FIGURE 7.1 Weak and strong μ-opioids were applied in the volar forearm of volunteers by dermal microdialysis. The intensity of opioid-induced maximum itch is shown in the left panel (visual analog scale: 0–10; mean ± SEM). Peak mast cell tryptase release during stimulation with the opioids is shown in the right panel (mean ± SEM). Only low-affinity opioids meperidine (40.4 mM) and morphine (3.11 mM) caused tryptase release from mast cells. The potent opioids alfentanil (1.2 mM), sufentanil (0.12 mM), and remifentanil (2.65 mM) provoked neither itch nor tryptase release. (Modified from Blunk, J. A, Schmelz, M., Zeck, S., Skov, P., Likar, R., and Koppert, W., Anesth Analg, 98, 364–370, 2004.)
Opioid Effects in Neurons and Keratinocytes
Opioid-induced itch has often been linked to peripheral release of histamine from mast cells as intradermally injected opioids can activate mast cells by a non-receptor-mediated mechanism (17). Accordingly, weak opioids, such as codeine, have been used as a positive control in skin prick tests. The opioid-induced release of histamine and mast cell tryptase can be specifically monitored by measuring tryptase concentration with dermal microdialysis following intraprobe delivery (18). In contrast to morphine, the highly potent μ-opioid agonist fentanyl does not provoke any mast cell degranulation, even if applied at concentrations having μ-agonistic effects exceeding those of morphine (Figure 7.1) (19). Thus, only high local concentrations of opioids are sufficient to degranulate mast cells and to induce itching regardless of their affinity to μ-opioid receptors. Therefore, local μ-opioid activation in the skin is not sufficient to provoke acute itch.
However, local opioid signaling in the skin has been identified as an important factor for epidermal homeostasis and including modulation of keratinocyte differentiation, skin barrier function, inflammation, and wound healing (20–22). Thus, therapeutic approaches using modulators of opioid signaling in the skin are not primarily directed toward neuronal effects but use these homeostatic interactions such as delta-opioids for improving skin barrier repair (23). Based on the close interactions between keratinocytes and sensory nerve endings in the epidermis, the cross talk between them (24–26) changes in keratinocyte differentiation, and mediator release based on changes in opioid signaling appears sufficient to cause sensitization and activation of the sensory nerves and thus underlie skin symptoms of itch and pain reported in sensitive skin.
Itch Induced by Nociceptors
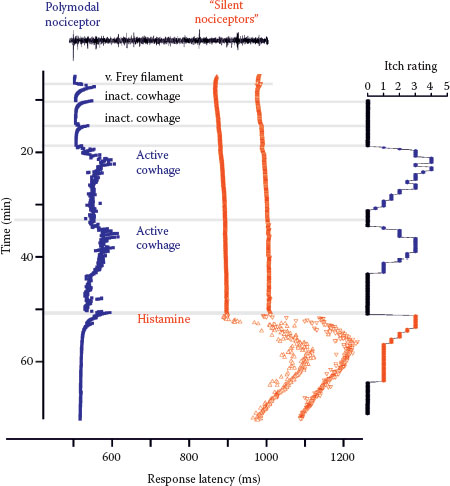
Cowhage spicules inserted into the human skin produce itch in an intensity which is comparable to that following histamine application (27,28) but is not accompanied by an axon reflex erythema and is unresponsive to histamine (H1) blocker (29). The active compound cysteine protease muconain has been identified and has shown to activate proteinase-activated receptor 2 (PAR 2) and even more potently PAR 4 (10). Interestingly, mechanoresponsive polymodal C fiber afferents, the most common type of afferent C nociceptors in the human skin (30), can be activated by cowhage in the cat (31), in nonhuman primates (11,29), and in human volunteers (32) (Figure 7.2).
FIGURE 7.2 Specimen of a microneurography recording from the peroneal nerve in humans (raw signal with marked action potentials on top) showing action potential responses to electrical stimulation to successive electrical stimulation in the receptive field plotted from top to bottom. Mechanical stimuli (von Frey filament, inactivated cowhage spicules) only activate the polymodal nociceptor (blue squares) as seen by irregular increases of response latency, whereas the two mechano-insensitive silent nociceptors (orange-red symbols) are not activated. The polymodal nociceptor is activated by the application of active cowhage in parallel to the itch rating of the subject shown on the right panel. In contrast, the silent nociceptors do not respond to cowhage stimulation but are activated following histamine ionotophoresis. Itch ratings are given on a numerical rating scale from 0 (0 = no itch) to 10 (10 = maximal imaginable itch). Active cowhage and histamine evoke similar itch; however, the first appears to be encoded by polymodal nociceptors whereas the latter by histamine-sensitive silent nociceptors. (Modified from Tominaga, M., and Takamori, K., J Dermatol, 41, 205–212, 2014.)
Given that cowhage spicules can activate a large proportion of polymodal nociceptors, we face a major problem to explain why the activation of these fibers by heat or by scratching actually inhibits itch, whereas the activation by cowhage produces it.
Encoding Itch by Subpopulations of Nociceptors
Considering nociceptors being involved in generating itch, a population code has been postulated (pattern theory) (1,6,33) in which only a subpopulation of nociceptors can also be activated by pruritic stimuli, whereas pure nociceptors are only responsive to algogens. Accordingly, itch will be felt when only the first subpopulation is responding but pain will be felt when both populations are active.
The encoding of itch by nociceptors has also been proposed to rely on a spatial code (34) based on the itch induced by capsaicin being locally applied on a cowhage spicule into the epidermis (28). The highly localized stimulation in the epidermis strongly activates some of the local nociceptors, while their immediate neighbors remain silent, resulting in a mismatch signal of activation and absence of activation from this site. It has thus been hypothesized that this mismatch might be perceived by the CNS as itch (32,34). Therefore, it needs to be pointed out that pruritus cannot only be explained by itch-specific or itch-selective neurons (7) along the specificity theory. In addition, the pure spatial pattern of activated nociceptors might similarly underlie the itch sensation without any requirement of itch-specific primary afferent neurons.
Peripheral Sensitization in Itch and Pain
Spontaneous itch and pain are of paramount clinical relevance, as they correlate with the main complaint of patients with chronic itch and pain. It is highly interesting that the patterns of sensitization linked to chronic pain and itch are remarkably similar.
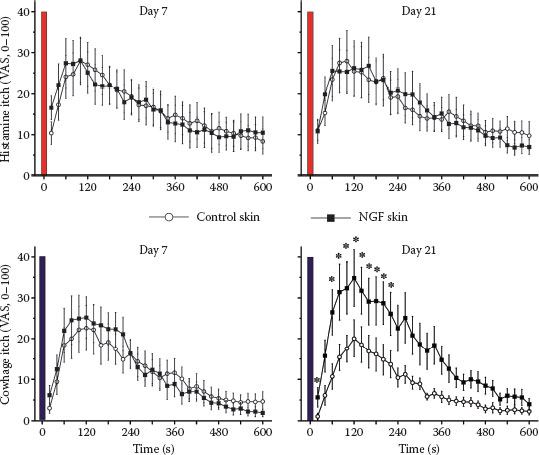
FIGURE 7.3 Itch ratings of human volunteers following stimulation with histamine (upper panels) and cowhage spicules (lower panels) are shown. The stimulations were applied in the forearm skin injected with saline (control) or NGF (1 μg in 50 μL) 7 days (left panels) or 21 days (right panels) after the injections. NGF injections did not sensitize histamine-induced itch, but cowhage-induced itch was increased by NGF at the maximum of the mechanical hyperalgesia (21 days; right lower panel). (Modified from Rukwied, R. R., Main, M., Weinkauf, B., and Schmelz, M., J Invest Dermatol, 133, 268–270, 2013.)
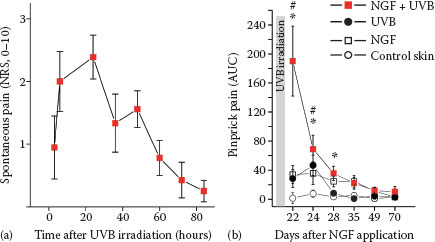
FIGURE 7.4 (a) Presensitization with NGF (1 μg) injected 3 weeks before UVB irradiation (threefold minimum erythema dose) provoked spontaneous pain in subjects with a time course matching the intensity of the UV-induced inflammation. (b) Hyperalgesia to pinprick stimuli develops following intradermal NGF injection and also for about 3 days after UVB irradiation. Combined sensitization with NGF and UVB irradiation causes a supraadditive increase of mechanical hyperalgesia. (Reprinted from Biochimie, 92, Okudaira, S., Yukiura, H., and Aoki, J., Biological roles of lysophosphatidic acid signaling through its production by autotaxin, 698–706, Copyright (2010), with permission from Elsevier.)
There is cumulative evidence for a prominent role of NGF-induced sensitization of primary afferents in both chronic itch and pain; increased levels of NGF were found in patients with chronic itch suffering from atopic dermatitis or psoriasis (35–38). Similarly, there is clear evidence for a major role of NGF in chronic inflammatory pain (39–41). Moreover, blocking NGF by specific antibodies proved to be analgesic in the patients with chronic pain (42,43). Anti-NGF strategies were also successful in animal models of chronic itch (44). It is therefore not surprising that intradermally injected NGF not only causes hyperalgesia to heat and mechanical stimuli in volunteers (45,46), but also sensitizes for cowhage-induced itch (47) (Figure 7.3).
Intracutaneous NGF injection does not induce visual inflammatory responses in humans (46), but interestingly, when combined with an inflammatory pain model (ultraviolet B [UVB] sunburn), the subjects report spontaneous pain (Figure 7.4) and pronounced hyperalgesia (48) that also includes axonal hyperexcitability (49). These results nicely match the analgesic effects of anti-NGF in chronic inflammatory pain that are not accompanied by reduced signs of inflammation (42). Therefore, it emerges that neurotrophic factors such as NGF can change expression patterns of primary afferent nociceptors such that their ability to signal pain or itch by local inflammatory mediators is increased. This increase might be based on higher discharge frequencies linked not only to sensitized transduction, but also to axonal hyperexcitability.
Links between Sensitization Mechanisms in Chronic Itch and Sensitive Skin
The structural damage of neurons can lead to hyperexcitability resulting in neuropathic itch and pain. The neuropathy of skin nerves can be quantified functionally by quantitative sensory testing or structurally by staining nerve fibers in skin biopsies (50). The correlation between structural loss of skin nerve fibers and reduced sensory function is well established (51). Unexpectedly, the degree of neuropathy does not predict the level of pain or itch symptoms (52,53). Functional and structural impairments are therefore essential to diagnose neuropathy (54). However, in most cases, neuropathy will simply lead to diminished sensory function without spontaneous pain or itch, for example, in diabetic neuropathy. Among patients with neuropathy, those with pain do not separate from those without pain based on the pattern or the degree of their neuropathy (52,55,56).
TABLE 7.1
Characteristics of Neuropathic and Inflammatory Pain as Compared to Sensitive Skin
|
Neuropathic Pain and Itch |
Inflammatory Pain and Itch |
Sensitive Skin |
||
|
Functional changes |
Sensitized |
+ |
+++ |
+++ |
|
evoked responses |
||||
|
Spontaneous |
+++ |
+++ |
+++ |
|
|
symptoms |
||||
|
Quantitative |
Sensitization and |
Sensitization |
||
|
sensory testing |
desensitization |
|||
|
Mechanical |
Mechanical and heat |
? |
||
|
hyperalgesia |
hyperalgesia |
|||
|
Structural changes |
Reduced nerve fiber |
Tendency to increased |
? |
|
|
density |
nerve fiber density |
|||
|
Biomarkers |
Methylglyoxal |
Autotaxin, |
? |
|
|
lysophosphatidic acid |
||||
|
Successful new treatment options |
Anti-NGF |
Recently, it has been suggested that small fiber neuropathy might contribute to sensitive skin (56) and could be regarded as similar to neuropathic pain (58). While questionnaires on neuropathic pain appear to be sensitive also for subjects with sensitive skin (58), it is unclear if sensitive skin is also linked to structural impairment of skin innervation. Measurements of epidermal innervation densities of sensitive skin in rosacea and its reduction following laser treatment (59) would suggest hyperinnervation rather than reduced fiber density in the epidermis as a correlate of increased sensitivity. Increased innervation density underlying skin hypersensitivity has been reported before in vulvodynia (60).
However, based on the experience from patients with neuropathic pain and neuropathic itch, it should be noted that levels of spontaneous pain and itch do not correlate with innervation density. Thus, measurements of innervation density in subjects with sensitive skin might not provide crucial pieces of information. The lack of correlation between evoked pain responses and occurrence of spontaneous pain in patients with neuropathic pain adds another key problem. In contrast, evoked and spontaneous symptoms are correlated and highly clinically relevant in chronic inflammatory hypersensitivity. In this respect, sensitive skin—when defined as increased sensitivity to evoked pain and itch—appears to be closely related to chronic inflammatory hypersensitivity (Table 7.1).
Biomarkers for Pain and Itch—Possible Overlap to Sensitive Skin?
Researchers have tried to identify biomarkers for pain and itch for decades. In more recent years, there has been some success as lysophosphatidic acid and its generating enzyme autotaxin have been found to correlate with the severity of pruritus in cholestatic pregnant women (61,62). Interestingly, lysophosphatidic acid has also been linked to the mechanisms of neuropathic pain (63). For neuropathic pain, there is evidence that methylglyoxal, a metabolite in the glucose metabolism, might serve as a biomarker for pain in painful diabetic neuropathy (64). Yet, there are no clinical trials that would use therapeutic approaches based on these two biomarkers. In terms of innovative mechanism-based therapies in the pain field, anti-NGF has proven to be clinically effective against chronic inflammatory pain (42,65). Interestingly, there are also suggestions to use the same approach in chronic itch (44). Antagonists of the neuropeptide SP have failed as a treatment for chronic pain (66) but appear promising as antipruritics (67,68). Thus, there are advances in itch and pain research that have resulted in the identification of first biomarkers and the successful development of therapies. The key question would be how this knowledge can be used for research in sensitive skin.
Perspectives
Our knowledge about mediators and mechanisms of chronic itch and pain has considerably increased in the last decade. In particular, specific markers for pruriceptors and first biomarkers for pain and itch have been found. It appears straightforward to simply use the functional and structural assessment tools validated for neuropathic and inflammatory conditions linked to chronic itch and pain also in subjects with sensitive skin. On the other hand, there are unsolved problems concerning the definition of sensitive skin. Moreover, the high percentage of around 50% of the population appears to be at odds with the assumption that the condition is a disease, unless one would assume that healthy subjects are a minority of the population. The dwindling percentage of healthy subjects as a consequence of the inflation of diagnoses has been criticized before, particularly in psychiatry (69). When based only on self-reports, it is questionable whether a sufficiently homogenous group of subjects that is suitable for mechanistic studies would evolve. Even in a seemingly homogeneous population of patients with neuropathic pain, current approaches try to cluster the patients according to sensory profiles as major mechanistic differences are assumed (70,71). Thus, for subjects reporting sensitive skin, even more heterogeneity is expected. It will be a highly interesting task in the future to define the path for the identification of those clusters for which identified mechanisms of chronic itch and pain are the underlying symptoms of sensitive skin.
REFERENCES
1. Handwerker HO. Itch hypotheses: From pattern to specificity and to population coding. In: Carstens E, Akiyama T, editors. Itch: Mechanisms and Treatment. Boca Raton, FL: Frontiers in Neuroscience; 2014.
2. Schmelz M, Schmidt R, Bickel A, Handwerker HO, Torebjörk HE. Specific C-receptors for itch in human skin. J Neurosci. 1997;17:8003–8008.
3. Schmelz M, Schmidt R, Weidner C, Hilliges M, Torebjörk HE, Handwerker HO. Chemical response pattern of different classes of C-nociceptors to pruritogens and algogens. J Neurophysiol. 2003;89:2441–2448.
4. Andrew D, Craig AD. Spinothalamic lamina 1 neurons selectively sensitive to histamine: A central neural pathway for itch. Nature Neuroscience. 2001;4:72–77.
5. Craig AD. How do you feel? Interoception: The sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666.
6. Akiyama T, Carstens E. Neural processing of itch. Neuroscience. 2013;250:697–714.
7. LaMotte RH, Dong X, Ringkamp M. Sensory neurons and circuits mediating itch. Nat Rev Neurosci. 2014;15:19–31.
8. Bautista DM, Wilson SR, Hoon MA. Why we scratch an itch: The molecules, cells and circuits of itch. Nat Neurosci. 2014;17:175–182.
9. Braz J, Solorzano C, Wang X, Basbaum AI. Transmitting pain and itch messages: A contemporary view of the spinal cord circuits that generate gate control. Neuron. 2014;82:522–536.
10. Reddy VB, Iuga AO, Shimada SG, LaMotte RH, Lerner EA. Cowhage-evoked itch is mediated by a novel cysteine protease: A ligand of protease-activated receptors. J Neurosci. 2008;28:4331–4335.
11. Johanek LM, Meyer RA, Friedman RM, Greenquist KW, Shim B, Borzan J et al. A role for polymodal C-fiber afferents in nonhistaminergic itch. J Neurosci. 2008;28:7659–7669.
12. Qu L, Fan N, Ma C, Wang T, Han L, Fu K et al. Enhanced excitability of MRGPRA3- and MRGPRD-positive nociceptors in a model of inflammatory itch and pain. Brain. 2014; 137(Pt 4):1039–1050.
13. Han L, Ma C, Liu Q, Weng HJ, Cui Y, Tang Z et al. A subpopulation of nociceptors specifically linked to itch. Nat Neurosci. 2012;16:174–182.
14. Liu Q, Sikand P, Ma C, Tang Z, Han L, Li Z et al. Mechanisms of itch evoked by beta-alanine. J Neurosci. 2012;32:14532–14537.
15. Wooten M, Weng HJ, Hartke TV, Borzan J, Klein AH, Turnquist B et al. Three functionally distinct classes of C-fibre nociceptors in primates. Nat Commun. 2014;5:4122.
16. Sikand P, Dong X, LaMotte RH. BAM8–22 peptide produces itch and nociceptive sensations in humans independent of histamine release. J Neurosci. 2011;31:7563–7567.
17. Ferry X, Brehin S, Kamel R, Landry Y. G protein-dependent activation of mast cell by peptides and basic secretagogues. Peptides. 2002;23:1507–1515.
18. Blunk JA, Seifert F, Schmelz M, Reeh PW, Koppert W. Injection pain of rocuronium and vecuronium is evoked by direct activation of nociceptive nerve endings. Eur J Anaesthesiol. 2003;20:245–253.
19. Blunk JA, Schmelz M, Zeck S, Skov P, Likar R, Koppert W. Opioid-induced mast cell activation and vascular responses is not mediated by mu-opioid receptors: An in vivo microdialysis study in human skin. Anesth Analg. 2004;98:364–370.
20. Slominski AT. On the role of the endogenous opioid system in regulating epidermal homeostasis. J Invest Dermatol. 2015;135:333–334.
21. Bigliardi PL, Neumann C, Teo YL, Pant A, Bigliardi-Qi M. Activation of the delta-opioid receptor promotes cutaneous wound healing by affecting keratinocyte intercellular adhesion and migration. Br J Pharmacol. 2015;172:501–514.
22. Neumann C, Bigliardi-Qi M, Widmann C, Bigliardi PL. The delta-opioid receptor affects epidermal homeostasis via ERK-dependent inhibition of transcription factor POU2F3. J Invest Dermatol. 2015;135:471–480.
23. Chajra H, Amstutz B, Schweikert K, Auriol D, Redziniak G, Lefevre F. Opioid receptor delta as a global modulator of skin differentiation and barrier function repair. Int J Cosmet Sci. 2015;37:386–394.
24. Lebonvallet N, Boulais N, Le Gall C, Pereira U, Gauche D, Gobin E et al. Effects of the re-innervation of organotypic skin explants on the epidermis. Exp Dermatol. 2012;21:156–158.
25. Pereira U, Boulais N, Lebonvallet N, Lefeuvre L, Gougerot A, Misery L. Development of an in vitro coculture of primary sensitive pig neurons and keratinocytes for the study of cutaneous neurogenic inflammation. Exp Dermatol. 2010;19:931–935.
26. Roggenkamp D, Falkner S, Stab F, Petersen M, Schmelz M, Neufang G. Atopic keratinocytes induce increased neurite outgrowth in a coculture model of porcine dorsal root ganglia neurons and human skin cells. J Invest Dermatol. 2012;132:1892–1900.
27. LaMotte RH, Shimada SG, Green BG, Zelterman D. Pruritic and nociceptive sensations and dysesthesias from a spicule of cowhage. J Neurophysiol. 2009;101:1430–1443.
28. Sikand P, Shimada SG, Green BG, LaMotte RH. Similar itch and nociceptive sensations evoked by punctate cutaneous application of capsaicin, histamine and cowhage. Pain. 2009;144:66–75.
29. Johanek LM, Meyer RA, Hartke T, Hobelmann JG, Maine DN, LaMotte RH et al. Psychophysical and physiological evidence for parallel afferent pathways mediating the sensation of itch. J Neurosci. 2007;27:7490–7497.
30. Schmidt R, Schmelz M, Forster C, Ringkamp M, Torebjörk HE, Handwerker HO. Novel classes of responsive and unresponsive C nociceptors in human skin. J Neurosci. 1995;15:333–341.
31. Tuckett RP, Wei JY. Response to an itch-producing substance in cat. II. Cutaneous receptor populations with unmyelinated axons. Brain Res. 1987;413:95–103.
32. Namer B, Carr R, Johanek LM, Schmelz M, Handwerker HO, Ringkamp M. Separate peripheral pathways for pruritus in man. J Neurophysiol. 2008;100:2062–2069.
33. McMahon SB, Koltzenburg M. Itching for an explanation. Trends Neurosci. 1992;15:497–501.
34. Schmelz M, Handwerker HO. Itch. Philadelphia, PA: Elsevier; 2013.
35. Toyoda M, Nakamura M, Makino T, Hino T, Kagoura M, Morohashi M. Nerve growth factor and substance P are useful plasma markers of disease activity in atopic dermatitis. BrJ Dermatol. 2002;147:71–79.
36. Tominaga M, Tengara S, Kamo A, Ogawa H, Takamori K. Psoralen-ultraviolet A therapy alters epidermal Sema3A and NGF levels and modulates epidermal innervation in atopic dermatitis. J Dermatol Sci. 2009;55:40–46.
37. Toyoda M, Nakamura M, Makino T, Morohashi M. Localization and content of nerve growth factor in peripheral blood eosinophils of atopic dermatitis patients. Clin Exp Allergy. 2003;33:950–955.
38. Yamaguchi J, Aihara M, Kobayashi Y, Kambara T, Ikezawa Z. Quantitative analysis of nerve growth factor (NGF) in the atopic dermatitis and psoriasis horny layer and effect of treatment on NGF in atopic dermatitis. J Dermatol Sci. 2009;53:48–54.
39. Chevalier X, Eymard F, Richette P. Biologic agents in osteoarthritis: Hopes and disappointments. Nat Rev Rheumatol. 2013;9:400–410.
40. Watanabe T, Inoue M, Sasaki K, Araki M, Uehara S, Monden K et al. Nerve growth factor level in the prostatic fluid of patients with chronic prostatitis/chronic pelvic pain syndrome is correlated with symptom severity and response to treatment. BJU Int. 2011;108:248–251.
41. Barcena de Arellano ML, Arnold J, Vercellino GF, Chiantera V, Ebert AD, Schneider A et al. Influence of nerve growth factor in endometriosis-associated symptoms. Reprod Sci. 2011;18:1202–1210.
42. Lane NE, Schnitzer TJ, Birbara CA, Mokhtarani M, Shelton DL, Smith MD et al. Tanezumab for the treatment of pain from osteoarthritis of the knee. N Engl J Med. 2010;363:1521–1531.
43. Sanga P, Katz N, Polverejan E, Wang S, Kelly KM, Haeussler J et al. Efficacy, safety, and tolerability of fulranumab, an anti-nerve growth factor antibody, in treatment of patients with moderate to severe osteoarthritis pain. Pain. 2013;154:1910–1919.
44. Tominaga M, Takamori K. Itch and nerve fibers with special reference to atopic dermatitis: Therapeutic implications. J Dermatol. 2014;41:205–212.
45. Hirth M, Rukwied R, Gromann A, Turnquist B, Weinkauf B, Francke K et al. NGF induces sensitization of nociceptors without evidence for increased intraepidermal nerve fiber density. Pain. 2013;154:2500–2511.
46. Rukwied R, Mayer A, Kluschina O, Obreja O, Schley M, Schmelz M. NGF induces non-inflammatory localized and lasting mechanical and thermal hypersensitivity in human skin. Pain. 2010;148:407–413.
47. Rukwied RR, Main M, Weinkauf B, Schmelz M. NGF sensitizes nociceptors for cowhage—But not histamine-induced itch in human skin. J Invest Dermatol. 2013;133:268–270.
48. Rukwied R, Weinkauf B, Main M, Obreja O, Schmelz M. Inflammation meets sensitization—An explanation for spontaneous nociceptor activity? Pain. 2013:10.
49. Rukwied R, Weinkauf B, Main M, Obreja O, Schmelz M. Axonal hyperexcitability after combined NGF sensitization and UV-B inflammation in humans. Eur J Pain. 2014;18:785–793.
50. Haanpaa M, Attal N, Backonja M, Baron R, Bennett M, Bouhassira D et al. NeuPSIG guidelines on neuropathic pain assessment. Pain. 2011;152:14–27.
51. Lauria G, Hsieh ST, Johansson O, Kennedy WR, Leger JM, Mellgren SI et al. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy: Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. Eur J Neurol. 2010;17:903–909.
52. Martinez V, Uceyler N, Ben Ammar S, Alvarez JC, Gaudot F, Sommer C et al. Clinical, histological and biochemical predictors of post-surgical neuropathic pain. Pain. 2015.
53. Uceyler N, Zeller D, Kahn AK, Kewenig S, Kittel-Schneider S, Schmid A et al. Small fibre pathology in patients with fibromyalgia syndrome. Brain. 2013;136:1857–1867.
54. Mellgren SI, Nolano M, Sommer C. The cutaneous nerve biopsy: Technical aspects, indications, and contribution. Handb Clin Neurol. 2013;115:171–188.
55. Kalliomaki M, Kieseritzky JV, Schmidt R, Hagglof B, Karlsten R, Sjogren N et al. Structural and functional differences between neuropathy with and without pain? Exp Neurol. 2011;231:199–206.
56. Wildgaard K, Ringsted TK, Hansen HJ, Petersen RH, Werner MU, Kehlet H. Quantitative sensory testing of persistent pain after video-assisted thoracic surgery lobectomy. Br J Anaesth. 2012;108:126–133.
57. Misery L, Bodere C, Genestet S, Zagnoli F, Marcorelles P. Small-fibre neuropathies and skin: News and perspectives for dermatologists. Eur J Dermatol. 2014;24:147–153.
58. Saint-Martory C, Sibaud V, Theunis J, Mengeaud V, Lauze C, Schmitt AM et al. Arguments for neuropathic pain in sensitive skin. Br J Dermatol. 2015;172:1120–1121.
59. Lonne-Rahm S, Nordlind K, Edstrom DW, Ros AM, Berg M. Laser treatment of rosacea: A pathoetiological study. Arch Dermatol. 2004;140:1345–1349.
60. Tympanidis P, Terenghi G, Dowd P. Increased innervation of the vulval vestibule in patients with vulvodynia. BrJ Dermatol. 2003;148:1021–1027.
61. Kremer AE, Martens JJ, Kulik W, Rueff F, Kuiper EM, Van Buuren HR et al. Lysophosphatidic acid is a potential mediator of cholestatic pruritus. Gastroenterology. 2010;139:1008–1018.
62. Kremer AE, Feramisco J, Reeh PW, Beuers U, Oude Elferink RP. Receptors, cells and circuits involved in pruritus of systemic disorders. Biochim Biophys Acta. 2014;1842:869–892.
63. Okudaira S, Yukiura H, Aoki J. Biological roles of lysophosphatidic acid signaling through its production by autotaxin. Biochimie. 2010;92:698–706.
64. Bierhaus A, Fleming T, Stoyanov S, Leffler A, Babes A, Neacsu C et al. Methylglyoxal modification of Na(v)1.8 facilitates nociceptive neuron firing and causes hyperalgesia in diabetic neuropathy. Nat Med. 2012;18:926–933.
65. Lewin GR, Lechner SG, Smith ES. Nerve growth factor and nociception: From experimental embryology to new analgesic therapy. Handb Exp Pharmacol. 2014;220:251–282.
66. Hill R. NK1 (substance P) receptor antagonists—Why are they not analgesic in humans? Trends Pharmacol Sci. 2000;21:244–246.
67. Stander S, Luger TA. NK-1 antagonists and itch. Handb Exp Pharmacol. 2015;226:237–255.
68. Stander S, Siepmann D, Herrgott I, Sunderkotter C, Luger TA. Targeting the neurokinin receptor 1 with aprepitant: A novel antipruritic strategy. PloS One. 2010;5:e10968. DOI: 10.1371/journal.pone.0010968.
69. Frances A. ICD, DSM and the Tower of Babel. Aust N Z J Psychiatry. 2014;48:371–373.
70. von Hehn CA, Baron R, Woolf CJ. Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron. 2012;73:638–652.
71. Baron R, Forster M, Binder A. Subgrouping of patients with neuropathic pain according to pain-related sensory abnormalities: A first step to a stratified treatment approach. Lancet Neurol. 2012;11:999–1005.