Introductory Embryology
Cardiac Development
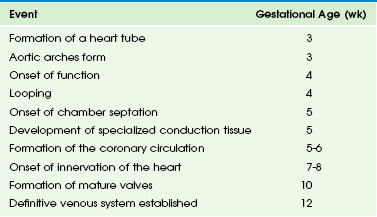
The major task in cardiac development is to form a four-chambered heart that functions in a coordinated fashion from a straight tube that functions merely by peristalsis. Cardiac development can be thought of as proceeding along various phases: fusion of myocardium and endocardium in the ventral midline to form a simple tube, onset of function, looping to the right side, specification and formation of chambers, development of specialized conduction tissue, formation of the coronary circulation, innervation of the heart, and formation of mature valves. The approximate times at which these various events occur in human development are shown in Table 62-1.
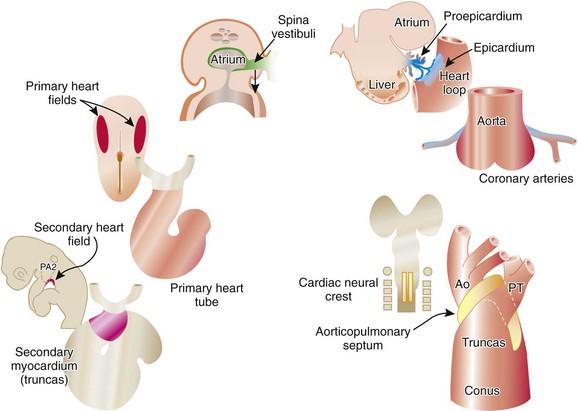
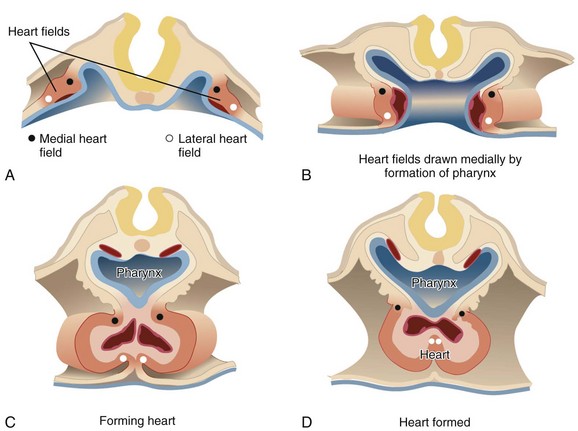
Three main groups of cells contribute to the morphogenesis of the heart. These main groups are the mesoderm of the primary heart fields (located in the splanchnic layer of lateral plate mesoderm bilaterally), the secondary heart fields (located in the pharyngeal mesenchyme), and the cardiac neural crest (a subdivision of the cranial portion of the neural crest). These tissues and their roles in the developing heart are illustrated in e-Figure 62-1, along with the two important extracardiac populations of cells described later in this chapter.
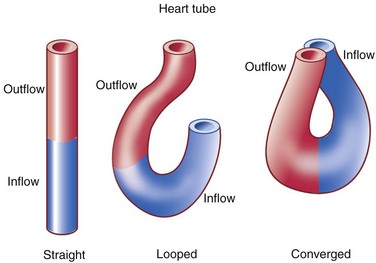
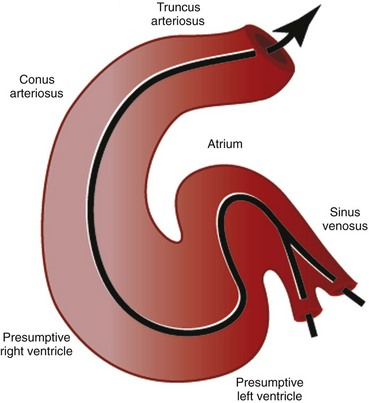
The first stage in the development of the heart is the formation of a single midline heart tube from the bilateral cardiogenic fields. This process is illustrated in e-Figure 62-2. The heart begins to beat even at this single tubular stage. Cells are then added to each end of the heart tube, and the tube begins to loop to the right. During looping, the tube is further lengthened by the addition of cells from the secondary heart field to the outflow pole. Looping and convergence bring the inflow and outflow poles of the heart into proximity (Fig. 62-3), setting the stage for septation and definitive chamber formation. The traditional names of the various segments of the recently looped heart are illustrated in Figure 62-4. Note that the heart in this configuration is set up to become a double-inlet left ventricle and a double-outlet right ventricle; if septation proceeds properly, these forms of congenital heart disease are avoided. Formation of a four-chamber heart requires the right ventricle to obtain an inlet and the left ventricle to obtain an outlet.
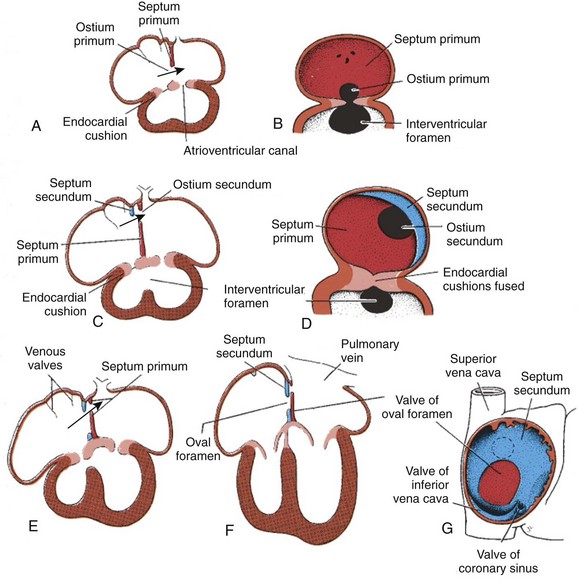
The atrioventricular canal of the heart is divided into right and left sides by endocardial cushions that develop at the atrioventricular junction and ultimately form the septum of the atrioventricular canal and the atrioventricular valves (Fig. 62-5). The right atrioventricular canal and right ventricle expand to the right, and the atria are septated from one another. Septum primum, the primary atrial septum, is led by the spina vestibuli (vestibular spine) and ultimately fuses with the endocardial cushions to close the first interatrial communication, or ostium primum. As the septum primum is growing, fenestrations begin to develop within it. These fenestrations coalesce to form the secondary interatrial communication, or ostium secundum (see Fig. 62-5). A second septum, or septum secundum, forms to the right of the septum primum much later, and postnatal fusion of the two septa obliterates any interatrial communication. Note that septum primum is ultimately a left atrial structure and that septum secundum is considered a right atrial structure, even though it expresses left-sided molecular markers. Note further that, in the common parlance of naming atrial septal defects, the defect is named for the embryonic ostium that persists, not for the embryonic tissue in which the defect exists. Thus, for example, a secundum atrial septal defect is persistence of the embryonic ostium secundum, even though the defect itself is most often in the septum primum. This naming practice is one of the more confusing points in the nomenclature of congenital heart disease.
The epicardium of the heart is derived from an extracardiac population of cells known as the proepicardium. These cells come from the mesenchyme of the septum transversum or liver and literally jump across the coelomic cavity to reach the heart (see e-Fig. 62-1). The proepicardium not only forms the definitive epicardium of the heart but also the endothelium and smooth muscles of the coronary arteries, as well as the connective tissue of the heart. The formation of the coronary arteries and the connective tissue of the heart is made possible by a process known as epithelial to mesenchymal transformation. The epicardium of the outflow tract is distinct from that of the remainder of the heart and is derived from the splanchnic mesoderm of the ventral pharynx. Because of the diversity of cells that the proepicardium provides, many investigators consider the proepicardium to be an important potential source of cardiac stem cells.
Vascular Development
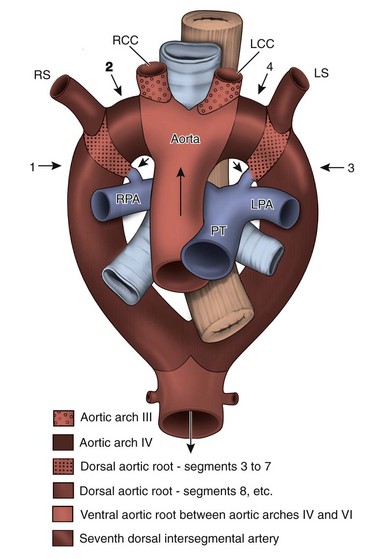
The central vessels, however, lose their symmetry and are extensively remodeled. The great arteries begin as bilaterally symmetric, paired arch arteries that connect the ventral aortic sac to the bilateral dorsal aortae. These symmetrical arch arteries undergo involution and remodeling to form the aortic arch, arch vessels, main and branch pulmonary arteries, and the ductus arteriosus (e-Fig. 62-6). This patterning is supported by cells from the cardiac neural crest. Abnormal arterial remodeling leads to aberrant origins of arch arteries and to vascular rings. To understand the embryonic origin of various arch anomalies, it is useful to consider a “totipotential arch” that includes all the relevant arch arteries and imagine how this arch may be “cut” to produce each anomaly. The “totipotential arch” is a theoretic construct. Such an arch never exists in fetal life; because of involution and remodeling, all of the represented components never exist simultaneously. However, the concept (illustrated in Fig. 62-7) is quite useful in understanding how various arch anomalies occur and which of them produce vascular rings.

e-Figure 62-6 Diagram of the bilaterally symmetrical aortic arch arteries that carry the early cardiac output to the dorsal aorta, which then distributes blood to the embryo.
The early symmetry is lost when these vessels are remodeled to be the adult great arteries of the thorax. Green, aortic arch artery 3; red, aortic arch artery 4; blue, aortic arch artery 6; A, aorta; BC, brachiocephalic; DA, ductus arteriosus; DC, ductus caroticus; LCC, left common carotid artery; LSC, left subclavian artery; LVA, left vertebral artery; P, pulmonary trunk; RCC, right common carotid artery; RSC, right subclavian artery; RVA, right vertebral artery. (From Kirby ML. Cardiac development. New York: Oxford University Press; 2006.)
The embryonic venous system is also extensively remodeled. Originally there are bilaterally symmetric vitelline, umbilical, and cardinal systems. The original systems drain to ipsilateral horns of the sinus venosus portion of the developing heart. Because the left sinus horn regresses and systemic venous return is directed solely to the right atrium, the left system of veins must involute or form anastomotic connections with the right system. The inferior vena cava is composed of elements of four separate systems (e-Fig. 62-8). When the inferior vena cava is interrupted, as in cases of the polysplenic form of heterotaxy, bilateral symmetry of all but the intrahepatic portion is largely maintained. The inferior venous drainage then proceeds to one or both superior venae cavae via azygous or hemiazygous veins.
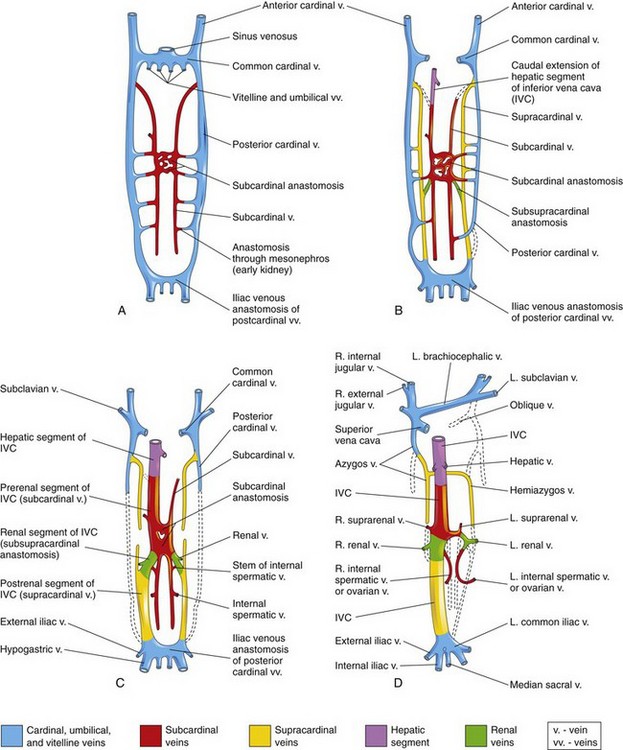
e-Figure 62-8 The primordial veins of the trunk in the human embryo (ventral views).
Initially, three systems of veins are present: the umbilical veins from the chorion, the vitelline veins from the umbilical vesicle (yolk sac), and the cardinal veins from the body of the embryo. Next, the subcardinal veins appear, and finally the supracardinal veins develop. A, At 6 weeks. B, At 7 weeks. C, At 8 weeks. D, Adult. This drawing illustrates the transformation that produces the adult venous pattern. (From Moore KL, Persaid TVN. The developing human: clinically oriented embryology, ed 8. Philadelphia: Saunders Elsevier; 2008. Fig. 13-4; as modified from Arey LB. Developmental anatomy, ed 7 revised. Philadelphia: WB Saunders; 1974.)
Bogers, AJJC, Gittenberger-de Groot, AC, Poelmann, RE, et al. Development of the origin of the coronary arteries, a matter of ingrowth or outgrowth. Anat Embryol (Berl). 1989;180:437–441.
Harvey RP, Rosenthal N, eds. Heart development. San Diego, CA: Academic Press, 1998.
Horsthuis, T, Christoffels, VM, Anderson, RH, et al. Can recent insights into cardiac development improve our understanding of congenitally malformed hearts? Clin Anat. 2009;22:4–20.
Hutson, MR, Kirby, ML. Neural crest and cardiovascular development: a 20-year perspective. Birth Defects Res. 2003;69:2–13.
Kirby, ML. Cardiac development. New York: Oxford University Press; 2007.
Moorman, AFM, Christoffels, VM. Cardiac chamber formation: development, genes and evolution. Physiol Rev. 2003;83:1223–1267.
Wessels, A, Pérez-Pomares, JM. The epicardium and epicardially derived cells (EPDCs) as cardiac stem cells. Anat Rec A Discov Mol Cell Evol Biol. 2004;276(1):43–57.