CHAPTER 59 Investigation of Human Cognition in Epilepsy Surgery Patients
Recent advancement of functional imaging technology has contributed significantly to an explosion of knowledge in this field. In particular, the advent of functional magnetic resonance imaging (fMRI) has made research into human brain function more approachable than ever before. This technique has made it possible to map each brain function with millimeter resolution in three-dimensional space.1,2 fMRI visualizes activities of the brain associated with a given brain function by detecting changes in the oxygenation level of local blood, which are in turn mediated by changes in metabolic demand and the subsequent response of blood flow to these metabolic changes. Because of the time lag of the hemodynamic response, this technique has inherent weaknesses in the speed of response.
Intracranial electrocorticography (ECoG) in patients with medically refractory epilepsy provides a unique opportunity to record human brain activity directly, with a high degree of spatial and temporal resolution.3,4 This technique complements the more widely available methods mentioned earlier. Although the spatial extent of ECoG investigation is limited to the area covered by the intracranial electrodes, this method is capable of localizing neuronal activity with far better spatial accuracy than the MEG or scalp EEG methods, and it can offer better temporal resolution than fMRI.
Methods
Subjects
Subjects are epilepsy patients whose seizures are refractory to nonsurgical treatment and who undergo invasive seizure monitoring in an effort to determine whether they are suitable candidates for resection surgery.5,6 Research protocols must be scrutinized and approved by the institutional review board where the research will be taking place according to the ethical guidelines of the institution’s governing bodies.7 In all cases, the plan for electrode placement is influenced exclusively by clinical criteria. Participation in this research does not change the risks associated with epilepsy surgery. The research plan is explained to research participants in detail, and informed consent for participation is obtained in advance. Particularly when chronic recordings are obtained over a period of days, this consent process is informally repeated before each experimental session. In most cases, patient-subjects wish to participate in these protocols but on certain days after surgery may elect to forgo testing. Because of this need for iterative informed consent and the practical considerations that accompany this requirement, most research of this type is carried out only with adult subjects.
Electrodes
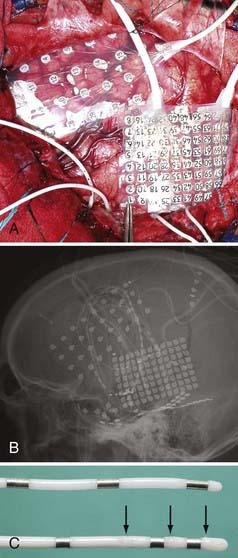
Several different types of clinical and combined clinical-research electrodes are available for invasive monitoring of seizure activity. Usually, the signals recorded from a given electrode contact can be split and used for both clinical monitoring and research purposes. This does not disrupt the clinical ECoG recording activity. There are two broad categories of intracranial electrodes: (1) subdural cortical surface electrodes in the form of either grids or strip electrodes and (2) depth electrodes (Fig. 59-1). The extent of coverage is decided solely by clinical necessity. Electrodes need to cover wide cortical surface areas and deep structures sufficiently to diagnose the seizure foci accurately. The specific implantation strategies used by different groups across the United States and elsewhere in the world to achieve this objective vary widely. Some programs use surface grids and strips almost exclusively, whereas other highly respected programs (e.g., Paris and Grenoble, France) use depth electrodes exclusively and implant more than 10 penetrating depth electrodes in a single hemisphere in many cases. There is no evidence proving the clinical superiority of any of these specific strategies, and in practice institutions have evolved to adopt a range of safe and effective approaches.
In addition to standard clinical electrodes, a variety of specially modified electrodes are available that can collect research data in addition to clinical ECoG data. Some manufacturers make customized electrodes to suit each researcher’s need (e.g., Ad Tech Corporation, Racine WI). Most clinical grid or strip electrodes are constructed with a center-to-center intercontact distance of 1 cm. High-density electrodes with less than 5 mm of interelectrode distance provide better spatial resolution and can be fabricated without altering the clinical risk profile of the grid.8 Custom depth electrodes have several high-impedance microwire contacts in addition to clinical low-impedance contacts (see Fig. 59-1).9–11 These high-impedance wires make it possible to record unit activity from the human brain. Some of the custom electrodes have more electrode contacts and lead cables attached to them than standard clinical electrodes do. The single-tailed electrode cables that some manufacturers provide can reduce the number of cables by combining multiple lead cables (up to 64 channels) into a single bundle, thus reducing the number of penetrations through the scalp.
Implantation Surgery
Accumulation of blood in the subdural space either beneath or above the grid electrodes sometimes occurs.6,12–15 Although the exact mechanism of such blood accumulation is unknown, it is presumed that direct contact between the base plate of the grid electrodes and the dura mater may disturb normal hemostatic and resorptive processes. At our institution, we perform the following procedures in an effort to prevent accumulation of blood in the subdural space:
Verification of Electrode Placement
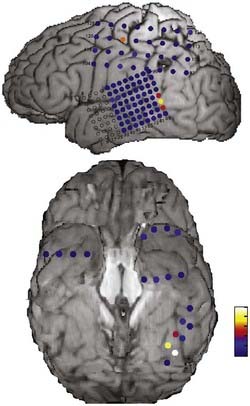
It is important to localize electrodes accurately in relation to surrounding brain structures to correctly interpret research data. Preimplantation and postimplantation computed tomography (CT), MRI, and photographs taken during both implantation and removal surgery are the three main tools used to localize the position of electrodes. Intracranially implanted electrodes create substantial artifact and distortion of images on CT and MRI, so extra caution is required when interpreting postimplantation imaging studies. The location of electrode contacts on a grid is best documented by photographs taken at the time of both implantation and explantation surgery. By matching the details of gyral and pial vessel anatomy, it is possible to localize surface contact locations with approximately millimeter accuracy. The position of electrodes is mapped onto a three-dimensional rendering of the brain surface drawn from each subject’s preoperative thin-slice MRI studies by referencing to a pattern of gyri and sulci on the cortical surface (Fig. 59-2).
Electrode contacts on a depth electrode can be localized in relation to surrounding brain structures by postimplantation MRI. Only the larger, low-impedance contacts can be clearly delineated on postimplantation MRI, but with knowledge of the spacing of microwires positioned between these contacts, it is possible to depict accurately where these recording sites are within the brain, and these locations can be depicted on the preimplantation MRI study.8,16,17
Electrical Stimulation
Electrical stimulation of the brain has been used for the investigation of brain function for close to a century and is still the “gold standard” for neurosurgical functional mapping to minimize the risk for postoperative functional deficits.18 Electrical stimulation of the cortex is believed to either facilitate or disrupt local brain function in the vicinity of a stimulation site, depending on the stimulation parameters. Exactly how the frequency of electrical stimulation modulates neural activity within the stimulated structure is poorly understood and is the subject of many ongoing experimental investigations. With that caveat, it is generally believed that high-frequency stimulation well above 50 Hz exerts an inhibitory effect on the firing of neurons in the brain region exposed to suprathreshold currents. Stimulation in the 50-Hz range may activate sensory cortices and cause a subject to experience a sensory perception, but stimulation of language critical sites will disrupt function without generating a percept. This technique is useful for the investigation of local cortical and subcortical function not only in the clinical setting but also as a research tool.
Averaged evoked potentials elicited by electrical stimulation can be used for the investigation of connectivity between remote brain sites. By manipulating stimulus parameters, stimulation site, and recording site, it is possible to delineate functional connections in various brain regions.19,20
Cooling
Cooling of brain tissue is known to modulate brain functions and has been used extensively in animal research,21–24 as well as in human clinical settings.25–27 Cortical cooling is a promising research tool to investigate local brain function.28,29 Cooling brain tissue is known to block synaptic transmission reversibly within a highly localized region.30 In contrast, electrical stimulation has the inherent problem of exerting effects well beyond the site of stimulation as a result of axonal conduction to functionally connected brain regions. The patterns of local spread of electrical stimulation currents within brain tissue are poorly understood. Neural activity induced by local stimuli may be transmitted to cortical and subcortical regions that are anatomically connected to the stimulation site, thereby disrupting or altering neuronal activity at these sites. Focal cooling can avoid the possibility of such remote effects. Brain blood flow exerts a robust effect on maintenance of tissue temperature. The area of low temperature is tightly restricted to within several millimeters of a brain cooling source, thus limiting the cooling effect to a local phenomenon.29,31
Measurement of Physiologic Phenomena
While subjects are performing cognitive tasks, various physiologic measures can be recorded along with the ECoG recording. Such measurements include eye tracking, the electrocardiogram, the respiratory rate, oxygen saturation, and skin conductance. These simultaneous recordings are useful objective measures that complement the ECoG data set and allow investigators to examine underlying cognitive processing in the brain in a more sensitive and controlled manner. Contemporary eye-tracking methods allow the investigator to accurately measure and record eye movements, as well as alterations in pupillary size; in many experimental studies such data have been demonstrated to reflect underlying neuropsychological processes in human subjects.32–36 Pupillary diameter readings are valuable measures of the status of the autonomic nervous system, along with skin conductance, heart rate, blood pressure, and respiratory rate.
Cognitive Studies
Cognitive Studies in the Medial Temporal Lobe
The medial temporal lobe is often involved in the generation of seizures in surgically treatable epilepsy patients37–39; thus, in many cases the ECoG recording electrodes are placed in this brain region. Neural structures in the medial temporal lobe play important roles in cognitive functions related to emotion, memory, and learning. For these reasons, the medial temporal lobe is one of the brain regions most extensively investigated by neurosurgeon-scientists who perform human cognitive research in epilepsy patients.4 Various recording techniques with modified electrodes permit finely detailed analysis of single-unit, multiunit, and local field potential activity in conjunction with sophisticated cognitive tasks. The combination of fine temporal and spatial resolution of these invasive recordings cannot be matched by any existing noninvasive research methods.
With this experimental approach, neurons in the medial temporal lobe, including the hippocampus, parahippocampal gyrus, and entorhinal cortex, were found to be activated by responding to visual stimuli of specific items.40 The activity of a subset of these neurons was correlated with behavioral performance, in this case it was whether the visual stimulus was successfully memorized. The response patterns of memory performance–related neurons were different between the hippocampus and entorhinal cortex, thus suggesting that different medial temporal subregions may contribute differently to different aspects of the memory and learning processes.41 In addition to item-specific neurons, category-specific neurons were found.42 Some of these neurons responded not to what subjects were seeing but to what subjects perceived, imagined, or attended.43,44 There were also neurons that were activated by spontaneous emergence of conscious recollection.45
Involvement of the medial temporal lobe in spatial navigation has also been investigated extensively. Two different kinds of neurons were found, one representing “place” cells that respond to specific spatial locations and the other representing “view” cells that respond to specific views of task-relevant landmarks.46 There was a difference in distribution of these cells between the hippocampus and entorhinal cortex. The activities of some place cells were modulated by specific combinations of goals of task and place; for example, certain cells were activated only when a particular house was found in a particular location. Another study revealed the existence of movement-related theta oscillations in the hippocampus and the neocortex, and these theta oscillations were significantly correlated with various cognitive functions.47
Local field potentials in the medial temporal lobe were used for the investigation of mental activity as well. Gamma-band activity in the parahippocampal gyrus and ripple oscillations (100 to 200 Hz) in the hippocampus and entorhinal cortex were found to be correlated to awake and sleep states.48 Ripples in the rhinal cortex were found to be correlated to memory performance.49 Functional coupling between these regions, which can be measured by coherence in gamma-band activities, was found to be correlated to successful memorization.50,51
The amygdala is known to be involved in defense mechanisms represented by the fight-or-flight response. A large volume of animal and human research has demonstrated that fearful emotion is modulated by activity of the amygdala.52–56 It has been shown that gamma-band activity in the amygdala is enhanced by aversive pictures.17 The amygdala-specific research carried out in neurosurgical research subjects provides unique insight into the precise timing and electrophysiologic nature of amygdala activation.
Cognitive Studies in the Ventral and Medial Prefrontal Cortex
The ventral and medial sector of the frontal lobe has undergone extensive evolutionary development in humans in comparison to other primates, not to mention other animals. The function of this region is implicated in highly human behavior, such as social interaction, moral judgment, fairness, self-control, prediction of the future, and decision making in conflict situations.57–59 A number of functional imaging studies have shown representation of these cognitive processes in this region of the brain.60–70 Individuals who sustain damage to this region of the brain do not usually have deficits in intellect that can be measured by conventional intellectual tests; however, they often exhibit inappropriate social behavior and poor judgment with regard to their social and financial well-being.59,71 Their choices are more influenced by immediate rewards even though the consequences of their choices are predicted to cause substantial disadvantage to them.72,73
Anatomic studies have shown that the ventral and medial sectors of the prefrontal cortices have abundant connections with the sensory areas of multiple modalities and subcortical structures, including the hypothalamus, thalamus, amygdala, and brainstem.74–76 The connectivity of this region supports the proposed function of this area, specifically, processing of various multimodal sensory inputs to modulate behavior, including visceral and autonomic function, to match behavior to fit appropriately to the situation in which an individual is placed.
Investigation of Emotion Representation
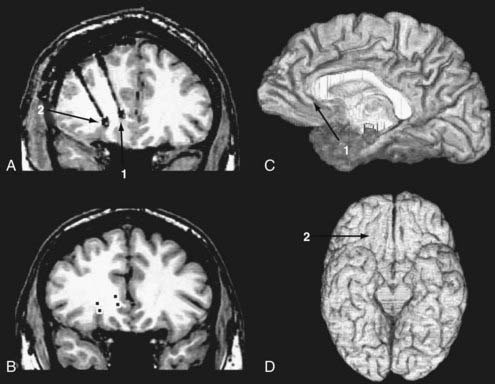
We have applied the single-unit recording technique to the investigation of responses of the ventromedial prefrontal cortex to pictures of complex emotional scenes.16,77 All patients suffered medically intractable epilepsy, and the preoperative epilepsy evaluation suggested a seizure focus residing in the frontal lobes; therefore, chronic EEG monitoring with depth and subdural electrodes covering the frontal lobes was warranted. We implanted custom-made depth electrodes that had eight high-impedance microcontacts incorporated in an electrode shaft along with four low-impedance clinical EEG contacts. Selection of target locations was based on preoperative MRI, and implantation of the electrodes was performed by using either the CRW stereotactic implantation system (Integra, Plainsboro, NJ) in earlier cases or the Stealth Station frameless stereotactic system (Medtronic, Minneapolis, MN) in later cases. We waited more than 5 days after the implantation surgery until we started conducting research protocols that involved complicated cognitive tasks. By the time we started research recordings, doses of analgesic and antiseizure medications had been tapered so that they did not affect subjects’ cognitive functions. At the time the studies were conducted, the subjects had fully recovered from the effects of the implantation surgery. Because the primary purpose of chronic seizure monitoring is to record seizure activity, it is not uncommon for subjects to have partial or complex seizures that may affect local brain activity. To avoid unwanted influence of such seizure activity on subjects’ cognitive function, we conducted research recordings only when subjects had not suffered seizures within the 12 hours preceding the recording session. After the chronic seizure monitoring was completed, we confirmed that the regions from which the recordings were obtained were distant from the seizure foci and also that the recordings were not contaminated by frequent interictal discharges.
Unit recording picks up field potentials associated with action potentials of neurons that are located within 100 µm from a high-impedance contact. The locations of recording sites were determined with the methods described earlier in this chapter, and the location of each electrode contact was delineated on the preimplantation MRI studies (Fig. 59-3).
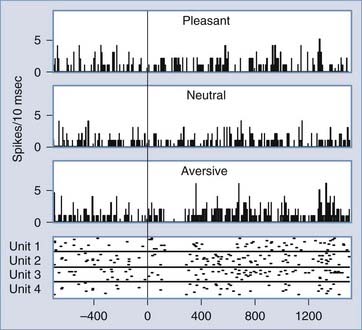
We investigated unit responses in the medial and ventral frontal cortex to visual stimuli that depicted pleasant, neutral, or aversive scenes. These scenes were part of the International Affective Picture System (IAPS), which has been used widely for studies of emotion.78 The aversive stimuli include pictures of wartime, mutilated bodies, burn victims, and so on. These pictures are visually heterogeneous and do not have any low-level or simple visual features in common that might explain the different responses to different emotion categories that we observed. In one subject, we found unit responses that were specific to aversive stimuli and very rapid in onset (Fig. 59-4).16 The latency of responses spanned between 120 and 140 msec. Rapid responses of this latency are most likely explained by feed-forward responses from the occipitotemporal visual cortices to the medial and ventral frontal cortex without any intervening feedback modulation.79 It is interesting to note that the unit responses to aversive pictures showed biphasic response patterns consisting of an initial rapid and brief decrease in firing and a later prolonged increase in activity. The later prolonged excitation is a possible reflection of recursive responses with multiple feed-forward and feedback interactions among multiple brain sites anatomically connected to the medial and ventral frontal cortex. These responses with different timing may reflect early coarse categorization of the emotional significance of visual scenes and later more detailed neural processing related to evaluation of the biologic value to an individual, prediction of consequences, triggering of autonomic responses, and planning of future action.
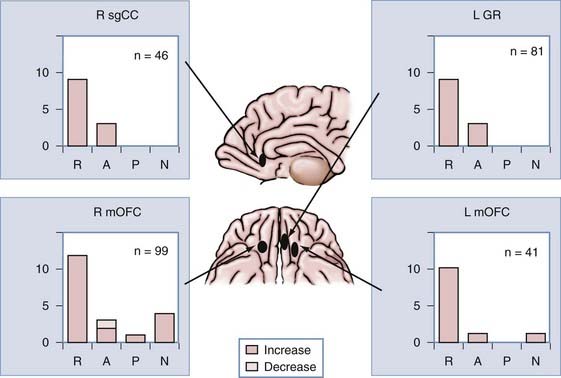
In addition to the subject just described, we recorded unit responses to pictures from the IAPS battery in the bilateral medial and ventral frontal cortices of three more subjects. Figure 59-5 shows the distribution of emotion-selective neurons at four different sites in these regions from a total of four subjects.77 Although aversive pictures recruited predominantly more neurons than did any other stimulus category in all sites, there is no clear topography of preferred emotional content. Because sampling of our unit recording is sparse (in other words, we recorded at only a few discrete brain sites in a small number of subjects), we might have missed an emotional topographic representation. It is premature to draw conclusions from our data about which hemisphere is predominantly involved in emotional processing, whether there is a preferred emotion that is processed in each hemisphere, or whether there is emotional topographic representation within a hemisphere. However, it is noteworthy that a minority of units responded to pleasant and neutral pictures at all locations. This provides evidence that information about the emotional content of visual scenes is intermingled within the medial and ventral frontal cortices.
Other investigative methods, including the blood oxygen level–dependent (BOLD) response, MEG, and ECoG, require many neurons to fire synchronously to be detected. Therefore, if a tiny fraction of neurons are active within a region, such activity cannot be detected by these methods. Conversely, it is possible to detect such underrepresented activities with unit recordings, as shown in the aforementioned study. Our findings are consistent with current knowledge about this region gained through a large amount of research in animal models and functional imaging studies in humans.80,81 Neurons within the orbitofrontal cortex have been shown to respond selectively to stimuli of a variety of sensory modalities based on their emotional significance, rewarding or punishing valence, or motivational value.
Investigation of Expectation of Reward and Punishment
The Iowa Gambling Task is a well-validated test for identifying and quantifying functional deficits associated with damage to the ventromedial prefrontal cortex. Deficits in this region have otherwise been very difficult to detect with other cognitive tasks designed to investigate frontal lobe function.58,82 In the Iowa Gambling Task, subjects choose cards from four decks in order to win or lose money. Each deck is assigned a different probability of gain and variance of win-loss value for each draw of a card. For example, one deck consists of a majority of a moderate amount of wins but occasional large losses, and in the long run the sum of draws would end up losing money. Another deck consists of many small wins and occasional moderate losses; however, the sum of draws from this deck would eventually end up winning money. Subjects do not know which deck is a winning deck and which one is a losing deck when they start the task. After drawing cards a number of times, subjects develop an expectation of what is likely to happen (win or lose) when they choose a card from a given deck on the basis of experience. Furthermore, subjects’ behavior comes to be influenced by the expectation. As such, subjects tend to choose more from decks where they feel that they will make more money in the long term and to avoid decks that may incur larger wins but lose money in the long term. Such expectation is probably unconscious in the early stages of its development. We applied a reinforcement-learning algorithm to model the process of development of expectation and choice selection behavior.83 The model incorporated the probability of selecting from each deck and the degree of expectation. The model estimated the value of all choices, which parallels the expectation from each deck. A reward prediction error is an index for measuring the discrepancy between expectation and reality and is derived by subtracting the estimation from the result of a draw.
We presented the Iowa Gambling Task to an epilepsy patient who had multiple electrodes in his frontal lobes.84 He performed the task as normal subjects would. He initially chose from all decks; however, with experience he learned to choose from good decks and to avoid choosing from bad decks. This provided evidence that the ventral and medial parts of the subject’s prefrontal cortex were functioning normally. While the subject was performing the task, we recorded local field potentials at three sites in the right frontal lobe with two custom depth electrodes: the granular paracingular cortex (area 10m), the middle frontal gyrus, and area 11l. The custom depth electrode had multiple high-impedance microwire contacts. To record local field potentials, we performed bipolar recording with a pair of closely positioned microcontacts at each recording site; as such, far field potentials would effectively cancel out.
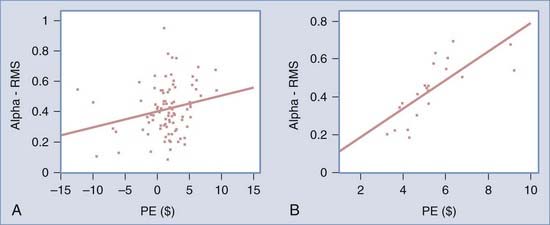
We found that reward prediction error correlated with the alpha-band component of the event-related potential recorded in the ventral and medial prefrontal cortex. No such correlation was found in other parts of the frontal lobe or in other frequency bands. Further analysis revealed that this correlation was significant only when a subject chose the risky decks but punishment was not delivered (Fig. 59-6). In other words, the alpha-band component of the EEG in this region was positively correlated with the magnitude of the difference between what the subject obtained and expectation only when the subject expected punishment but was unexpectedly rewarded. These findings are consistent with other studies suggesting that the ventral and medial prefrontal cortex is involved in updating the expectations of punishment or reward and in reinforcement learning.85–90
Conclusion
Intracranial recording of human epilepsy patients provides an invaluable platform for conducting cognitive neuroscience research. The data collected during these experiments cannot be obtained with alternative, noninvasive methods. Because of an abundance of opportunities to record from the medial temporal lobe, the majority of research up to now has been focused on the activities of neurons in this region in relation to learning and memory, spatial navigation, and emotional responses. Recently, application has been expanded to recordings of other regions and to the investigation of more higher order cognitive processes such as imagery,43 awareness,44 and consciousness.91,92 The development of hybrid depth electrodes that allow unit recording and the availability of more than a few hundred channels of multichannel ECoG recording, affordable high-speed personal computers, and advanced analytic techniques will permit investigation of more complicated cognitive functions that involve multiple distributed brain locations and multiple scales of neural signals. Analysis of the dynamic interaction of distributed neural systems on a millisecond scale with good spatial specificity is possible only with intracranial recording. This technique has promising applications in the investigation of such higher order human functions as the neuroscience of economy, ethics, social interaction, altruism, mood, motivation, the construction of ideas, language, and so on. The data obtained with this technique will be potentially applicable to the treatment of disease conditions such as pathologic gambling, antisocial behavior, autism, anxiety disorders, and memory disorders, to name a few.
Adolphs R. Fear, faces, and the human amygdala. Curr Opin Neurobiol. 2008;18:166-172.
Cameron KA, Yashar S, Wilson CL, et al. Human hippocampal neurons predict how well word pairs will be remembered. Neuron. 2001;30:289-298.
Ekstrom AD, Kahana MJ, Caplan JB, et al. Cellular networks underlying human spatial navigation. Nature. 2003;425:184-188.
Engel AK, Moll CK, Fried I, et al. Invasive recordings from the human brain: clinical insights and beyond. Nat Rev Neurosci. 2005;6:35-47.
Fell J, Klaver P, Lehnertz K, et al. Human memory formation is accompanied by rhinal-hippocampal coupling and decoupling. Nat Neurosci. 2001;4:1259-1264.
Fried I, MacDonald KA, Wilson CL. Single neuron activity in human hippocampus and amygdala during recognition of faces and objects. Neuron. 1997;18:753-765.
Fried I, Wilson CL, Maidment NT, et al. Cerebral microdialysis combined with single-neuron and electroencephalographic recording in neurosurgical patients. Technical note. J Neurosurg. 1999;91:697-705.
Gelbard-Sagiv H, Mukamel R, Harel M, et al. Internally generated reactivation of single neurons in human hippocampus during free recall. Science. 2008;322:96-101.
Greenlee JD, Oya H, Kawasaki H, et al. Functional connections within the human inferior frontal gyrus. J Comp Neurol. 2007;503:550-559.
Howard MA, Volkov IO, Granner MA, et al. A hybrid clinical-research depth electrode for acute and chronic in vivo microelectrode recording of human brain neurons. Technical note. J Neurosurg. 1996;84:129-132.
Howard MA, Volkov IO, Mirsky R, et al. Auditory cortex on the human posterior superior temporal gyrus. J Comp Neurol. 2000;416:79-92.
Kawasaki H, Adolphs R, Oya H, et al. Analysis of single-unit responses to emotional scenes in human ventromedial prefrontal cortex. J Cogn Neurosci. 2005;17:1509-1518.
Kawasaki H, Kaufman O, Damasio H, et al. Single-neuron responses to emotional visual stimuli recorded in human ventral prefrontal cortex. Nat Neurosci. 2001;4:15-16.
Kreiman G, Koch C, Fried I. Category-specific visual responses of single neurons in the human medial temporal lobe. Nat Neurosci. 2000;3:946-953.
Kreiman G, Koch C, Fried I. Imagery neurons in the human brain. Nature. 2000;408:357-361.
Oya H, Adolphs R, Kawasaki H, et al. Electrophysiological correlates of reward prediction error recorded in the human prefrontal cortex. Proc Natl Acad Sci U S A. 2005;102:8351-8356.
Oya H, Kawasaki H, Howard MA, et al. Electrophysiological responses in the human amygdala discriminate emotion categories of complex visual stimuli. J Neurosci. 2002;22:9502-9512.
Quiroga RQ, Mukamel R, Isham EA, et al. Human single-neuron responses at the threshold of conscious recognition. Proc Natl Acad Sci U S A. 2008;105:3599-3604.
Reale RA, Calvert GA, Thesen T, et al. Auditory-visual processing represented in the human superior temporal gyrus. Neuroscience. 2007;145:162-184.
Van Gompel JJ, Worrell GA, Bell ML, et al. Intracranial electroencephalography with subdural grid electrodes: techniques, complications, and outcomes. Neurosurgery. 2008;63:498-505.
1 Mukamel R, Gelbard H, Arieli A, et al. Coupling between neuronal firing, field potentials, and FMRI in human auditory cortex. Science. 2005;309:951-954.
2 Nir Y, Fisch L, Mukamel R, et al. Coupling between neuronal firing rate, gamma LFP, and BOLD fMRI is related to interneuronal correlations. Curr Biol. 2007;17:1275-1285.
3 Engel J. Research on the human brain in an epilepsy surgery setting. Epilepsy Res. 1998;32:1-11.
4 Engel AK, Moll CK, Fried I, et al. Invasive recordings from the human brain: clinical insights and beyond. Nat Rev Neurosci. 2005;6:35-47.
5 Nair DR, Burgess R, McIntyre CC, et al. Chronic subdural electrodes in the management of epilepsy. Clin Neurophysiol. 2008;119:11-28.
6 Van Gompel JJ, Worrell GA, Bell ML, et al. Intracranial electroencephalography with subdural grid electrodes: techniques, complications, and outcomes. Neurosurgery. 2008;63:498-505.
7 World Medical Association declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. JAMA. 1997;277:925-926.
8 Reale RA, Calvert GA, Thesen T, et al. Auditory-visual processing represented in the human superior temporal gyrus. Neuroscience. 2007;145:162-184.
9 Fried I, Wilson CL, Maidment NT, et al. Cerebral microdialysis combined with single-neuron and electroencephalographic recording in neurosurgical patients. Technical note. J Neurosurg. 1999;91:697-705.
10 Howard MA, Volkov IO, Granner MA, et al. A hybrid clinical-research depth electrode for acute and chronic in vivo microelectrode recording of human brain neurons. Technical note. J Neurosurg. 1996;84:129-132.
11 Howard MA, Volkov IO, Mirsky R, et al. Auditory cortex on the human posterior superior temporal gyrus. J Comp Neurol. 2000;416:79-92.
12 Lee WS, Lee JK, Lee SA, et al. Complications and results of subdural grid electrode implantation in epilepsy surgery. Surg Neurol. 2000;54:346-351.
13 Burneo JG, Steven DA, McLachlan RS, et al. Morbidity associated with the use of intracranial electrodes for epilepsy surgery. Can J Neurol Sci. 2006;33:223-227.
14 Mocco J, Komotar RJ, Ladouceur AK, et al. Radiographic characteristics fail to predict clinical course after subdural electrode placement. Neurosurgery. 2006;58:120-125.
15 Fountas KN, Smith JR. Subdural electrode-associated complications: a 20-year experience. Stereotact Funct Neurosurg. 2007;85:264-272.
16 Kawasaki H, Kaufman O, Damasio H, et al. Single-neuron responses to emotional visual stimuli recorded in human ventral prefrontal cortex. Nat Neurosci. 2001;4:15-16.
17 Oya H, Kawasaki H, Howard MA, et al. Electrophysiological responses in the human amygdala discriminate emotion categories of complex visual stimuli. J Neurosci. 2002;22:9502-9512.
18 Penfield W, Boldrey E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain. 1937;60:389-443.
19 Greenlee JD, Oya H, Kawasaki H, et al. A functional connection between inferior frontal gyrus and orofacial motor cortex in human. J Neurophysiol. 2004;92:1153-1164.
20 Greenlee JD, Oya H, Kawasaki H, et al. Functional connections within the human inferior frontal gyrus. J Comp Neurol. 2007;503:550-559.
21 Wang C, Waleszczyk WJ, Burke W, et al. Modulatory influence of feedback projections from area 21a on neuronal activities in striate cortex of the cat. Cereb Cortex. 2000;10:1217-1232.
22 Javedan SP, Fisher RS, Eder HG, et al. Cooling abolishes neuronal network synchronization in rat hippocampal slices. Epilepsia. 2002;43:574-580.
23 Noga BR, Kriellaars DJ, Brownstone RM, et al. Mechanism for activation of locomotor centers in the spinal cord by stimulation of the mesencephalic locomotor region. J Neurophysiol. 2003;90:1464-1478.
24 Hashemi-Nezhad M, Wang C, Burke W, et al. Area 21a of cat visual cortex strongly modulates neuronal activities in the superior colliculus. J Physiol. 2003;550:535-552.
25 Hill MW, Wong M, Amarakone A, et al. Rapid cooling aborts seizure-like activity in rodent hippocampal-entorhinal slices. Epilepsia. 2000;41:1241-1248.
26 Karkar KM, Garcia PA, Bateman LM, et al. Focal cooling suppresses spontaneous epileptiform activity without changing the cortical motor threshold. Epilepsia. 2002;43:932-935.
27 Tanaka N, Fujii M, Imoto H, et al. Effective suppression of hippocampal seizures in rats by direct hippocampal cooling with a Peltier chip. J Neurosurg. 2008;108:791-797.
28 Volgushev M, Vidyasagar TR, Chistiakova M, et al. Synaptic transmission in the neocortex during reversible cooling. Neuroscience. 2000;98:9-22.
29 Bakken HE, Kawasaki H, Oya H, et al. A device for cooling localized regions of human cerebral cortex. Technical note. J Neurosurg. 2003;99:604-608.
30 Yang XF, Ouyang Y, Kennedy BR, et al. Cooling blocks rat hippocampal neurotransmission by a presynaptic mechanism: observations using 2-photon microscopy. J Physiol. 2005;567:215-224.
31 Quinn KJ, O’Brien JH. Isotherm of alcohol-cooled cryoprobe. Brain Res Bull. 1979;4:119-121.
32 Adolphs R, Gosselin F, Buchanan TW, et al. A mechanism for impaired fear recognition after amygdala damage. Nature. 2005;433:68-72.
33 Doi H, Ueda K. Searching for a perceived stare in the crowd. Perception. 2007;36:773-780.
34 Pecchinenda A, Pes M, Ferlazzo F, et al. The combined effect of gaze direction and facial expression on cueing spatial attention. Emotion. 2008;8:628-634.
35 Balslev D, Miall RC. Eye position representation in human anterior parietal cortex. J Neurosci. 2008;28:8968-8972.
36 Hoehl S, Wiese L, Striano T. Young infants’ neural processing of objects is affected by eye gaze direction and emotional expression. PLoS ONE. 2008;3:e2389.
37 Penfield W, Flanigan H. Surgical therapy of temporal lobe seizures. AMA Arch Neurol Psychiatry. 1950;64:491-500.
38 Earle KM, Baldwin M, Penfield W. Temporal lobe seizures; the anatomy and pathology of the probable cause. J Neuropathol Exp Neurol. 1953;12:98-99.
39 McKhann GM, Schoenfeld-McNeill J, Born DE, et al. Intraoperative hippocampal electrocorticography to predict the extent of hippocampal resection in temporal lobe epilepsy surgery. J Neurosurg. 2000;93:44-52.
40 Fried I, MacDonald KA, Wilson CL. Single neuron activity in human hippocampus and amygdala during recognition of faces and objects. Neuron. 1997;18:753-765.
41 Cameron KA, Yashar S, Wilson CL, et al. Human hippocampal neurons predict how well word pairs will be remembered. Neuron. 2001;30:289-298.
42 Kreiman G, Koch C, Fried I. Category-specific visual responses of single neurons in the human medial temporal lobe. Nat Neurosci. 2000;3:946-953.
43 Kreiman G, Koch C, Fried I. Imagery neurons in the human brain. Nature. 2000;408:357-361.
44 Kreiman G, Fried I, Koch C. Single-neuron correlates of subjective vision in the human medial temporal lobe. Proc Natl Acad Sci U S A. 2002;99:8378-8383.
45 Gelbard-Sagiv H, Mukamel R, Harel M, et al. Internally generated reactivation of single neurons in human hippocampus during free recall. Science. 2008;322:96-101.
46 Ekstrom AD, Kahana MJ, Caplan JB, et al. Cellular networks underlying human spatial navigation. Nature. 2003;425:184-188.
47 Ekstrom AD, Caplan JB, Ho E, et al. Human hippocampal theta activity during virtual navigation. Hippocampus. 2005;15:881-889.
48 Bragin A, Engel J, Wilson CL, et al. High-frequency oscillations in human brain. Hippocampus. 1999;9:137-142.
49 Axmacher N, Elger CE, Fell J. Ripples in the medial temporal lobe are relevant for human memory consolidation. Brain. 2008;131:1806-1817.
50 Fell J, Klaver P, Lehnertz K, et al. Human memory formation is accompanied by rhinal-hippocampal coupling and decoupling. Nat Neurosci. 2001;4:1259-1264.
51 Fell J, Fernández G, Klaver P, et al. Rhinal-hippocampal coupling during declarative memory formation: dependence on item characteristics. Neurosci Lett. 2006;407:37-41.
52 Adolphs R, Tranel D, Damasio H, et al. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature. 1994;372:669-672.
53 Adolphs R, Tranel D, Damasio H, et al. Fear and the human amygdala. J Neurosci. 1995;15:5879-5891.
54 Adolphs R, Tranel D, Hamann S, et al. Recognition of facial emotion in nine individuals with bilateral amygdala damage. Neuropsychologia. 1999;37:1111-1117.
55 Adolphs R. Neural systems for recognizing emotion. Curr Opin Neurobiol. 2002;12:169-177.
56 Adolphs R. Fear, faces, and the human amygdala. Curr Opin Neurobiol. 2008;18:166-172.
57 Bechara A, Tranel D, Damasio H, et al. Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science. 1995;269:1115-1118.
58 Bechara A, Damasio H, Tranel D, et al. Deciding advantageously before knowing the advantageous strategy. Science. 1997;275:1293-1295.
59 Hornak J, Bramham J, Rolls ET, et al. Changes in emotion after circumscribed surgical lesions of the orbitofrontal and cingulate cortices. Brain. 2003;126:1691-1712.
60 Moll J, de Oliveira-Souza R, Bramati IE, et al. Functional networks in emotional moral and nonmoral social judgments. Neuroimage. 2002;16:696-703.
61 O’Doherty JP, Deichmann R, Critchley HD, et al. Neural responses during anticipation of a primary taste reward. Neuron. 2002;33:815-826.
62 O’Doherty J, Critchley H, Deichmann R, et al. Dissociating valence of outcome from behavioral control in human orbital and ventral prefrontal cortices. J Neurosci. 2003;23:7931-7939.
63 O’Doherty J, Winston J, Critchley H, et al. Beauty in a smile: the role of medial orbitofrontal cortex in facial attractiveness. Neuropsychologia. 2003;41:147-155.
64 Coricelli G, Critchley HD, Joffily M, et al. Regret and its avoidance: a neuroimaging study of choice behavior. Nat Neurosci. 2005;8:1255-1262.
65 Schaich Borg J, Hynes C, Van Horn J, et al. Consequences, action, and intention as factors in moral judgments: an FMRI investigation. J Cogn Neurosci. 2006;18:803-817.
66 Moll J, Krueger F, Zahn R, et al. Human fronto-mesolimbic networks guide decisions about charitable donation. Proc Natl Acad Sci U S A. 2006;103:15623-15628.
67 Weber B, Aholt A, Neuhaus C, et al. Neural evidence for reference-dependence in real-market-transactions. Neuroimage. 2007;35:441-447.
68 Padoa-Schioppa C, Assad JA. The representation of economic value in the orbitofrontal cortex is invariant for changes of menu. Nat Neurosci. 2008;11:95-102.
69 Takahashi H, Kato M, Matsuura M, et al. Neural correlates of human virtue judgment. Cereb Cortex. 2008;18:1886-1891.
70 Zahn R, Moll J, Paiva M, et al. The neural basis of human social values: evidence from functional MRI. Cereb Cortex. 2009;19:276-283.
71 Berlin HA, Rolls ET, Kischka U. Impulsivity, time perception, emotion and reinforcement sensitivity in patients with orbitofrontal cortex lesions. Brain. 2004;127:1108-1126.
72 Bechara A, Damasio AR, Damasio H, et al. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7-15.
73 Anderson SW, Bechara A, Damasio H, et al. Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nat Neurosci. 1999;2:1032-1037.
74 Seltzer B, Pandya DN. Frontal lobe connections of the superior temporal sulcus in the rhesus monkey. J Comp Neurol. 1989;281:97-113.
75 Ongür D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206-219.
76 Ongür D, Ferry AT, Price JL. Architectonic subdivision of the human orbital and medial prefrontal cortex. J Comp Neurol. 2003;460:425-449.
77 Kawasaki H, Adolphs R, Oya H, et al. Analysis of single-unit responses to emotional scenes in human ventromedial prefrontal cortex. J Cogn Neurosci. 2005;17:1509-1518.
78 Lang PJ. The emotion probe. Studies of motivation and attention. Am Psychol. 1995;50:372-385.
79 Liu J, Harris A, Kanwisher N. Stages of processing in face perception: an MEG study. Nat Neurosci. 2002;5:910-916.
80 Hikosaka K, Watanabe M. Delay activity of orbital and lateral prefrontal neurons of the monkey varying with different rewards. Cereb Cortex. 2000;10:263-271.
81 Rolls ET. The orbitofrontal cortex and reward. Cereb Cortex. 2000;10:284-294.
82 Bechara A, Damasio H, Tranel D, et al. The Iowa Gambling Task and the somatic marker hypothesis: some questions and answers. Trends Cogn Sci. 2005;9:159-162.
83 Sutton RS, Barto AG. Reinforcement learning: an introduction. IEEE Trans Neural Netw. 1998;9:1054.
84 Oya H, Adolphs R, Kawasaki H, et al. Electrophysiological correlates of reward prediction error recorded in the human prefrontal cortex. Proc Natl Acad Sci U S A. 2005;102:8351-8356.
85 Schoenbaum G, Chiba AA, Gallagher M. Orbitofrontal cortex and basolateral amygdala encode expected outcomes during learning. Nat Neurosci. 1998;1:155-159.
86 O’Doherty J, Kringelbach ML, Rolls ET, et al. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci. 2001;4:95-102.
87 O’Doherty JP, Dayan P, Friston K, et al. Temporal difference models and reward-related learning in the human brain. Neuron. 2003;38:329-337.
88 Roesch MR, Olson CR. Neuronal activity related to reward value and motivation in primate frontal cortex. Science. 2004;304:307-310.
89 Rolls ET. The functions of the orbitofrontal cortex. Brain Cogn. 2004;55:11-29.
90 Schoenbaum G, Roesch M. Orbitofrontal cortex, associative learning, and expectancies. Neuron. 2005;47:633-636.
91 Crick F, Koch C, Kreiman G, et al. Consciousness and neurosurgery. Neurosurgery. 2004;55:273-281.
92 Quiroga RQ, Mukamel R, Isham EA, et al. Human single-neuron responses at the threshold of conscious recognition. Proc Natl Acad Sci U S A. 2008;105:3599-3604.