Intraosseous Infusion

Review Box 25-1 Intraosseous infusion: indications, contraindications, complications, and equipment.
In a review of vascular access success rates in children in cardiac arrest, the average time needed to establish percutaneous peripheral IV access was 7.9 ± 4.2 minutes, with only a 17% success rate.1 Some authors advocate the use of central venous lines in children, but such lines are also difficult to place and are associated with risk for arterial injury, infection, and pneumothorax. Alternative routes of drug administration, such as the endotracheal and rectal routes, are not reliable in patients in shock or cardiac arrest.2
Peripheral IV access can also be difficult in adults, including those who are obese, have burn injuries, are volume-depleted, or are in shock.3 This difficulty is compounded in the prehospital or military setting, where environmental factors and the need for rapid transport can challenge even the most skilled practitioners.4
Intraosseous (IO) access can provide rapid, lifesaving intravascular access in challenging environments and in both pediatric and adult patients. The American Heart Association, the American Academy of Pediatrics, and the American College of Surgeons all recommend IO access in children in emergency situations when IV access is not immediately possible.5,6 The latest edition of the advanced trauma life support course of the American College of Surgeons also notes that IO access using specially designed equipment is an important option in adult trauma patients.5 IO access is often faster than IV access, and the success rate after failed IV attempts is high. In a retrospective study of pediatric cardiac arrest patients, time to IO access was significantly shorter than time to IV access.7 In a similar review of intravascular access during pediatric cardiac arrest, Brunette and Fischer2 noted that when compared with central venous access and venous cutdown, IO access and IV access were faster, and the success rate for IO access was 83% versus 17% for IV access. IO access can also be a rapid and successful technique in adults.8 Because of their success under difficult battlefield conditions, the U.S. military has adopted the use of IO infusion devices.5
IO access is not always successful. In a 5-year review of prehospital IO needle placement, success rates were higher in children younger than 3 years (85%) than in older children or adults (50%).7 The main causes of failure were errors in identification of landmarks and bending of the needles. Better needle design and new devices have helped overcome problems with bone penetration (see “Equipment and Setup,” later).
Historical Perspective
One of the earliest references describing the IO route is attributed to Drinker and colleagues,9 who in 1922 examined the circulation of the sternum and suggested it as a site for transfusion. The route was not used clinically until 1934, when Josefson,10 a Swedish clinician, injected liver concentrate into the sternum of 12 adult patients with pernicious anemia and reported that all 12 improved. Subsequently, the technique became widespread in Scandinavian countries. In 1944, British physician Hamilton Bailey11 described the utility of sternal IO access in blackout conditions in London during World War II.
In 1940, the technique was introduced to American clinicians by Tocantins and associates,12–14 who described a series of animal and clinical studies demonstrating that fluid is rapidly transported from the medullary cavity of long bones to the heart. They recommended using the manubrium in older children and adults and the upper part of the tibia or lower part of the femur in children 3 years or younger. Over the next 2 decades, thousands of cases of IO infusion of blood, crystalloid substances, and drugs were reported.15–19 The procedure was more commonly used in children because of the difficulty in achieving other forms of IV access. Nevertheless, during the 1940s, IO infusion was also used extensively in adults, and a sternal puncture kit for bone marrow infusion was a common component of emergency medical supplies during World War II.4,20 This resulted in more than 4000 reported cases of successful IO access in wounded soldiers.19 During this time, relatively few complications were reported despite the fact that the needles were often left in place for 24 to 48 hours. Heinild and coworkers in 194717 reviewed 982 cases of IO infusion and reported only 18 failures and 5 cases of osteomyelitis. None of the cases of osteomyelitis occurred in patients who received isotonic solutions.
With the introduction of plastic catheters and improved cannulation techniques, the need for IO infusion as an alternative route for IV access diminished, and the technique was all but abandoned. In the mid-1980s, James Orlowski brought about a renaissance in the use of IO access for pediatric resuscitation. While traveling through India during a cholera epidemic, he observed emergency health care workers using IO access to deliver fluids and medications. In 1984 he wrote an editorial, “My Kingdom for an Intravenous Line,”20 in which he advocated the use of IO access during pediatric resuscitation. After Orlowski’s editorial, others began promoting the use of IO access to allow rapid drug delivery during cardiopulmonary resuscitation (CPR) in children.1,21,22 In 1988, IO techniques were adopted by the American Heart Association and included in the pediatric advance life support guidelines.6 Since then, the technique has become widespread throughout the United States and is recognized as an accepted alternative to IV access in pediatric emergencies and, increasingly, in neonatal and adult emergencies. In addition, the safety, ease, and effectiveness of the technique have led to its use in prehospital emergency care.7,23–26
Anatomy and Physiology
Long bones are richly vascular structures with a dynamic circulation. They are capable of accepting large volumes of fluid and rapidly transporting fluid or drugs to the central circulation. The bone, like most organs, is supplied by a major artery (nutrient artery). The artery pierces the cortex and divides into ascending and descending branches, which further subdivide into arterioles that pierce the endosteal surface of the stratum compactum to become capillaries. The capillaries drain into medullary venous sinusoids throughout the medullary space, which in turn drain into a central venous channel (Fig. 25-1). The medullary sinusoids accept fluid and drugs during IO infusion and serve as a route for transport to the central venous channel, which exits the bone as nutrient and emissary veins.27 The medullary cavity thus functions as a rigid, noncollapsible vein, even in the presence of profound shock or cardiopulmonary arrest.28 Radiographic studies have demonstrated that radiopaque dye spreads only a few centimeters in the medullary space before being transported to the venous system.29
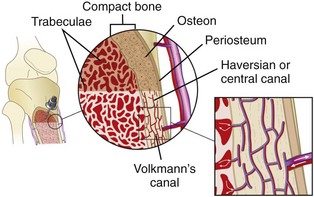
Figure 25-1 Schematic diagram illustrating the vascular anatomy of a long bone with an intraosseous needle in place.
Almost every drug and fluid commonly used during resuscitation has been reported upon in clinical and preclinical IO studies. Medications and fluids that have been administered through IO infusion are listed in Box 25-1. Crystalloid infusion studies in animals have demonstrated that infusion rates of 10 to 17 mL/min may be achieved with gravity infusion and rates as high as 42 mL/min with pressure infusion.30–32 In a swine model of hemorrhagic shock, Neufeld and colleagues31 found that the IO delivery rate of crystalloid was similar to that with both peripheral and central venous administration. IO crystalloid infusions have also been shown to produce a significant increase in blood pressure in a hemorrhagic shock model in rabbits.32 In small animals (7 to 8 kg), the size of the marrow cavity is the rate-limiting factor, whereas in larger animals (12 to 15 kg), the size of the needle determines the flow.33,34 Blood under pressure can be infused approximately two thirds as fast as crystalloid fluids.33 However, in a swine model evaluating IO infusion for mild therapeutic hypothermia, IO infusion of ice-cold saline was not as efficacious as intravenous infusion.35
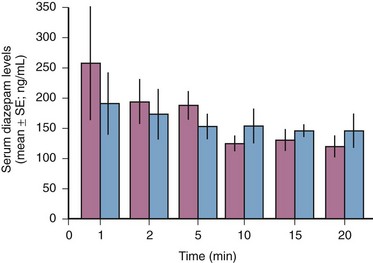
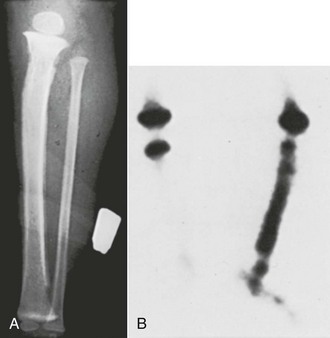
Comparisons of IO and IV infusion of drugs have demonstrated that the drugs reach the central circulation by both routes in similar concentrations and at the same time (Fig. 25-2).14,36 This holds true even during CPR, during which sodium bicarbonate has been shown to provide greater buffering capacity when administered by the IO route than by the peripheral IV route.37 Early IO access and infusion of epinephrine improved 24-hour survival in a swine model of ventricular fibrillation versus delayed IV administration.38 IO infusion of iodinated computed tomographic contrast material has also been reported as being successful,39 as has scorpion antivenin.40
Voelckel and associates36 demonstrated that bone marrow blood flow responds to both the physiologic stress of hemorrhagic shock and vasopressors given during resuscitation after hypovolemic cardiac arrest in dogs. After successful resuscitation, bone marrow blood flow decreased after high-dose epinephrine but was maintained after high-dose vasopressin. These findings in animal models suggest the need for pressurized IO infusion techniques during hemorrhagic shock and certain drug therapies.
Indications
Obtaining venous access can be a difficult task even under the best circumstances. This difficulty is compounded during high-stress situations or low-flow states such as cardiac arrest. Studies have shown that IO devices provide a rapid and effective means of fluid and drug administration during pediatric CPR.1 This is also true during resuscitation of critically injured infants and children, in whom IO infusion of blood, colloids, or crystalloids (or any combination thereof) may be lifesaving. IO infusion is also beneficial in the management of children with other medical conditions, including those with respiratory distress, neurologic insults, dehydration and sepsis.42–44 The widespread use of IO devices has led to improved prehospital vascular access and a marked reduction in critical transport with failed vascular access.45
IO access is not commonly used in infants, but it is recommended as an alternative for medication and crystalloid administration when venous access is not readily obtained.46 IO infusion has been used with success in both premature and term infants.47–49 In one study, 30 IO lines were placed in 20 preterm and 7 full-term neonates with a variety of illnesses (e.g., respiratory distress syndrome, perinatal asphyxia, congenital cardiac anomalies) in whom conventional venous access had failed.48 All survived resuscitation with no long-term effects from IO line placement. Gestational age ranged from 32 to 41 weeks and birth weight ranged from 515 to 4050 g.48 In 2000, Abe and coworkers49 reported on the speed and ease of establishing newborn emergency vascular access by using turkey bones and plastic infant legs to simulate IO access and fresh umbilical cord to simulate umbilical venous catheterization. They demonstrated that for individuals who do not perform newborn resuscitation frequently, IO placement was easier and quicker than umbilical venous catheterization. A comparison of umbilical venous versus IO access in a simulated model of neonatal resuscitation showed that IO access was faster with no difference in error rate or perceived difference in ease of use.50
IO infusion is also indicated for adult patients in whom attempts at peripheral or central venous access have been unsuccessful. This may include adult patients with burns, trauma, shock, dehydration, or status epilepticus.51 Multiple sites, including the iliac crest, femur, proximal and distal ends of the tibia, radius, clavicle, and calcaneus may be used.52–55 Of these, the tibia may be less desirable because red marrow is replaced by less vascular yellow marrow or fat by the fifth year of life. In contrast, the sternum has been advocated as the best site to establish IO access in adults because it is large and flat and can readily be located.56 In addition, the sternum’s cortical bone is thin (1 to 2 mm) and the marrow space relatively uniform (6 to 11 mm).57
There has also been renewed interest in IO access by the U.S. military. In addition to logistic constraints that limit the volume of isotonic crystalloid fluids available to resuscitate injured soldiers, hypotension, environmental and tactical conditions, and the presence of mass casualties can lead to excessive delay in obtaining vascular access.4 The Army Institute for Research has compared several IO infusion devices, including the FAST-1 Intraosseous Infusion System (PYNG Medical Corporation, Richmond, British Columbia, Canada), the Bone Injection Gun (BIG; Waismed, Yokenam, Israel), the Sur-Fast Hand-Driven Threaded-Needle (Cook Critical Care, Bloomington, IL), and the Jamshidi Straight-Needle (Allegence Health Care, McGaw Park, IL).56 Success rates with these devices were similar (94% to 97%), and all were inserted in less than 2 minutes. The participants rated no individual device as being significantly better than the others. It was concluded that each device was easy to master and could be used appropriately during special operations when IV access could not be accomplished.56 A recent randomized controlled trial (RCT) of the BIG device versus the EZ-IO showed no significant differences in success rates or overall ease of use. Of 40 adults in the prehospital setting, vascular access was successfully achieved on the first attempt in 80% to 90% of patients within 2 minutes.58 Another recent RCT showed that a Jamshidi 15-gauge needle could be placed significantly faster than the FAST-1 device but had similar success and complication rates, as well as perceived ease of use.59
In addition to serving as a route for fluid administration, the IO needle may be used to obtain blood for typing, crossmatching, and determining blood chemistry in the marrow cavity. Serum electrolyte, blood urea nitrogen, creatinine, glucose, and calcium levels are very similar to those in samples obtained from an IO aspirate.60,61 Blood gas values obtained from the IO site were similar to those obtained from central venous sites during steady and low-flow states in one animal model.62 Brickman and colleagues63 demonstrated that bone marrow aspirates obtained from an IO needle in the iliac crest could be used reliably to type and screen blood for transfusion. A complete blood cell count may not be reliable because it reflects the marrow cell count rather than the cell count in the peripheral circulation. Furthermore, the aspirated blood usually clots within seconds, even if placed in a tube that contains heparin.
Contraindications
Relatively few contraindications to IO infusion exist. Osteoporosis and osteogenesis imperfecta are associated with a high potential for fracture; therefore, unless absolutely necessary, the procedure should be avoided when these diagnoses are known. A fractured bone should be avoided because as fluid is infused, it increases intramedullary pressure and forces fluid to extravasate at the fracture site. This may slow the healing process, cause nonunion of the bone, or lead to a compartment syndrome. Similar extravasation of fluid can occur through recent IO puncture sites placed in the same bone. Hence, recent previous use of the same bone for IO infusion represents a relative contraindication to IO line placement. Needle insertion through areas of cellulitis, infection, or burns should also be avoided. Patients with right-to-left intracardiac shunts (e.g., tetralogy of Fallot, pulmonary atresia) may be at higher risk for fat or bone marrow embolization.64,65
Equipment and Setup
IO Needles (Fig. 25-3)
Needles used for IO access range in size from 13 to 20 gauge and must be sturdy enough to penetrate bone without bending or breaking. They must also be long enough to reach the marrow cavity. Standard needles for drawing blood or administering medications are not adequate for IO infusions; they are not sturdy enough to penetrate bone and do not have a stylet to prevent bone from plugging the lumen. A cadaver study of IO puncture suggested that nonstyletted needles (2.5-cm, 18-gauge phlebotomy needles and 7.6-cm, 14-gauge IV needles) enter the marrow space successfully only about half the time.66 In the past, an 18-gauge spinal needle was commonly used in children younger than 12 to 18 months. This needle, though readily available in most EDs, often bends, is too long for rapid infusion of fluid, and has a greater risk for occlusion from clotted blood.67 Very small “butterfly” needles have been used with success in preterm infants.68

Figure 25-3 Various intraosseous needles.
Jamshidi Disposable Sternal/Iliac Aspiration Needle
Like the Illinois Sternal/Iliac Aspiration Needle, the Jamshidi Disposable Sternal/Iliac Aspiration Needle (Cardinal Health, Dublin, OH) was designed for bone marrow aspiration, but it has a shorter shaft and smaller handle, which makes it easier to use. It comes in either 15 or 18 gauge and also features an adjustable plastic sleeve to prevent overpenetration. Once inserted, the needle protrudes approximately 2 inches from the skin, which increases the risk for accidental dislodgment. In a study using a turkey bone model, participants rated the Jamshidi needle easier to use than the Cook IO needle.69
Sur-Fast Needle
The Sur-Fast Needle (Cook Critical Care, Bloomington, IN) is also specifically designed for IO insertion and infusion. It has a threaded shaft that helps secure the needle in the bone and a detachable handle that may be reused with multiple needles. In a study by Jun and associates,70 the Sur-Fast IO needle had a success rate similar to that of a standard bone marrow aspiration needle.
IO Devices
FAST-1 Intraosseous Infusion System (Fig. 25-4)
The FAST-1 Intraosseous Infusion System (PYNG Medical, Richmond, British Columbia, Canada) uses an impact-driven device designed for sternal placement only. The FAST-1 has been used successfully by both military and prehospital care providers.56,71 In one prehospital study, flow rates of 80 and 150 mL/min were obtained by using gravity and a pressure bag, respectively.72 In a prospective prehospital evaluation, the mean time to successful placement was 67 seconds.73 The device has a series of stabilizing probes that help maintain good contact with the sternum and serve as the depth control mechanism for insertion of the needle. These probes use the surface of the manubrium rather than the patient’s skin to ensure the proper depth of insertion. Once the device is positioned against the sternum, additional pressure triggers the release of a hollow needle into the medullary space. The needle comes preconnected to IV tubing. The handle is automatically released from the stylet and infusion tubing once the needle has met its preset depth. Removal of the needle requires a threaded tool provided with the device. The FAST-1 is larger and heavier than other IO devices and, once triggered, cannot be reused.
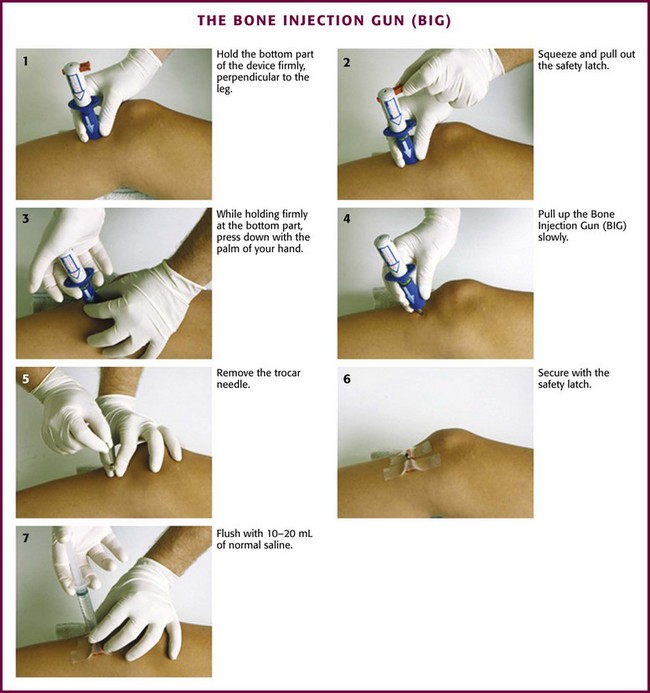
Bone Injection Gun—BIG (Fig. 25-5)
The BIG (Waismed, Yokenam, Israel) is another spring-loaded, impact-driven device that comes in both pediatric and adult sizes. Like the FAST-1 system, this device is designed for single use only. An advantage of the BIG is the ability to adjust the depth of insertion, which allows it to be used at different sites (e.g., tibia, humerus). However, if the device is not carefully stabilized before and during insertion, incorrect placement can easily occur. In addition, there is the potential for operator and patient injury if the device is accidentally triggered or mistargeted.71 In a prospective, prehospital setting the BIG device was shown to have a 71% success rate in children and a 73% success rate in adults.74 In another prehospital study, 91% of 181 patients had successful insertion on the first attempt.75
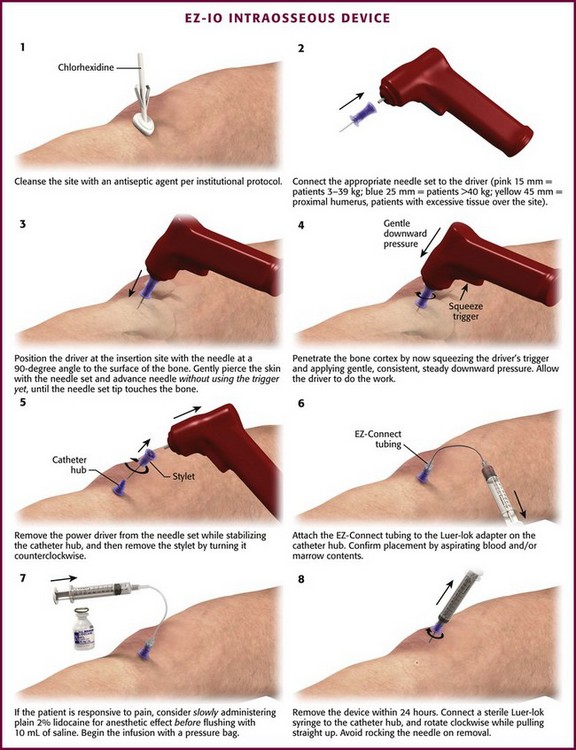
EZ-IO Device (Fig. 25-6)
The EZ-IO Device (Vida-Care, San Antonio, TX) handheld, battery-powered device that drills an IO needle to the appropriate depth in the IO space. The EZ-IO device allows the operator to control the pressure or force used during insertion.76 Placement can be achieved in less than 10 seconds in the vast majority of patients, with first-time successful insertion rates ranging from 77% to 97%.77–80

Figure 25-6 EZ-IO Device.
Procedure
The patient’s age and size are the two most important factors when choosing the best site for needle penetration. In infants and children younger than 6 years, the proximal end of the tibia is the preferred site, followed by the distal ends of the tibia and femur. Other sites such as the clavicle and humerus have been used, but neither has gained popularity. In adults, the distal part of the tibia has been the most common site for IO access. However, with the introduction of spring-loaded and drill devices, IO locations once reserved only for children are now potential sites in adults as well. In addition, the FAST-1 System makes the sternum a simple and effective location for IO access in adults.78
Proximal Tibia
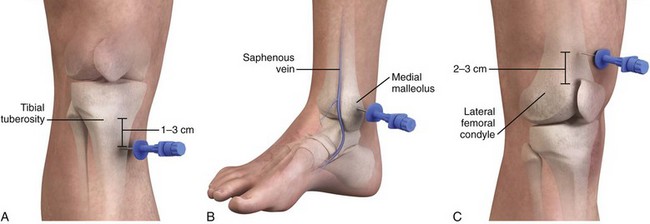
The tibia is a large bone with a thin layer of overlying subcutaneous tissue that allows landmarks to readily be palpated. Insertion here does not interfere with airway management or CPR. This is one of the most common infusion sites. On the proximal end of the tibia, the broad, flat, anteromedial surface is used and the tibial tuberosity serves as a landmark. The site of IO cannulation is approximately 1 to 3 cm (2 finger widths) below the tuberosity (Fig. 25-8A). This location is far enough away from the growth plate to prevent damage, but it is in an area where the bone is still soft enough to allow easy penetration with the needle. In adults, penetrating the thick bone in the proximal end of the tibia is much more difficult and requires a 13- to 16-gauge needle. A spring-loaded device such as the BIG or a battery-powered drill such as the EZ-IO can make penetration much easier and allows the use of smaller-gauge needles. In a comparison of first-attempt success between tibial and humeral IO insertion during out-of-hospital cardiac arrest, tibial placement was significantly more successful (90%) than humeral placement (60%) with a lower rate of needle dislodgement.81
Distal Tibia
The distal end of the tibia, though a preferred site in adults, may be used in children as well.67,82 The cortex of the bone and the overlying tissue are both thin. The site of needle insertion is the medial surface at the junction of the medial malleolus and the shaft of the tibia, posterior to the greater saphenous vein (see Fig. 25-8B). The needle is inserted perpendicular to the long axis of the bone or 10 to 15 degrees cephalad to avoid the growth plate.83
Sternum
The sternum has several advantages over peripheral bones but is rarely used in the ED. Its advantages include a large, relatively flat body that can be readily located; retention of a high proportion of red marrow, which allows rapid transfer of infused fluids and drugs to the central circulation; and thinner, more uniform cortical bone overlying a relatively uniform marrow space. In addition, the sternum is less likely to be fractured in major trauma.72 Introduction of the FAST-1 System, which allows safe and effective penetration of the sternum, has led to increased use and popularity of sternal IO insertion in adults.
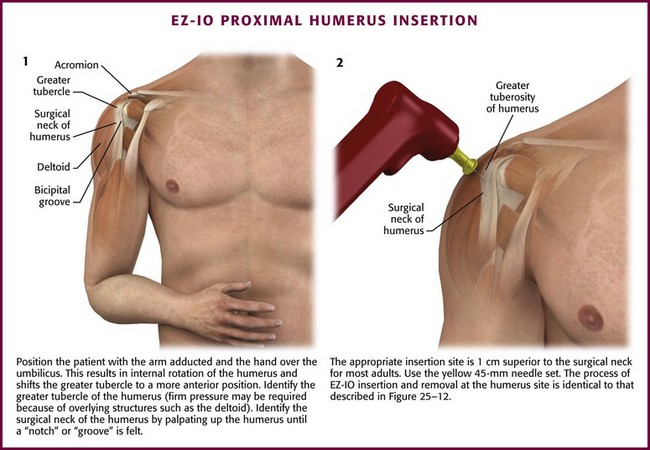
Humerus
The proximal end of the humerus is a relatively new option for IO access, but it is well tolerated and easily accessed. The close proximity of the greater tubercle of the humerus to the heart provides rapid infusion of medication and fluid into the general circulation. The humerus has been shown to be an effective site relative to peripheral or central access in a prospective resuscitation model.84,85
Other Sites
The distal portion of the femur is occasionally used as an alternative site in children, but because of thick overlying muscle and soft tissue, it is more difficult to palpate bony landmarks (Fig. 25-8C). If chosen, the needle should be inserted 2 to 3 cm above the femoral condyles in the midline and directed cephalad at an angle of 10 to 15 degrees from the vertical.85 Other sites, including the clavicle and calcaneus, can be used as alternatives, but these sites are less popular.
Manual Needle Insertion (Fig. 25-9)
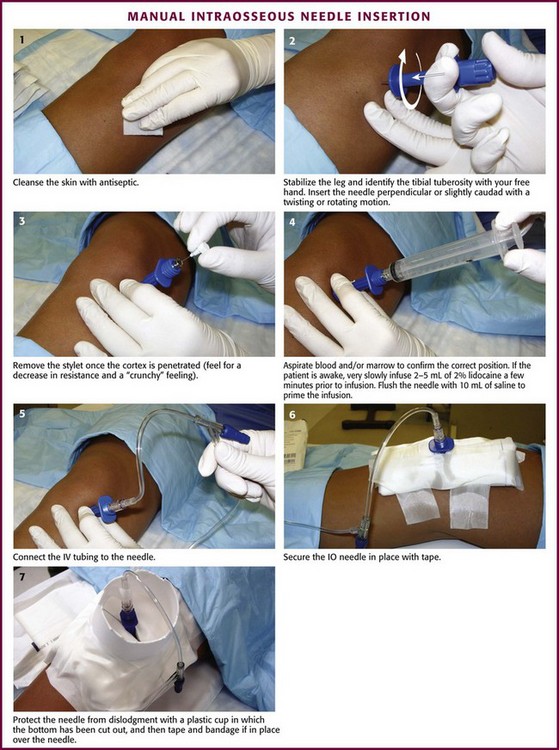
Before insertion, stabilize the site with the free hand (i.e., the hand not holding the IO needle) and use it to identify the landmarks. For example, during proximal tibial insertion, stabilize the proximal end of the tibia with the thumb and index finger of the free hand and use them to palpate the tibial tuberosity (the main bony landmark for proximal tibial insertion). During insertion, avoid injury to this hand by keeping it out of the plane of insertion and clear of the puncture site. Direct the IO needle perpendicular (90 degrees) to the bone’s long axis and slightly caudad (60 to 75 degrees). Directing the needle slightly caudad helps avoid penetration of the growth plate. Advance the needle with a twisting or rotating motion (but not a rocking motion) to drive it into the bone and to puncture the cortex. Once the cortex has been penetrated, there will be a sudden decrease in bony resistance and a “crunchy” feeling as the needle enters the marrow cavity. Penetration of the inner cortex usually occurs at a depth of approximately 1 cm. Aspirate for blood or marrow contents (or both) to confirm correct placement. Other signs of correct placement include the needle’s ability to remain upright without support and to have free-flowing fluid without signs of extravasation into surrounding tissue.86,87
Once proper placement is confirmed, secure the needle and tubing with tape. Fastening the leg to an appropriately sized leg board helps further stabilize a lower extremity insertion site in infants and small children. Protect the needle from accidental dislodgment by cutting the bottom out of a plastic cup and taping and bandaging the cup in place over the device. Commercially made shields are also available for this purpose. Remove the IO needle as soon as IV access has been secured, and apply a sterile dressing over the site. Control excessive bleeding by applying direct pressure over the site for 5 minutes.88
Use of Specific IO Devices
The FAST-1 device was designed specifically to penetrate the sternum.56,89 It is prepackaged with alcohol and iodine and comes with a protective dressing to hold the device in place. It also includes a threaded tip remover for easy removal of the metal tip and infusion tubing.
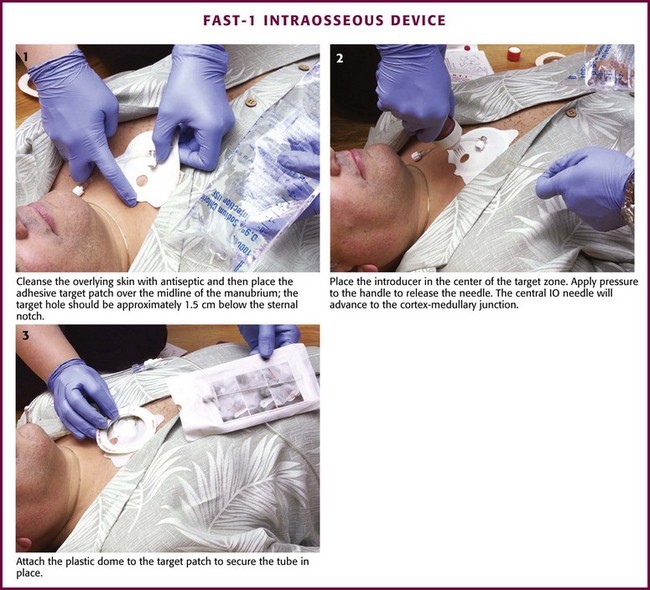
Figure 25-10 Insertion of the FAST-1 Intraosseous Device.
After disinfecting the skin site over the sternum, place the target patch over the midline of the manubrium with the hole in the middle of the target approximately 1.5 cm below the sternal notch. Next, place the FAST-1 introducer in the center of the target zone. The introducer has a “bone cluster” of needles that form a circle. These needles “sense” the cortex of the sternum and help ensure proper needle depth. Once the introducer is in position over the target zone, apply pressure to the handle to release an inner needle located in the center of the bone cluster. This needle has a small metal tip that is preconnected to plastic infusion tubing. After release, the central IO needle advances 5 mm beyond the circular cluster of needles, stops at the bony cortex, and positions the metal tip at the cortex-medullary junction. At this point, withdraw the handle so that only the plastic infusion tube is left protruding from the insertion site. Marrow aspiration and rapid flow of fluid help verify the appropriate position. Attach the plastic dome to the target patch via Velcro fasteners and secure the tubing in place. Removal of the infusion tube requires the use of a threaded-tip remover, which is included. The tube can also be removed by directly pulling it; however, the metal tip is sometimes left behind and must be extracted through a small incision.56
Complications
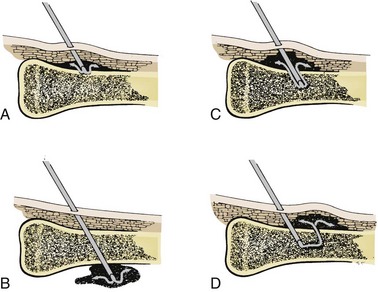
Technical difficulties are the most common complications, but they decrease as familiarity with the technique increases (Fig. 25-14). The most common mistake is to place excessive pressure on the needle during insertion and force it entirely through the bone and out the other side (Fig. 25-15). Minimize this risk by using appropriate landmarks and keeping the needle perpendicular to the long axis of the bone. In addition, hold the needle with the index finger approximately 1 cm from the bevel. When this finger touches the skin, the needle should be in the marrow cavity and no further pressure needs to be applied. Some IO needles have a mark 1 cm from the bevel (e.g., Cook IO Needle), whereas others have a special guide or mechanism to ensure proper insertion and depth of penetration (e.g., Illinois Sternal/Iliac Aspiration Needle). If available, use of these adjuncts will also help prevent overpenetration.
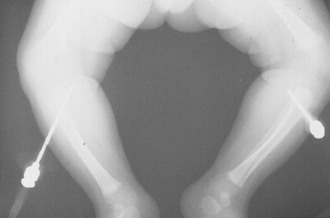
Figure 25-15 Radiograph of bilaterally misplaced intraosseous needles with penetration through the posterior tibial cortices.
Extravasation of fluid is less common but may be associated with a number of adverse events.90,91 Extravasation may be caused by fluids being infused under excessive pressure and with prolonged use of an IO site.92 As noted previously, extravasation may also result from incomplete needle penetration or penetration through the opposite cortex. Even when an IO needle has been positioned properly, fluid can leak out through holes made by previous IO attempts or through an insertion site made too large from “rocking” during insertion or from an improperly secured needle that becomes loose with movement.91–93 Interestingly, the type of needle used does not appear to influence extravasation rates.94 Regardless of the cause, if extravasation occurs, remove the needle quickly and apply pressure to the site. If left unchecked, extravasation can lead to a number of adverse events (see “Soft Tissue and Bony Complications” below). In addition, though not directly harmful, extravasation of fluid through multiple cortical defects from previous IO attempts has been associated with lower serum levels of infused drugs.95
Soft Tissue and Bony Complications
In the past, concerns about infection have led clinicians to shy away from using the IO route. Although the potential for infection is real, its actual incidence is low. A literature review of more than 4000 cases from 1942 to 1977 found a 0.6% infection rate.3 Although most of the affected access sites were not placed under emergency conditions, the needles were often left in place for 1 to 2 days, thus increasing the likelihood of infection. A survey of more than 1000 U.S. and foreign medical schools found that the incidence of infection for IO needles placed in emergency conditions was less than 3%.96 The most common infection is cellulitis at the puncture site, which usually responds well to antibiotics. Osteomyelitis is less common but also usually responds well to antibiotics. Heinild and coworkers17 reported three cases of osteomyelitis in 25 patients who received infusions of undiluted 50% dextrose in water. More recently, Platt and coworkers97 reported a case of fungal osteomyelitis and sepsis secondary to an IO infusion device. A case of tibial osteomyelitis with IO abscess was also recently reported.98
In addition to infection, inflammatory reactions of the bone may be seen. Such reactions are most common when hypertonic or sclerosing agents are used and may produce an elevation of the periosteum on plain radiographs or a positive bone scan (Fig. 25-16). Unlike patients with osteomyelitis from bacteria, a child with a sterile inflammatory reaction should not appear ill. One hypertonic sclerosing drug that may be used during cardiac arrest is sodium bicarbonate. Heinild and coworkers17 reported 78 cases of bicarbonate infusion with no complications. Animal studies have reported a decrease in cellularity with edema and destruction of some cells, but these changes are temporary and resolve completely in a few weeks.99–102 A case of systemic fibrinolysis through IO vascular access in a patient with ST-elevation myocardial infarction has recently been reported.103
Skin Sloughing
Skin sloughing and myonecrosis have been reported secondary to extravasation of infused fluids and medications.102 This occurs if fluid or drugs extravasate from the puncture site into the surrounding tissue. When drugs such as calcium chloride, epinephrine, and sodium bicarbonate are infused, care should be taken to prevent dislodgment of the needle and extravasation into tissue. In addition, it is best to infuse such drugs only by gravity because infusion under pressure increases the risk for extravasation.
Compartment Syndrome
Compartment syndrome may occur when fluids leak out of the bone into a closed compartment such as the anterior or deep posterior compartment of the lower leg.104–107 The risk for compartment syndrome can be reduced by carefully placing and securing the IO needle, limiting the number of attempts in the same bone, and removing the needle once IV access has been obtained. In addition, it is prudent to check the insertion site frequently, especially when fluids are being infused under pressure.
Epiphyseal Injuries
Injury to the growth plate and subsequent developmental abnormalities of the bone are ongoing concerns with the IO route. Regardless, these fears are largely unsupported in the available literature. In fact, there have been no reports of growth plate damage or permanent abnormalities of the bone. Two animal studies specifically examined damage to the epiphysis. Sodium bicarbonate was injected directly into the epiphysis and no radiologic evidence of epiphyseal injury was found.108,109 In addition, two prospective radiologic analyses failed to identify any growth abnormalities 1 year after tibial IO insertion.110,111 By pointing the needle away from the joint space and using the previously mentioned landmarks for insertion, the risk for epiphyseal injury is remote.
Whereas growth plate abnormalities appear to be very rare, tibial fractures have been reported after IO placement.112 Hence, it is appropriate to take follow-up radiographs of patients who have undergone IO needle attempts or placement. Cortical defects may be seen on radiographs for up to 40 days after injection.113
Fat Embolism
Fat embolism is another potential complication of IO insertion.3,67 This condition is rare, however, and has been reported only in adult patients.64 Animal studies have found no changes in blood gases during IO infusion and limited evidence of fat globule collections in the lungs.64,65 In a swine cardiac arrest model, there was no difference in the risk for fat emboli in pigs that had an IO line inserted versus those receiving IV medications.114,115 Because the marrow in infants and children is primarily hematopoietic, this potential complication is unlikely to occur in this population.
Pain with Infusion
Most patients undergoing IO infusion will not be in a condition to sense pain, but infusion into bone marrow can be quite painful. Infusing 2 to 5 mL of 2% lidocaine before infusion has been suggested to relieve pain in awake patients. Medications intended to remain in the medullary space, such as local anesthetics, must be injected very slowly until the desired anesthetic effect if achieved. A recent study suggests that lidocaine is efficacious in attenuating the pain related to IO infusion.116
Training
Procedural training can be performed on simulation models, animal bones (e.g., chicken or turkey legs), or cadavers. IO insertion techniques are part of the curriculum of the pediatric advanced life support, advanced pediatric life support, and advanced trauma life support courses. Training typically consists of an hour of didactics followed by a hands-on session. Novice users have achieved success rates of 93% to 97% for manual and battery-powered IO placement in a variety of simulated settings.* Battery-powered IO placement has also been shown to have high success rates after training.
References
1. Brunette, D, Fischer, R. Intravenous access in pediatric cardiac arrest. Am J Emerg Med. 1988;6:557.
2. Orlowski, JP, Gallagher, JM, Porembka, DT. Endotracheal epinephrine is unreliable. Resuscitation. 1990;19:103.
3. Rosetti, V, Thompson, BM, Miller, J, et al. Intraosseous infusion: an alternative route of pediatric intravascular access. Ann Emerg Med. 1985;14(9):885–888.
4. Dubick, M, Holcomb, J. A review of intraosseous vascular access: current status and military application. Mil Med. 2000;165:552.
5. ATLS Student Manual, 7th ed. Chicago, American College of Surgeons, 2004.
6. PALS Provider Manual. Dallas: American Heart Association; 2002
7. Glaeser, P, Hellmich, T, Szewczuga, D, et al. 5-Year experience in pre-hospital intraosseous infusions in children and adults. Ann Emerg Med. 1993;22:1119–1124.
8. Waisman, M, Waisman, D. Bone marrow infusion in adults. J Trauma. 1997;42:288.
9. Drinker, CK, Drinker, KR, Lund, CC. The circulation in the mammalian bone marrow. Am J Physiol. 1922;62:1.
10. Josefson, A. A new method of treatment—intraosseous injections. Acta Med Scand. 1934;81:550.
11. Bailey, H. Bone marrow as a site for the reception of infusions, transfusion, and anesthetic agents. BMJ. 1944;2:181.
12. Tocantins, LM. Rapid absorption of substances injected into the bone marrow. Proc Soc Exp Biol Med. 1940;45:292.
13. Tocantins, LM, O’Neil, JF. Infusions of blood and other fluids into the general circulation via the bone marrow. Surg Gynecol Obstet. 1941;73:281.
14. Tocantins, LM, O’Neil, JF, Jones, HW. Infusions of blood and other fluids via the bone marrow. JAMA. 1941;117:1229.
15. Arbeiter, HI, Greengard, J. Tibial bone marrow infusion in infancy. J Pediatr. 1944;25:1.
16. Elston, JT, Jayne, RV, Kaump, DH, et al. Intraosseous infusions in infants. Am J Clin Pathol. 1947;17:143.
17. Heinild, S, Sondergaard, T, Tudvad, F. Bone marrow infusion in childhood: experiences from a thousand infusions. J Pediatr. 1947;30:400.
18. Foex, BA. Discovery of the intraosseous route for fluid administration. J Accid Emerg Med. 2000;17:136.
19. Timboe, HL, Bruttig, SP, Ruemmler, MW. Adult IO in the combat zone: the past, present and future use of intraosseous infusion by the U.S. military. JEMS. 2005;30(suppl 27):8.
20. Orlowski, JP. My kingdom for an intravenous line. Am J Dis Child. 1984;138:803.
21. Parrish, GA, Turkewitz, D, Skiendzizlewski, JJ. Intraosseous infusions in the emergency department. Am J Emerg Med. 1986;4:59.
22. Anderson, TE, Arthur, K, Klienman, M, et al. Intraosseous infusion: success of a standardized regional training program for prehospital advanced life support providers. Ann Emerg Med. 1994;23:52.
23. Fuchs, S, LaCovey, D, Paris, P. A prehospital model of intraosseous infusion. Ann Emerg Med. 1991;20:371.
24. Lillis, KA, Jaffe, DM. Prehospital intravenous access in children. Ann Emerg Med. 1992;21:1430.
25. Kelly, PJ, eds. Handbook of Physiology: The Cardiovascular System, Vol 3. London: Oxford University Press, 1985.
26. Berg, RA. Emergency infusion of catecholamines into bone marrow. Am J Dis Child. 1984;138:810.
27. Begg, AC. Intraosseous venography of the lower limb and pelvis. Br J Radiol. 1954;27:318.
28. Hodge, D, Delgado-Paredes, C, Gleisher, G. Intraosseous infusion flow rates in hypovolemic “pediatric” dogs. Ann Emerg Med. 1987;16:305.
29. Schoffstall, JM, Spivey, WH, Davidheiser, S, et al. Intraosseous crystalloid and blood infusion in a swine model. J Trauma. 1989;29:384.
30. Shoor, PM, Berryhill, RE, Benumof, JL. Intraosseous infusions—pressure flow relationship in pharmacokinetics. J Trauma. 1979;19:772.
31. Neufeld, JD, Marx, JA, Moore, EE, et al. Comparison of intraosseous, central, and peripheral routes of crystalloid infusion for resuscitation of hemorrhagic shock in a swine model. J Trauma. 1993;34:422.
32. Morris, RE, Schonfeld, N, Haftel, AJ. Treatment of hemorrhagic shock with intraosseous administration of crystalloid fluid in the rabbit model. Ann Emerg Med. 1987;16:27.
33. Spivey, WH, Unger, HD, Lathers, CM, et al. Intraosseous diazepam suppression of pentylenetetrazol-induced epileptogenic activity in pigs. Ann Emerg Med. 1987;16:156.
34. Spivey, WH, Lathers, CM, Malone, DR, et al. Comparison of intraosseous, central, and peripheral routes of sodium bicarbonate administration during CPR in pigs. Ann Emerg Med. 1985;14:135.
35. Larabee, TM, Cambell, JA, Severyn, FA, et al. Intraosseous infusion of ice cold saline is less efficacious than intravenous infusion for induction of mild therapeutic hypothermia in a swine model of cardiac arrest. Resuscitation. 2011;82:603–606.
36. Voelckel, WG, Luri, KG, McKnite, S, et al. Comparison of epinephrine with vasopressin on bone marrow blood flow in an animal model of hypovolemic shock and subsequent cardiac arrest. Crit Care Med. 2001;29:1587.
37. Atkins, DL, Chamedies, L, Fallat, ME, et al. Resuscitation science of pediatrics. Ann Emerg Med. 2001;37:41.
38. Zuercher, M, Kern, KB, Indik, JH, et al. Epinephrine improves 24 hour survival in a swine model of prolonged ventricular fibrillation demonstrating that early intraosseous is superior to delayed intravenous administration. Anesth Analg. 2011;112:884–890.
39. Knuth, TE, Paxton, JH, Myers, D. Intraosseous injection of iodinated computed tomography contrast agent in an adult blunt trauma patient. Ann Emerg Med. 2010;57:382–386.
40. Hiller, K, Jarrod, MM, Franke, HA, et al. Scorpion antivenom administered by alternative infusions. Ann Emerg Med. 2010;56:309–310.
41. Guy, J, Haley, K, Zuspan, S. Use of intraosseous infusion in the pediatric trauma patient. J Pediatr Surg. 1993;28:158.
42. Banerjee, S, Singhi, SC, Singh, S. The intraosseous route is a suitable alternative to intravenous route for fluid resuscitation in severely dehydrated children. Indian Pediatr. 1994;31:1511.
43. Fiorito, B, Mirza, F, Doran, T, et al. Intraosseous access in the setting of pediatric critical care transport. Pediatr Crit Care. 2005;6:50.
44. Niermayer, S, Kattwinkel, J, Van Reempts, P, et al. International guidelines for neonatal resuscitation: an excerpt from the 2000 Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care: international consensus on science, contributors, and reviewers for the neonatal resuscitation guidelines. Pediatrics. 2000;106:E29.
45. Sommer, A, Weiss, M, Deanovic, D, et al. Intraosseous infusion in the pediatric emergency medical service. Analysis of emergency medical missions 1990-2009. Anaesthetist. 2011;60:125–131.
46. Möller, JC, Reiss, I, Schaible, T. Vascular access in neonates and infants—indications, routes, techniques and devices, complications. Intensive Care World. 1995;12:48.
47. Kelsal, AW. Resuscitation with intraosseous lines in neonatal units. Arch Dis Child. 1993;68:324.
48. Ellemunter, H, Simma, B, Trawoger, R, et al. Intraosseous lines in preterm and full term neonates. Arch Dis Child Fetal Neonatal Ed. 1999;80:F74.
49. Abe, K, Blum, G, Yamamoto, L. Intraosseous is faster and easier than umbilical access in newborn emergency vascular models. Am J Emerg Med. 2000;18:126.
50. Rajani, AK, Chitkara, R, Oehlert, et al. Comparison of umbilical venous and intraosseous access during simulated neonatal resuscitation. Pediatrics. 2011;28:954–958.
51. Frascone, R, Kaye, K, Dries, D, et al. Successful placement of an adult sternal intraosseous line through burned skin. J Burn Care. 2003;24:306.
52. Valdes, M. Intraosseous fluid administration in emergencies. Lancet. 1977;235:1235.
53. McCarthy, G, Buss, P. The calcaneum as a site for intraosseous infusion. J Accid Emerg Med. 1998;15:421.
54. Calkins, M, Fitzgerald, G, Bentley, T, et al. Intraosseous infusion devices: a comparison for potential use in special operations. J Trauma. 2000;48:1068.
55. Clem, M, Tierney, P. Intraosseous infusions via the calcaneus. Resuscitation. 2004;62:107.
56. Johnson, D, Findlay, J, Macnab, A. Cadaver testing to validate design criteria of an adult intraosseous infusion system. Mil Med. 2005;170:251.
57. Kramer, GC. Intraosseous Resuscitation Progress Report. Davis, CA: University of California; 1990.
58. Leidel, BA, Kirchhoff, C, Braunstein, V, et al. Comparison of two intraosseous access devices in adult patients under resuscitation in the emergency department; a prospective, randomized study. Resuscitation. 2010;81:994–999.
59. Harholt, KA, van Leishout, EM, Thies, WC, et al. Intraosseous devices: a randomized controlled trial comparing three intraosseous devices. Prehosp Emerg Care. 2010;14:6–13.
60. Orlowski, JP, Porembka, DT, Gallagher, JM, et al. The bone marrow as a source of laboratory studies. Ann Emerg Med. 1989;18:1348.
61. Spivey, WH, McNamara, RM, Lathers, CM. Comparison of intraosseous and intravenous CBC and ASTRA 8 in swine [abstract]. Ann Emerg Med. 1986;15:647.
62. Kissoon, N, Rosenberg, H, Gloor, J, et al. Comparison of the acid-base status of blood obtained from intraosseous and central venous sites during steady and low flow states. Crit Care Med. 1993;21:1765.
63. Brickman, KR, Krupp, K, Rega, P, et al. Typing and screening of blood from intraosseous access. Ann Emerg Med. 1992;21:414.
64. Orlowski, JP, Julius, CJ, Petras, RE, et al. The safety of intraosseous infusions: risk of fat and bone marrow emboli to the lungs. Ann Emerg Med. 1989;18:1062.
65. Fiallos, M, Kissoon, N, Abdelmonheim, T, et al. Fat embolism with the use of intraosseous infusion during cardiopulmonary resuscitation. Am J Med Sci. 1997;314:73.
66. Totten, VY. Intraosseous infusions through non-styletted needles. Emerg Med. 1995;7:85.
67. Iserson, KV, Criss, E. Intraosseous infusions, a usable technique. Am J Emerg Med. 1986;14:540.
68. Lake, W, Emmerson, AJ. Use of a butterfly as an intraosseous needle in an edematous preterm infant. Arch Dis Child Fetal Neonatal Ed. 2003;88:F409.
69. Halm, B, Yamamoto, LG. Comparing ease of intraosseous needle placement: Jamshidi versus Cook. Am J Emerg Med. 1998;16:420.
70. Jun, H, Haruyama, AZ, Chang, KS, et al. Comparison of a new screw-tipped intraosseous needle versus a standard bone marrow aspiration needle for infusion. Am J Emerg Med. 2000;18:135.
71. Fowler, R, Gallagher, JW, Isaacs, SM, et al. The role of intraosseous vascular access in the out of hospital environment (resource document to the NAEMSP position statement). Prehosp Emerg Care. 2007;11:63.
72. Macnab, A. A new system for sternal intraosseous infusion in adults. Prehosp Emerg Care. 2000;4:173.
73. Byars, DV, Tsuchitatni, SN, Erwin, E, et al. Evaluation of success rate and access time for an adult sternal intraosseous device deployed in the prehospital setting. Prehosp Disaster Med. 2011;26:127–129.
74. Gerritse, BM, Scheffer, GJ, Draaisma, JM. Prehospital intraosseous access with the bone injection gun by a helicopter-transported emergency medical team. J Trauma. 2009;66:1739.
75. Schwartz, D, Amir L. Dichter, R, et al. The use of a powered device for intraosseous and fluid administration in a national EMS: a 4-year experience. J Trauma. 2008;64:650–654. [discussion 654-655].
76. Miller, L, Morrisette, C. Vidaport: an advanced easy IO device. Prehosp Emerg Care. 2004;8:110.
77. Davidoff, J, Fowler, R, Gordon, D, et al. Clinical evaluation of a novel intraosseous device for adults: prospective, 250-patient multicentered trial. JEMS. 2005;30(suppl):20.
78. Gillum, L, Kovar, J. Powered intraosseous access in the prehospital setting. JEMS. 2005;30:24.
79. Frascone, RJ, Jensen, JP, Kaye, K, et al. Consecutive field trials using two different intraosseous devices. Prehosp Emerg Care. 2007;11:164.
80. Horton, MA, Beamer, C. Powered intraosseous insertion provides safe and effective vascular access for pediatric emergency patients. Pediatr Emerg Care. 2008;24:347.
81. Reades, R, Studnek, JR, Garrett, JS, et al. Comparison of first attempt success between tibial and humeral intraosseous insertions during out of hospital cardiac arrest. Prehosp Emerg Care. 2011;15:278–281.
82. Iserson, KV. Intraosseous infusions in adults. J Emerg Med. 1989;7:587.
83. Spivey, WH. Intraosseous infusions. J Pediatr. 1987;111:6339.
84. Glaeser, PW, Losek, JD. Emergency intraosseous infusions in children. Am J Emerg Med. 1986;4:34.
85. Paxton, JH, Knuth, TE, Klausner, HA. Proximal humerus intraosseous insertion: a preferred emergency venous access. J Trauma. 2009;67:606–611.
86. Stone, MB, Teisman, NA, Wang, R. Ultrasonographic confirmation of intraosseous needle placement in an adult unembalmed cadaver model. Ann Emerg Med. 2007;49:515.
87. Nicks, BA, McGinnis, HD. Ultrasound confirmation of intraosseous line placement using a cadaveric teaching model. Ann Emerg Med. 2006;46:52.
88. Mofenson, HC, Tascone, A, Caraccio, TR. Guidelines for intraosseous infusions. J Emerg Med. 1988;6:143.
89. Vardi, A, Berkenstadt, H, Levin, I, et al. Intraosseous vascular access in the treatment of chemical warfare casualties assessed by advanced simulation: proposed alteration of treatment protocol. Anesth Analg. 2007;98:1753.
90. Galpin, RD, Kronick, JB, Willis, RB, et al. Bilateral lower extremity compartment syndromes secondary to intraosseous fluid resuscitation. J Pediatr Orthop. 1991;11:773.
91. Simmons, CM, Johnson, NE, Perkin, RM, et al. Intraosseous extravasation complication reports. Ann Emerg Med. 1994;23:363.
92. Tenenbein, M. Intraosseous infusion and compartment syndrome. Pediatr Emerg Care. 1992;5:4.
93. Quilligan, JJ, Turkel, H. Bone marrow infusions and its complications. Am J Dis Child. 1946;71:457.
94. LaSpada, J, Kissoon, N, Melker, R, et al. Extravasation rates and complications of intraosseous needles during gravity and pressure infusion. Crit Care Med. 1995;23:2023.
95. Brickman, K, Rega, P, Choo, M, et al. Comparison of serum phenobarbital levels after single versus multiple attempts at intraosseous infusion. Ann Emerg Med. 1990;19:31.
96. Spivey, WH, Hodge, D. Survey of intraosseous complications [unpublished manuscript]. Philadelphia: Medical College of Philadelphia; 1988.
97. Platt, SL, Notterman, DA, Winchester, P. Fungal osteomyelitis and sepsis from intraosseous infusion. Pediatr Crit Care. 1993;9:149.
98. Henson, NL, Payan, JM, Terk, MR. Tibial subacute osteomyelitis with intraosseous abscess: an unusual complication of intraosseous infusion. Skeletal Radiol. 2011;40:239–242.
99. Walden, TM, Lennart, W. On injuries of bone marrow after intraosseous injections: an experimental investigation. Acta Chir Scand. 1947;96:152.
100. Pollack, CV, Pender, ES, Woodall, BN, et al. Long-term effects of intraosseous infusion on tibial bone marrow in the weanling pig model. Am J Emerg Med. 1992;10:27.
101. Spivey, WH, Unger, HD, McNamara, RM, et al. The effect of intraosseous sodium bicarbonate on bone in swine. Ann Emerg Med. 1987;16:773.
102. Alam, HB, Punzalan, CM, Koustova, E. Hypertonic saline: intraosseous infusion causes myonecrosis in a dehydrated swine model of uncontrolled hemorrhagic shock. J Trauma. 2002;52:18.
103. Ruiz-Hornillos, PJ, Martinez-Camara, F, Elizondo, M. Systemic fibrinolysis through intraosseous vascular access in ST-segment elevation myocardial infarction. Ann Emerg Med. 2011;57:572–574.
104. Gunal, I, Kose, N, Gurer, D. Compartment syndrome after intraosseous infusion: an experimental study in dogs. J Pediatr Surg. 1996;31:1491.
105. Vidal, R, Kissoon, N, Gayle, M. Compartment syndrome following intraosseous infusion. Pediatrics. 1993;91:1201.
106. Gayle, M, Kisson, N. A case of compartment syndrome following intraosseous infusions. Pediatr Emerg Care. 1994;10:157.
107. Kissoon, N. A case of compartment syndrome following intraosseous infusions. Pediatr Emerg Care. 1994;10:378.
108. Brickman, KR, Rega, P, Koltz, M, et al. Analysis of growth plate abnormalities following intraosseous infusion through the proximal tibial epiphysis in pigs. Ann Emerg Med. 1988;17:121.
109. Dedrick, DK, Mase, C, Ranger, W, et al. The effects of intraosseous infusion on the growth plate in a nestling rabbit model. Ann Emerg Med. 1992;21:494.
110. Fiser, RT, Walker, WM, Selbert, JJ, et al. Tibial length following intraosseous infusion: a prospective, radiological analysis. Pediatr Emerg Care. 1997;13:1986.
111. Claudet, I, Baunin, C, Laporte-Turpin, E, et al. Long term effects on tibial growth after intraosseous infusion: a prospective, radiographic analysis. Pediatr Emerg Care. 2003;19:397.
112. Bowley, DM, Loveland, J, Pitcher, GL. Tibial fracture as a complication of intraosseous infusion during pediatric resuscitation. J Trauma. 2003;55:786.
113. Barron, BJ, Tran, HD, Lamki, LM. Scintigraphic findings of osteomyelitis after intraosseous infusion in a child. Clin Nucl Med. 1994;19:307.
114. Thomas, ML, Tighe, JR. Death from fat embolism as a complication of intraosseous phlebography. Lancet. 1973;2:1415.
115. Plewa, MC, King, RW, Fenn-Buderer, ND, et al. Hematologic safety of intraosseous blood transfusion in a swine model of pediatric hemorrhagic hypovolemia. Acad Emerg Med. 1995;2:799.
116. Philbeck, TE, Miller, LJ, Montez, D, et al. Hurts so good: easing IO pain and pressure. JEMS. 2010;35:58–69.
117. Levitan, RM, Bortle, CD, Snyder, TA. Use of a battery-operated needle driver for intraosseous access by novice users: skill acquisition with cadavers. Ann Emerg Med. 2009;54:692–694.
118. Cooper, BR, Mahoney, PF, Hodgetts, TJ. Intra-osseous access (EZ-IO) for resuscitation: UK military combat experience. R Army Med Corps. 2007;153:314–316.