Chapter 449 Iron-Deficiency Anemia
Clinical Manifestations
Iron deficiency has nonhematologic systemic effects. The most concerning effects in infants and adolescents are impaired intellectual and motor functions that can occur early in iron deficiency before anemia develops. There is evidence that these changes might not be completely reversible after treatment with iron, increasing the importance of prevention. Pica, the desire to ingest non-nutritive substances, and pagophagia, the desire to ingest ice, are other systemic symptoms of iron deficiency. The pica can result in the ingestion of lead-containing substances and result in concomitant plumbism (Chapter 702).
Laboratory Findings
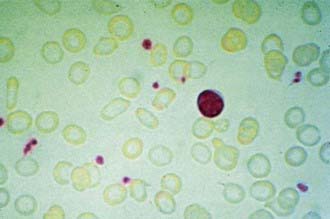
In progressive iron deficiency, a sequence of biochemical and hematologic events occurs (Tables 449-1 and 449-2). Clinically, iron deficiency anemia is not difficult to diagnose. First, tissue iron stores are depleted. This depletion is reflected by reduced serum ferritin, an iron-storage protein, which provides an estimate of body iron stores in the absence of inflammatory disease. Next, serum iron levels decrease, the iron-binding capacity of the serum (serum transferrin) increases, and the transferrin saturation falls below normal. As iron stores decrease, iron becomes unavailable to complex with protoporphyrin to form heme. Free erythrocyte protoporphyrins (FEPs) accumulate, and hemoglobin synthesis is impaired. At this point, iron deficiency progresses to iron-deficiency anemia. With less available hemoglobin in each cell, the red cells become smaller. This morphologic characteristic is best quantified by the decrease in mean corpuscular volume (MCV) and mean corpuscular hemoglobin (MCH). Developmental changes in MCV require the use of age-related standards for diagnosis of microcytosis (see Table 441-1). Increased variation in cell size occurs as normocytic red cells are replaced by microcytic ones; this variation is quantified by an elevated RBC distribution width (RDW). The red cell count (RBC) also decreases. The reticulocyte percentage may be normal or moderately elevated, but absolute reticulocyte counts indicate an insufficient response to the degree of anemia. The blood smear reveals hypochromic, microcytic red cells with substantial variation in cell size. Elliptocytic or cigar-shaped red cells are often seen (Fig. 449-1). Detection of increased transferrin receptor and decreased reticulocyte hemoglobin concentration provides supporting diagnostic information when these studies are available.
| INDICATOR | SELECTED CUTOFF VALUES TO DEFINE IRON DEFICIENCY | COMMENTS |
|---|---|---|
| Hemoglobin (g/L) | 6 mo-5 yr <110 | When used alone, it has low specificity and sensitivity |
| 6-11 yr <115 | ||
| Nonpregnant women <120 | ||
| Pregnant women <110 | ||
| Mean corpuscular volume (MCV) (µm3) | Children older than 11 yr and adults <82 | A reliable, but late indicator of iron deficiency Low values can also be due to thalassemia |
| Reticulocyte hemoglobin content (CHr) (pg) | In infants and young children <27.5 In adults ≤28.0 |
A sensitive indicator that falls within days of onset of iron-deficient erythropoiesis False normal values can occur when MCV is increased and in thalassemia Wider use is limited because it can only be measured on a few analyzer models |
| Erythrocyte zinc protoporphyrin (ZPP) (µmol/mol heme) | ≤5 yr >70 Children >5 yr >80 Children >5 yr on washed red cells >40 |
It can be measured directly on a drop of blood with a portable hematofluorometer A useful screening test in field surveys, particularly in children, in whom uncomplicated iron deficiency is the primary cause of anemia Red cells should be washed before measurement because circulating factors, including serum bilirubin, can spuriously increase values Lead poisoning can increase values, particularly in urban and industrial settings |
| Transferrin saturation | <16% | It is inexpensive, but its use is limited by diurnal variation in serum iron and by many clinical disorders that affect transferrin concentrations |
| Serum ferritin (SF) (µg/L) | ≤5 yr <12 Children >5 yr <15 In all age groups in the presence of infection <30 |
It is probably the most useful laboratory measure of iron status; a low value of SF is diagnostic of iron-deficiency anemia in a patient with anemia In healthy persons, SF is directly proportional to iron stores: 1 µg/L SF corresponds to 8-10 mg body iron or 120 µg storage iron per kg body weight As an acute-phase protein, SF increases independent of iron status by acute or chronic inflammation; it is also unreliable in patients with malignancy, hyperthyroidism, liver disease, or heavy alcohol intake |
| Serum transferrin receptor (sTfR) | Cutoff varies with assay and with patient’s age and ethnic origin | Main determinants are the erythroid mass in the bone marrow and iron status; thus, sTfR is increased by enhanced erythropoiesis and iron deficiency sTfR is not substantially affected by the acute-phase response, but it might be affected by malaria, age, and ethnicity Its application is limited by high cost of commercial assays and lack of an international standard |
| sTfR : SF ratio | This ratio is a quantitative estimate of total body iron; the logarithm of this ratio is directly proportional to the amount of stored iron in iron-replete patients and the tissue iron deficit in iron deficiency In elderly people, this ratio might be more sensitive than other laboratory tests for iron deficiency This ratio cannot be used in patients with inflammation because SF might be high independent of iron stores This ratio is assay specific Although it is only validated for adults, this ratio has been used in children |
From Zimmermann MB, Hurrell RF: Nutritional iron deficiency, Lancet 370:511–520, 2007.
Differential Diagnosis
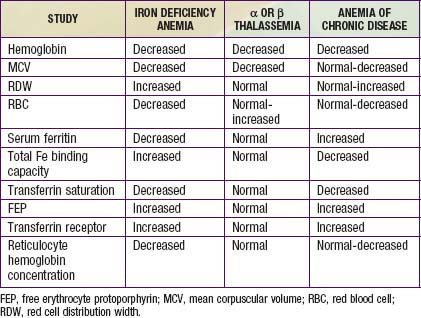
The most common alternative causes of microcytic anemia are α or β thalassemia and hemoglobinopathies, including hemoglobin E and C (Chapter 456). The thalassemia traits are most common and are associated with an elevated as opposed to decreased red blood cell count and a normal as opposed to elevated RDW. The anemia of chronic disease is usually normocytic but can be microcytic in a minority of cases (Chapter 445). Lead poisoning can cause microcytic anemia, but more often iron deficiency anemia causes pica, which then results in lead intoxication (Chapter 702). Table 449-1 compares the use of laboratory studies in the diagnosis of the most common microcytic anemias. Other etiologies of microcytic anemia are found in Table 449-3.
Treatment
The regular response of iron-deficiency anemia to adequate amounts of iron is a critical diagnostic and therapeutic feature (Table 449-4). Oral administration of simple ferrous salts (most often ferrous sulfate) provides inexpensive and effective therapy. There is no evidence that the addition of any trace metal, vitamin, or other hematinic substance significantly increases the response to simple ferrous salts. Aside from the unpleasant taste of iron, intolerance to oral iron is uncommon in young children. In contrast, older children and adolescents sometimes have GI complaints.
Table 449-4 RESPONSES TO IRON THERAPY IN IRON-DEFICIENCY ANEMIA
| TIME AFTER IRON ADMINISTRATION | RESPONSE |
|---|---|
| 12-24 hr | Replacement of intracellular iron enzymes; subjective improvement; decreased irritability; increased appetite |
| 36-48 hr | Initial bone marrow response; erythroid hyperplasia |
| 48-72 hr | Reticulocytosis, peaking at 5-7 days |
| 4-30 days | Increase in hemoglobin level |
| 1-3 mo | Repletion of stores |
In addition to iron therapy, dietary counseling is usually necessary. Excessive intake of milk, particularly bovine milk, should be limited. Iron deficiency in adolescent girls secondary to abnormal uterine blood flow loss is treated with iron and hormone therapy (Chapter 110.2).
If the anemia is mild, the only additional study is to repeat the blood count approximately 4 wk after initiating therapy. At this point the hemoglobin has usually risen by at least 1-2 g/dL and has often normalized. If the anemia is more severe, earlier confirmation of the diagnosis can be made by the appearance of a reticulocytosis usually within 48-96 hr of instituting treatment. The hemoglobin will then begin to increase 0.1-0.4 g/dL per day depending on the severity of the anemia. Iron medication should be continued for 8 wk after blood values normalize to re-establish iron stores. Good follow-up is essential to ensure a response to therapy. When the anemia responds poorly or not at all to iron therapy, there are multiple considerations, including diagnoses other than iron deficiency (see Table 449-3).
American Academy of Pediatrics. Recommendations for preventive pediatric health care (website). http://brightfutures.aap.org/pdfs/AAP%20Bright%20Futures%20Periodicity%20Sched%20101107.pdf. Accessed June 29, 2010
Arlet JB, Hermine O, Darnige L, et al. Iron-deficiency anemia in Castleman disease: implication of the interleukin 6/hepcidin pathway. Pediatrics. 2010;126:e1608-e1612.
Auerbach M, Ballard H, Glaspy J. Clinical update: intravenous iron for anaemia. Lancet. 2007;369:1502-1504.
Baker PN, Wheeler SJ, Sanders TA, et al. A prospective study of micronutrient status in adolescent pregnancy. Am J Clin Nutr. 2009;89:1114-1124.
Baker RD, Greer FR, Committee on Nutrition. Clinical report: diagnosis and prevention of iron deficiency and iron deficiency anemia in infants and young children (0-3 years of age). Pediatrics. 2010;126(5):1040-1050.
Beard JL. Why iron deficiency is important in infant development. J Nutr. 2008;138:2534-2536.
Buchanan GR. The tragedy of iron deficiency during infancy and early childhood. J Pediatr. 1998;135:413-415.
Committee on Nutrition American Academy of Pediatrics. Nutritional needs of the preterm infant. In: Kleinman RE, editor. Pediatric nutrition handbook. ed 6. Elk Grove Village, IL: American Academy of Pediatrics; 2009:403-419.
Finberg KE, Heeney MM, Campagna DR, et al. Mutations in TMPRSS6 cause iron-refractory iron deficiency anemia (IRIDA). Nat Genet. 2008;40:569-571.
Galloway MJ, Smellie WS. Investigating iron status in microcytic anaemia. BMJ. 2006;333:791-793.
Lozoff B, Clark KM, Jing Y, et al. Dose-response relationships between iron deficiency with or without anemia and infant social-emotional behavior. J Pediatr. 2008;152:696-702.
Lozoff B, Jimenez E, Smith JB. Double burden of iron deficiency in infancy and low socioeconomic status. Arch Pediatr Adolesc Med. 2006;160:1108-1112.
Medical Letter: Ferumoxytol (Feraheme)—a new parenteral iron formulation. 52:23–24, 2010.
Mercer J, Erickson-Owens D. Delayed cord clamping increases infants’ iron stores. Lancet. 2006;367:1956-1957.
Moy RJ. Prevalence, consequences and prevention of childhood nutritional iron deficiency: a child public health perspective. Clin Lab Haematol. 2006;28:291-298.
Sutcliffe TL, Khambalia A, Westergard S, et al. Iron depletion is associated with daytime bottle-feeding in the second and third years of life. Arch Pediatr Adolesc Med. 2006;160:1114-1120.
Zimmermann MB, Hurrell RF. Nutritional iron deficiency. Lancet. 2007;370:511-520.