Intravascular ultrasound
(CONSULTANT-LEVEL EXAMINATION)
Overview
Since its advent in 1977, percutaneous coronary intervention (PCI) has become a dominant treatment modality for ischemic coronary artery disease, especially unstable angina and acute myocardial infarction. During the last 2 decades, intravascular ultrasound (IVUS), a catheter-based technique that provides tomographic images perpendicular to the length of the coronary arteries, has been used widely in clinical research and has contributed to technologic improvements in interventional cardiology because it provides invaluable information on the coronary vascular lumen and wall. IVUS uses high-frequency catheter-based transducers to visualize all basic components of the vessel: the cross-sectional luminal size, shape, and vessel wall, as well as the various layers of the wall such as the intima, media, and adventitia and perivascular structures. IVUS examination of the carotid arteries may enable the morphologic characteristics of the carotid lesions to be assessed and thus treatment to be optimized. IVUS evaluation of the venous circulation is still limited. However, the method has been used to study arteriovenous malformations and mainly to demonstrate inferior vena cava compression or thrombosis, as well as to guide stent and filter placement.1
Clinical applications of intravascular ultrasound for percutaneous coronary interventions
In the bare metal stent (BMS) era, the major use of IVUS has been for optimization of stent deployment, particularly for complex lesions such as bifurcations, left main lesions, in-stent restenosis, and saphenous vein graft lesions. A challenging problem after BMS implantation was in-stent restenosis, and IVUS predictors of this phenomenon include smaller minimal stent area (MSA), stent underexpansion, stent edge dissection, incomplete stent apposition, and incomplete lesion coverage.2 IVUS is superior to coronary angiography in assessing vessel size, calcium content, and lesion severity.3 Therefore IVUS can be used before PCI to assess reference lumen dimensions and lesion length for appropriate stent sizing and to identify superficial calcium, which may lead to prestent rotational atherectomy. Poststent IVUS assessment may detect complications of PCI and suboptimal stent deployment.4
Previous studies have shown a beneficial effect of IVUS guidance on postprocedural angiographic results and stent restenosis during long-term follow-up as a result of a larger MSA with a higher postdilation balloon pressure.2,3,5 Stent under expansion (identified by IVUS) can be treated with appropriate postballoon dilation. IVUS allows more aggressive intervention with a larger-diameter balloon with confidence in terms of safety; thus BMS implantation under IVUS guidance can provide a bigger MSA and more favorable clinical outcomes than angiographically guided PCI can. Although studies have differed regarding the best cutoff value for MSA (ranging from 6.5 to 9.0 mm2), larger post-PCI areas consistently predict lower rates of restenosis.6,7 In a registry of 1706 patients, the risk for restenosis with BMSs decreased 19% for every 1-mm2 increase in MSA.8
Routine use of IVUS for BMS implantation is still controversial. Several meta-analyses have shown that when compared with angiographically guided PCI, IVUS-guided PCI results in an improvement in acute postinterventional results (larger minimal luminal diameter) and a lower frequency of repeated revascularization, angiographic restenosis, and main adverse coronary event rates, but no difference in the incidence of death or myocardial infarction during the follow-up period.9–11
The advent of drug-eluting stents (DESs) has markedly reduced the rate of in-stent restenosis. However, rapid implementation of DESs in standard practice also led to expansion of the indications for PCI to high-risk patients and complex lesions, and in-stent restenosis still occurs in 3% to 20% of patients, depending on patient and lesion characteristics and DES type.12 Indeed, several retrospective investigations have showed the potential of IVUS in optimizing stent deployment, even in the DES era. In a study of 449 patients (543 lesions) who completed 6-month angiographic follow-up after the implantation of sirolimus-eluting stents, the postprocedural minimum stent lumen area and stent length on IVUS emerged as the only predictors of stent restenosis.13 In another study, unselected patients undergoing DES implantation under IVUS guidance were identified and compared with those undergoing angiographically guided PCI,14 and it was suggested that IVUS-guided DES implantation may significantly decrease rates of definite stent thrombosis at 30 days and 12 months. However, the few randomized controlled trials evaluating IVUS guidance for PCI with DESs showed that IVUS-guided PCI with DESs may not influence rates of restenosis. One published randomized trial, HOME DES (Long-Term Health Outcome and Mortality Evaluation after Invasive Coronary Treatment Using Drug Eluting Stents With or Without IVUS Guidance), randomized 210 patients to an IVUS-guided PCI strategy versus an angiographically guided strategy.15 In this study the IVUS-guided strategy led to more frequent postdilation, higher balloon inflation pressure, and larger balloon size, but it did not result in lower rates of target vessel revascularization or major adverse cardiac events.
The reduced risk for in-stent restenosis in patients undergoing DES implantation is offset by concerns about stent thrombosis.16 A study featuring a propensity-matched analysis in 884 patients treated with DESs showed a significant reduction in the stent thrombosis rate at both 30 days (0.5% vs. 1.4%, P = .046) and 12 months (0.7% vs. 2.0%, P = .014) in the IVUS-guided PCI group.14
IVUS may play a potential role in the assessment of coronary lesions classified as intermediate based on angiography, especially those located in the left main coronary artery (Figure 17-1). Management of intermediate lesions remains a therapeutic dilemma for interventional cardiologists. Even experienced interventional cardiologists cannot accurately assess the hemodynamic significance of intermediate or moderate lesions with stenosis of between 40% and 70% via angiography.17 In this case, fractional flow reserve (FFR) is considered the “gold standard” for assessment of lesions, but several studies have reported fairly good correlation between IVUS-derived anatomic data and ischemia by physiologic assessment. FFR can be predicted accurately by using established equations and accurate three-dimensional IVUS imaging,18 and several studies have suggested that with non–left main lesions, a minimal lumen area (MLA) of 4.0 mm2 or greater can accurately identify nonischemic lesions for which PCI can be safely deferred, whereas an MLA of less than 4.0 mm2 does not accurately predict a hemodynamically significant lesion and should not be used to justify revascularization. Because accurate assessment of intermediate left main lesions is important to optimize outcomes, IVUS has been widely used for the assessment of intermediate left main coronary artery lesions.19 In a study of 55 patients with moderate left main stenosis, an MLA cutoff value of 5.9 mm2 (sensitivity of 93% and specificity of 95%) and a minimal lumen diameter of 2.8 mm (sensitivity of 93% and specificity of 98%) best correlated with an FFR of less than 0.75.20 In a recent study of 354 patients with intermediate left main stenoses, an MLA value greater than 6.0 mm2 identified patients at low risk for adverse events with deferred revascularization.21
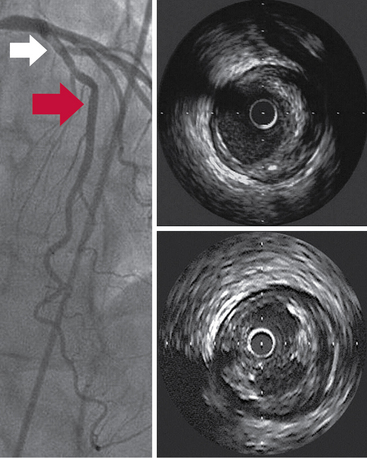
Figure 17-1 A cross-sectional tomographic image obtained by intravascular ultrasound examination (top right) within the proximal segment (white arrow on angiogram) revealed substantial atheroma within the arterial wall. The bottom right segment illustrates a tomographic image of atheroma containing significant ulceration (5 o’clock) at the site of the red arrow on the angiogram.
Intravascular ultrasound for carotid revascularization
Carotid artery stenosis is the cause of about 20% to 25% of strokes.22 Carotid stenosis of 75% to 94% is associated with a risk for stroke of 18.5% in asymptomatic patients and 27% in symptomatic patients.23 Large-scale randomized trials have established the benefit of carotid endarterectomy over medical management in patients with symptomatic and, to a lesser degree, asymptomatic carotid artery disease. In the last decade, carotid artery stenting has been increasingly advocated as less invasive treatment than surgery.24
In contrast to the large amount of literature evaluating the potential of IVUS guidance for PCI, to date, published studies evaluating the utility of IVUS for carotid artery stenting are limited. Unlike the coronary artery, the extracranial carotid artery can be examined directly by B-mode and Doppler ultrasound. Current guidelines recommend duplex ultrasonography as the initial diagnostic test to detect hemodynamically significant carotid stenosis in asymptomatic patients with known or suspected carotid stenosis.25
However, several observational studies have suggested that preoperative IVUS evaluation of the internal carotid artery may provide useful information on the characteristics of the lesions and aid in stent selection, and it may be used to assess postprocedural results.26–28 IVUS may be used to assess the quality of performance during open surgical procedures.29 Finally, virtual histology IVUS allows improved characterization of plaque composition and may play a potential role in the preoperative or intraoperative evaluation of the lesion to be treated by identifying patients with unstable plaque and, consequently, at high risk for intraoperative cerebral embolism.30
Pearls and highlights
• IVUS guides coronary stent deployment efficiently, particularly for complex lesions such as bifurcations, left main lesions, and saphenous vein graft lesions.
• It may be used as a complementary tool for assessing intermediate coronary lesion severity (left main artery).
• Color flow IVUS facilitates real-time dynamic assessment of carotid plaque morphology and aids in stent selection. Its use in the evaluation of veins is still limited; however, it has been used to guide inferior vena cava stent and filter placement.
References
1. Gasparis, AP, Kokkosis, A, Labropoulos, N, et al. Venous outflow obstruction with retroperitoneal Kaposi’s sarcoma and treatment with inferior vena cava stenting. Vasc Endovasc Surg. 2009; 43:295–300.
2. Fitzgerald, PJ, Oshima, A, Hayase, M, et al. Final results of the Can Routine Ultrasound Influence Stent Expansion (CRUISE) study. Circulation. 2000; 102:523–530.
3. Waller, BF, Pinkerton, CA, Slack, JD, Intravascular ultrasound: a histological study of vessels during lifethe new ‘gold standard’ for vascular imaging. Circulatio. 1992; 85:2305–2310.
4. Gorge, G, Haude, M, Ge, J, et al. Intravascular ultrasound after low and high inflation pressure coronary artery stent implantation. J Am Coll Cardiol. 1995; 26:725–730.
5. Albiero, R, Rau, T, Schlüter, M, et al. Comparison of immediate and intermediate-term results of intravascular ultrasound versus angiography-guided Palmaz-Schatz stent implantation in matched lesions. Circulation. 1997; 96:2997–3005.
6. Kuntz, RE, Safian, RD, Carrozza, JP, et al. The importance of acute luminal diameter in determining restenosis after coronary atherectomy or stenting. Circulation. 1992; 86:1827–1835.
7. de Feyter, PJ, Kay, P, Disco, C, et al. Reference chart derived from post–stent-implantation intravascular ultrasound predictors of 6-month expected restenosis on quantitative coronary angiography. Circulation. 1999; 100:1777–1783.
8. Kasaoka, S, Tobis, JM, Akiyama, T, et al. Angiographic and intravascular ultrasound predictors of in-stent restenosis. J Am Coll Cardiol. 1998; 32:1630–1635.
9. Berry, E, Kelly, S, Hutton, J, et al, Intravascular ultrasound–guided interventions in coronary artery disease: a systematic literature review, with decision-analytic modelling, of outcomes and cost-effectiveness. Health Technol Asses. 2000; 4:1–117.
10. Casella, G, Klauss, V, Ottani, F, et al, Impact of intravascular ultrasound–guided stenting on long-term clinical outcome: a meta-analysis of available studies comparing intravascular ultrasound–guided and angiographically guided stenting. Catheter Cardiovasc Inter. 2003; 59:314–321.
11. Parise, H, Maehara, A, Stone, GW, et al. Meta-analysis of randomized studies comparing intravascular ultrasound versus angiographic guidance of percutaneous coronary intervention in pre–drug-eluting stent era. Am J Cardiol. 2011; 107:374–382.
12. Dangas, GD, Claessen, BE, Caixeta, A, et al. In-stent restenosis in the drug-eluting stent era. J Am Coll Cardiol. 2010; 56:1897–1907.
13. Hong, MK, Mintz, GS, Lee, CW, et al. Intravascular ultrasound predictors of angiographic restenosis after sirolimus-eluting stent implantation. Eur Heart J. 2006; 27:1305–1310.
14. Roy, P, Steinberg, DH, Sushinsky, SJ, et al. The potential clinical utility of intravascular ultrasound guidance in patients undergoing percutaneous coronary intervention with drug-eluting stents. Eur Heart J. 2008; 29:1851–1857.
15. Jakabcin, J, Spacek, R, Bystron, M, et al. Long-term health outcome and mortality evaluation after invasive coronary treatment using drug eluting stents with or without the IVUS guidance. Randomized control trial. HOME DES IVUS. Catheter Cardiovasc Interv. 2010; 75:578–583.
16. Okabe, T, Mintz, GS, Buch, AN, et al. Intravascular ultrasound parameters associated with stent thrombosis after drug-eluting stent deployment. Am J Cardiol. 2007; 100:615–620.
17. Tobis, J, Azarbal, B, Slavin, L. Assessment of intermediate severity coronary lesions in the catheterization laboratory. J Am Coll Cardiol. 2007; 49:839–848.
18. Takayama, T, Hodgson, JM, Prediction of the physiologic severity of coronary lesions using 3D IVUS: validation by direct coronary pressure measurements. Catheter Cardiovasc Inter. 2001; 53:48–55.
19. Leesar, MA, Masden, R, Jasti, V. Physiological and intravascular ultrasound assessment of an ambiguous left main coronary artery stenosis. Catheter Cardiovasc Interv. 2004; 62:349–357.
20. Jasti, V, Ivan, E, Yalamanchili, V, et al. Correlations between fractional flow reserve and intravascular ultrasound in patients with an ambiguous left main coronary artery stenosis. Circulation. 2004; 110:2831–2836.
21. de la Torre Hernandez, JM, Hernández Hernandez, F, Alfonso, F, et al, for the LITRO Study Group: Prospective application of pre-defined intravascular ultrasound criteria for assessment of intermediate left main coronary artery lesionsresults from the multicenter LITRO study. J Am Coll Cardio. 2011; 58:351–358.
22. Roger, VL, Go, AS, Lloyd-Jones, DM, et al, Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation. 2011;123(4):e18–e209.
23. Inzitari, D, Eliasziw, M, Gates, P, et al. The causes and risk of stroke in patients with asymptomatic internal-carotid-artery stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 2000; 342:1693–1700.
24. Roffi, M, Mukherjee, D, Clair, DG. Carotid artery stenting vs. endarterectomy. Eur Heart J. 2009; 30:2693–2704.
25. Brott, TG, Halperin, JL, Abbara, S, et al. ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease. a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of NeuroInterventional Surgery, Society for Vascular Medicine, and Society for Vascular Surgery. Circulation. 2011; 124:e54–e130.
26. Wilson, EP, White, RA, Kopchok, GE. Utility of intravascular ultrasound–guided carotid artery stenting. J Endovasc Surg. 1996; 3:63–68.
27. Clark, DJ, Lessio, S, O’Donoghue, M, et al. Safety and utility of intravascular ultrasound–guided carotid artery stenting. Catheter Cardiovasc Interv. 2004; 63:355–362.
28. Joan, MM, Moya, BG, AgustÃ, FP, et al. Utility of intravascular ultrasound examination during carotid stenting. Ann Vasc Surg. 2009; 23:606–611.
29. Kawamata, T, Okada, Y, Kondo, S, et al. Extravascular application of an intravascular ultrasound (IVUS) catheter during carotid endarterectomy to verify distal end of stenotic lesions. Acta Neurochir (Wien). 2004; 146:1205–1209.
30. Dietrich, E, Margolis, P, Reid, D, et al, Virtual histology intravascular ultrasound assessment of carotid artery disease: the Carotid Artery Plaque Virtual Histology Evaluation (CAPITAL) study. J Endovasc The. 2007; 14:676–686.