Chapter 7 Investigation, discipline and legal proceedings
INTRODUCTION
It is also important that the public has confidence in the investigatory, disciplinary and legal processes, and these are consistent and transparent with outcomes that reflect the gravity and nature of the matter. This chapter will focus on the processes of investigation and discipline as well as a summary of legal actions that may flow from them and also the impact of a national system of regulation.
INVESTIGATIONS AND DISCIPLINE
In addition to the registration process, pharmacy registering authorities have the responsibility for the investigation and discipline of pharmacists. This includes the authority to receive complaints, investigate allegations of misconduct by pharmacists, and to take appropriate disciplinary action. In addition to the disciplinary processes of registering authorities, there are also the health complaints commissions in each state and territory which can also handle service complaints from the public. These commissions were established as part of a general move towards the recognition of greater consumer rights.[1] Complaints are managed through legislative arrangements and/or memorandums of understanding between the relevant commission and registration board, being dealt with by either body depending on the nature and seriousness of the complaint.
Table 7.1 lists the eight pharmacy registering authorities and the legislation providing for the management of complaints, investigations and discipline of pharmacists.
Table 7.1 Pharmacy regulation legislation dealing with discipline
| Jurisdiction | Authority | Legislation specifying disciplinary process |
|---|---|---|
| ACT | Pharmacy Board of the Australian Capital Territory | Health Professionals Act 2004 (ACT) |
| NSW | Pharmacy Board of New South Wales | Pharmacy Practice Act 2006 (NSW) |
| NT | Pharmacy Board of the Northern Territory | Health Practitioners Act 2004 (NT) |
| Qld | Pharmacists Board of Queensland | Health Practitioner (Professional Standards) Act 1999 (Qld) |
| SA | Pharmacy Board of South Australia | Pharmacy Practice Act 2007 (SA) |
| Tas | Pharmacy Board of Tasmania | Pharmacists Registration Act 2001 (Tas) |
| Vic | Pharmacy Board of Victoria | Health Professions Registration Act 2005 (Vic) |
| WA | Pharmaceutical Council of Western Australia | Pharmacy Act 1964 (WA) |
In Queensland the legislative provisions directed to the discipline of registrants has been separated from the legislation dealing with the registration of pharmacists. The Health Practitioner (Professional Standards) Act 1999 (Qld) applies to all health professionals, except nurses. The objective of dealing with all health professionals under one piece of legislation is to improve consistency across disciplines with regard to disciplinary processes and outcomes.
The following is a brief outline of the current relevant sections of legislation in each jurisdiction that address complaints, investigations and discipline. There have been recent arrangements as part of the proposed national registration scheme for health professionals (further discussed in Chapter 5) that will impact on disciplinary processes.[2]
OVERVIEW OF LEGISLATIVE FRAMEWORK
Australian Capital Territory
Section 18 of the Health Professionals Act 2004 defines the required standard of practice for a health professional as ‘the exercise of professional judgment, knowledge, skill and conduct at a level that maintains public protection and safety’.
New South Wales
Northern Territory
Queensland
Pursuant to section 48 of the Health Practitioners (Professional Standards) Act 1999 a complaint to the board may be about ‘… any aspect of a registrant’s conduct or practice, or another matter relating to the registrant that appears to provide grounds for disciplinary action against the registrant’. Also, if the board reasonably believes that an aspect of a registrant’s conduct or practice may provide grounds for disciplinary action it may conduct an investigation on its own initiative; that is, without having received a complaint. In addition, the board may immediately suspend or impose conditions on a pharmacist’s registration if the board reasonably believes that: (a) the registrant poses an imminent threat to the wellbeing of vulnerable persons; and (b) immediate action to suspend, or impose conditions on, the registrant’s registration is necessary to protect the vulnerable persons.
Grounds for disciplinary action are included in section 124 of the Act and include the following:
‘Unsatisfactory professional conduct’ is defined in the Schedule dictionary of the Act as:
The Act also empowers the board to manage impaired practitioners, including persons suffering from mental or physical illness and/or drug addiction. The impaired practitioner may be managed without recourse to disciplinary action, although disciplinary action may be invoked by the board depending on the circumstances.
South Australia
‘Unprofessional conduct’ is defined in the dictionary section of the Act as including the following:
Section 51 of the Act imposes the responsibility on the pharmacist, or health professional that treats a pharmacist, or a university where a pharmacy student is enrolled, to notify the board if they are of the opinion that a student or pharmacist is ‘medically unfit’ to practise. The board then conducts an inquiry to determine whether the pharmacist or student is mentally or physically unfit to practise.
Tasmania
While the matter of impairment is not specifically mentioned in the Act, the board may, under section 43(c), investigate a pharmacist who, it is alleged ‘… lacks sufficient physical capacity, mental capacity or skill to engage in the practice of pharmacy …’
Victoria
VCAT may also cancel the registrant’s registration.
Section 36 of the Act requires medical practitioners to notify the board if they are treating a pharmacist or a registered pharmacy student who suffers from an illness or condition which, in their opinion, impairs or may seriously impair that person’s ability to practise as a pharmacist or that student’s ability to undertake clinical training, and may result in the public being at risk. Sections 51 to 54 provide the board with the power to initiate a health assessment of the registrant or student by a registered health practitioner following the outcome of an investigation.
Western Australia
Section 32(1) of the Act details as follows the grounds for disciplinary action:
PROPOSED ARRANGEMENTS FOR HANDLING COMPLAINTS (‘NOTIFICATIONS’) UNDER A NATIONAL REGISTRATION SCHEME
As part of the establishment of a national registration and accreditation scheme for health professions (discussed in Chapter 5), there have been proposed arrangements for the handling of complaints or ‘notifications’ (to use the term favoured by the National Health Workforce Taskforce) and disciplinary issues in order to improve national consistency. Under the scheme regulatory tools will be implemented that will deal with practitioner competence, health and performance matters, and mechanisms to support a robust and publicly accountable notifications management and disciplinary system.[2]
Health practitioner boards in the various jurisdictions will maintain the oversight of decisions made in the management of notifications against registered practitioners. It has been recommended to implement three streams for the handling of health practitioner notifications, including complaints, namely:
COMPLAINTS AND INVESTIGATIONS
Natural justice
The notion of natural justice is to ensure that the proceedings are conducted fairly, impartially and without prejudice. The pharmacy registering authority must therefore provide the pharmacist, against whom the accusations are made, with a clear statement of the actual charge, adequate time to prepare a submission in response to the charge and the right to be heard on all allegations.
Hearings
Evidence and standard of proof
The rules of evidence do not strictly apply in disciplinary proceedings involving health professionals.[3] Therefore, a board, disciplinary committee, panel and even a tribunal may admit and inform itself of matters which, in the judicial adversarial process, would be excluded from consideration based on the inadmissibility of the content. The disciplinary body therefore has the potential to admit and consider a broader range of information or materials than that which may come before a court. However, although the rules of evidence do not need to be followed in disciplinary proceedings, they generally provide guidance in terms of fairness and the general conduct of proceedings.[3]
In all jurisdictions the standard of proof required for making a finding is the civil standard, ‘on the balance of probabilities’, and not the more onerous criminal standard of ‘beyond reasonable doubt’. This is in accordance with the Briginshaw test that was defined in Briginshaw v Briginshaw (1938) 60 CLR 336, and is the standard followed in Australia in regard to all disciplinary proceedings. The rationale for the lower standard is that the jurisdiction is protective towards the public, and a professional may need to be excluded from practice in order to protect the public on the basis of facts that are impossible to prove beyond reasonable doubt.[3] However, according to the Briginshaw case, the clarity of proof required to discharge the burden must reflect the seriousness of the charge.
OUTCOMES OF DISCIPLINARY ACTION
Mutual recognition legislation gives effect to the results of disciplinary action in other jurisdictions. Therefore, in the case where a pharmacist’s registration is cancelled or suspended by a registering authority as a result of disciplinary action, authorities in other jurisdictions, whether interstate or overseas, may give effect to that order. The jurisdictions have similar stepwise processes in place whereby less serious breaches are managed through streamlined procedures and more serious breaches are referred to committees or panels. However, a significant difference exists between the powers of the various authorities to impose sanctions. In Queensland, Western Australia, Victoria, New South Wales and the Australian Capital Territory, the cancellation or suspension of registration is reserved for tribunals, presided over by a judge. In comparison, the Pharmacy Boards of South Australia, Tasmania and the Northern Territory have the power to both suspend registrations for up to 3 years and to cancel registrations.
In general, disciplinary bodies are able to apply a broader range of penalties that are more remedial than are those available through the courts.[3] Disciplinary sanctions imposed on health professionals through regulatory authorities do not follow a punitive approach, but rather seek to protect the public by specific and general deterrence. Therefore, one of the aims of disciplinary actions is to deter other pharmacists from similar behaviour. Additionally, disciplinary outcomes also serve to maintain the reputation and standing of health professionals.[4] These two outcomes need to be balanced.
Severity of penalties imposed
The court instead imposed a 4-week suspension.
The case of Ha v Pharmacy Board (Vic) 18 VAR 465; [2002] VSC 322, did not directly involve the practice of pharmacy but rather, as stated by Gillard J (at [89]), was brought to ‘uphold the law’. Mr Ha, a pharmacist, indecently assaulted two young females (14 and 20 years old) during job interviews. In the Supreme Court of Victoria, Gillard J acknowledged (at [84]) that, in determining:
Gillard J further observed (at [86]) that the inappropriate behaviour of the appellant:
Another interesting decision was the Supreme Court of Tasmania’s decision to increase the penalty imposed on Mr Adamson in Adamson v Pharmacy Board (Tas) [2004] TASSC 32. This case involved a dispensing error whereby the pharmacist dispensed Panafcortelone® (prednisolone) 25mg instead of 5mg and thereafter incorrectly dispensed a repeat supply of the same tablets. The Pharmacy Board of Tasmania’s order was to allow Mr Adamson to continue to practise but only under the supervision of another pharmacist. However, Mr Adamson was the owner of the pharmacy and the court indicated that it would therefore not be appropriate to make him work under the supervision of an employee. As Mr Adamson was already 80 years old, the court held that his name be removed from the register, but in order to enable the selling of his pharmacy, deferred the deregistration until a later date. Mr Adamson was not allowed to dispense medication during the deferral of his deregistration.
Publicity of outcomes
An important function of professional disciplinary mechanisms is the publicity given to breaches.[5] It is important that pharmacists are informed of disciplinary outcomes to enable them to predict the consequences of unprofessional conduct. The publication of disciplinary decisions therefore serves to educate other pharmacists regarding practice requirements and the disciplinary process. An analysis of the impact of the publication of the Royal Pharmaceutical Society of Great Britain Statutory Committee’s decisions in the Pharmaceutical Journal indicated that publication served to inform and deter other pharmacists from similar conduct and played a role in keeping the number of persistent offenders low.[6]
Case examples
Dispensing errors
A 2005 survey of complaints received by the Pharmacy Board of Victoria indicated that over a 78-month period covering 1 July 1998 to 31 December 2004, 45% (73) of the 162 complaints received by the board were associated with dispensing errors.[7] Labelling errors accounted for 21% and selection errors for 74% of the complaints. The remaining errors were either the dispensing of expired medicines, or the wrong quantity.
More than 50% of dispensing errors reported to Guild Insurance relate to human error.[8] With regard to the most common pharmacy dispensing errors, PDL identified the two most frequent causes as selection of the incorrect strength of a medicine, and selection of the incorrect product.[9]
The pharmacist was suspended from the register for a period of 3 years.
A New South Wales coroner made specific comments about a pharmacist’s responsibility to scrutinise prescriptions and intervene if necessary. These comments followed the death of a 17-year-old female after being prescribed and dispensed an overdose of the opioid Kapanol® with the active ingredient morphine:[10]
The complaint of professional misconduct concerned the dispensing of a drug of addiction, morphine tartrate ampoules 120mg/1.5mL by the pharmacist on or about 29 September 2004, for Mr Wayne Ritchie on a prescription written by Dr Garry Gow. The pharmacist was employed at the relevant time as the pharmacist in charge and is an experienced pharmacist with no prior disciplinary history. Shortly after the morphine tartrate ampoules were dispensed by the pharmacist, Mr Ritchie self injected an ampoule and died. The direct cause of death was a morphine overdose.
Mr Justice Keith confirmed the court’s approach towards pharmacists’ liability to ensure the dosage is correct in the recent High Court case of Horton v Lloyds Pharmacy Ltd (2006). In this case the plaintiff, a USA lawyer, Cathy Horton, had an incorrect prescription prescribed by a UK doctor in July 2001, and the pharmacist at Lloyds Pharmacy dispensed the prescription without questioning the dose. The prescription was for dexamethasone 4mg daily instead of her maintenance dose of 0.5mg daily — eight times the dose that she had taken for a number of years. On her return to the USA, a doctor continued to prescribe 4mg daily after reading the dispensing label. By the end of October 2001, Ms Horton had developed Cushing’s syndrome and subsequently required multiple hospital admissions and was unfit for work for many months. She claimed that the negligent over-dispensing ‘wrecked’ her life and robbed her of the chance of making millions from a new business venture. The judge ruled that the accepted wisdom was that pharmacists should consider whether a prescribed medication was suitable for the patient. It should have occurred to the pharmacist that the dose was eight times the strength of those that had been dispensed on seven previous occasions. It was accepted that the deterioration in Ms Horton’s health did not result from the tablets dispensed by Lloyds Pharmacy. However, it was ruled that there was a direct causal link between the pharmacist’s failure to question the prescription and the American doctor providing the 4mg daily dose. Ms Horton claimed £5 million in damages.
Generic substitution
Generic substitution increases the risk that medication errors will not be identified by patients as patients may assume that the incorrectly dispensed medicine is a generic version of the correct medicine. A Victorian pharmacy error, which led to the hospitalisation of a 7-year-old asthmatic boy, involved the incorrect dispensing of Risperdal® (risperidone) instead of Redipred® (prednisolone). The boy’s parents subsequently gave him large doses of risperidone for his asthma.[11] An important factor that contributed to this potentially fatal incident was the fact that the parents did not identify the error, even though the boy’s father said that they had been issued at least two bottles of the wrong medication by their pharmacy. He further commented:
It comes in the same size bottle, it’s liquid and looks the same. We just thought this other drug was a generic brand of the same drug.[12]
These comments suggest system failures in both the dispensing and patient counselling processes and the need to educate patients to focus on active ingredients rather than on the brand names.
It has been suggested in the USA that courts should more closely examine pharmacists’ expanded role with regard to cases that involve generic substitution. This is a result of two reported appellate court cases involving drug product selection in which pharmacists were sued for damages.[13] In Ulman v Grant (1982) 450 NYS 2d 955, the patient presented a prescription to a pharmacy for Septra DS®, a specific brand of sulphamethoxazole/trimethoprim. The prescriber wrote ‘substitution permitted’ on the prescription, and the pharmacy dispensed Bactrim DS®. The plaintiff suffered an adverse reaction and sued the pharmacy. In Bichler v Willing (1977) 397 NYS 2d 57 a pregnant mother was prescribed diethylstilbestrol (DES) and the pharmacy dispensed the Eli Lilly brand. The daughter of the mother claimed severe and permanent injury due to the medicine, and sued the pharmacy.
It is important to note that claims against health professionals in the USA are more common than negligence claims in Australia and the UK. This is mainly because in the USA there are no patient cost disincentives to initiate legal action; for example, an absence of the ‘loser pays’ legal cost rule. However, these cases do provide some insight as the courts had to consider whether the pharmacist’s choice of a specific brand would have made a difference in determining their liability. The courts held that a pharmacist is not negligent unless the pharmacist knowingly dispenses a medication that is inferior or defective. Hence, if the generic medicine is not inferior or defective, the injury is not foreseeable. Therefore, both plaintiffs in these cases were unsuccessful in establishing pharmacist liability. However, it has been argued that these cases were considered before the role of pharmacists had expanded, and that today’s courts would give closer examination to pharmacists’ expanded role in selecting an appropriate generic product.[13] An in-depth analysis of the theories of potential pharmacist liability and claims of professional negligence had subsequently been undertaken by legal and pharmacy practice experts in the USA.[13] The authors concluded that pharmacists undertake new responsibilities under medication selection law (common law and legislation), and might be exposed to liability if injuries were to occur when generic medicines were substituted for prescribed brand medicines. Three possible theories were identified under which pharmacies might be held liable for injuries sustained in medication product selection situations, namely: (1) negligence; (2) express or implied warranties; or (3) strict product liability.
Lack of advice or written information
The provision of advice fulfils an important risk management activity. However, a 2004 review of 51 investigations involving dispensing errors undertaken by the Pharmacists Board of Queensland found that in all but one case, counselling had not been provided by the pharmacist.[14] Similarly, the Pharmacy Board of New South Wales, in investigating more than 6000 complaints over 10 years, estimated that, had the patient been counselled, at least 25% of medication errors might have been detected before handing out the medication.[15] Both the Queensland and New South Wales boards indicated that they accept there is little need for counselling to be provided on every occasion a prescription is dispensed, and that pharmacists need to use professional judgment.
In the case where a pharmacist has not counselled a patient or carer and the patient suffers a medication adverse event, it would be reasonable in a disciplinary investigation to establish whether the lack of counselling is professional conduct ‘of a lesser standard than those expected’, or whether the pharmacist’s conduct demonstrates a lack of judgment or care.[14] In a 2005 Pharmacists Board of Queensland investigation involving the dispensing of the cytotoxic medicine methotrexate, it was found that lack of counselling by the pharmacist and the failure to provide any written information had significantly contributed to the patient’s dosing error.[16]
In the matter of Sedrak [2007] NSWPB 4 (10 October 2007), the Pharmacy Board of New South Wales brought disciplinary action against a pharmacist, in part for the lack of adequate counselling on three occasions that lead to complaints. The board report on the matter stated:[17]
The complaint of professional misconduct encompassed events arising from the dispensing of paediatric prescriptions by the pharmacist, the sole proprietor and pharmacist in charge of the pharmacy. In the first incident the medication for a 20 months old infant was placed in a box containing a directions label for another adult patient. The child had an adverse reaction to the medication. The complaint alleged certain failures in the pharmacist’s dispensing process, including a failure to counsel. In the second incident the pharmacist dispensed a medication for a 10 year old girl, which had an approved indication for the treatment of a prostatic condition, without verifying with the prescriber the intended use of the medication in a female child or satisfying himself as to the therapeutic purpose of such supply contrary to clause 53 of the Poisons and Therapeutic Goods Regulation 2002. The pharmacist had also failed to counsel the mother of the patient as to the dosage and administration of the medication. In the third incident the pharmacist had supplied antibiotic eye drops and eye ointment for a 20 months old child, without a prescription from a medical practitioner, contrary to Section 10(3) of the Poisons and Therapeutic Goods Act 1966. The pharmacist admitted some of the particulars of the complaint. No witnesses were called for cross examination by the Respondent.
Supply of pseudoephedrine and anabolic steroids
A number of matters involving pseudoephedrine were considered by the Pharmacy Board of New South Wales from 2004 to the present day, including: Paek [2008] NSWPB 3 (12 March 2008); Huynh [2008] NSWPB 2 (9 January 2008); Moleta [2007] NSWPB 2 (12 September 2007); War [2008] NSWPB 5 (11 June 2008); Rodger [2007] NSWPB 3 (12 September 2007); Waskin [2005] NSWPB 3 (14 December 2005); Barone [2004] NSWPB 1 (14 July 2004); Ton [2004] NSWPB 2 (8 December 2004). The penalties imposed in these cases ranged from caution and reprimand to suspension and cancellation, in many cases with the addition of a fine. Where caution, reprimand and suspension were involved the pharmacist was often also required to undertake an ethics course, undergo forensic assessment, undertake continuing education and prepare a paper for presentation to the board.
Medication reviews
Significant duties and responsibilities are imposed on both community pharmacists and accredited pharmacists in providing medication review services. Pharmaceutical Defence Limited (PDL) identified potential risk areas in the provision of Home Medicines Review (HMR) services, which indicate ways in which a pharmacist may be deficient during the HMR process:[18]
Participation by the pharmacist in HMR activity results in increased responsibility and the potential for professional liability on accredited pharmacists. Litigation involving HMRs had already been reported, namely:[19]
Extemporaneous compounding
The Pharmacy Board of Victoria in July 2007 found a pharmacist, who extemporaneously compounded a preparation that caused the patient to suffer a serious adverse reaction, guilty of professional misconduct and subsequently cancelled his registration. The facts of this case were that the pharmacist, on his own order, dispensed and supplied an extemporaneously prepared mouth ulcer gel by combining the contents of a 500mg amoxycillin capsule/crushed tablet and the contents of a 500 000 units nystatin capsule/crushed tablet with Bonjela, a proprietary medicine available without prescription. The amoxycillin was scheduled as a prescription only medicine, for which the pharmacist did not have a prescription. The patient, who was allergic to penicillin, upon using the mouth ulcer, suffered an episode of acute penicillin anaphylaxis for which she had to be hospitalised.[20]
PROFESSIONAL NEGLIGENCE
Disciplinary proceedings through the pharmacy registering authorities to the tribunals are distinct from proceedings initiated through the adversarial court system. Action through the adversarial court system in the main involves a claim of medical negligence, which is a civil action initiated under the law of torts. The focus of civil litigation is to seek financial compensation. Although health care negligence litigation in Australia has historically mostly involved medical practitioners, the case law principles are applicable to all health professionals, and all health professionals are potentially liable for damage or injury sustained by a patient while under their care.[21]
Whether a medical practitioner carries out a particular form of treatment in accordance with the appropriate standard of care is a question in the resolution of which responsible professional opinion will have an influential, often a decisive, role to play; whether the patient has been given all the relevant information to chose between undergoing and not undergoing the treatment is a question of a different order. Generally speaking, it is not a question the answer to which depend upon medical standards or practices. Except in those cases where there is a particular danger that the provision of all relevant information will harm an unusually nervous, disturbed or volatile patient, no special medical skill is involved in disclosing the information, including the risks attending the proposed treatment. Rather, the skill is communicating the relevant information to the patient in terms which are reasonably adequate for that purpose having regard to the patient’s apprehended capacity to understand that information.
As advances in technology, such as computer systems that maintain patient profiles and automatically warn of drug interactions, expand the capabilities and responsibilities of pharmacists, it is important to evaluate potential liability in an effort to make the profession aware of potential litigation scenarios and to assist the profession to develop risk management procedures. However, Australian litigation involving negligent claims regarding pharmacists’ expertise in the more recently evolved areas of practice do not exist, and pharmacists’ legal liability towards patient care services has therefore not been well defined yet.[22]
Review of Australian negligence law
The Review of the Law of Negligence in 2002 followed the Australian medical indemnity crisis, which was caused by an increased number of claims and amounts awarded in damages as the principal source of compensation for those injured through the fault of medical practitioners. The review was an attempt by Australian governments to reform common law and balance the scales between the interests of both plaintiffs and defendants. The objective was to implement the recommendations into a single statute that could be adopted uniformly in the various states and territories, thereby creating a consistent approach to the law governing liability and damages for personal injury and death resulting from negligence.[23]
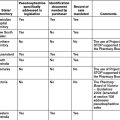
As a result of the review, civil liability legislation was introduced in all Australian jurisdictions, as summarised in Table 7.2.
| Jurisdiction | Civil liability legislation |
|---|---|
| ACT | Civil Law (Wrongs) Act 2003 (ACT); Civil Law (Wrongs) Amendment Act 2003 (ACT) |
| NSW | Civil Liability Act 2002 (NSW); Civil Liability Amendment (Personal Responsibility) Act 2002 (NSW) |
| NT | Personal Injuries (Liabilities and Damages) Act 2003 (NT); Personal Injuries (Civil Claims) Act 2003 (NT) |
| Qld | Civil Liability Act 2003 (Qld); Personal Injuries Proceedings Act 2002 (Qld) |
| SA | Volunteers Protection Act 2001 (SA); Recreational Services (Limitation of Liability) Act 2002 (SA); Wrongs (Liability and Damages for Personal Injury) Amendment Act 2002 (SA) |
| Tas | Duties Act 2001 (Tas); Civil Liability Act 2002 (Tas) |
| Vic | Wrongs and Other Acts (Law of Negligence) Act 2003 (Vic); Wrongs and Other Acts (Public Liability Insurance Reform) Act 2002 (Vic); Wrongs and Limitation of Actions Act (Insurance Reform) Act 2003 (Vic) |
| WA | Civil Liability Act 2002 (WA); Volunteers (Protection from Liability) Act 2002 (WA); Insurance Commission of WA Amendment Act 2002 (WA) |
Proactive and reactive duty of a doctor to warn of risk
Standard of care for professionals
Section 49B covers failed contraceptive procedure or contraceptive advice:
The impact of the new legislation on health professionals, and specifically on pharmacists, has not yet been tested in the courts, and it has been suggested that the real impact of the changes effected by the civil liability Acts will not be apparent for some years.[24] As the professional responsibility and subsequent legal liability of pharmacists in the evolving areas of practice is less predictable than the more routine focused technical functions that were primarily and traditionally a pharmacist’s main role, pharmacists’ potential liability with regard to the expanding role has yet to be determined.
Availability of pharmacy incident data
As mentioned, there are very few reported Australian cases initiated through the adversarial court system that involve pharmacists in claims of professional negligence. This does not mean cases have not been initiated, but rather reflect the fact that pharmacist indemnity insurers tend to settle out of court.[20] PDL or Guild Insurance do not make available incident data. The reason quoted for this is because incident report information is considered ‘commercially sensitive’.[20] Tito already highlighted the unavailability of data in 1994 through the Australian Review of Professional Indemnity Arrangements:[5]
PDL reports only limited data regarding incident reports filed, and the reported information does not give statistics on the seriousness of errors.[25] Therefore, Australian data involving pharmacists’ incidents and claims are not publicly available. However, incident data provide valuable insights into the vulnerabilities of dispensing procedures and identifies areas for improvement. The data should be used to develop measures and systems to prevent medication errors; the lack of available information precludes regulatory authorities or the profession from identifying practice shortcomings that need to be urgently addressed to prevent similar incidents in the future.
Procedures to follow after a mistake
Complainants are often annoyed because of a perception of an arrogant or off-hand manner by the pharmacist and feel that their concerns have been neither appreciated nor acknowledged. As a result, matters that could have been resolved in a professional manner by the pharmacist become the subject of a disciplinary investigation or civil action. The complainant is often left with the perception that he or she has been treated by the pharmacist in an ‘uncaring or dismissive’ manner; and that the concern experienced by the patient, especially when some or all of the incorrectly dispensed medicine has been taken, has not been addressed by the pharmacist. This causes patients to lose trust in the profession.
To provide some guidance to pharmacists in case of a dispensing error, PDL has developed procedures to follow (see Box 7.1).[25]
Box 7.1 Pharmaceutical Defence Limited procedures for mistakes
Has any harm been suffered? Has any expense been incurred?
If so, it may be sensible at this stage to say that you will, of course, cover these expenses.
DO NOT OFFER COMPENSATION — This may be regarded as an attempt to bribe your way out of trouble.
Jurisdictional disciplinary case reports and outcomes, available through the pharmacy registering authorities
Pharmaceutical Defence Limited annual reports. Online. Available: at www.pdl.org.au/
Practitioner Regulation Subcommittee HWPC. Consultation paper: Proposed arrangements for handling complaints, and dealing with performance, health and conduct matters. 7 October 2008
1 Wilson B. Health Complaints: What do consumers really want? Health Issues. Summer 2006;89:40-44.
2 Practitioner Regulation Subcommittee HWPC. Consultation paper: Proposed arrangements for handling complaints, and dealing with performance, health and conduct matters. 7 October 2008
3 Forbes J.R.S. Justice in Tribunals, 2nd edn. Sydney: The Federation Press; 2006.
4 Freckelton I. Regulation of health practitioners: Grappling with temptations and transgressions. Journal of Law and Medicine. 2004;11(1):401-408.
5 Tito F. Review of professional indemnity arrangements for health care professionals. Compensation and professional indemnity in health care: An interim report. Canberra: Australian Government Publishing Service; February 1994.
6 Tullett J., Rutter P., Brown D. A longitudinal study of United Kingdom pharmacists’ misdemeanours — trials, tribulations and trends. Pharmacy World & Science. April 2003;25(2):43-51.
7 Newgreen D.B., Pressley J.A., Marty S.H. A survey of dispensing errors reported to the Pharmacy Board of Victoria, July 1998 to December 2004. Australian Pharmacist. August 2005;24(8):644-648.
8 Guild Insurance/Guildwatch. Liability & Dispensing Guide for Pharmacists. Guild Insurance. 2006.
9 Pharmaceutical Defence Limited (PDL). Annual Report. PDL, 2005
10 Pharmacy Board of New South Wales. Responsibility to scrutinise and intervene. Bulletin. Sydney: Pharmacy Board of New South Wales; August 2003.
11 Fleming T. Error seriously injures boy. Pharmacy News. 30 August 2007.
12 The Bendigo Advertiser. Ethan’s nightmare — boy rushed to hospital drug mix-up. The Bendigo Advertiser. 21 August 2007.
13 Christensen T.P., Kirking D.M., Ascione F.J., Welage L.S., Gaither C.A. Drug product selection: Legal issues. Journal of American Pharmaceutical Association. 2001;41(6):868-874.
14 Low J. Criteria for Counselling. Pharmaceutical Society of Australia (Qld branch). April/May 2004; 6
15 Pharmacy Board of New South Wales. Criteria for counselling: the role of counselling in error minimisation. Sydney: Pharmacy Board of New South Wales; August 2003.
16 Brand P. Labelling methotrexate tablets. Letter. Pharmacists Board of Queensland, 2005
17 17NSW Pharmacy Board. Sedrak [2007] NSWPB 4. Online. Available: www.austlii.edu.au/au/cases/nsw/NSWPB/2007/4.html [accessed 27 May 2009
18 Pharmaceutical Defence Limited (PDL). Legal liability for delay during the HMR process. Annual Report. PDL, 2004
19 Baker P. HMRs – Opportunity or legal liability? Retail Pharmacy. July 2006;15(7):17-22.
20 Pharmacy Board of Victoria. Formal hearing: Robert Wesley Symons, 4 July 2007
21 Forrester K., Griffiths D. Essentials of Law for Health Professionals, 2nd edn. Sydney: Elsevier Australia; 2005.
22 Kiel H. Pharmacist misconduct: The pitfalls of practice. Journal of Law and Medicine. 2005;12:348-353.
23 Chambers K., Krikorian R. Review of the final Ipp Report and its impact on health claims. Australian Health Law Bulletin. December 2002/January 2003;11(4):37-44.
24 Madden B. Changes to the definition of negligence. Australian Health Law Bulletin. August/September 2003;12(1):6-12.
25 Pharmaceutical Defence Limited (PDL). Annual Report. PDL, 2005