50 Intrathecal Catheter Implantation
Placement
Position
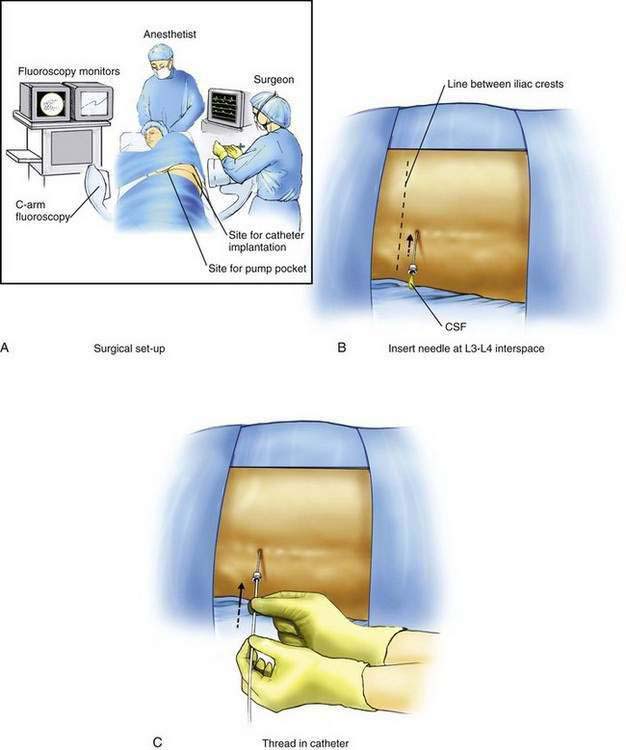
Implantation of an intrathecal drug delivery system is a minor surgical procedure that is carried out in the operating room using aseptic precautions, including skin preparation, sterile draping, and full surgical attire (Fig. 50-1A). The procedure can be conducted under regional anesthesia or general anesthesia using dedicated anesthesia personnel. Performing the initial spinal catheter placement under general anesthesia is controversial, and concerns about neural injury are similar to those when performing any neuraxial technique under general anesthesia.
The patient is positioned on a radiolucent table in the lateral decubitus position with the patient’s side for the pump pocket nondependent (see Fig. 50-1A). The arms are extended at the shoulders and secured so that they are well away from the surgical field. The skin is prepared and sterile drapes are applied. The fluoroscopic C-arm is positioned across the lumbar region to provide a cross-table anteroposterior view of the lumbar spine. Care must be taken to ensure that the radiographic view is not rotated by observing that the spinous processes are in the midline, halfway between the vertebral pedicles (see Fig. 50-1E).
Procedure for Intrathecal Catheter Placement
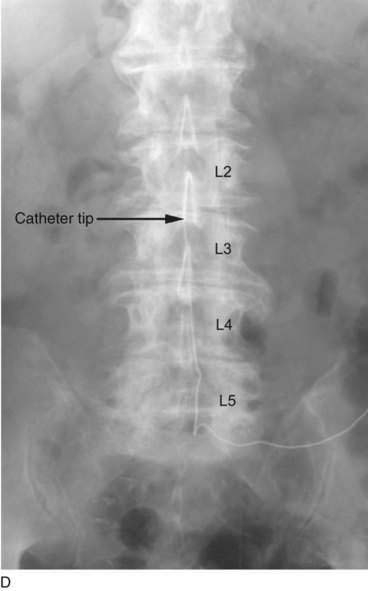
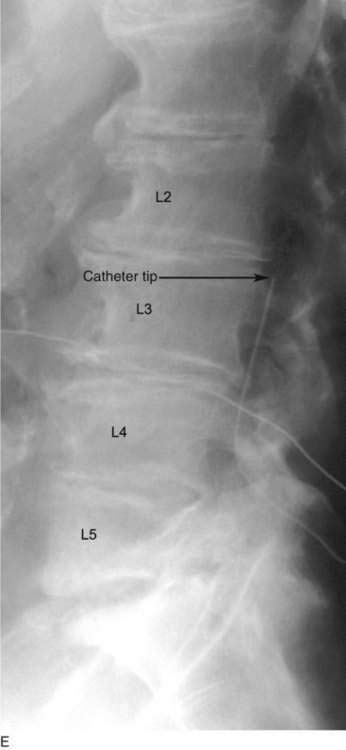
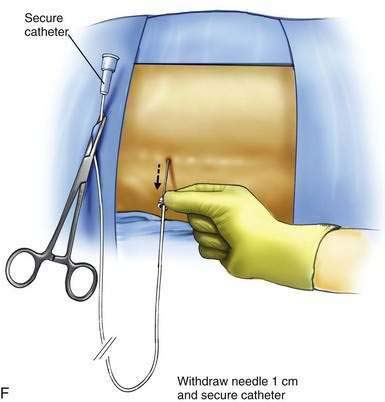
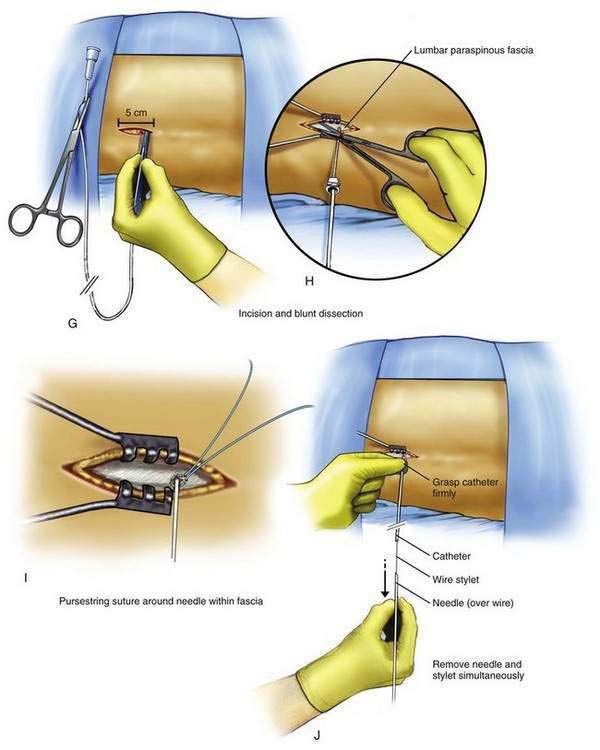
The L3-L4 interspace is identified using fluoroscopy The spinal needle supplied by the intrathecal device manufacturer must be used to ensure that the catheter can advance through the needle without damage. The needle is advanced using a paramedian approach starting 1 to 1.5 cm lateral to the spinous processes. The needle is directed to enter the spinal space in the midline; the stylet is removed to ensure adequate flow of CSF (Fig. 50-1B). The spinal catheter is then advanced through the needle until the tip is well into the spinal space but below L2 in the lumbar cistern (Fig. 50-1C). The position of the catheter tip is verified using fluoroscopy in the anteroposterior and lateral planes (Fig. 50-1D and E). The needle is then withdrawn slightly (about 1 to 2 cm) but left in place around the catheter in the subcutaneous tissues to protect the catheter during the subsequent incision and dissection (Fig. 50-1F). The catheter is secured to the surgical field using a small clamp to ensure that it does not fall from the sterile field (see Fig. 50-1F).
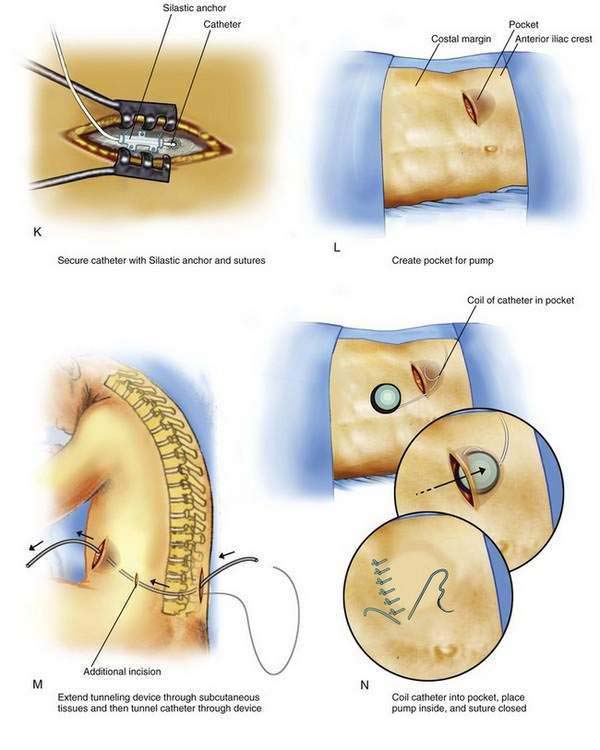
A 5- to 8-cm incision parallel to the axis of the spine is extended from just cephalad to just caudad to the needle, extending directly through the needle’s entry point on the skin (Fig. 50-1G). The subcutaneous tissues are divided using blunt dissection until the lumbar paraspinous fascia is visible surrounding the needle shaft (Fig. 50-1H). A pursestring suture is created in the fascia surrounding the needle shaft site (Fig. 50-1I). This suture is used to tighten the fascia around the catheter and prevent backflow of CSF, which may lead to a chronic subcutaneous CSF collection. The needle and stylet are then removed simultaneously, using care not to dislodge the spinal catheter (Fig. 50-1J). Free flow of CSF from the catheter should be evident; if there is no CSF flowing from the catheter, a blunt needle can be inserted in the end of the catheter and gentle aspiration used to ensure that the catheter remains in the thecal sac. If CSF cannot be aspirated from the catheter, the catheter is removed and replaced. The catheter is then secured to the paraspinous fascia using a specific anchoring device supplied by the manufacturer (Fig. 50-1K).
Attention is now turned to creating the pocket in the patient’s abdominal wall. A 10- to 12-cm transverse incision is made along the previously marked line, and a subcutaneous pocket is created using blunt dissection (Fig. 50-1L). The pocket should always be created caudad to the incision; if the pocket is placed cephalad to the incision, the weight of the pump on the suture line is likely to cause wound dehiscence. In many patients, the blunt dissection can be accomplished using gentle but firm pressure with the fingers. It is simpler and less traumatic to use a small surgical scissors to perform the blunt dissection, using repeated opening motions rather than closing or cutting motions that are likely to cut vascular structures and provoke marked bleeding. An alternative to blunt dissection is the use of a monopolar electrocautery device in the “cut” mode, an effective means to carry out the necessary dissection without excessive tissue trauma or blood loss. After the pocket has been created, the pump is placed in the pocket to ensure that the pocket is large enough. The pump should fit completely within the pocket without any part of the device extending into the incision. With the device in place, the wound margins should fall into close apposition. There should be no tension on the sutures during closure of the incision, or the wound is more likely to undergo dehiscence.
After the pocket has been created, a tunneling device is extended within the subcutaneous tissues between the paraspinous incision and the pocket (Fig. 50-1M). The catheter is then advanced through the tunnel, leaving a small tension-relief loop of catheter in the subcutaneous area of the paraspinous dissection (commonly used tunneling devices place a hollow plastic sleeve through which the catheter can be advanced from the patient’s back to the pump pocket). The catheter is then trimmed to a length that allows a small loop of catheter to remain deep to the pump and attached to it. The pump is placed in the pocket with a loop of catheter deep to the device (Fig. 50-1N). This loop and the small loop of catheter in the paraspinous region allow patient movement without placing tension on the distal catheter, causing it to be pulled from the thecal sac. Two or more sutures are then placed through the suture loops or mesh enclosure surrounding the pump and are used to secure the pump to the abdominal fascia. These simple retaining sutures prevent the pump from rotating or flipping within the pocket. The skin incisions are then closed in two layers: a series of interrupted subcutaneous sutures to close the fascia securely overlying the pump and the catheter, followed by skin closure using suture or staples.
Procedure for Permanent Epidural Catheter Placement
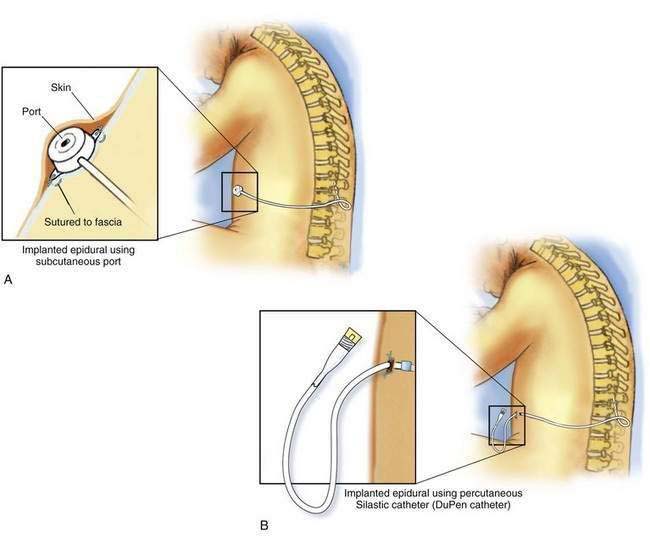
To place a permanent epidural system with a subcutaneous port, a 6- to 8-cm transverse incision is made overlying the costal margin halfway between the xiphoid process and the anterior axillary line. A pocket is created overlying the rib cage using blunt dissection (Fig. 50-2A). The catheter is then tunneled from the paraspinous region to the pocket, as described previously for intrathecal catheter placement, and secured to the port. The port must then be sutured securely to the fascia over the rib cage. Care must be taken to ensure that the port is secured firmly in a region that overlies the rib cage; if the port migrates inferiorly to lie over the abdomen, it becomes difficult to access. The rigid support of the rib cage holds the port firmly from behind, allowing easier access to the port. The skin incisions are then closed in two layers: a series of interrupted subcutaneous sutures to close securely the fascia overlying the catheter, followed by skin closure using sutures or staples.
To place a permanent epidural system without a subcutaneous port, a tunneling device is extended from the paraspinous incision to the right upper abdominal quadrant just inferior to the costal margin. A small incision (about 0.5 cm) is made to allow the tunneling device to exit the skin. Percutaneous epidural catheters are supplied in two parts: the proximal portion of the catheter that is placed in the epidural space and the distal portion of the catheter that enters the abdominal wall and connects with the proximal portion of the catheter. The distal portion of the catheter is now secured to the tunneling device and pulled through the incision in the abdominal wall subcutaneously to emerge from the paraspinous incision (Fig. 50-2B). Many catheters are supplied with an antibiotic-impregnated cuff that is designed to arrest entry of bacteria along the track of the catheter. This cuff should be placed about 1 cm from the catheter’s exit site along the subcutaneous catheter track. The proximal and distal portions of the catheter are then trimmed, leaving enough catheter length to ensure that there is no traction on the catheter with movement. The two ends of the catheter are connected using a stainless steel union supplied by the manufacturer and sutured securely. The paraspinous skin incision is then closed in two layers: a series of interrupted subcutaneous sutures to securely close the fascia overlying the catheter, followed by skin closure using suture or staples. The skin incision at the epidural catheter’s exit site in the right upper quadrant is closed around the base of the catheter using one or two simple interrupted sutures.