ESSENTIALS OF ELECTROCARDIOGRAM READING
Interpretation of ECG
Acute (transmural) myocardial infarction
Subendocardial ischaemia
Left ventricular hypertrophy
Right ventricular hypertrophy
Right atrial hypertrophy/strain
Right bundle branch block
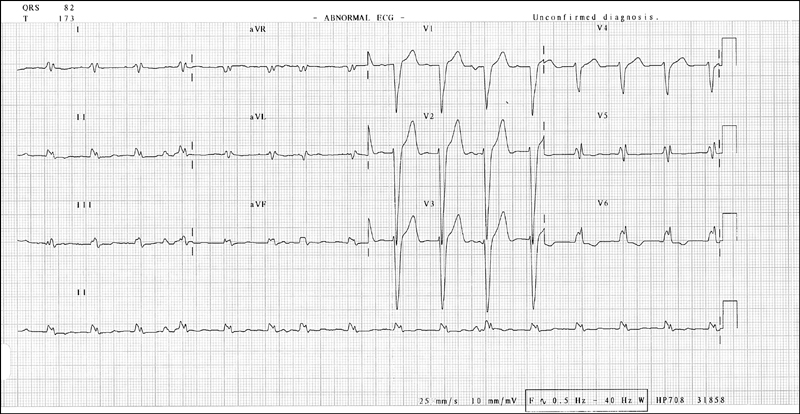
Left bundle branch block
Left anterior hemiblock
Left posterior hemiblock
Bifascicular block
First-degree heart block
Second-degree heart block
Type I (Wenckebach phenomenon)
Type II
Complete heart block
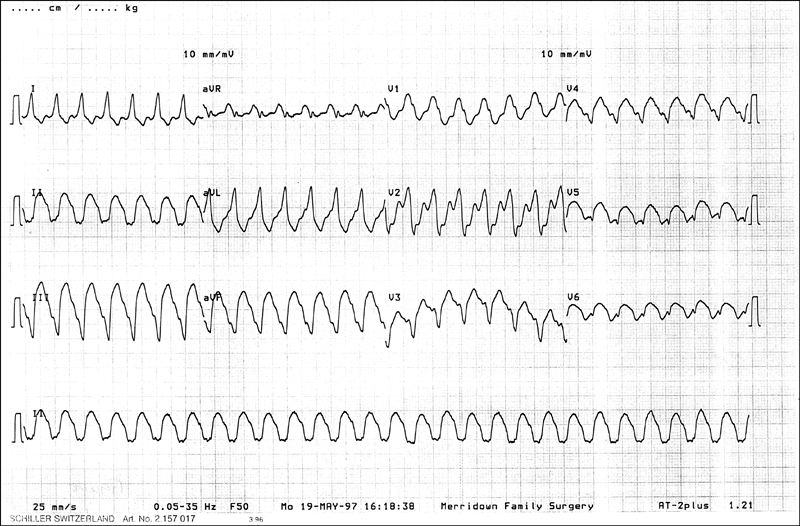
Tachyarrhythmias
Ventricular tachycardia
Atrial tachycardia
Atrial flutter
Junctional tachycardia (AV nodal re-entry tachycardia)
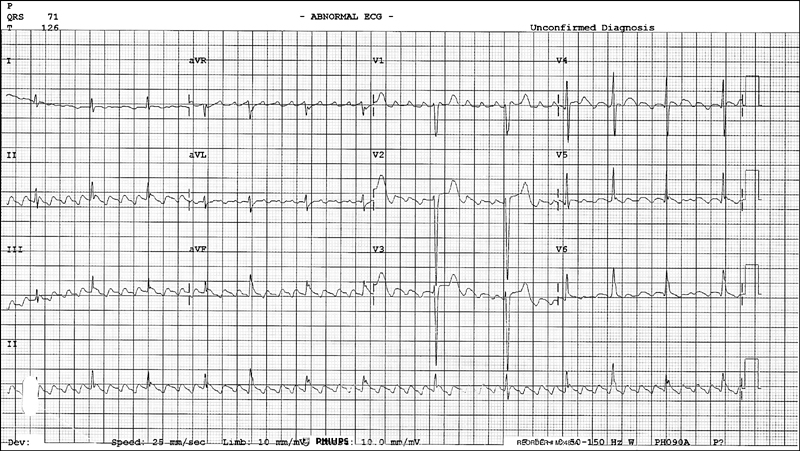
Wolff-Parkinson-White syndrome
Hypertrophic obstructive cardiomyopathy
HAEMATOLOGICAL STUDIES
Full blood count
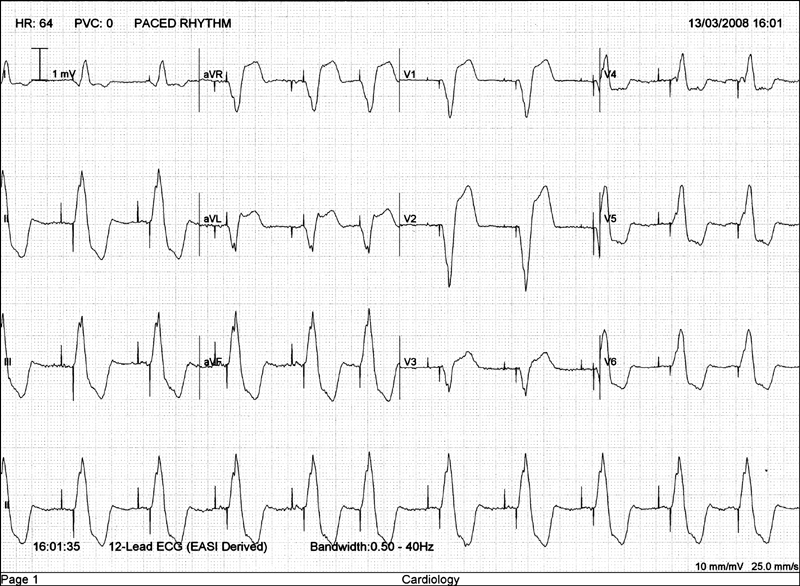
Figure 14.1 ECG showing atrial and ventricular paced rhythm with pacing spikes clearly visible before each atrial and ventricular complex. Also note the left bundle branch block (LBBB) pattern due to the early stimulation of the right ventricle by the pacing lead located there.
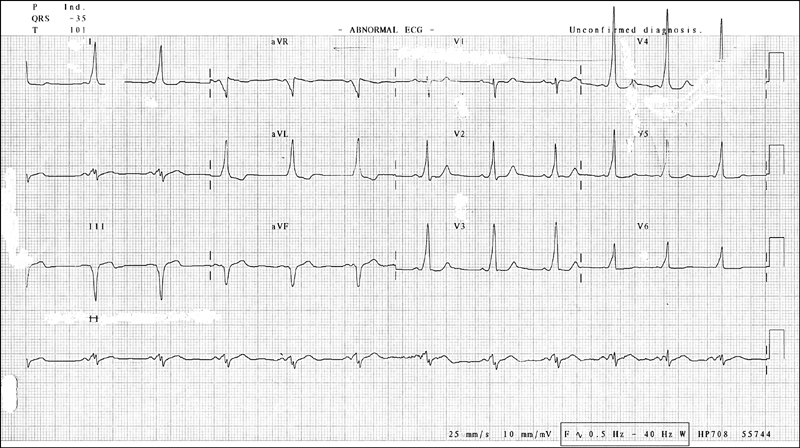
Figure 14.3 ECG showing Wolff-Parkinson-White (WPW) syndrome. Notice the short P-R interval and the delta wave visible in most QRS complexes.
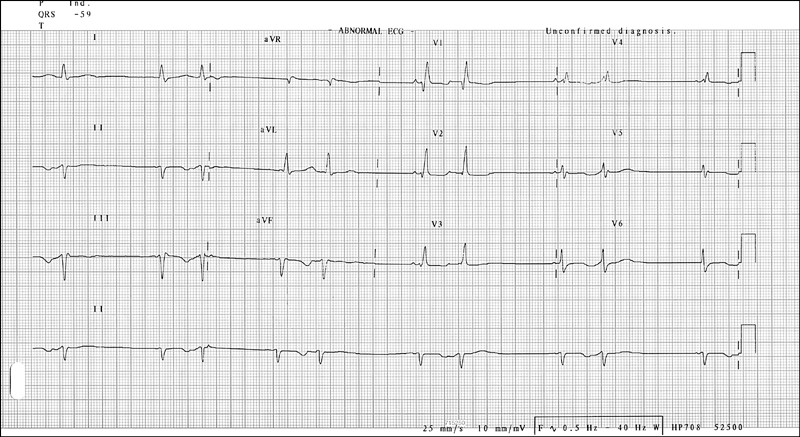
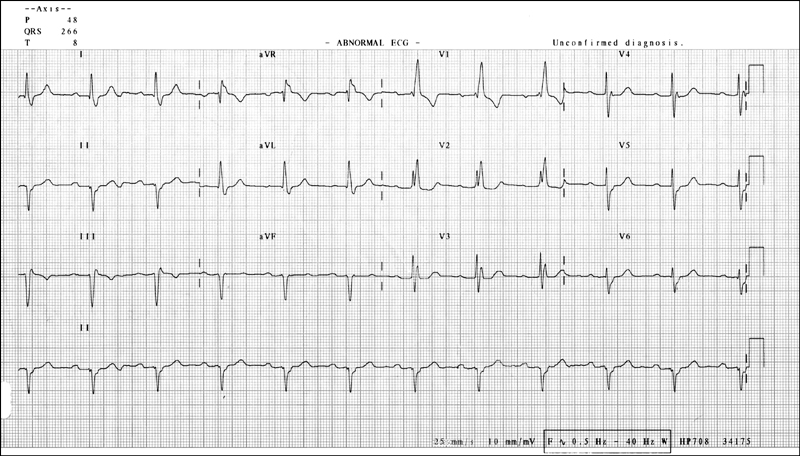
Figure 14.5 ECG showing supraventricular bigeminy and left axis deviation
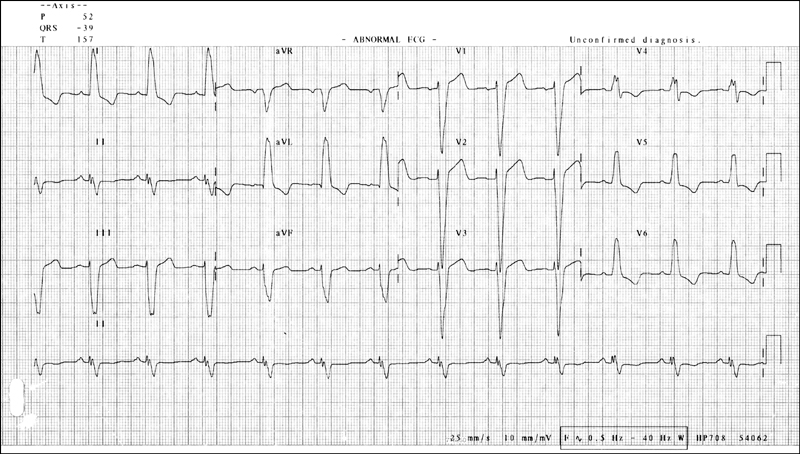
Figure 14.7 ECG showing left bundle branch block (LBBB)
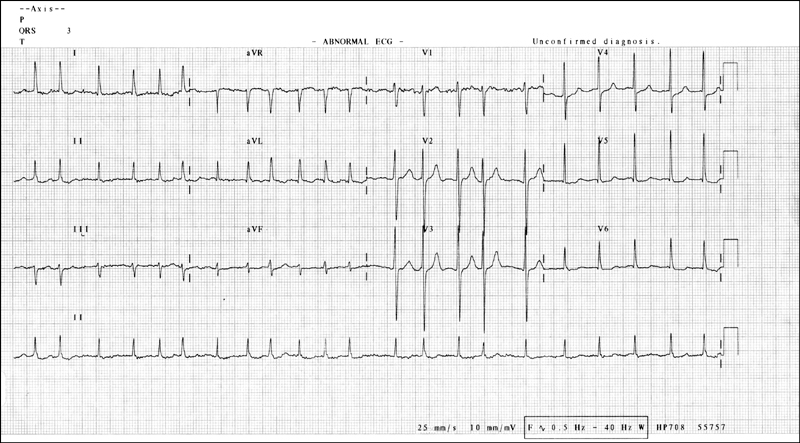
Figure 14.9 ECG showing atrial fibrillation
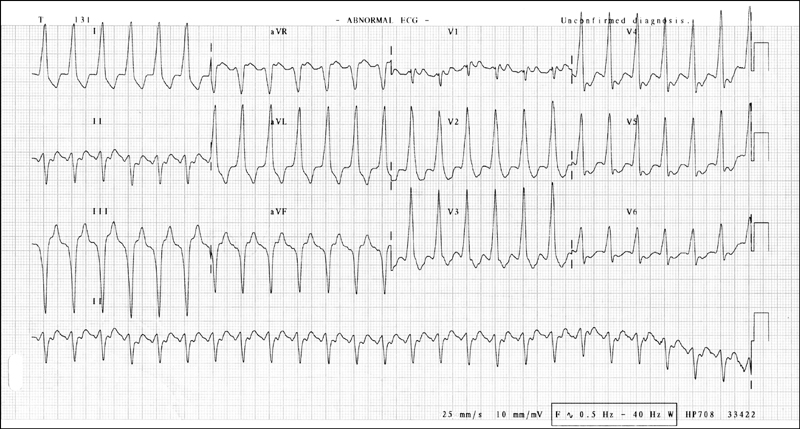
Figure 14.10 ECG showing supraventricular tachycardia with aberrancy
Neutrophilia
Lymphocytosis
Eosinophilia
Basophilia
Leucopenia
Thrombophilic screen
Electrolyte profile
Sodium
Hypernatraemia
Hyponatraemia
Potassium
Hyperkalaemia
Hypokalaemia
Calcium
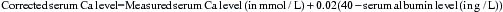
Hypercalcaemia
Hypocalcaemia
Acid–base status
Metabolic acidosis
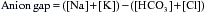
Metabolic alkalosis
Respiratory acidosis
Respiratory alkalosis
RENAL FUNCTION INDICES
LIVER FUNCTION TESTS
Aminotransferases (ALT/AST)
Alkaline phosphatase (ALP)
Gammaglutamyl transpeptidase (GGT)
Serum albumin level
Bilirubin
Autoimmune markers of liver disease
ENDOCRINOLOGICAL STUDIES
Thyroid function tests
Direct test of thyroid function
Adrenal function tests
Cushing’s syndrome (excess secretion of corticosteroids)
Tests for adrenal insufficiency
Test for hyperaldosteronism
Test for phaeochromocytoma
Parathyroid hormone tests
Test for hyperparathyroidism
Pituitary function tests
LUNG FUNCTION TESTS
NUCLEAR IMAGING
Lung scan
Ventilation-perfusion (V/Q) scan
Thyroid scan
Adrenal scan
Renal scans
Cardiac scans
Perfusion imaging
Acute myocardial infarction
Gated heart pool scan
RADIOLOGICAL IMAGING STUDIES
Chest X-ray
CT scan of the chest
High-resolution CT scan
Normal-resolution CT scans
Helical CT scan
Abdominal imaging
Abdominal ultrasound
Abdominal X-ray
Barium swallow
Abdominal CT scan
Abdominal MRI scan
Cranial imaging
Cranial CT scans
Cranial MRI
Skeletal imaging and arthritis
Figure 14.11 This is a frontal projection, postero-anterior view chest X-ray. It is of normal appearance.
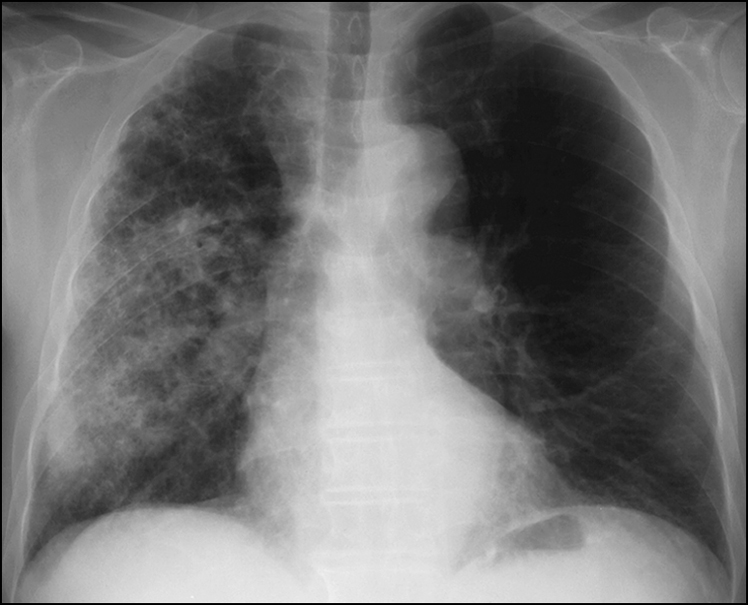
Figure 14.12 Diffuse alveolar opacification of the right lung with evidence of preexisting CAL and normal heart size. The appearance is consistent with atypical pneumonia, especially due to Legionella. Other differential diagnoses include unilateral pulmonary oedema, pulmonary haemorrhage and unilateral bronchiolitis obliterans with organising pneumonia (BOOP).
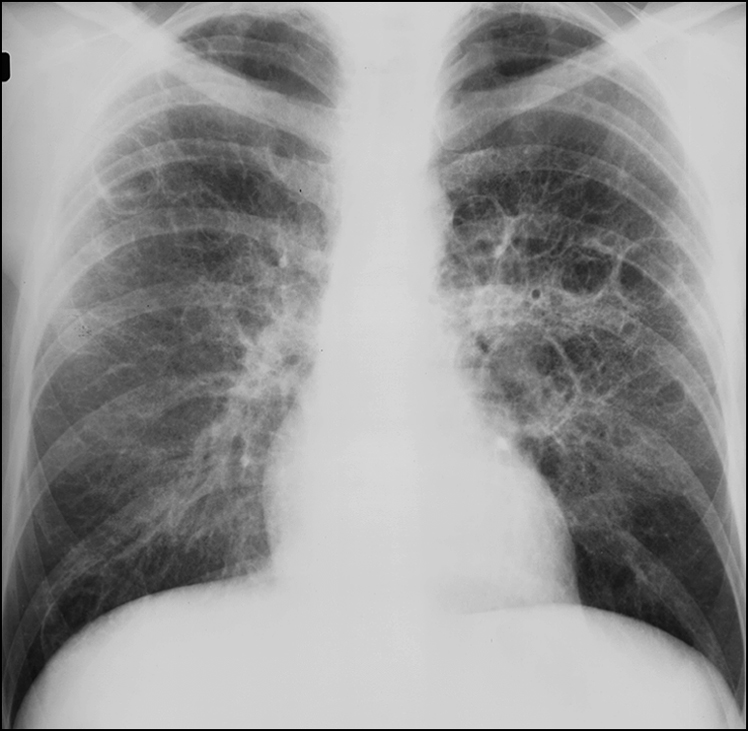
Figure 14.13 There is a coarse interstitial pattern with central and basal predominance. Cystic spaces are a prominent feature. Apices are relatively spared. Mediastinum is normal. Appearance of this chest X-ray is consistent with Pneumocystis carinii pneumonia in a patient with HIV infection.
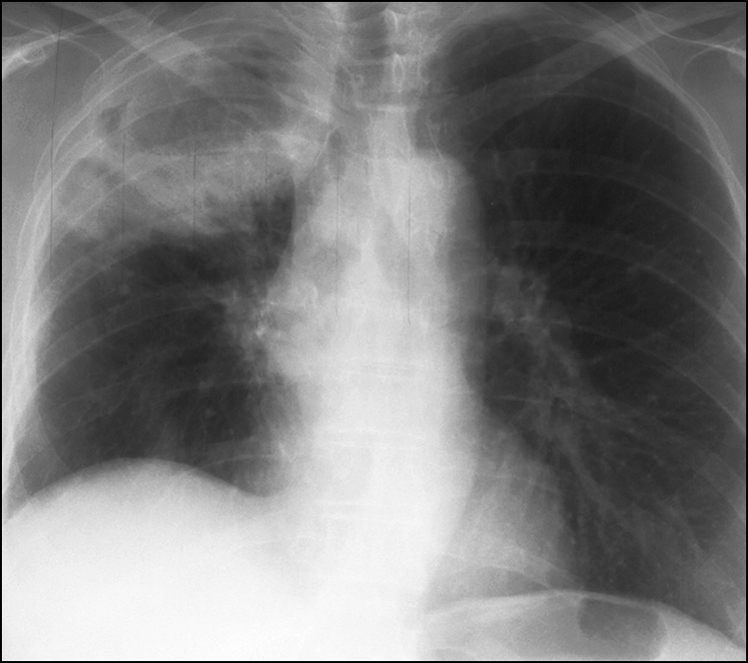
Figure 14.14 Extensive irregular opacity in the right lung apex, with cavitation and a fluid level. This has the appearance of a cavitating abscess. There is elevation of right hemidiaphragm. Other differential diagnoses include post-primary tuberculosis of right apex, Klebsiella pneumonia, and right apical malignancy with cavitation.
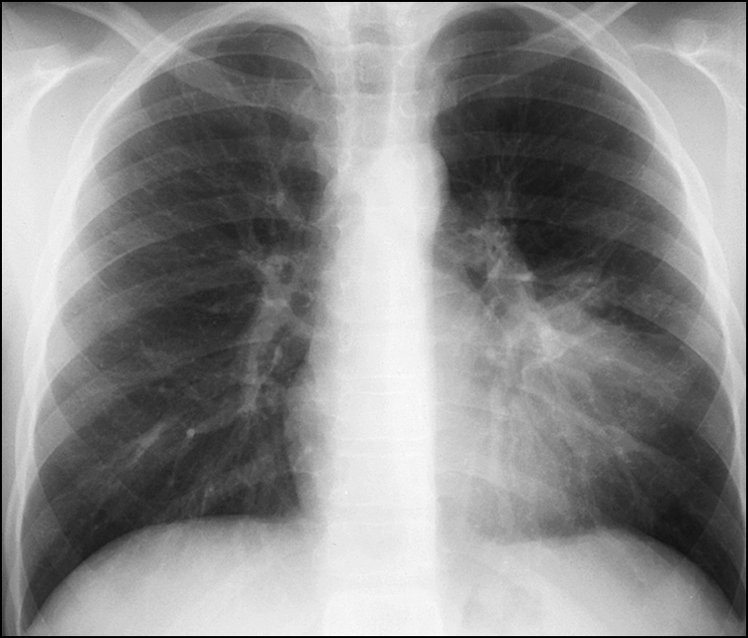
Figure 14.15 There is obliteration of the upper segment of the left heart border and opacification of the mid-segment of the left lung. There are air bronchograms. This is consolidation of the lingula.
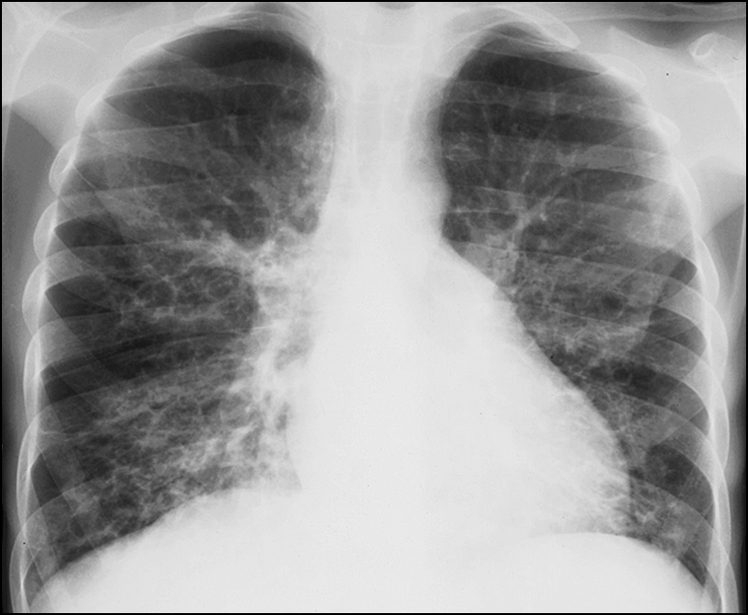
Figure 14.16 There is hyperinflation of both lung fields and increased lung markings. Multiple cystic areas are present in both lungs, suggesting bronchiectasis. Appearance of a large main pulmonary artery segment suggests pulmonary arterial hypertension. This picture shows diffuse bronchiectasis consistent with cystic fibrosis.
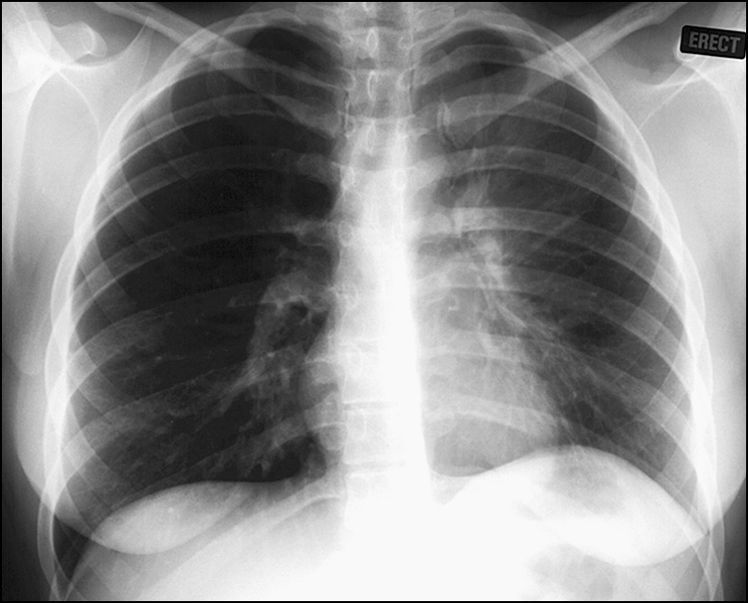
Figure 14.17 There is a band of opacification behind and running parallel to the sternum, thicker superiorly. There is slight elevation of the left hemidiaphragm and mediastinal shift to the left. These features suggest left upper lobe collapse.
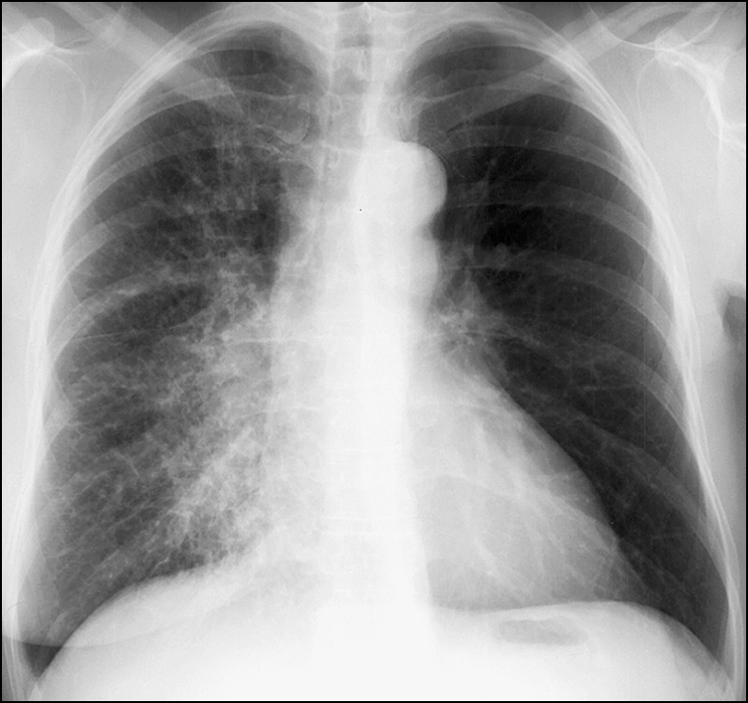
Figure 14.18 There is absence of the left breast shadow. There is a coarse reticular pattern throughout the right lung with perihilar predominance. The left lung is clear. This picture is highly suggestive of lymphangitis carcinomatosis in a patient who has had left-sided mastectomy for cancer of the breast.
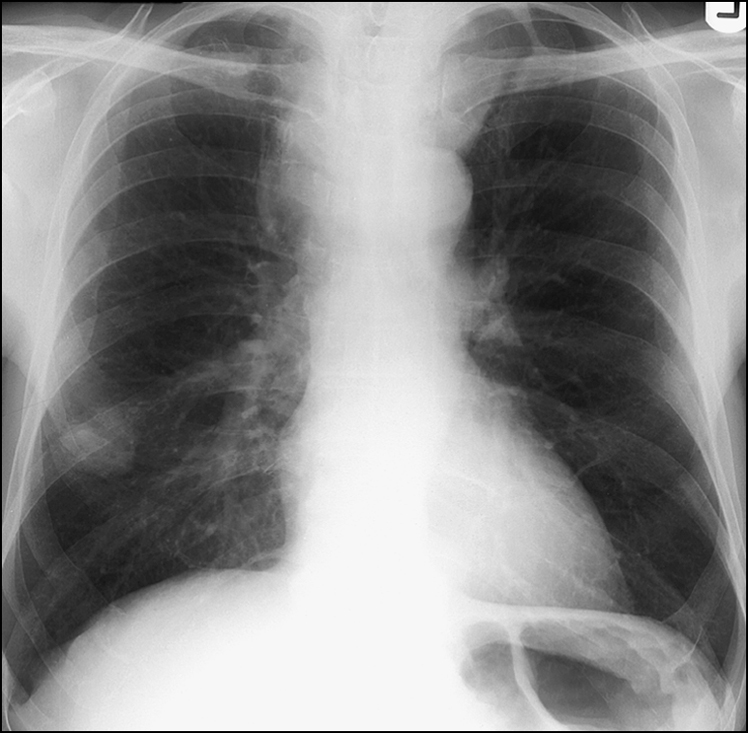
Figure 14.19 This chest X-ray shows a mass in the lateral basal segment of the right lower lobe with widening of the upper mediastinum and irregularity of the outline of the trachea. This appearance is consistent with metastatic carcinoma of the lung.
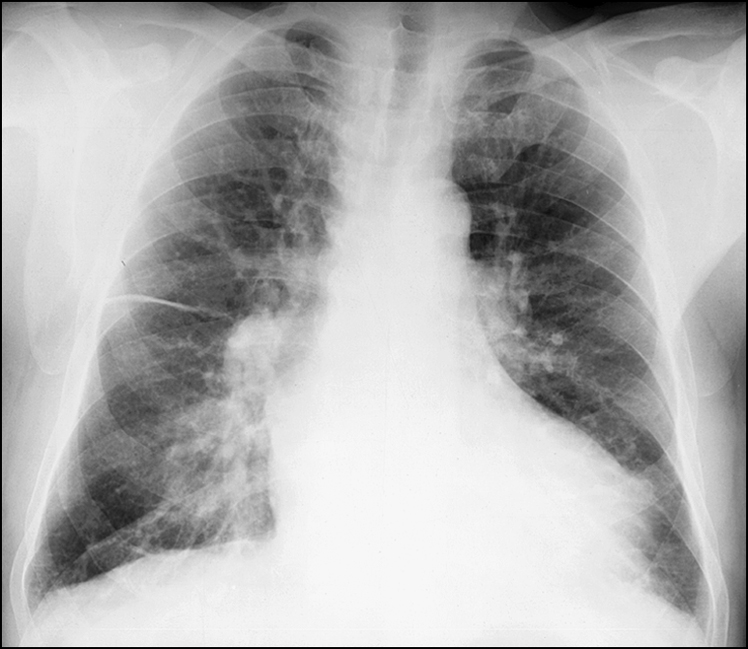
Figure 14.20 This chest X-ray shows an interstitial pattern throughout the lung fields with septate lines (Kerley A and B) and perihilar haze. There is interstitial oedema, suggesting high left atrial pressures. There are widespread areas of confluence, suggesting alveolar pulmonary oedema, and therefore left atrial pressure should be above 40 mmHg.
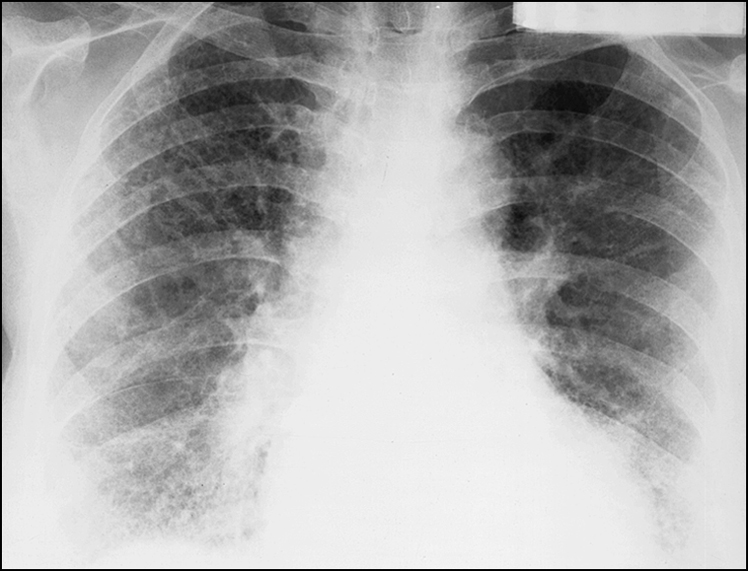
Figure 14.21 There are reticulonodular markings in the lower zones bilaterally. This is the honeycomb pattern of pulmonary fibrosis. The differential diagnoses include rheumatoid arthritis, asbestosis, scleroderma, idiopathic pulmonary fibrosis and chronic aspiration with fibrosis.
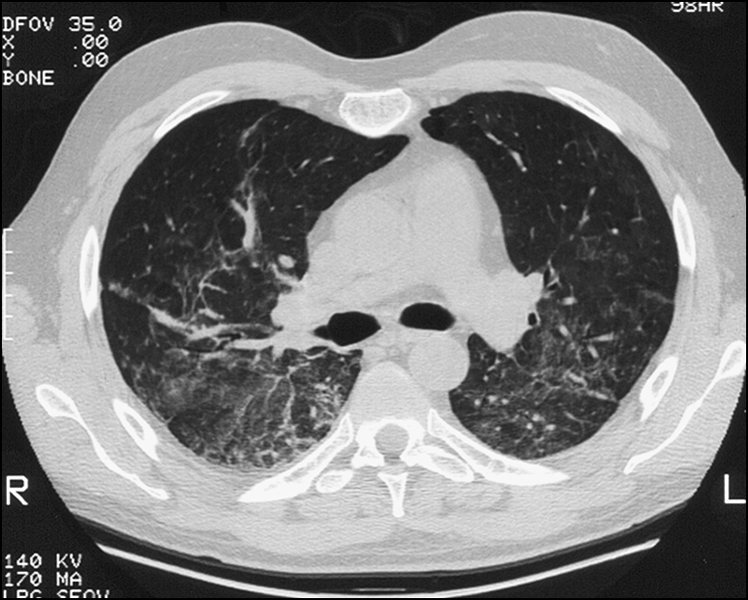
Figure 14.22 This high-resolution CT scan of the lung shows widespread air space opacification bilaterally. This opacification has the ground glass appearance suggestive of active interstitial inflammation.
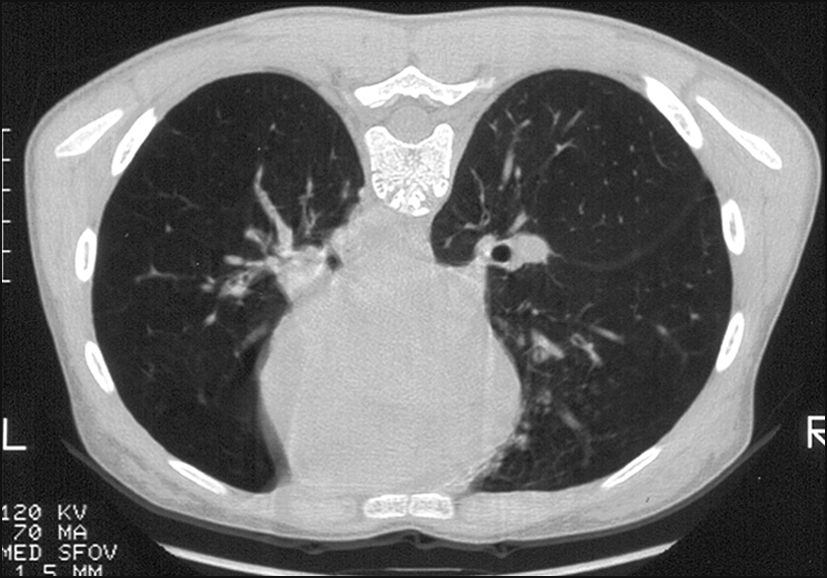
Figure 14.23 This high-resolution CT scan of the lung shows asymmetrical transradiancy between the two lungs. There are peribronchial cuffing, tramline markings and cystic spaces. This is bronchiectasis of the left lower lobe.
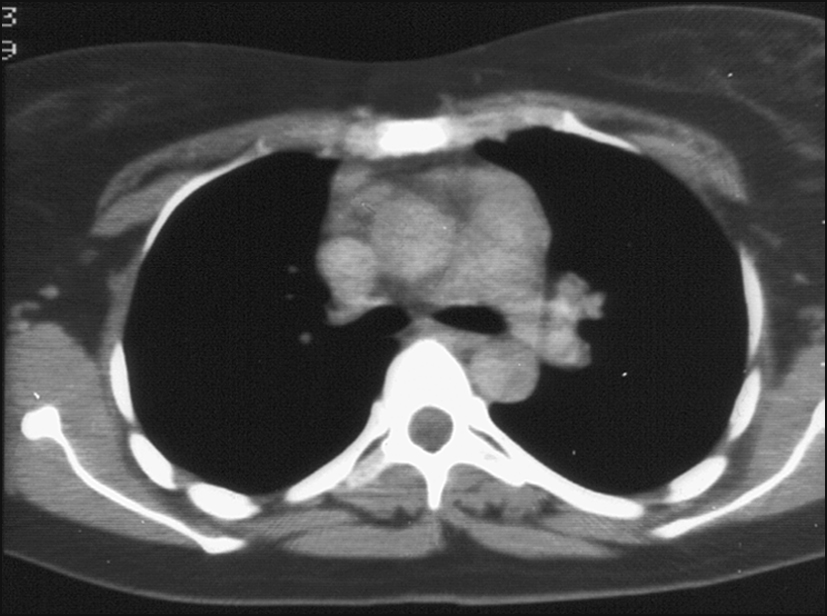
Figure 14.24 This thoracic CT scan performed in the mediastinal window shows mediastinal lymphadenopathy in both preaortic and aortopulmonary areas. The differential diagnoses include soft tissue malignancy, metastatic lung cancer and lymphoma.
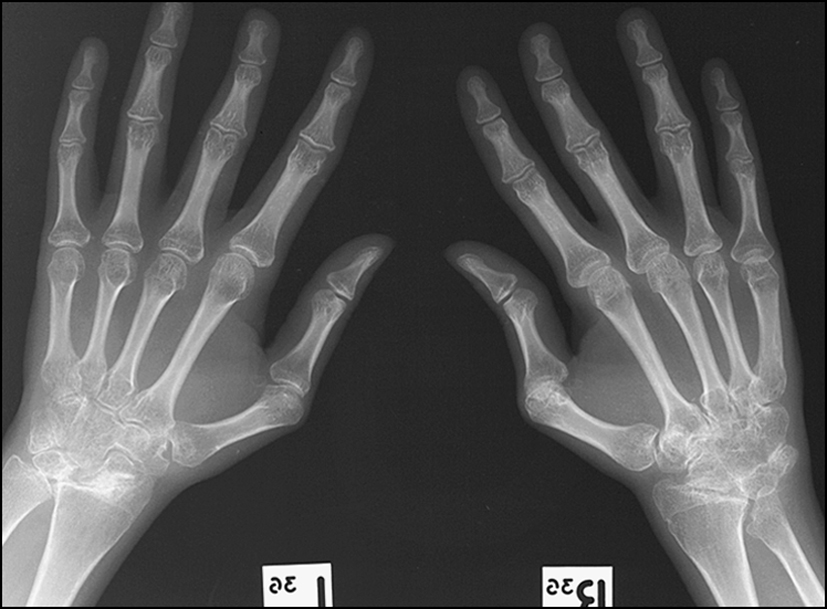
Figure 14.25 This X-ray of left and right hands shows periarticular osteopenia, ankylosis and deformity of the carpus, subluxation and deformity of the radiocarpal and distal radioulnar joints. There are periarticular erosions and erosion of the ulnar styloid. There is loss of joint space at the first metacarpophalangeal joint. This picture is consistent with rheumatoid arthritis of the hands.
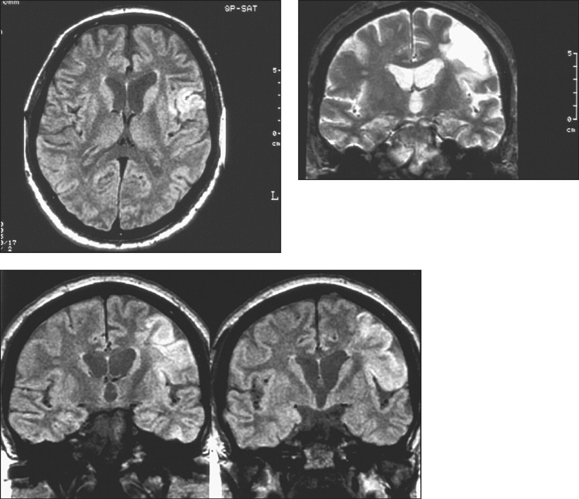
Figure 14.26 The MRIs show an area of abnormality in the left parietal lobe. There is a wedge-shaped area that is slightly hypointense on the T1-weighted picture and hyperintense on the T2-weighted picture. This is consistent with an area of infarction. In addition there are areas that are hyperintense on T1-weighted, T2-weighted and intermediate images consistent with extracellular methaemoglobin. This picture is consistent with subacute haemorrhage.
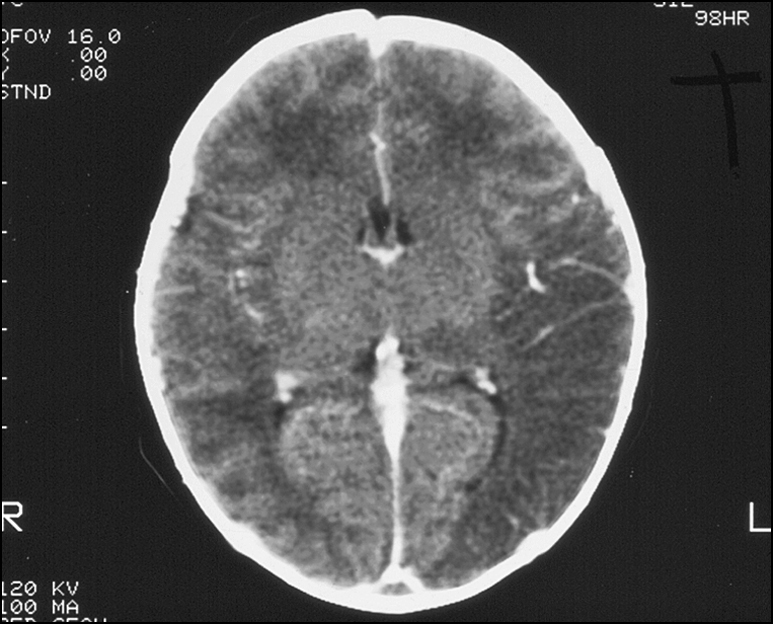
Figure 14.27 There are moderately well-defined low-density areas in the left posterior temporal, parietal and occipital cerebral lobes and similar smaller areas in the right posterior parieto-occipital regions. This picture is consistent with areas of watershed infarction.
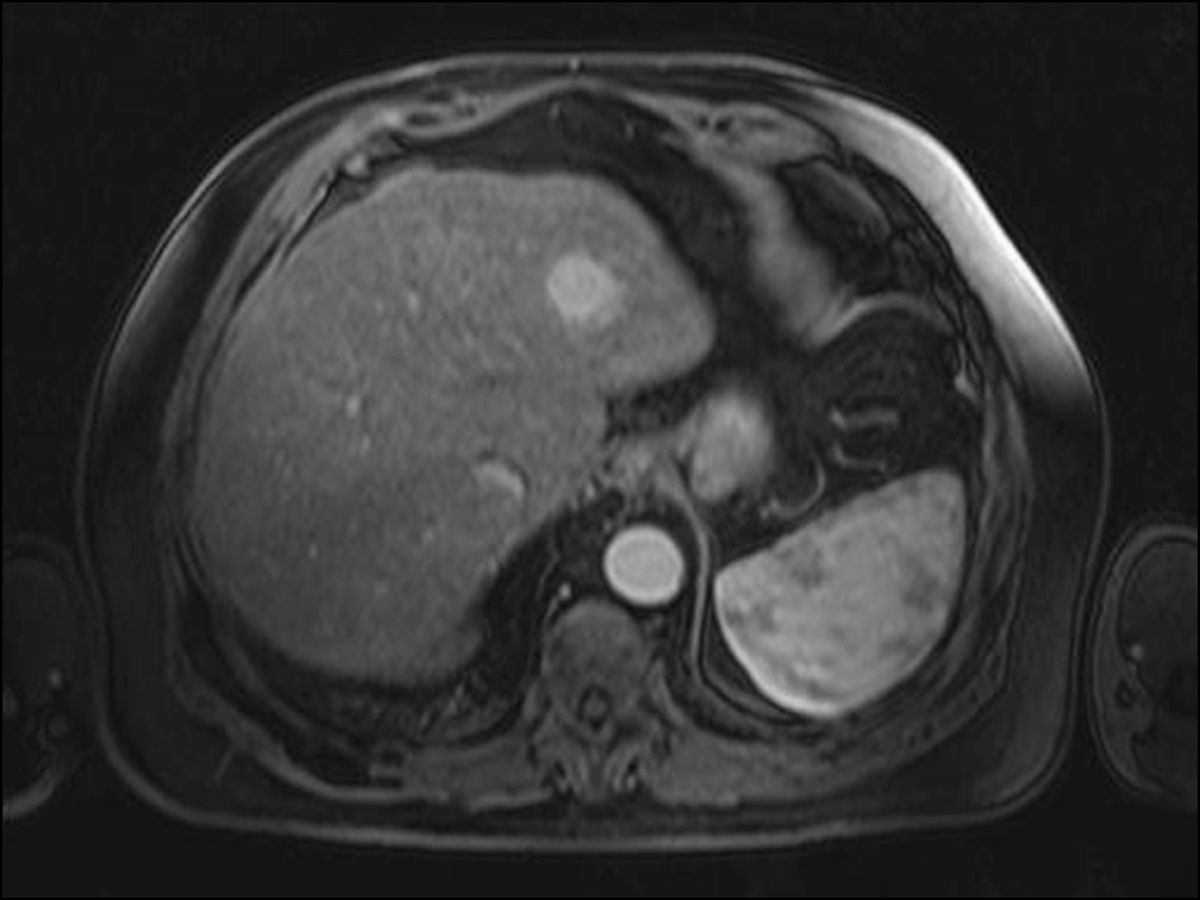
Figure 14.28 Cirrhotic liver with a contrast enhancing mass lesion
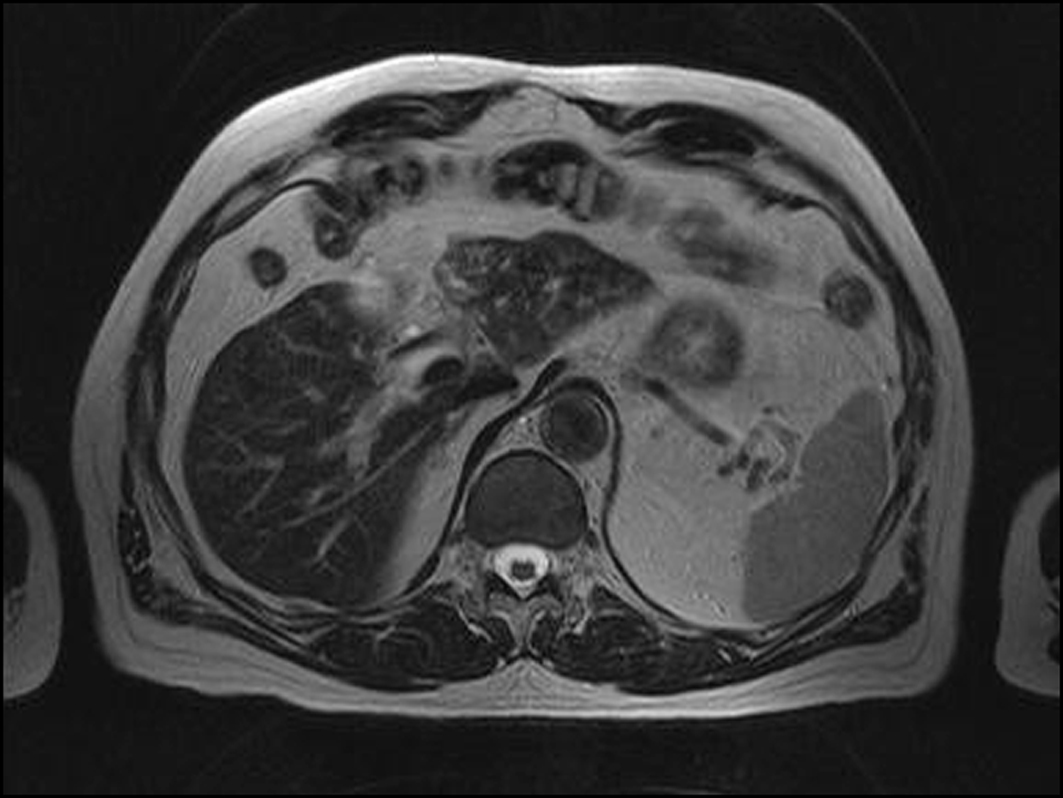
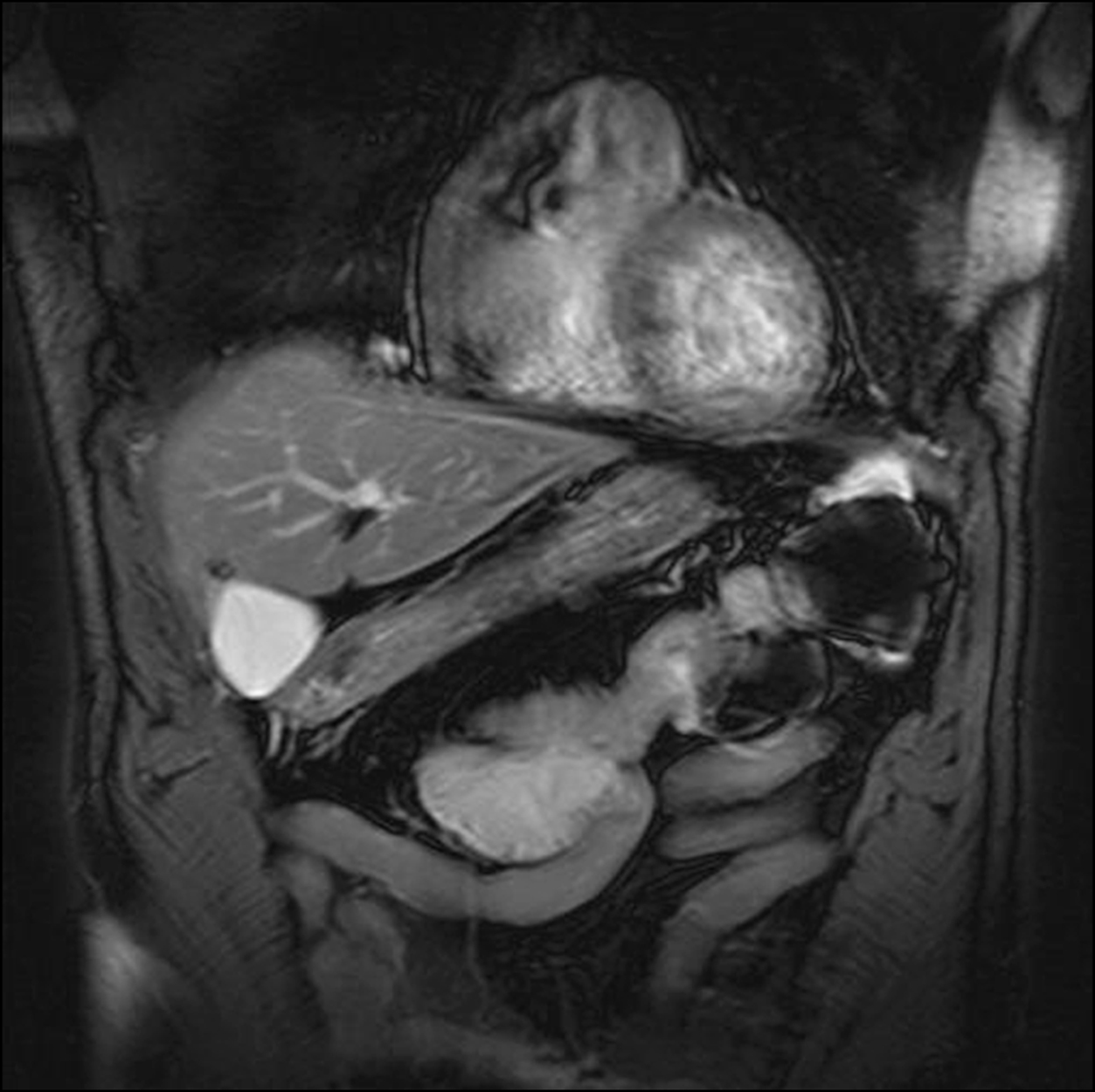
Figure 14.30 MRI of primary sclerosing cholangitis
CORONARY AND CARDIAC IMAGING
Coronary angiography
Echocardiogram
Coronary CT
Cardiac MRI
Intracoronary ultrasound
Intracardiac ultrasound
Electrophysiological study/RF ablation
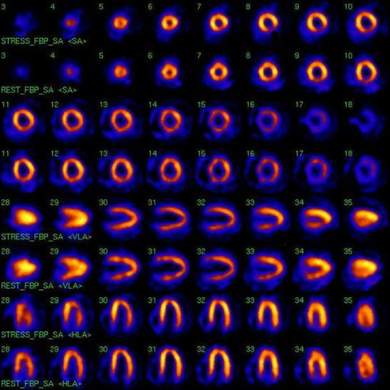
Figure 14.31 Technetium-sestamibi scan showing normal coronary perfusion at rest and impaired perfusion during stress. The top row of each view depicts perfusion during activity and the bottom row depicts perfusion at rest. In this scan the stress images show a defect in the left ventricular wall that normalises during rest.
(reprinted from Niederkohr R D, Daniels C, Raman S V 2008 Concordant findings on myocardial perfusion SPECT and cardiac magnetic resonance imaging in a patient with myocarditis. Journal of Nuclear Cardiology 15(3):466–8)
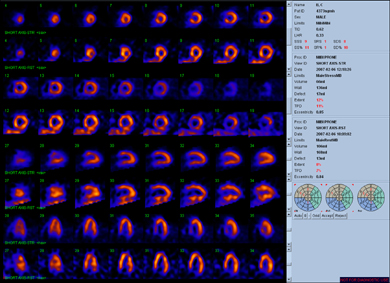
Figure 14.32 An abnormal sestamibi scan. The stress and rest tomographic images are labelled and the equivalent stress views are above the corresponding resting views. In this scan there is reversible ischaemia in the anterior wall.
(Image courtesy of Dr P Sullivan)
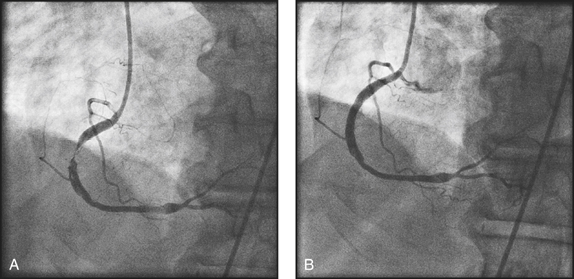
Figure 14.33 A 65-year-old man presented with exertional angina with a background history of hypertension and hypercholesterolaemia. His exercise stress test was positive for reversible coronary ischaemia. (a) Subsequent coronary angiography showed a significant stenosis of the right coronary artery. (b) This lesion was treated with angioplasty and stent placement.
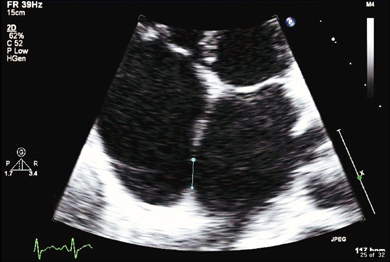
Figure 14.34 A 31-year-old woman presented with exertional dyspnoea. On examination she had fixed splitting of the second heart sound. On echocardiography there was a septum secundum type atrial septal defect (ASD). She was treated with percutaneous closure of the defect.
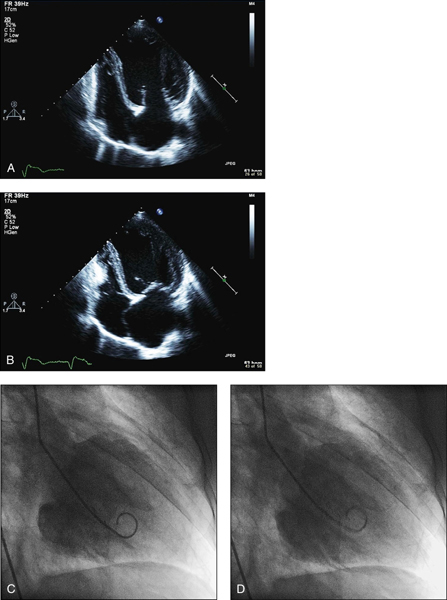
Figure 14.35 A 44-year-old man presented with progressive dyspnoea and peripheral oedema in the background of chronic alcoholism. On examination there was bilateral pitting oedema of the ankles and an elevated JVP. There was an audible S3 gallop in the precordium. There were bibasilar crepitations in the lung fields. Echocardiogram showed (a) left ventricular dilation and (b) impairment of systolic function. His coronary angiography was normal. (C and D) Left ventriculography showed severe impairment of systolic function.
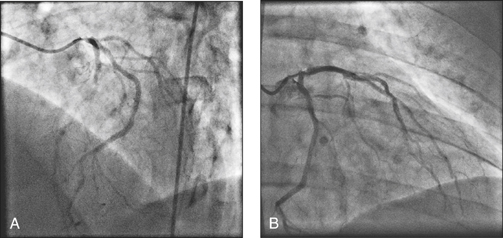
Figure 14.36 A 71-year-old man was brought in by ambulance after a cardiac arrest and successful resuscitation in the local shopping mall. Upon presentation the ECG showed deep T wave inversion in the septal and left lateral leads. Urgent coronary angiography showed significant narrowing at the osteum of the left main coronary artery (A and B). The patient was referred to the cardiac surgeon for urgent coronary artery bypass surgery, and in the meantime was anticoagulated with heparin.
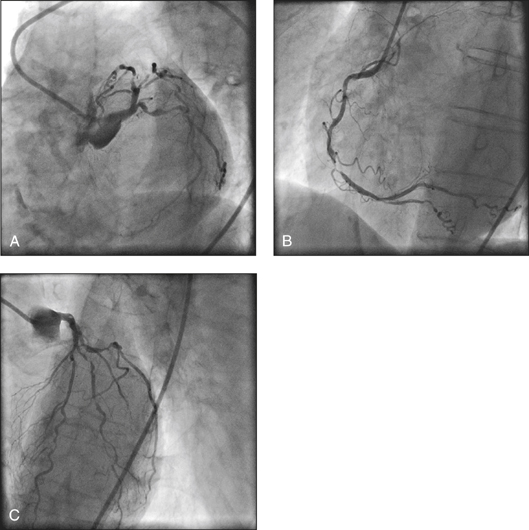
Figure 14.37 A 75-year-old woman with diabetes presented with 2 hours of retrosternal ache radiating down the right arm. She also complained of nausea. Her ECG showed ST segment depression of 2 mm in the inferior leads. She had cardiac biomarker positivity. Coronary angiography showed diffused, critical stenoses in all three coronary arteries (triple vessel disease). The patient was referred to the cardiac surgeon for coronary artery bypass surgery.
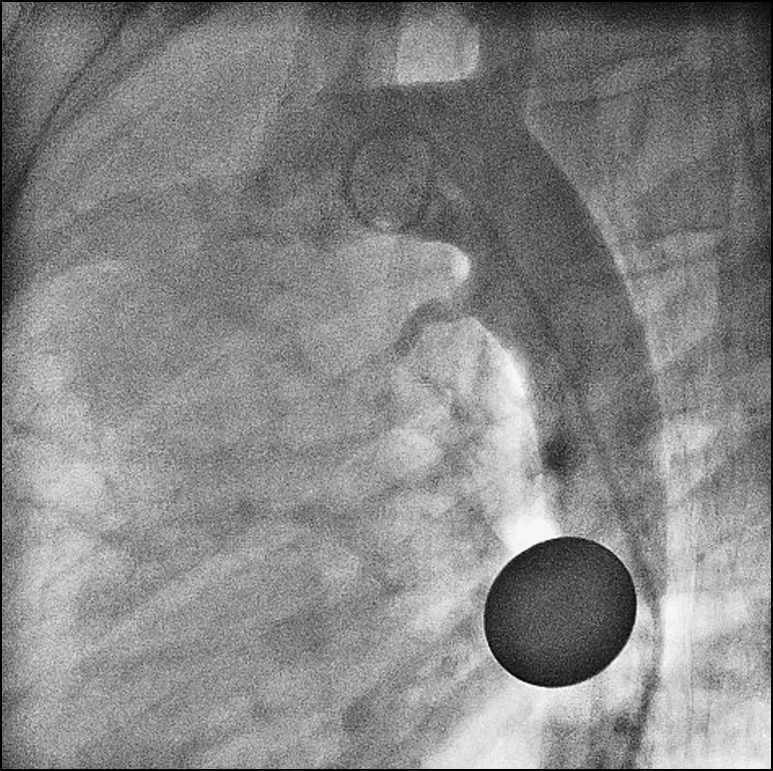
Figure 14.38 A 20-year-old woman was referred by the family practitioner upon the incidental finding of a machinery murmur heard all over the precordium. The murmur was loudest in the left subclavian region. Echocardiography showed a patent ductus arteriosus (PDA). This was confirmed by ascending aortography. Note the 10-cent coin placed over the patient’s chest during aortography to help estimate the size of the PDA. This PDA was closed percutaneously with a ductal occluder.
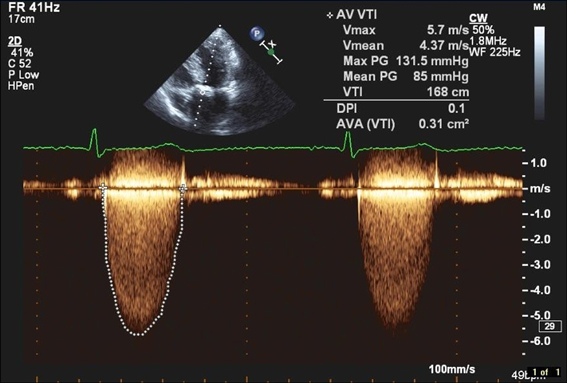
Figure 14.39 An 86-year-old woman presented with sudden loss of consciousness. On examination she had a slow rising pulse and a harsh ejection systolic murmur that radiated to her carotids bilaterally. Echocardiography demonstrated a calcified aortic valve with a critical gradient on Doppler assessment. Note the maximum and mean pressure gradients of significance. She was referred to the cardiac surgeon for valve replacement.
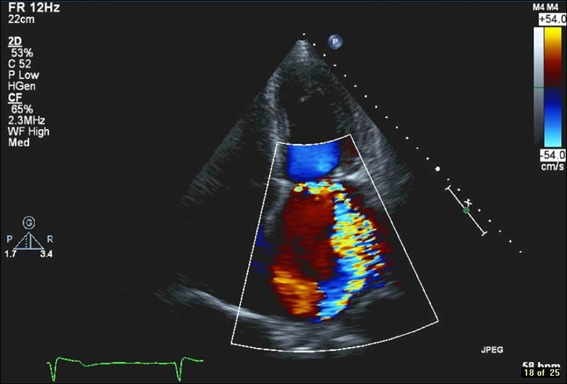
Figure 14.40 A 60-year-old man who presented with retrosternal chest pain was diagnosed with acute coronary syndrome. There were deep T wave inversions in the lateral lead in the ECG. He had significant troponin level elevation. He subsequently developed an apical systolic murmur that radiated to his left axilla. Echocardiography showed an eccentric jet of mitral regurgitation on colour flow Doppler assessment. Coronary angiography showed a tight stenosis of the large left circumflex coronary artery. This lesion was treated with angioplasty and stent placement. Subsequent echocardiography showed significant improvement of the mitral regurgitation. The valve defect was due to papillary muscle ischaemia associated with the circumflex artery stenosis.
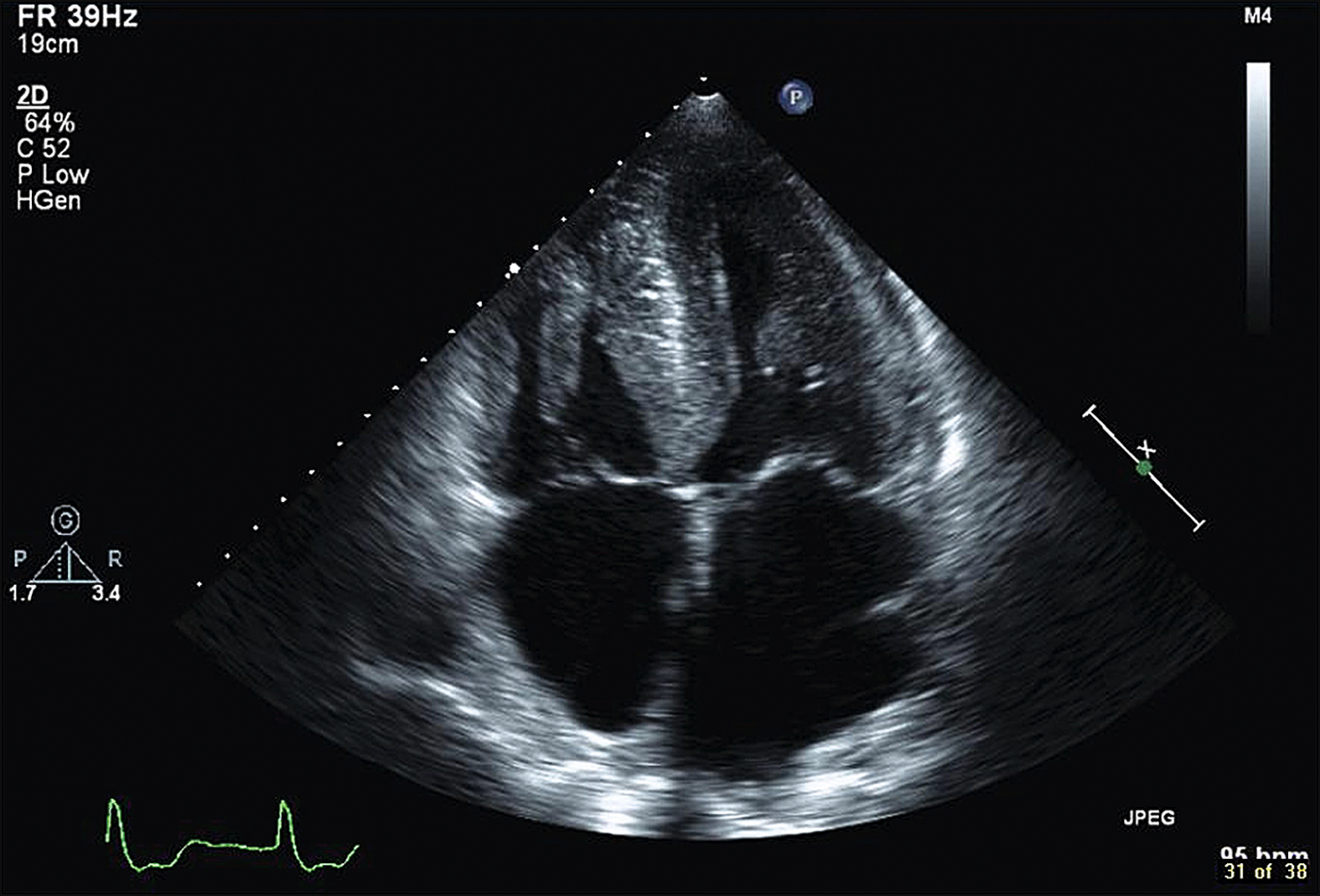
Figure 14.41 A 74-year-old man presented with dyspnoea, orthopnoea and paroxysmal nocturnal dyspnoea. On echocardiography there was severe hypertrophy of the left ventricle with impaired diastolic filling.
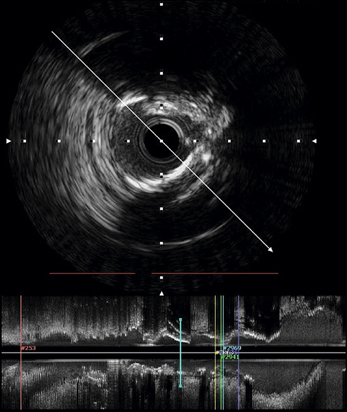
Figure 14.42 A 60-year-old smoker presented with angina on exertion. Coronary angiography showed stenosis of the left anterior descending (LAD) artery. This lesion was further characterised and measured with intravascular ultrasound (IVUS) imaging. The lesion was subsequently treated with a coronary stent
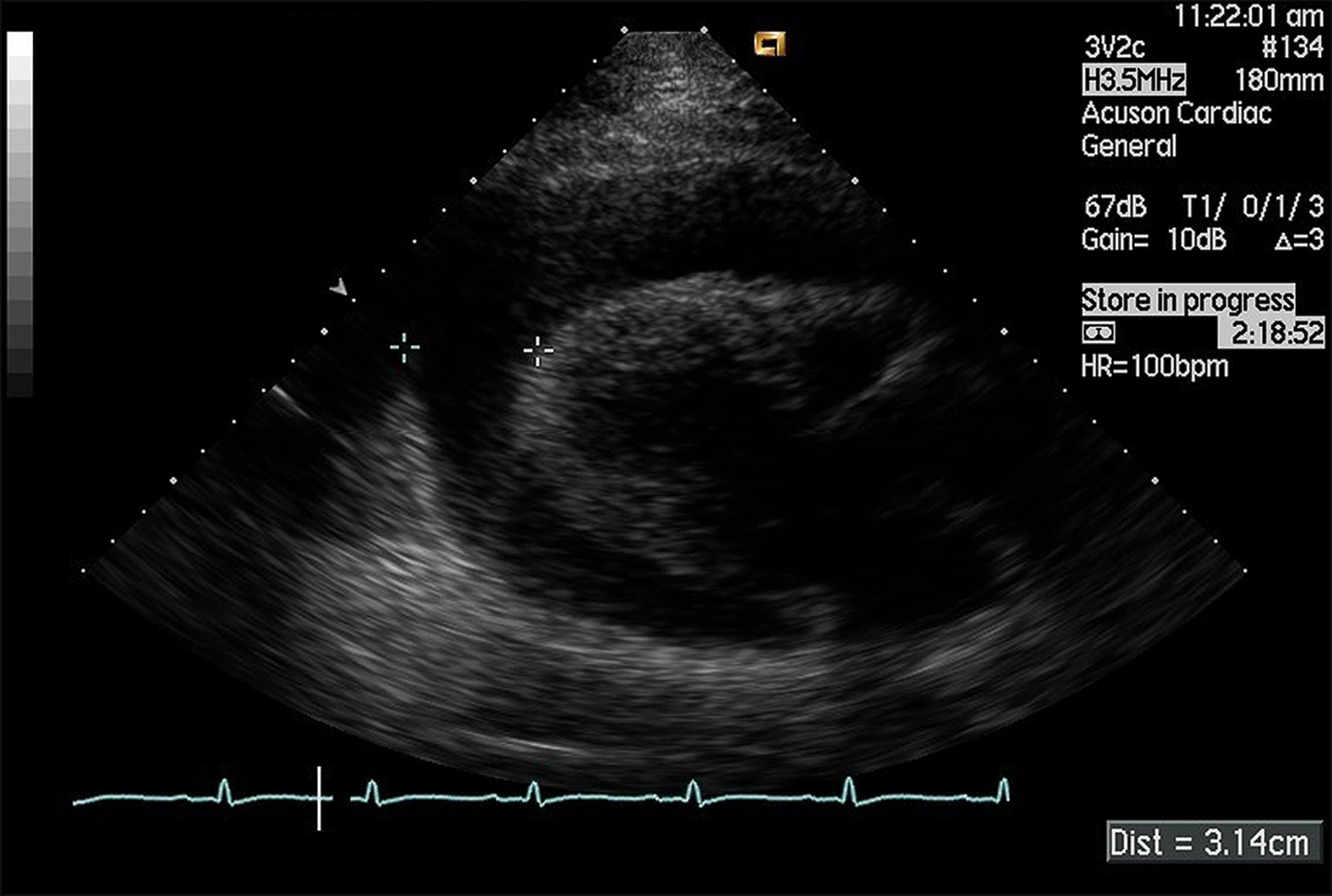
Figure 14.43 A 47-year-old woman with a past history of breast cancer presented with syncope. On examination she was hypotensive at 80/40 mmHg and her pulse rate was 120 bpm. Her JVP was elevated with evident Kussmaul’s sign. There was pulsus paradoxus. Echocardiography revealed a significant pericardial effusion with evidence of cardiac tamponade. Note the large collection of fluid around the left ventricle.
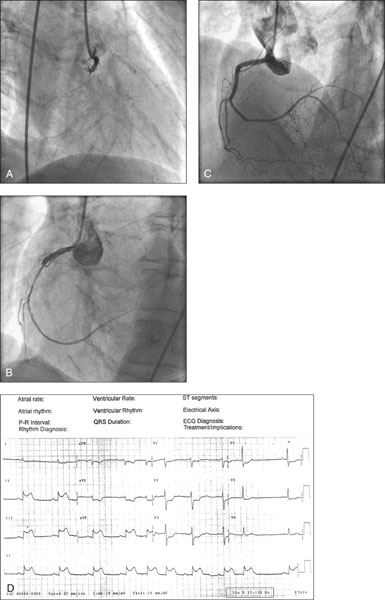
Figure 14.44 A 46-year-old heavy smoker presented with severe retrosternal chest tightness, nausea, vomiting and diaphoresis of 1 hour’s duration. He was hypotensive on assessment. The 12-lead ECG showed ST segment elevation of 2 mm in leads II, III and AVF, confirming the diagnosis of ST elevation myocardial infarction (STEMI) (see figure D). The patient was referred to the interventional cardiology team for primary percutaneous intervention (primary PCI). Angiography revealed an occluded right coronary artery (RCA) which was reopened with balloon angioplasty and stenting.
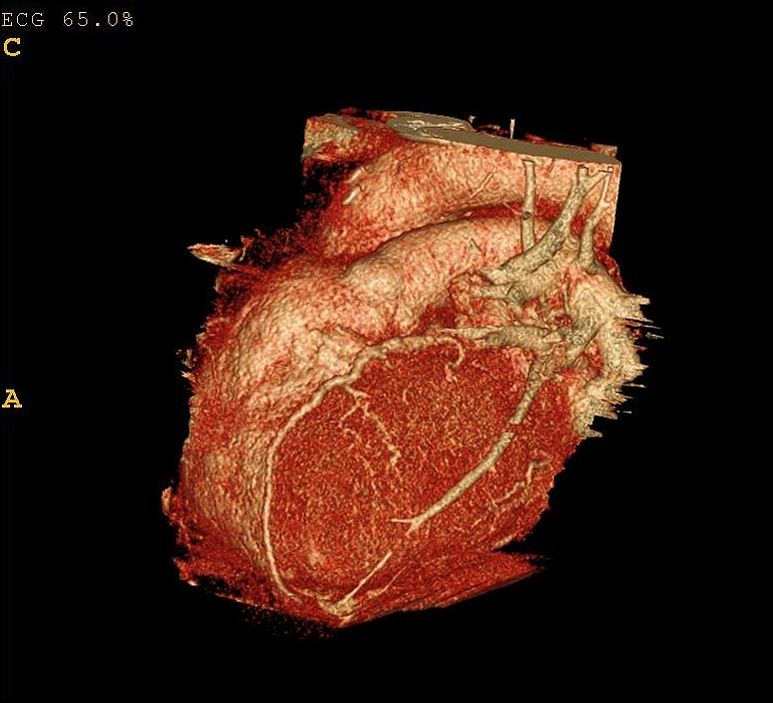
Figure 14.45 CT coronary angiogram
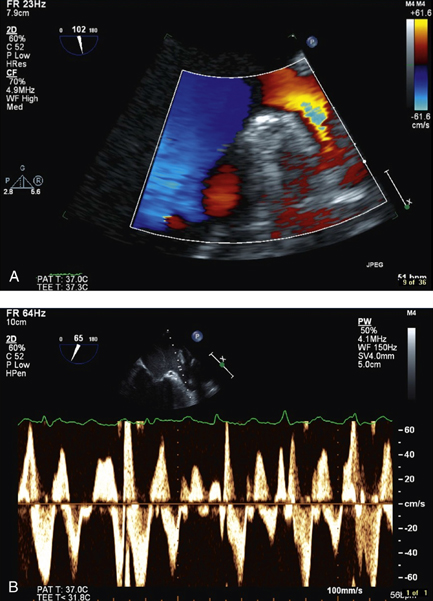
Figure 14.46 A 52-year-old man with new-onset atrial fibrillation underwent transoesophageal echocardiography (TOE) prior to direct current cardioversion. There was no thrombus seen within the left atrial appendage and the Doppler flow assessment ruled out stasis in the vicinity.