63.
60 Gidding S.S. Late onset valvular dysfunction in Kawasaki disease. Prog Clin Biol Res. 1987;250:305–309.
61 Gidding S.S., Shulman S.T., Ilbawi M., et al. Mucocutaneous lymph node syndrome (Kawasaki disease): delayed aortic and mitral insufficiency secondary to active valvulitis. J Am Coll Cardiol. 1986;7(4):894–897.
62 Ravekes W.J., Colan S.D., Gauvreau K., et al. Aortic root dilation in Kawasaki disease. Am J Cardiol. 2001;87(7):919–922.
63 Ozdogu H., Boga C. Fatal cardiac tamponade in a patient with Kawasaki disease. Heart Lung. 2005;34(4):257–259.
64 Kuppuswamy M., Gukop P., Sutherland G., et al. Kawasaki disease presenting as cardiac tamponade with ruptured giant aneurysm of the right coronary artery. Interact Cardiovasc Thorac Surg. 2010;10(2):317–318.
65 Imai Y., Sunagawa K., Ayusawa M., et al. A fatal case of ruptured giant coronary artery aneurysm. Eur J Pediatr. 2005;165(2):1–4.
66 Maresi E., Passantino R., Midulla R., et al. Sudden infant death caused by a ruptured coronary aneurysm during acute phase of atypical Kawasaki disease. Hum Pathol. 2001;32(12):1407–1409.
67 Council on Cardiovascular Disease in the Young Committee on Rheumatic Fever Endocarditis and Kawasaki Disease American Heart Association. Diagnostic guidelines for Kawasaki disease. Circulation. 2001;103(2):335–336.
68 Research Committee on Kawasaki Disease. Report of subcommittee on standardization of diagnostic criteria and reporting of coronary artery lesions in Kawasaki disease. Tokyo, Japan: Ministry of Health and Welfare, 1984.
69 de Zorzi A., Colan S.D., Gauvreau K., et al. Coronary artery dimensions may be misclassified as normal in Kawasaki disease. J Pediatr. 1998;133(2):254–258.
70 Tierney E.S., Newburger J.W., Graham D., et al. Diastolic function in children with Kawasaki Disease. Int J Cardiol. 2009;148(3):309–312.
71 Greil G.F., Stuber M., Botnar R.M., et al. Coronary magnetic resonance angiography in adolescents and young adults with Kawasaki disease. Circulation. 2002;105(8):908–911.
72 Danias P.G., Stuber M., Botnar R.M., et al. Coronary MR angiography: clinical applications and potential for imaging coronary artery disease. Magn Reson Imaging Clin N Am. 2003;11(1):81–99.
73 Mavrogeni S., Papadopoulos G., Douskou M., et al. Magnetic resonance angiography is equivalent to x-ray coronary angiography for the evaluation of coronary arteries in Kawasaki disease. J Am Coll Cardiol. 2004;43(4):649–652.
74 Sohn S., Kim H.S., Lee S.W. Multidetector row computed tomography for follow-up of patients with coronary artery aneurysms due to Kawasaki disease. Pediatr Cardiol. 2004;25(1):35–39.
75 Schmidt W.A. Use of imaging studies in the diagnosis of vasculitis. Curr Rheumatol Rep. 2004;6(3):203–211.
76 Muhling O., Jerosch-Herold M., Nabauer M., et al. Assessment of ischemic heart disease using magnetic resonance first-pass perfusion imaging. Herz. 2003;28(2):82–89.
77 Kaul S., Ito H. Microvasculature in acute myocardial ischemia: part I: evolving concepts in pathophysiology, diagnosis, and treatment. Circulation. 2004;109(2):146–149.
78 Zilberman M.V., Witt S.A., Kimball T.R. Is there a role for intravenous transpulmonary contrast imaging in pediatric stress echocardiography? J Am Soc Echocardiogr. 2003;16(1):9–14.
79 Ishii M., Himeno W., Sawa M., et al. Assessment of the ability of myocardial contrast echocardiography with harmonic power Doppler imaging to identify perfusion abnormalities in patients with Kawasaki disease at rest and during dipyridamole stress. Pediatr Cardiol. 2002;23(2):192–199.
80 Kato H., Sugimura T., Akagi T., et al. Long-term consequences of Kawasaki disease. A 10- to 21-year follow-up study of 594 patients. Circulation. 1996;94(6):1379–1385.
81 Kamiya T., Suzuki A., Ono Y., et al. Angiographic follow-up study of coronary artery lesion in the cases with a history of Kawasaki disease – with a focus on the follow-up more than ten years after the onset of the disease. In: Kato H., ed. Kawasaki disease. Proceedings of the 5th International Kawasaki Disease Symposium, Fukuoka, Japan, May 22-25, 1995. The Netherlands: Elsevier Science B.V; 1995:569–573.
82 Suda K., Iemura M., Nishiono H., et al. Long-term prognosis of patients with Kawasaki disease complicated by giant coronary aneurysms: a single-institution experience. Circulation. 2011;123(17):1836–1842.
83 Tsuda E., Kamiya T., Kimura K., et al. Coronary artery dilatation exceeding 4.0 mm during acute Kawasaki disease predicts a high probability of subsequent late intima-medial thickening. Pediatr Cardiol. 2002;23(1):9–14.
84 Iemura M., Ishii M., Sugimura T., et al. Long term consequences of regressed coronary aneurysms after Kawasaki disease: vascular wall morphology and function. Heart. 2000;83(3):307–311.
85 Tsuda E., Kamiya T., Ono Y., et al. Incidence of stenotic lesions predicted by acute phase changes in coronary arterial diameter during Kawasaki disease. Pediatr Cardiol. 2005;26(1):73–79.
86 Kato H., Ichinose E., Kawasaki T. Myocardial infarction in Kawasaki disease: clinical analyses in 195 cases. J Pediatr. 1986;108(6):923–927.
87 Tsuda E., Hirata T., Matsuo O., et al. The 30-year outcome for patients after myocardial infarction due to coronary artery lesions caused by Kawasaki disease. Pediatr Cardiol. 2010;32(2):176–182.
88 Sugimura T., Kato H., Inoue O., et al. Intravascular ultrasound of coronary arteries in children. Assessment of the wall morphology and the lumen after Kawasaki disease. Circulation. 1994;89(1):258–265.
89 Sasaguri Y., Kato H. Regression of aneurysms in Kawasaki disease: a pathological study? J Pediatr. 1982;100(2):225–231.
90 Muzik O., Paridon S.M., Singh T.P., et al. Quantification of myocardial blood flow and flow reserve in children with a history of Kawasaki disease and normal coronary arteries using positron emission tomography. J Am Coll Cardiol. 1996;28(3):757–762.
91 Mitani Y., Okuda Y., Shimpo H., et al. Impaired endothelial function in epicardial coronary arteries after Kawasaki disease. Circulation. 1997;96:454–461.
92 Yamakawa R., Ishii M., Sugimura T., et al. Coronary endothelial dysfunction after Kawasaki disease: evaluation by intracoronary injection of acetylcholine. J Am Coll Cardiol. 1998;31(5):1074–1080.
93 Cheung Y.F., Wong S.J., Ho M.H. Relationship between carotid intima-media thickness and arterial stiffness in children after Kawasaki disease. Arch Dis Child. 2007;92(1):43–47.
94 McCrindle B.W., McIntyre S., Kim C., et al. Are patients after Kawasaki disease at increased risk for accelerated atherosclerosis? J Pediatr. 2007;151(3):244–248.
95 Deng Y.B., Li T.L., Xiang H.J., et al. Impaired endothelial function in the brachial artery after Kawasaki disease and the effects of intravenous administration of vitamin C. Pediatr Infect Dis J. 2003;22(1):34–39.
96 Gupta-Malhotra M., Gruber D., Abraham S.S., et al. Atherosclerosis in survivors of Kawasaki disease. J Pediatr. 2009;155(4):572–577.
97 Gersony W.M. The adult after Kawasaki disease: the risks for late coronary events. J Am Coll Cardiol. 2009;54(21):1921–1923.
98 Durongpisitkul K., Gururaj V.J., Park J.M., et al. The prevention of coronary artery aneurysm in Kawasaki disease: a meta-analysis on the efficacy of aspirin and immunoglobulin treatment. Pediatrics. 1995;96(6):1057–1061.
99 Furusho K., Kamiya T., Nakano H., et al. High-dose intravenous gammaglobulin for Kawasaki disease. Lancet. 1984;2(8411):1055–1058.
100 Red Book. Report of the Committee on Infectious Disease. Kawasaki Disease. Pickering L.K., ed 26. Elk Grove Village, IL: American Academy of Pediatrics. 2003:394.
101 Tse S.M., Silverman E.D., McCrindle B.W., et al. Early treatment with intravenous immunoglobulin in patients with Kawasaki disease. J Pediatr. 2002;140(4):450–455.
102 Marasini M., Pongiglione G., Gazzolo D., et al. Late intravenous gamma globulin treatment in infants and children with Kawasaki disease and coronary artery abnormalities. Am J Cardiol. 1991;68(8):796–797.
103 Newburger J.W., Sleeper L.A., McCrindle B.W., et al. Randomized trial of pulsed corticosteroid therapy for primary treatment of Kawasaki disease. N Engl J Med. 2007;356(7):663–675.
104 Sundel R.P., Baker A.L., Fulton D.R., et al. Corticosteroids in the initial treatment of Kawasaki disease: report of a randomized trial. J Pediatr. 2003;142(6):611–616.
105 Okada Y., Shinohara M., Kobayashi T., et al. Effect of corticosteroids in addition to intravenous gamma globulin therapy on serum cytokine levels in the acute phase of Kawasaki disease in children. J Pediatr. 2003;143(3):363–367.
106 Shinohara M., Sone K., Tomomasa T., et al. Corticosteroids in the treatment of the acute phase of Kawasaki disease. J Pediatr. 1999;135(4):465–469.
107 Nonaka Z., Maekawa K., Okabe T., et al. Randomized controlled study of intravenous prednisolone and gamma globulin treatment in 100 cases with Kawasaki disease. Proceedings of the Fifth International Symposium on Kawasaki Disease. 1995. pp 328–331
108 Asano T., Sudoh M., Watanabe M., et al. Transient thrombocytopenia with large platelets in Kawasaki disease. Pediatr Hematol Oncol. 2007;24(7):551–554.
109 Wooditch A.C., Aronoff S.C. Effect of initial corticosteroid therapy on coronary artery aneurysm formation in Kawasaki disease: a meta-analysis of 862 children. Pediatrics. 2005;116(4):989–995.
110 Dale R.C., Saleem M.A., Daw S., et al. Treatment of severe complicated Kawasaki disease with oral prednisolone and aspirin. J Pediatr. 2000;137(5):723–726.
111 Wallace C.A., French J.W., Kahn S.J., et al. Initial intravenous gammaglobulin treatment failure in Kawasaki disease. Pediatrics. 2000;105(6):E78.
112 Hashino K., Ishii M., Iemura M., et al. Re-treatment for immune globulin-resistant Kawasaki disease: a comparative study of additional immune globulin and steroid pulse therapy. Pediatr Int. 2001;43(3):211–217.
113 Imagawa T., Mori M., Miyamae T., et al. Plasma exchange for refractory Kawasaki disease. Eur J Pediatr. 2004;163(4–5):263–264.
114 Williams R.V., Wilke V.M., Tani L.Y., et al. Does abciximab enhance regression of coronary aneurysms resulting from Kawasaki disease? Pediatrics. 2002;109(1):E4.
115 Weiss J.E., Eberhard B.A., Chowdhury D., et al. Infliximab as a novel therapy for refractory Kawasaki disease. J Rheumatol. 2004;31(4):808–810.
116 Burns J.C., Best B.M., Mejias A., et al. Infliximab treatment of intravenous immunoglobulin-resistant Kawasaki disease. J Pediatr. 2008;153(6):833–838.
117 Son M.B., Gauvreau K., Burns J.C., et al. Infliximab for intravenous immunoglobulin resistance in Kawasaki disease: a retrospective study. J Pediatr. 2011;158(4):644–649.
118 Kuijpers T.W., Biezeveld M., Achterhuis A., et al. Longstanding obliterative panarteritis in Kawasaki disease: lack of cyclosporin A effect. Pediatrics. 2003;112(4):986–992.
119 Manlhiot C., Brandao L.R., Somji Z., et al. Long-term anticoagulation in Kawasaki disease: initial use of low molecular weight heparin is a viable option for patients with severe coronary artery abnormalities. Pediatr Cardiol. 2010;31(6):834–842.
120 Sugahara Y., Ishii M., Muta H., et al. Warfarin therapy for giant aneurysm prevents myocardial infarction in Kawasaki disease. Pediatr Cardiol. 2008;29(2):398–401.
121 Tsuda E., Ishihara Y., Kawamata K., et al. Pregnancy and delivery in patients with coronary artery lesions caused by Kawasaki disease. Heart. 2005;91(11):1481–1482.
122 Tsuda E., Kitamura S., Kimura K., et al. Long-term patency of internal thoracic artery grafts for coronary artery stenosis due to Kawasaki disease: comparison of early with recent results in small children. Am Heart J. 2007;153(6):995–1000.
123 Tsuda E., Kitamura S. National survey of coronary artery bypass grafting for coronary stenosis caused by Kawasaki disease in Japan. Circulation. 2004;110(11 Suppl 1):II61–II66.
124 Kitamura S., Tsuda E., Kobayashi J., et al. Twenty-five-year outcome of pediatric coronary artery bypass surgery for Kawasaki disease. Circulation. 2009;120(1):60–68.
125 Akagi T. Interventions in Kawasaki disease. Pediatr Cardiol. 2005;26(2):206–212.
126 Subcommittee of Cardiovascular Sequelae, Subcommittee of Surgical Treatment, Kawasaki Disease Research Committee. Guidelines for treatment and management of cardiovascular in Kawasaki disease. Heart Vessels. 1987;3(1):50–54.
127 Ishii M., Ueno T., Akagi T., et al. Guidelines for catheter intervention in coronary artery lesion in Kawasaki disease. Pediatr Int. 2001;43(5):558–562.
128 Ishii M., Ueno T., Ikeda H., et al. Sequential follow-up results of catheter intervention for coronary artery lesions after Kawasaki disease: quantitative coronary artery angiography and intravascular ultrasound imaging study. Circulation. 2002;105(25):3004–3010.
129 Muta H., Ishii M. Percutaneous coronary intervention versus coronary artery bypass grafting for stenotic lesions after Kawasaki disease. J Pediatr. 2010;157(1):120–126.
130 Takahashi K., Oharaseki T., Naoe S. Pathological study of postcoronary arteritis in adolescents and young adults: with reference to the relationship between sequelae of Kawasaki disease and atherosclerosis. Pediatr Cardiol. 2001;22(2):138–142.
131 Noto N., Okada T., Yamasuge M., et al. Noninvasive assessment of the early progression of atherosclerosis in adolescents with Kawasaki disease and coronary artery lesions. Pediatrics. 2001;107(5):1095–1099.
132 Cheung Y.F., Ho M.H., Tam S.C., et al. Increased high sensitivity C reactive protein concentrations and increased arterial stiffness in children with a history of Kawasaki disease. Heart. 2004;90(11):1281–1285.
133 Mitani Y., Sawada H., Hayakawa H., et al. Elevated levels of high-sensitivity c-reactive protein and serum amyloid-A late after Kawasaki disease. Association between inflammation and late coronary sequelae in Kawasaki disease. Circulation. 2005;111(1):38–43.
134 Kavey R.E., Allada V., Daniels S.R., et al. Cardiovascular risk reduction in high-risk pediatric patients: a scientific statement from the American Heart Association Expert Panel on Population and Prevention Science; the Councils on Cardiovascular Disease in the Young, Epidemiology and Prevention, Nutrition, Physical Activity and Metabolism, High Blood Pressure Research, Cardiovascular Nursing, and the Kidney in Heart Disease; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research. Circulation. 2006;114(24):2710–2738.
135 Graham T.P.Jr, Driscoll D.J., Gersony W.M., et al. Task Force 2: congenital heart disease. J Am Coll Cardiol. 2005;45(8):1326–1333.