Chapter 24
Intravascular Ultrasound
Donald B. Reid, Frank R. Arko III,
Based on a chapter in the seventh edition by Katja C. Vogt and Torben V. Schroeder
Intravascular ultrasound (IVUS) is a catheter-based guidance system that is used during endovascular procedures. Whereas it was originally introduced in interventional cardiology, IVUS is now used in a wide variety of peripheral vascular interventions.1–6 The IVUS probe is passed into the vessel lumen, and because the ultrasound probe is in such proximity, great resolution of detail is possible with significant magnification. IVUS provides histologic detail of the vessel wall and also demonstrates blood flow within the lumen.7,8 It has two main clinical roles. It provides a diagnostic ability to assess and to measure the severity of disease before treatment and also demonstrates the completeness of treatment after intervention.9,10
The main limitation of catheter angiography is that it defines only the lumen and not the disease in the vessel wall.7 The increased complexity of endovascular interventions has required improved vascular imaging. Whereas computed tomographic angiography (CTA), magnetic resonance imaging, and duplex ultrasonography allow excellent imaging, these modalities have little to offer at the time of intervention. Duplex ultrasound has been used to assess the completeness of treatment after open surgical procedures and some peripheral endoluminal interventions. However, it is technician dependent and limited in thoracic, abdominal, and pelvic examinations.11–13
The first clinical use of IVUS began in the late 1980s in the coronary circulation because it provided additional information to that obtained by arteriography, namely, detailed arterial wall characteristics with a close relationship between the histologic section and the corresponding ultrasonic cross section (with regard to the location, plaque thickness, and extent of atherosclerosis along the circumference of the vessel wall).1 Arteriography provides the endovascular surgeon with details of the vessel contour, collateral circulation, quality of flow, and inflow and outflow. IVUS, however, allows greater appreciation of the vessel wall disease than only the lumen and can distinguish between soft plaque and calcification.14 Intimal flaps, thrombus formation, and ulceration are visible with IVUS, and the luminal diameter and cross-sectional areas can also be measured.15 IVUS can also detect lesions missed on conventional arteriography.9 Given that it can detect such clinically important findings, IVUS has been used to guide percutaneous transluminal angioplasty, stenting, atherectomy, laser, thrombolysis, and endoluminal grafting.4,5,10,16–21
Its clinical value within the operating room at assessing the severity of disease and subsequently the completeness of treatment was quickly appreciated. This very practical assistance within the operating room environment has become much easier to use with the developments of three-dimensional, color-flow, and virtual histology IVUS (VH IVUS). Its use is described in detail in this chapter together with the latest technical advances of IVUS and its application in different peripheral situations.
Technical Considerations
Intravascular Ultrasound Systems
There are two commercially available types of IVUS systems. The first is a mechanical system in which the ultrasound transducer is located at the tip of a flexible high-torque catheter. This allows fast rotation of the transducer. Older systems were subject to the imaging artifact of the adjacent wire. However, newer systems have a dual-purpose channel that allows catheter access over a guide wire that can then be withdrawn and replaced with the IVUS transducer. This mechanical rotation system is commercially available through Boston Scientific Corporation (Natick, Mass).
The second IVUS system type sweeps the ultrasound signal around the circumference of the probe electronically using 64 miniaturized transducer elements positioned circumferentially around the tip of the catheter. The transducers are electronically activated in sequence to produce an array of images in a plane perpendicular to the long axis of the catheter. This phased array system is currently manufactured by Volcano Corporation (Rancho Cordova, Calif).
Both types of catheters transmit sound waves covering 360 degrees of the vessel wall and create an axial image of the lumen and the vessel wall at the tip of the catheter.
Catheters and Probes
All IVUS catheters are connected to a separate console that displays the images and allows recording of still images as well as video loops. Measurements including diameter, circumference, and area can then be obtained on the workstation.
The resolution of IVUS is determined by the frequency of its beam. The higher the frequency, the greater the resolution is between two juxtaposed points of an image. When the frequency is increased, however, penetration distance is decreased because of the effects of absorption of the ultrasound beam as it propagates deeper into tissue. Thus, the appropriate catheter for a particular vessel depends on choosing the appropriate frequency for its size and depth. For example, with the Volcano system, three catheters are available: 0.014-inch and 0.018-inch catheters, which are 20 MHz, and a 0.035-inch probe at 10 MHz (Fig. 24-1). Catheters with higher frequencies between 20 and 45 MHz enable resolutions of 0.2 to 0.5 mm in the lateral direction. Frequencies between 8 and 12 MHz are best suited for the aorta, inferior vena cava (IVC), and iliac aneurysms; frequencies around 20 MHz are best suited for visualizing smaller vessels, such as occlusive iliac arteries and carotid, superficial femoral, popliteal, and tibial vessels. Higher frequencies, such as 45 MHz, are best suited for the very small diameter coronary vessels.
Lower frequency catheters, such as the 10-MHz catheter, generally require a larger 9F sheath; the higher frequency catheters can be delivered through 7F and smaller. All catheters are delivered over a guide wire. The larger catheters with lower frequency are delivered over the wire; the small catheters with higher frequencies are often on a rapid exchange system.
Another specialized catheter that is available is the Pioneer catheter (Medtronic, Minneapolis, Minn). This catheter is also on a rapid exchange system using a 0.014-inch wire. It works on a Volcano workstation and is used to gain reentry in treating aortic dissections or crossing chronic total occlusions in the subintimal space. The dual-lumen catheter integrates a phased array IVUS probe for site control and a reentry needle made of nitinol in which the depth of penetration can be controlled on the catheter. Color-flow ultrasound is used to direct the needle in the 12-o’clock position. Once reentry is obtained, a second 0.014-inch wire is then passed through the second channel into the true lumen.22
Intravascular Ultrasound Images
IVUS creates a two-dimensional gray-scale image that allows complete visualization of the three layers of the arterial wall. The intima appears as bright and echogenic, whereas the media is darker and more echolucent. The adventitia is brighter than the intima. However, the demarcation of the adventitia to the surrounding tissue is much more difficult to discern. There is a demarcation of the blood-filled lumen and the vessel wall in both arteries and veins. This allows quantification of the free luminal area and evaluation of the degree of stenosis and the distribution of plaque.
As is seen with other forms of ultrasound, calcified lesions evaluated with IVUS are bright and cause characteristic posterior shadowing. This is due to the lack of penetration of the ultrasound through calcification. In comparison, soft plaque containing lipids is more echolucent and appears darker, whereas fibrotic plaques (associated with restenosis) are brighter but without the posterior shadowing seen in heavily calcified lesions.9
Color-Flow Intravascular Ultrasound
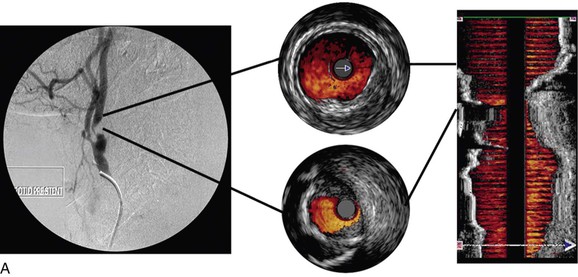
Color-flow IVUS is currently available only on the Volcano system. ChromaFlo is the computer software that detects blood flow and colors it red.8,23,24 The software detects differences between adjacent IVUS frames. As the blood cells move through the artery, they move through the IVUS image frames. The software detects differences between adjacent frames where there has been movement of blood and colors this red. ChromaFlo also detects faster flowing blood and colors it yellow. However, at present, flow velocities cannot be measured by this technique. ChromaFlo does not use the Doppler effect.25 Color-flow IVUS has been a helpful addition because it shows clearly where the lumen meets the vessel wall (Fig. 24-2).
Three-Dimensional Intravascular Ultrasound
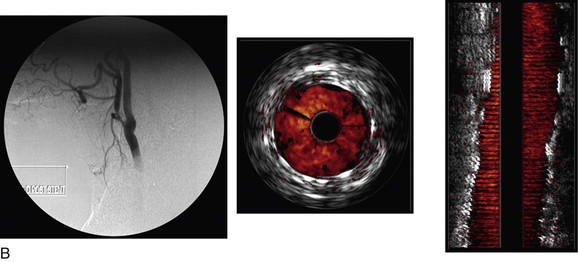
A longitudinal reconstruction provides an image similar to an angiogram. The longitudinal image is created by performing a “pullback” of the IVUS probe through the vessel. Computer software that automatically detects the borders of layers of the artery (intima/lumen and intima/media) or the vein (intima/lumen) facilitates reconstruction of the image. Longitudinal images are generally used in conjunction with axial IVUS images to interpret the pullback. However, whereas axial images allow accurate diameter measurements, a longitudinal reconstruction is not anatomic. In tortuous vessels, the catheter tip is not always in the center of the lumen. This causes distortion as the reconstruction assumes that the catheter follows a straight path. Length measurements with the longitudinal reconstruction are therefore not accurate.26
Virtual Histology Intravascular Ultrasound
Conventional gray-scale IVUS images are created by use of the intensity (amplitude) of the returning signal. The vessel wall is either brightly reflective (echogenic, white), dark (echolucent), or in between (gray).8
VH IVUS creates an image using the frequency as well as the amplitude of the returning signal: different tissues reflect ultrasound at different frequencies.27,28 Meticulous correlation of IVUS frequency data from diseased coronary arteries with subsequent tissue histopathologic sections of the explanted vessels has enabled virtual histology data to be classified into four histologic components: fibrous, fibrofatty, calcified, and necrotic lipid core.29,30 A color-coded map of atherosclerotic plaque is produced by the VH IVUS software: dark green, fibrous; yellow/green, fibrofatty; white, calcified; and red, necrotic lipid core plaque (Fig. 24-3).
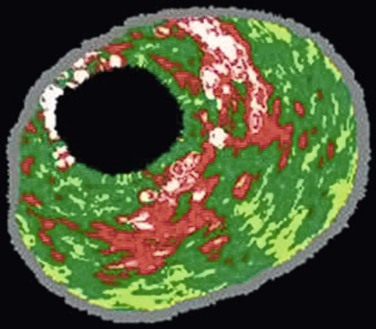
Figure 24-3 Virtual histology IVUS produces a color-coded map of the histologic components of the plaque. Dark green, fibrous; yellow/green, fibrofatty; white, calcified; red, necrotic.
VH IVUS is available only on the Volcano system and with the Eagle Eye catheter (Volcano Corporation). This catheter requires a 0.014-inch guide wire. Therefore, it is possible to use virtual histology only in relatively small vessels, such as the carotid, renal, and superficial femoral arteries. After a pullback of the probe through an arterial stenosis, an assessment of the plaque type can be made.31,32
The diagnostic accuracy of VH IVUS to correctly identify plaque types in peripheral vascular disease was investigated and validated in the Carotid Artery Plaque Virtual Histology Evaluation (CAPITAL) study, in which VH IVUS interpretation was compared with the true histopathology of carotid plaque.33 In a Food and Drug Administration–approved study and with institutional review board ethical approval, patients undergoing carotid endarterectomy had an antegrade puncture of the common carotid artery after surgical exposure of the carotid artery bifurcation. An access sheath was placed and VH IVUS was performed with use of a cerebral protection filter device. Carotid endarterectomy was then completed and the endarterectomy specimen sent for histopathologic examination. In this double-blinded study, 153 VH IVUS images were compared with the true histopathology sections from the endarterectomy specimens. The predictive accuracy of VH IVUS to agree with true histology in the different carotid plaque types was 99.4% for thin-cap fibroatheroma, 96.1% for calcified thin-cap fibroatheroma, 85.9% for fibroatheroma, 85.5% for fibrocalcific, 83.4% for pathologic intimal thickening, and 72.4% for calcified fibroatheroma. This study found that VH IVUS accurately diagnoses the different carotid artery plaque types. It was most accurate in “vulnerable plaque” types (thin-cap fibroatheroma and calcified thin-cap fibroatheroma (Fig. 24-4).
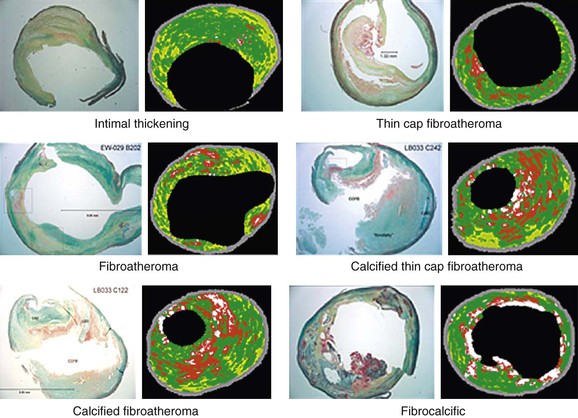
Figure 24-4 The six plaque types with examples of true histologic sections compared with the corresponding images of virtual histology IVUS that was performed immediately before carotid endarterectomy during the CAPITAL study. (From Diethrich EB, et al: Virtual histology intravascular ultrasound assessment of carotid artery disease: the Carotid Artery Plaque Virtual Histology Evaluation [CAPITAL] study. J Endovasc Ther 14:676-686, 2007.)
An interesting finding relating to plaque characterization in the CAPITAL study was that proportionally less necrotic core plaque was found in patients taking aspirin (this was statistically significant; P < .05). Similarly, the finding that nodules projecting into the lumen were statistically significantly associated with previous neurologic symptoms gives an indication of the clinical importance of these different morphologic characteristics of carotid plaque (Fig. 24-5).
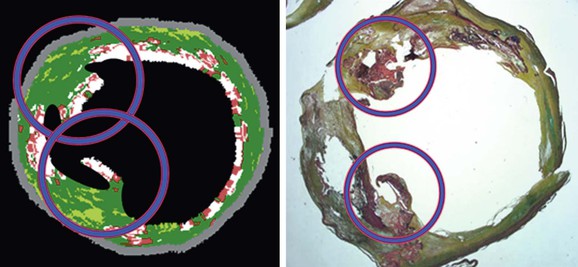
Figure 24-5 Virtual histology IVUS image and true histopathology section of carotid artery plaque show calcified projections (circled). The presence of such calcified projections was statistically associated with previous neurologic symptoms. (From Diethrich EB, et al: Virtual histology intravascular ultrasound assessment of carotid artery disease: the Carotid Artery Plaque Virtual Histology Evaluation [CAPITAL] study. J Endovasc Ther 14:676-686, 2007.)
The clinical value of VH IVUS is that with knowledge of the plaque type, the interventionalist can anticipate how the plaque is likely to behave at the moment of treatment: will it resist complete stent expansion or will it break up and embolize debris?28 VH IVUS has been used mostly in carotid artery stenting, where such information guides the way in which the patient is treated in the operating room, and it has the potential to make carotid stenting a safer procedure.34–38 However, it requires considerable experience and understanding of VH IVUS imaging and histopathology (Fig. 24-6).39–44
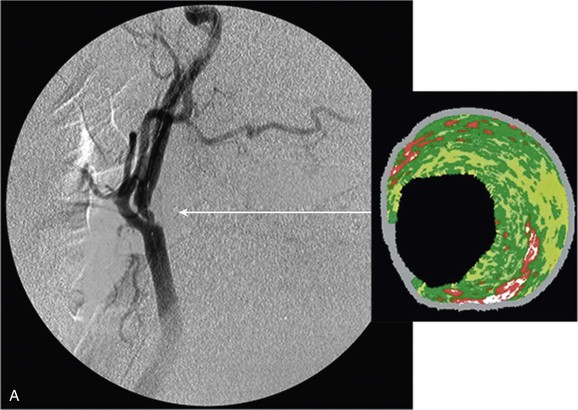
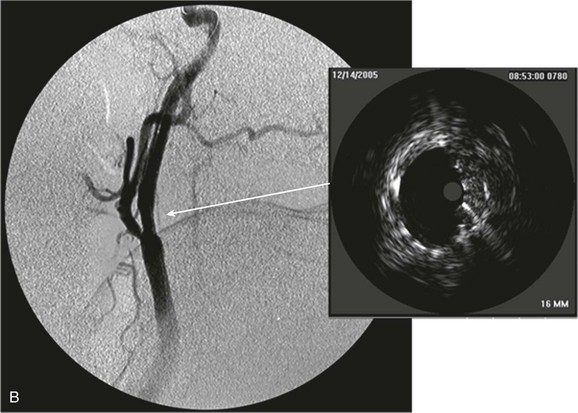
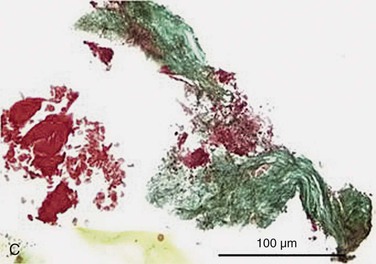
Figure 24-6 A, Angiography and IVUS demonstrate a focal stenosis at the origin of the right internal carotid artery in an asymptomatic 70-year-old man. At the narrowest point of the stenosis, the virtual histology IVUS shows predominantly fibrous (dark green) and calcified (white) areas. B, As anticipated, after stenting, there was incomplete stent expansion with midstent “waisting” despite repeated dilation at high pressures. C, Light microscopy of fibrous collagen fragments and blood clot from particles collected in the cerebral protection filter after treatment. (From Irshad K, et al: Virtual histology intravascular ultrasound in carotid interventions. J Endovasc Ther 14:198-207, 2007.)
Clinical Applications
Balloon Angioplasty and Stenting
IVUS acts as a guidance system during balloon angioplasty and stenting. It can determine the exact diameter of the vessel to be dilated as well as the composition of the plaque that is present. Furthermore, it can accurately calculate the degree of stenosis.45,46 This allows appropriate sizing of the balloon as well as of the stent to be implanted. This is probably more important with the placement of a balloon-expandable stent compared with a self-expanding stent, which is typically oversized. The use of IVUS after balloon angioplasty alone can assess the results of the intervention and determine the amount of dissection and whether a stent is required. In peripheral balloon angioplasty, evaluation of the size of the free luminal area and any residual stenosis has been found to predict patency; residual stenosis of greater than 60% is associated with unfavorable patency rates.47
The importance of appropriate balloon dilatation with stent to vessel wall apposition has been clearly demonstrated in both coronary and peripheral vessels.2,9 IVUS can readily identify the stent struts as echogenic structures and determine the degree of stent expansion and apposition to the vessel wall. If the stent is not fully apposed to the vessel wall, it can be further dilated with a larger balloon. This is frequently not visualized with arteriography alone.47 It has been shown that in the iliac vessels, the use of IVUS has improved patency of percutaneous transluminal angioplasty and stenting compared with angiography alone. In one study, 40% of patients had an underexpanded stent after IVUS.9 They were then expanded further with a larger balloon to ensure stent to vessel wall apposition and to enhance luminal diameter.
Endovascular Stent-Grafts for Abdominal Aortic Aneurysms
In the last 15 years, there has been a substantial clinical shift toward endovascular aneurysm repair of abdominal aortic aneurysms.48–50 With these procedures, there is a significant amount of information that is required before intervention.51 The most important is accurate assessment of the proximal and distal landing zones, particularly diameter and length measurements, as well as detection of arterial wall calcium and thrombus formation.20,52 Such assessment is generally performed preoperatively with the use of CTA, and this has become the imaging modality of choice. However, in certain cases, IVUS can be used in addition to preoperative CTA. IVUS is particularly helpful in further assessing cases that are technically challenging on the computed tomography scan. In addition, IVUS can be used instead of CTA in patients who have renal impairment, for whom contrast material cannot be safely given, or in patients with an allergy to contrast agents.20 Studies have demonstrated that IVUS can accurately determine the length and diameter of the landing zones in choosing the endograft size, can accurately identify the side branches, and can be used to deploy the stent-graft by visualizing the lowest renal artery while using fluoroscopy to watch the stent-graft open.52,53 In this way, intravenous contrast agents can, where appropriate, be avoided completely. One helpful use of IVUS in endovascular aneurysm repair is to check whether the contralateral gate has been accurately cannulated.
In patients with difficult access, IVUS can be used to assess occlusive disease and to guide ballooning as well as to determine whether continued efforts at percutaneous endografting are warranted or whether a conduit should be used. In the experience of one of the authors, access vessels that are heavily calcified as seen on IVUS can sometimes benefit from the use of rotational atherectomy to increase luminal diameter before balloon angioplasty. IVUS can also assess whether there has been an improvement in the access-related issues rather than opening the endograft to see if it will pass. This can therefore limit access-related complications, such as dissection and iliac artery rupture.
After repair, IVUS can demonstrate whether there is good apposition of the stent-graft with the proximal and distal landing zones. This can help determine whether an endoleak may or may not be a type I endoleak from poor seal. If there is poor apposition of the stent-graft or infolding of the stent-graft, it can help guide further therapy by determining whether to balloon, to place a balloon-expandable stent, or to place a more proximal cuff to achieve seal. This can all be done while limiting the amount of contrast material that has to be used.
Finally, after the procedure, access vessels can be inspected with IVUS to assess for luminal patency and to see if there are any irregularities, such as dissection, to the vessels. If they are identified, IVUS can guide the appropriate intervention and assess the result of treatment.
Thoracic Aortic Disease
There have been an increasing number of interventions for thoracic aortic disease with the advent of thoracic aortic stent-grafts.54 These grafts can be used to treat aneurysms, penetrating aortic ulcers, intramural hematomas, and aortic dissections.55–57 Currently, thoracic aortic stent-grafts require larger sheath diameters than the abdominal aortic stent-grafts. As such, they can create more access-related complications.
Often, when a patient presents with thoracic aortic disease, CTA of the chest has been performed without CTA of the abdomen and pelvis. If an intervention is planned, IVUS can be used at the time of the procedure to determine the size of the access vessels to limit the amount of radiation and to reduce the need for another load of contrast material.
IVUS can clearly identify the nature and extent of aortic disease, including dissections, penetrating aortic ulcers, intramural hematoma, and aneurysms. In cases of Stanford type B aortic dissection, it can clearly identify the true and false lumen and guide therapy with accurate assessment of the size of the graft to be implanted.58 It can identify the exact location of the entry tear and determine whether the left subclavian artery needs to be covered. The exact location of the innominate, left carotid, and left subclavian arteries can be clearly visualized. The thickness, movement of the dissection flap, and number of fenestrations between the true and false lumen are also readily seen, albeit that experience with IVUS is important (Fig. 24-7). Furthermore, each visceral vessel can be seen and assessed as to whether it is perfused by the true or false lumen. Because flap movement can be identified, dynamic obstruction of any of the visceral arteries can also be diagnosed and used to determine whether further therapy other than coverage of the entry tear is required, such as a stent in the visceral artery. The amount of coverage required for a thoracic aortic dissection can also be determined with IVUS. Whereas more coverage of the thoracic aorta is associated with increased false lumen thrombosis, there is a slight increase in the risk of spinal cord ischemia. If extension is planned to extend near the celiac artery, we find that IVUS is accurate in determining the origin of this vessel. It can often be difficult to accurately identify the origin of the celiac artery by aortography alone.58
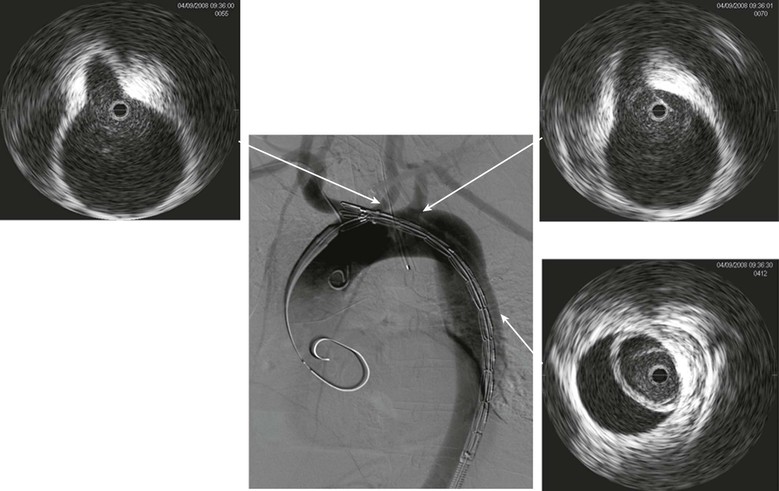
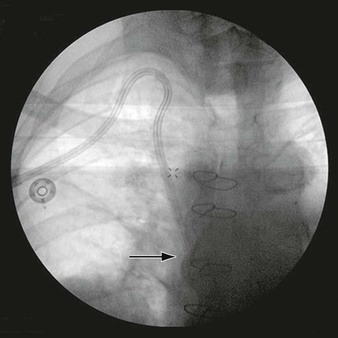
Figure 24-7 Digital subtraction aortography shows a dissection of the aorta being treated by endoluminal grafting. The corresponding IVUS images accurately locate the origins of the left carotid and left subclavian arteries, which are healthy. The IVUS probe is in the true lumen surrounded by the circle of intima that has separated, causing a false lumen.
After deployment of the stent-graft, the thoracic aorta can again be visualized. IVUS can assess the proximal deployment of the stent-graft and ensure that the innominate and left carotid arteries are patent. It can assess the ascending aorta for the presence of a retrograde dissection. Stent-graft expansion and graft–vessel wall apposition can also be determined with IVUS. Within the dissected aorta, the expansion of the true lumen can be determined and the false lumen assessed after therapy. Further evaluation of the visceral vessels is then performed to assess flow to these vessels. If a dynamic flap is still seen, IVUS can guide therapy to cannulate these visceral vessels and stent them.
With penetrating aortic ulcers, the exact location of the ulcer can be visualized with IVUS; appropriate proximal and distal landing zones of between 15 and 20 mm can clearly be seen with IVUS, and the appropriate graft size and length can be chosen on the basis of these images. After intervention, stent apposition can be clearly seen, ensuring that the ulcer is completely covered.
Intramural hematomas, like dissections and penetrating ulcers, can clearly be seen with IVUS, and therapy is guided on the basis of IVUS. Appropriate sizing to the inner wall is easily measured. As with dissections, IVUS offers the same benefits in treatment of thoracic aneurysms. Before intervention, it can assess the access vessels, determine the size and length of the landing zones, identify branch vessels, and identify any calcific plaques or mobile atheroma in the aortic arch that may be a potential source of embolic stroke. When IVUS detects potentially embolic material, we limit any ballooning in the arch.59 After the procedure, IVUS can assess the proximal and distal landing zones of the graft and assess patency of the access vessels.60
Venous Imaging
The importance of IVUS in the imaging of venous structures cannot be emphasized enough. IVUS is superior to single-plane and multiplanar venography in detecting the extent and type of morphologic lesions seen in the vein.61 IVUS has proved superior in showing trabeculations and webs that may be hidden in contrast medium. Venous wall thickness, neointimal hyperplasia, venous movement, and degree of thrombolysis can accurately be determined, and in this regard IVUS is superior to venography.62–65 This is essential in the diagnosis and endovascular management of iliac vein compression as well as of chronic deep venous thrombosis.66,67 The degree of venous stenosis can be calculated precisely by measuring the cross-sectional areas and diameters of normal and compressed or diseased veins with the software built into the IVUS system. It can guide stent insertion as well.
The deployment of IVC filters has increasingly been performed with IVUS instead of contrast venography.68 Bedside IVC filter placement can eliminate the need to transport critically ill patients to the endovascular suite. IVUS can clearly identify the renal veins, accurately measure the size of the vena cava, and thus allow the accurate placement of the filter without the need for fluoroscopy or contrast enhancement.69
IVUS has also been used recently to visualize venous abnormalities and to guide treatment in patients with chronic cerebrospinal venous insufficiency. In this situation, IVUS demonstrates valve abnormalities as well as luminal constrictions in the jugular and azygos veins.70
Cost versus Benefit
IVUS does add a cost to the procedure, especially because the catheters are disposable. However, in selective imaging of complex cases and in cases of critical limb ischemia, its benefit generally outweighs the issue of cost.9
Whereas there are no cost-effectiveness studies of IVUS in the periphery, there are some studies of its clinical value. In a retrospective study, an improved clinical outcome was found in patients who underwent aortoiliac artery stenting with IVUS guidance compared with those who had no IVUS.71 Another study of 100 consecutive peripheral interventions reported that IVUS detected unsatisfactory stent deployment in 34% of patients despite a satisfactory completion angiogram. After re-treatment, the cross-sectional area through the stent was increased by 42% (52 of these patients were undergoing carotid stenting).9
In another study, 131 patients underwent renal artery stenting with IVUS evaluation. IVUS detected unsuspected maldeployment of the stent in 23.5% of cases. The IVUS findings included 22 (14.4%) instances of incomplete stent apposition/expansion, 8 (5.2%) dissections, and 6 (3.9%) incompletely covered ostia.72
A favorable limb salvage rate in 50 consecutive patients undergoing IVUS-guided treatment for critical limb ischemia also reported its benefit. IVUS was crucial in 32% of cases, discovering unsuspected disease and inaccurately deployed stents. Limb salvage rate was 79% at 3 years despite the fact that nearly 25% of the patients were diabetic.73
Such studies certainly lend support to the clinical outcome benefit of IVUS, but no randomized controlled trial in peripheral vascular disease has yet been undertaken. We have found IVUS to be especially helpful in complex cases. However, the operating room team needs to regularly use IVUS so that when it is needed (e.g., an emergency case), it is quickly available and used with experience. Technology has advanced IVUS with great benefit, especially the additions of color flow and three-dimensional reconstruction, which make it much more user-friendly in the operating room. It is not surprising, therefore, that the clinical use of IVUS continues to increase.
Limitations
All imaging techniques have their limitations. IVUS requires a large (8F-9F) sheath size for evaluation of the aorta and IVC. IVUS can also be associated with artifacts that are important to appreciate. The near-field artifact present in phased array catheters means that a small portion of the vessel lumen immediately adjacent to the probe cannot be visualized because of a dead space that is caused by acoustic incoherence. This is called the Fresnel zone and is determined by the diameter of the transducer and the wavelength of the ultrasound. Fortunately, it is clinically unimportant.
Heavily calcified vessels produce bright echoes with posterior shadowing, which limits visualization of structures beyond. This is also seen with metallic stent struts, with shadowing appearing behind the struts.
Mechanical systems, unlike electronic systems, require flushing of the transducer chamber to avoid air bubbles that can distort the image. Also, in mechanical systems, when the rotating transducer inside the catheter is exposed to frictional forces, the drive cable undergoes a variation in velocity and portions of the image become distorted.
One potential risk of performing IVUS is embolization caused by dislodging of disease from the vessel wall. However, in practice, this is a rare phenomenon, and it is not thought to be clinically important in most arterial situations, with the exception of carotid angioplasty and stenting.31,74
Future Advances
Technical limitations of ultrasound may one day be superseded, either by technologic advances in transducers or by use of other imaging waveforms. Optical coherence tomography has recently been proposed as a possible alternative to IVUS.75 It uses light at a specific wavelength that is transmitted from an intra-arterial catheter with a transducer. The resolution is far superior to IVUS imaging; however, the depth of view is only a few millimeters, and the vessel needs to be washed with saline so that the light can be visualized by the probe. At present, optical coherence tomography is not a viable clinical alternative to IVUS.76,77
Currently, IVUS images the vessel wall with axial slices at 90 degrees to the probe. One possible future advance is “forward-looking” IVUS, which could be potentially useful in guiding wires through chronic total occlusions. However, there is no commercially available forward-looking IVUS system at present.78
Selected Key References
Arko F, Mettauer M, McCollough R, Patterson D, Manning L, Lee S, Buckley CJ. Use of intravascular ultrasound improves long-term clinical outcome in the endovascular management of atherosclerotic aortoiliac occlusive disease. J Vasc Surg. 1998;27:614–623.
Diethrich EB. Endovascular treatment of abdominal occlusive disease: the impact of stents and intravascular ultrasound imaging. Eur J Surg. 1993;7:228–236.
The use of IVUS to accurately deploy aortic stents was quickly recognized..
Diethrich EB, Margolis P, Reid DB, Burke A, Ramaiah V, Rodriguez-Lopez JA, Wheatley G, Olsen D, Virmani R. Virtual histology intravascular ultrasound assessment of carotid artery disease: the Carotid Artery Plaque Virtual Histology Evaluation (CAPITAL) study. J Endovasc Ther. 2007;14:676–686.
Gussenhoven EJ, Essed CE, Frietman P, van Egmond F, Lancée CT, van Kappellen WH, Roelandt J, Serruys PW, Gerritsen GP, van Urk H. Intravascular ultrasonic imaging: histologic and echographic correlation. Eur J Vasc Surg. 1989;3:571–576.
Irshad K, Reid DB, Miller PH, Velu R, Kopchok GE, White RA. Early clinical experience with color three-dimensional intravascular ultrasound in peripheral interventions. J Endovasc Ther. 2001;8:329–338.
Isner JM, Rosenfield K, Losordo DW, Kelly S, Palefski P, Langevin RE, Razvi S, Pastore JO, Kosowsky BD. Percutaneous intravascular US as adjunct to catheter-based interventions: preliminary experience in patients with peripheral vascular disease. Radiology. 1990;175:61–70.
Kopchok GE, White RA, Guthrie C, Hsiang Y, Rosenbaum D, White GH. Intraluminal vascular ultrasound: preliminary report of dimensional and morphological accuracy. Ann Vasc Surg. 1990;4:291–296.
Laskey WK, Brady ST, Kussmaul WG, Waxler AR, Krol J, Herrmann HC, Hirshfeld JW Jr, Sehgal C. Intravascular ultrasonographic assessment of the results of coronary artery stenting. Am Heart J. 1993;125:1576–1583.
The reference list can be found on the companion Expert Consult website at www.expertconsult.com.
References
1. Gussenhoven EJ, et al. Intravascular ultrasonic imaging: histologic and echographic correlation. Eur J Vasc Surg. 1989;3:571–576.
2. Isner JM, et al. Percutaneous intravascular US as adjunct to catheter-based interventions: preliminary experience in patients with peripheral vascular disease. Radiology. 1990;175:61–70.
3. Laskey WK, et al. Intravascular ultrasonographic assessment of the results of coronary artery stenting. Am Heart J. 1993;125:1576–1583.
4. White R, et al. Innovations in vascular imaging: arteriography, three-dimensional CT scans, and two- and three-dimensional intravascular ultrasound evaluation of an abdominal aortic aneurysm. Ann Vasc Surg. 1994;8:285–289.
5. Gussenhoven EJ, et al. Intravascular ultrasound predictors of outcome after peripheral balloon angioplasty. Eur J Vasc Endovasc Surg. 1995;10:279–288.
6. Diethrich EB. Endovascular treatment of abdominal occlusive disease: the impact of stents and intravascular ultrasound imaging. Eur J Surg. 1993;7:228–236.
7. Irshad K, et al. The role of intravascular ultrasound and peripheral endovascular interventions. Heuser RR, et al. Textbook of peripheral vascular interventions. Martin Dunitz: London; 2004:25–34.
8. Irshad K, et al. Early clinical experience with color three-dimensional intravascular ultrasound in peripheral interventions. J Endovasc Ther. 2001;8:329–338.
9. Reid DB, et al. Clinical application of intravascular ultrasound in peripheral vascular disease. Seigel RJ. Intravascular ultrasound imaging in coronary artery disease. Marcel Dekker: New York; 1998:309–341.
10. Cavaye DM, et al. Intravascular ultrasound imaging: an essential component of angioplasty assessment and vascular stent deployment. Int Angiol. 1993;12:212–220.
11. Barnes RW, et al. Intraoperative assessment of arterial reconstruction by Doppler ultrasound. Surg Gynecol Obstet. 1978;146:896–900.
12. Baril DT, et al. Duplex criteria for determination of in-stent stenosis after angioplasty and stenting of the superficial femoral artery. J Vasc Surg. 2009;49:133–138.
13. Mitchell DG. Color Doppler imaging: principles, limitations and artifacts. Radiology. 1990;177:1–10.
14. Subramanian M, et al. Intravascular ultrasound in endovascular repair of abdominal aortic aneurysm. Pererdikides TP, et al. Advances in endovascular management of abdominal aortic aneurysms. PMP Limited: Athens; 2009:15–20.
15. Kopchok GE, et al. Intraluminal vascular ultrasound: preliminary report of dimensional and morphological accuracy. Ann Vasc Surg. 1990;4:291–296.
16. Katzen, et al. Role of intravascular ultrasound in peripheral atherectomy and stent deployment. Circulation. 1991;84:2152.
17. Reid DB, et al. Endovascular significance of the external carotid artery in the treatment of cerebrovascular insufficiency. J Endovasc Ther. 2004;11:727–733.
18. Scoccianti M, et al. Intravascular ultrasound guidance for peripheral vascular interventions. J Endovasv Surg. 1995;2:356–364.
19. Van Sambeek MR, et al. Intravascular ultrasound in endovascular stent-grafts for peripheral aneurysm: a clinical study. J Endovasc Surg. 1998;5:106–112.
20. White RA, et al. Intravascular ultrasound: the ultimate tool for abdominal aortic aneurysm assessment and endovascular graft delivery. J Endovasc Surg. 1997;4:45–55.
21. Navarro F, et al. Intravascular ultrasound assessment of iliac stent procedures. J Endovasc Ther. 2000;7:315–319.
22. Krishnamurthy VN, et al. Intravascular ultrasound–guided true lumen reentry device for recanalization of unilateral chronic total occlusion of iliac arteries: technique and follow-up. Ann Vasc Surg. 2010;24:487–497.
23. Carlier SG, et al. Blood assessment with intravascular ultrasound catheters: the ideal tool for simultaneous assessment of the coronary hemodynamics and vessel wall? Semin Intervent Cardiol. 1998;3:21–29.
24. Van der Steen AF, et al. Flow estimation using an intravascular imaging catheter. Ultrasonics. 2000;38:363–368.
25. Neilson TR, et al. The Doppler signal: where does it come from and what does it mean? AJR Am J Roentgenol. 1988;151:439–447.
26. Reid DB, et al. The clinical value of three-dimensional intravascular ultrasound imaging. J Endovasc Surg. 1995;2:356–364.
27. Reid DB. Early clinical experience with virtual histology intravascular ultrasound. J Endovasc Ther. 2006;13:1–31.
28. Irshad K, et al. Virtual histology intravascular ultrasound in carotid interventions. J Endovasc Ther. 2007;14:198–207.
29. Nair A, et al. Assessing spectral algorithms to predict atherosclerotic plaque composition with normalized and raw intravascular ultrasound data. Ultrasound Med Biol. 2001;27:1319–1331.
30. Nair A, et al. Coronary plaque classification with intravascular ultrasound radiofrequency data analysis. Circulation. 2002;106:2200–2206.
31. Diethrich EB, et al. Virtual histology and color flow intravascular ultrasound in peripheral interventions. Semin Vasc Surg. 2007;19:155–160.
32. González A, et al. Carotid plaque characterization by virtual histology intravascular ultrasound related to the timing of carotid intervention. J Endovasc Ther. 2012;19:764–773.
33. Diethrich EB, et al. Virtual histology intravascular ultrasound assessment of carotid artery disease: the Carotid Artery Plaque Virtual Histology Evaluation (CAPITAL) study. J Endovasc Ther. 2007;14:676–686.
34. Diethrich EB, et al. Stenting in carotid surgery: initial experience in 110 patients. J Endovasc Surg. 1996;2:42–62.
35. Diethrich EB, et al. Percutaneous techniques for endoluminal carotid interventions. J Endovasc Surg. 1996;3:182–202.
36. Reid DB. Carotid plaque characterization: helpful to endarterectomy and endovascular surgeons. J Endovasc Surg. 1998;5:247–250.
37. Biasi GM, et al. Carotid plaque echolucency increases the risk of stroke in carotid stenting: the Imaging in Carotid Angioplasty and Risk of Stroke (ICAROS) study. Circulation. 2004;110:756–762.
38. Reid DB, et al. Intravascular ultrasound assessment in carotid interventions. J Endovasc Surg. 1996;3:203–210.
39. Virmani R, et al. Atherosclerotic plaque progression and vulnerability to rupture. Arterioscler Thromb Vasc Biol. 2005;25:2054–2061.
40. Virmani R, et al. Pathology of the thin-cap fibroatheroma. J Interv Cardiol. 2003;16:267–272.
41. Stary HC, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1995;92:1355–1374.
42. Naghavi M, et al. From vulnerable plaque to vulnerable patient. Circulation. 2003;108:1664–1672.
43. Spagnoli LG, et al. Extracranial thrombotically active carotid plaque as a risk factor for ischaemic stroke. JAMA. 2004;292:1845–1852.
44. Reid DB, et al. Intravascular ultrasound: plaque characterization. Nicolaides A, et al. Ultrasound and carotid bifurcation atherosclerosis. Springer: London; 2012:551–561.
45. Clair D. Pros and cons for intravascular ultrasound in stenting. Phlebology. 2013;28(Suppl 1):129–134.
46. Marrocco CJ, et al. Intravascular ultrasound. Semin Vasc Surg. 2012;25:144–152.
47. Arko F, et al. Use of intravascular ultrasound in the endovascular management of atherosclerotic aortoiliac occlusive disease. Am J Surg. 1996;172:546–549.
48. Greenhalgh RM, et al. Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30 day operative mortality results: randomised controlled trial. [EVAR trial participants] Lancet. 2004;364:843–848.
49. Prinssen M, et al. A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. N Engl J Med. 2004;351:1607–1618.
50. Lederle FA, et al. Outcomes following endovascular vs open repair of abdominal aortic aneurysms: a randomized trial. JAMA. 2009;302:1535–1542.
51. Reid AW, et al. Vascular imaging: an unparalleled decade. J Endovasc Ther. 2004;11(Suppl II):II163–II179.
53. Pearce BJ, et al. Using IVUS during EVAR and TEVAR: improving patient outcomes. Semin Vasc Surg. 2009;22:172–180.
54. Grabenwoger M, et al. Thoracic endovascular aortic repair (TEVAR) for the treatment of aortic diseases: a position statement from the European Association for Cardio-Thoracic Surgery (EACTS) and the European Society of Cardiology (ESC), in collaboration with the European association of percutaneous cardiovascular interventions. Eur Heart J. 2012;33:1558–1563.
55. Dake MD, et al. Transluminal placement of endovascular stent grafts for the treatment of descending thoracic aortic aneurysms. N Engl J Med. 1994;331:1729–1734.
56. Dake MD, et al. Endovascular stent graft placement for treatment of acute aortic dissection. N Engl J Med. 1999;340:1546–1552.
57. Nienaber CA, et al. Nonsurgical reconstruction of thoracic aortic dissection by stent-graft placement. N Engl Med. 1999;340:1538–1545.
58. Kobayashi N, et al. Intravascular ultrasound–guided endovascular stenting for celiac artery complicated with hepatic hypoperfusion after acute type B aortic dissection. J Am Coll Cardiol. 2012;59:1568 [24] .
59. Kim K, et al. Predictability of cerebral embolization from aortic arch manipulations during thoracic endovascular repair. Am Surg. 2011;77:1399–1409.
60. Koschyk DH, et al. Value of intravascular ultrasound for endovascular stent-graft placement in aortic dissection and aneurysm. J Card Surg. 2003;18:471–477.
61. McLafferty RB. The role of intravascular ultrasound in venous thromboembolism. Semin Intervent Radiol. 2012;29:10–15.
62. Neglen P, et al. Intravascular ultrasound scan evaluation of the obstructed vein. J Vasc Surg. 2002;35:694–700.
63. Hingorani A, et al. Role of IVUS versus venograms in assessment of ilio-femoral vein stenosis. J Vasc Surg. 2001;52:804.
64. Arko FR, et al. Aggressive percutaneous mechanical thrombectomy of deep venous thrombosis: early clinical results. Arch Surg. 2007;142:513–518.
65. Murphy EH, et al. Device and imaging-specific volumetric analysis of clot lysis after percutaneous mechanical thrombectomy for iliofemoral DVT. J Endovasc Ther. 2010;17:423–433.
66. Cockett FB, et al. Iliac vein compression. Its relation to iliofemoral thrombosis and the post-thrombotic syndrome. Br Med J. 1967;2:14–19.
67. Forauer AR, et al. Intravascular ultrasound in the diagnosis and treatment of iliac vein compression (May-Thurner) syndrome. J Vasc Interv Radiol. 2002;13:523–527.
68. Aidinian G, et al. Intravascular ultrasound–guided inferior vena cava filter placement in the military multitrauma patients: a single-center experience. Vasc Endovascular Surg. 2009;43:497–501.
69. Hodgkiss-Harlow K, et al. Technical factors affecting the accuracy of bedside IVC filter placement using intravascular ultrasound. Vasc Endovascular Surg. 2012;46:293–299.
70. Sclafani SJ. Intravascular ultrasound in the diagnosis and treatment of chronic cerebrospinal venous insufficiency. Tech Vasc Inter Radiol. 2012;15:131–143.
71. Arko F, et al. Use of intravascular ultrasound improves long-term clinical outcome in the endovascular management of atherosclerotic aortoiliac occlusive disease. J Vasc Surg. 1998;27:614–623.
72. Dangas G, et al. Intravascular ultrasound guided renal artery stenting. J Endovasc Ther. 2001;8:238–247.
73. Irshad K, et al. The role of intravascular ultrasound and peripheral endovascular interventions. Heuser RR, et al. Textbook of peripheral vascular interventions. Martin Dunitz: London; 2004:25–34.
74. Timaran CH, et al. Atherosclerotic plaque composition assessed by virtual histology intravascular ultrasound and cerebral embolization after carotid stenting. J Vasc Surg. 2010;52:1188–1194.
75. Setacci C, et al. Safety and feasibility of intravascular optical coherence tomography using a nonocclusive technique to evaluate carotid plaques before and after stent deployment. J Endovasc Ther. 2012;19:303–311.
76. Reimers B, et al. Commentary: optical coherence tomography: a valuable tool to improve carotid artery stenting. J Endovasc Ther. 2012;19:312–313.
77. Yoshimura S, et al. Visualization of internal carotid artery atherosclerotic plaques in symptomatic and asymptomatic patients: a comparison of optical coherence tomography and intravascular ultrasound. Am J Neuroradiol. 2012;33:308–313.
78. Back MR, et al. Forward-looking intravascular ultrasonography: in-vitro imaging of normal and atherosclerotic human arteries. Ann Surg. 1994;60:738–743.