16 Intraoperative Radiation Therapy
Intraoperative irradiation therapy (IORT) in its broadest sense refers to the delivery of irradiation at the time of an operation. This can involve the use of electrons (intraoperative electron irradiation therapy; IOERT), high dose rate brachytherapy (HDR-IORT) or orthovoltage (used by select institutions) in conjunction with surgical exploration and resection with or without external beam irradiation therapy (EBRT) and chemotherapy. IORT evolved as an attempt to achieve higher effective doses of irradiation while dose-limiting structures are surgically displaced.1–4
Rationale for Intraoperative Irradiation
Influence of Dose on Local Control
In many animal experiments, local tumor control increases sharply with increasing irradiation dose, and the shape of the curve closely follows the theoretical model.5 The animal data also show that the irradiation dose needed to control a certain percentage of tumors will increase as the tumor volume increases and, conversely, that the percentage of tumors that will be controlled at a certain dose level will decrease as the volume of the tumor increases. Although a given irradiation dose may be able to control a small tumor mass with high probability and acceptable morbidity, that same dose may be ineffective against larger volume tumors that contain a larger number of clonogenic cells.
Fletcher and colleagues6 performed an extensive evaluation of dose–response curves of human tumors emphasizing adenocarcinoma of the breast and squamous cell carcinomas (SCCs) of the head and neck. In breast cancer, the probability of controlling subclinical nodal or chest wall disease was 60% to 70% with a dose of 30 to 35 Gray (Gy), 85% with 40 Gy, and 95% with 45 to 50 Gy (the usual fractionation was 10 Gy per week in 2.0-Gy fractions). For locally advanced breast cancers, LC required much higher doses. For these large tumors, 70% LC was achieved with doses of 90 Gy (with protracted fraction) by Fletcher6 versus 35% LC with doses of 50 to 60 Gy in the series of Griscom and Wang.7
The most extensive information on LC versus dose exists for SCCs of the head and neck. These data have been summarized by Fletcher and Shukovsky6 and Tepper.8 For microscopic disease in lymph nodes, a dose of 30 to 40 Gy produces LC in 60% to 70% of patients, compared to greater than 90% control at doses of 50 Gy in 25 fractions over 5 weeks. For early-stage primary tumors of the head and neck, a strong dose–response curve has not been demonstrated, because of good control at virtually all doses commonly used. Data compiled by Tepper for higher stage tumors indicate that 20% LC results after a dose of 46 Gy, 50% control with 58.5 Gy, and 80% control only with a very high dose of 75.5 Gy. Thus, a marked improvement in LC results from the ability to increase the tumor dose significantly.
Local Tumor Control versus Complications
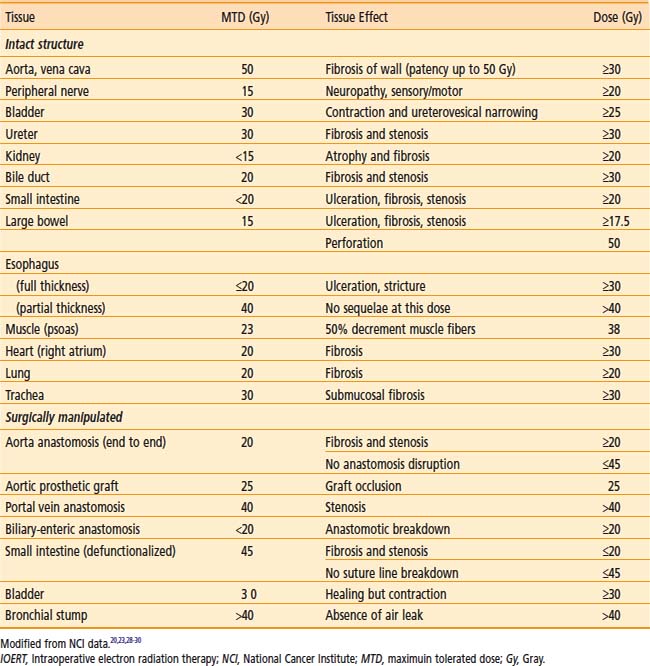
For patients with locally advanced abdominal or pelvic malignancies in whom all disease cannot be removed surgically with negative margins, EBRT (with or without chemotherapy) is often only palliative, because doses greater than 45 to 50 Gy in 25 to 28 fractions cannot be delivered safely. Gastrointestinal tolerance to fractionated EBRT is demonstrated in Table 16-1.9 If treated with tolerable doses, patients often have local persistence or relapse of disease with secondary complications that may require hospitalization and/or reoperation for small bowel obstruction, ureteral obstruction, bowel perforation, and so on.
Impact of Local Control on Distant Metastases
In the ASTRO Gold Medal paper of Dr. Herman Suit,10 the theme of distant metastases developing from a locally recurrent tumor was discussed as a component of the overall premise that LC benefits survival. Data were presented from several spontaneous tumor systems to suggest that the rate of distant metastases was related to both tumor size and disease presentation as primary versus locally recurrent disease. In both the spontaneous fibrosarcoma FSaII and SCC VII lines in the C3H/Sed mouse, Ramsey et al. reported increased rates of distant metastases with 6-mm versus 12-mm tumor size and primary versus recurrent tumors.11 Ramsey’s work confirmed an earlier evaluation by Suit et al.12 in which 12-mm isotransplants of C3H mouse mammary tumors were treated with single-dose irradiation and evaluated for disease control both locally and distantly. The rate of distant metastases increased with lack of LC. The incidence of distant metastases was 31% (16 of 52) in mice with LC, 50% (9 of 18) in those with local relapse who were salvaged with further resection, and 80% (12 of 15) in mice with local relapse in whom salvage was not attempted.
Human data were also quoted to support the thesis of metastases arising from the local relapse. For patients with squamous cell cancers of the cervix, prostate, and head and neck cancers, the metastatic frequency was higher in patients with local relapse than in those with LC.13–16
Treatment Issues: Sequencing, Irradiation Dose, and Technique
Technique and Dose: IORT
The technical aspects of both the surgical and irradiation components of IORT procedures have been discussed in detail in previous publications1–3 and will not be reiterated in detail. A carefully constructed team including surgeons, radiation oncologists, anesthesiologists, operating room nursing staff, radiation physics and dosimetry staff, and radiation therapists must jointly oversee IORT procedures.
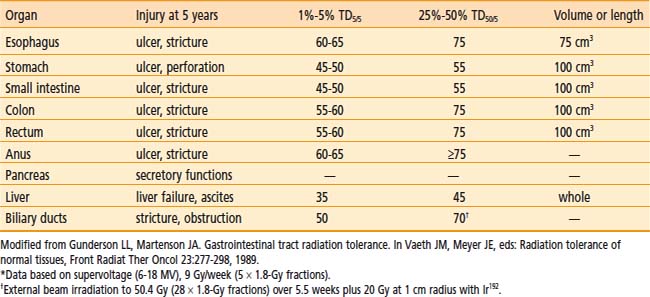
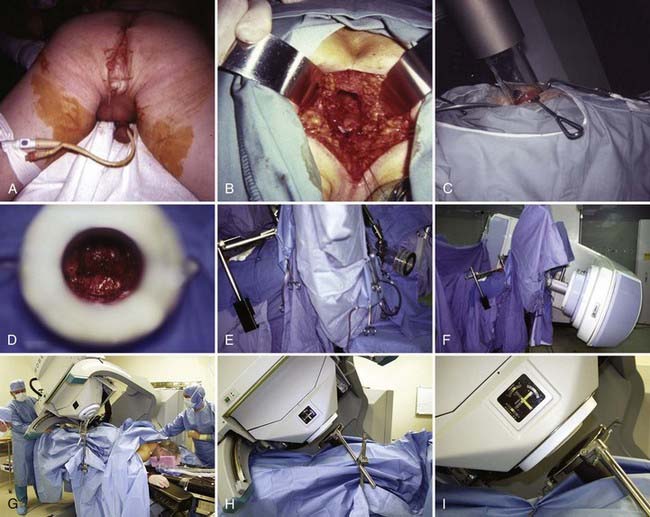
Following surgical exploration and maximal resection, the radiation oncologist joins the surgeon in the operating room to determine whether IORT is indicated and technically feasible, based on a review of surgical-pathologic findings. The site of the IORT treatment field is determined and marked with sutures or clips. If the IORT treatment machine is in the radiation oncology department, the patient will then be transferred to a stretcher for transport after temporary closure of incisions, and placed on the linear accelerator table in the radiation oncology department for IOERT treatment. If the institution has a mobile X-band accelerator (Mobetron, electron energies of 4 to 12 MeV) that can be brought to the operating room, the IOERT applicator is immobilized in position with a modified Buchwalter retractor after the applicator size and shape has been selected to encompass the tumor bed or unresected disease with a margin of ∼1 cm (Fig. 16-1A-F). The operating room table is then shifted adjacent to the IOERT machine and the gantry angle of the mobile accelerator is adjusted to provide alignment between the IOERT applicator and the accelerator head (Fig. 16-1G-I). The IORT team exits the room and IOERT is delivered while the patient and anesthesia equipment are monitored. The operating room table is then shifted back to the original location for surgical reconstruction and closure of incisions.
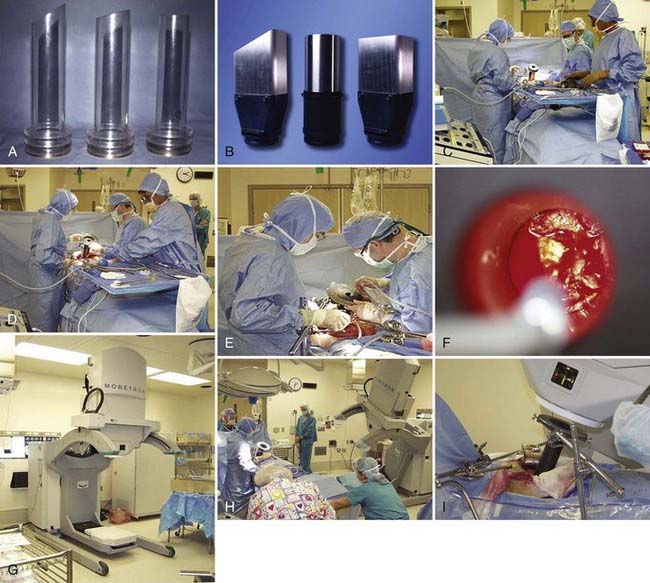
The IOERT energy and dose are dependent on the amount of residual disease remaining after maximal resection and on the EBRT component that is feasible (Figs. 16-2 and 16-3). When an R0 or R1 resection has been accomplished, 6 or 9 MeV electrons would provide adequate depth dose, but for R2 resection or unresectable disease, 9- to 12-MeV electron energies or higher would be necessary to ensure adequate depth dose coverage (Fig. 16-2). If previously unirradiated patients have received preoperative doses of 45 to 54 Gy (1.8-Gy fractions 5 days per week), the IORT dose usually varies from 10 to 20 Gy: microscopic residual disease or less, 10 to 12.5 Gy; gross residual disease, 15 to 20 Gy. In previously irradiated patients, the IORT dose is usually 15 to 20 Gy if EBRT doses of 20 to 30 Gy can safely be given pre- or postoperatively. IORT doses of 25 to 30 Gy have been given to patients in whom no or limited EBRT is planned, but such doses have higher risks of nerve intolerance. The biologic effectiveness of single-dose IORT is considered equivalent to 1.5 to 2.5 times the same total dose of fractionated EBRT.17 The effective dose in the IOERT field, when added to 45 to 50 Gy given with EBRT, is 60 to 70 Gy for 10-Gy IOERT, 75 to 87.5 Gy with a 15-Gy boost, and 85 to 100 Gy with 20-Gy IOERT.
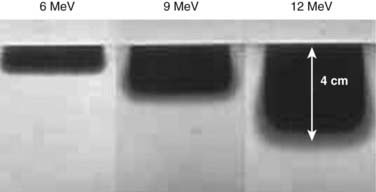
FIGURE 16-2 • Depth dose of 6, 9, and 12 MeV electrons with nonbevel applicator on the Mobetron mobile accelerator using film measurements.
IORT is given with IOERT, HDR-IORT, or orthovoltage machines, depending on institutional preference and available technology. Some institutions have both IOERT and HDR-IORT equipment and choose which to use according to anatomical constraints and amount of residual disease after maximal resection (i.e., HDR-IORT is not ideal if thickness of residual disease exceeds 0.5 cm).4 If both are technically feasible, IOERT is often chosen because of shorter set-up and treatment times, better depth dose, and more homogeneous dose distribution. Since IOERT is delivered with rigid Lucite or metal applicators, some locations may be anatomically challenging, and HDR-IORT with malleable flab applicators may be preferable, if available.
In IOERT only institutions that have a wide variety of applicators including 15° and 30° beveled ends (Fig. 16-1A,B), the combination of applicator choice and patient position can usually result in a successful treatment. Most abdominal sites are usually easily treated with IOERT approaches using either flat or 15° bevel applicators (Fig. 16-1C-F). For pelvic lesions, the availability of 30° bevel applicators usually allows successful approximation of the IOERT applicator to the tumor bed whether treated through the abdominal incision (Fig. 16-4) or the perineal incision after abdominal-perineal resection (Fig. 16-5).
Dose-Limiting Structures and IORT Tolerance
In patients with locally advanced malignancies, the issue of morbidity following aggressive treatment that includes IORT is placed into clearer perspective by a comparison with tumor-related morbidity.18–34 For instance, when EBRT is used as the main treatment modality for locally advanced rectal cancers, more than 90% of patients have local relapse or persistence of disease and most are dead in 2 to 3 years (nearly 100% tumor-related morbidity/mortality).
IOERT tolerance for intact or surgically manipulated organs or structures in animals is shown in Table 16-2.
Dose-sensitive structures include ureter and bile duct.20–22,26,29–31 Neither structure is dose-limiting, because stents can be inserted as indicated to overcome obstruction and preserve renal or liver function. In an early Mayo Clinic Rochester (MCR) analysis, 44% of previously unobstructed ureters became partially or totally obstructed when included in the IOERT field.22 A later MCR analysis of 146 patients in whom the ureter was within the IOERT field, found that the risk of subsequent obstruction was highest in patients receiving >15 Gy IOERT.31
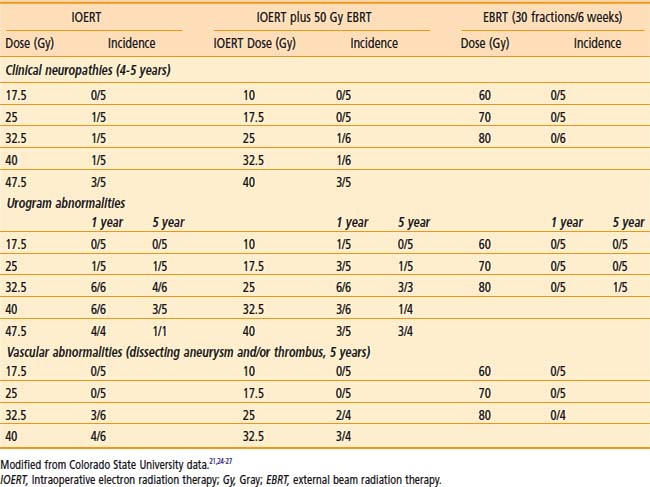
Peripheral nerve is the principal dose-limiting normal tissue for IOERT in the pelvis or retroperitoneum20–27,30,32–34 in animal studies from the National Cancer Institute (NCI) and Colorado State University (CSU) (Tables 16-2 and 16-3) and clinical analyses from MCR (Table 16-4), the NCI and other institutions. The anatomic location of peripheral nerve tissue often makes it adjacent to or involved by tumor. Even when nerve is uninvolved, it is usually impossible to move or shield nerve from the IOERT field.
An in-depth analysis of nerve and ureteral tolerance of IOERT was published on 51 patients who received IOERT at MCR as a component of treatment for the management of locally advanced primary or recurrent pelvic malignancies.22 Treatment consisted of EBRT (median 50.4 Gy), maximal resection when feasible, and an IOERT boost (range: 10 to 25 Gy) that utilized 9 to 18 MeV electrons. Fifty of the 51 were eligible for peripheral neurotoxicity analysis. Sixteen (32%) developed grade 1 to 3 peripheral neuropathy (unilateral pelvic or extremity pain, leg weakness, numbness or tingling). Pain was severe (grade 3) in only 3 (6%). Neuropathy incidence by IOERT location was as follows: pelvic sidewall 15 of 32 (47%), presacrum 1 of 12 (8%), central pelvis 0 of 6 (0%).
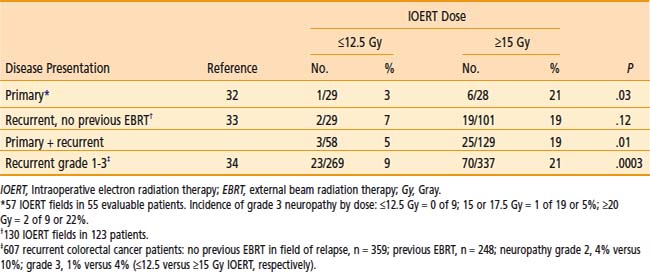
In MCR analyses of IOERT regimens in 187 patients with locally advanced colorectal cancer,32,33 tolerance data suggest a relationship between IOERT dose and the incidence of grade 2 or 3 neuropathy (see Table 16-4; EBRT factors appeared constant). With an IOERT dose of 10 to 12.5 Gy, the incidence of grade 2 to 3 neuropathy was 5%; with doses of ≥15 Gy, the incidence was 19% (P = .01). The incidence of grade 3 neuropathy was ∼5% in both primary and locally recurrent patients and the incidence of grade 1 to 3 neuropathy was ∼32%. This trend is consistent with animal data that suggest a correlation between IOERT dose and the incidence of clinical and electrophysiologic neuropathy in dogs.
In the most recent MCR analysis of 607 patients with locally recurrent colorectal cancer, the incidence of grade 1 to 3 neuropathy was 15% (grade 1: 32 patients [5.3%]; grade 2: 43 patients [7.1%]; grade 3: 18 patients [3.0%]; see Table 16-4).34 For IOERT doses of ≤12.5 Gy (versus ≥15 Gy), the incidence of grade 2 neuropathy was 4% (versus 10%) and the incidence of grade 3 neuropathy was 1% (versus 4%), P < .0003.
Pancreas IOERT
EBRT Plus Chemotherapy
For unresectable lesions, the use of EBRT plus concurrent 5-FU or gemzar-based chemotherapy results in a doubling of median survival rate (SR) compared with surgical bypass or stents alone (median SR: 3 to 6 months vs 9 to 13 months) and an increase in 2-year SR from 0%-5% to 10%-20%.35–39 However, 5-year SR is rare and LC is low. In a series from Thomas Jefferson University (TJUH) using EBRT doses of 60 to 70 Gy in 1.8- to 2.0-Gy fractions over 7 to 8 weeks, LC was achieved in less than 20% of patients treated with EBRT alone.35–36 With EBRT plus chemotherapy, LC was achieved in ∼30% of patients.
IORT Alone or Plus EBRT
The combination of EBRT plus IOERT has resulted in an improvement in LC in IOERT series from MGH, Mayo, and TJUH37,39–45 (Table 16-5). This has not, however, translated into major improvements in either median or 2-year survival. The delivery of EBRT plus concurrent chemotherapy before restaging and laparotomy plus IOERT or resection plus IOERT translates into improved patient selection and some improvement in median and 2-year survival.41,45
Table 16-5 Pancreas: EBRT With or Without IOERT or Brachytherapy for Unresectable/Borderline Resectable Cancers
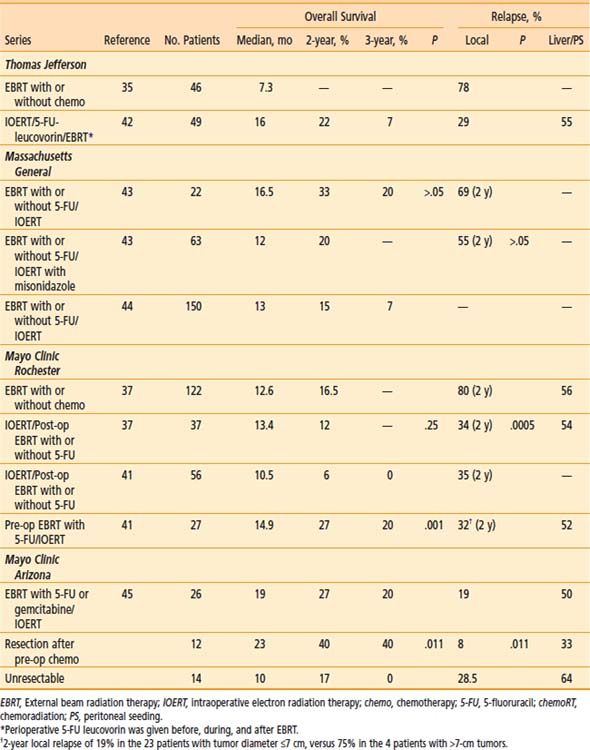
Massachusetts General Hospital
In the 1970s and 1980s, the treatment regimen at MGH was a combination of low-dose pre-op EBRT, IOERT, and high-dose post-op EBRT.40 Patients with locally unresectable disease (no distant metastases) received 10 to 15 Gy of pre-op EBRT (pancreas and nodes). If metastases were not found and the primary was unresectable, IOERT was given (15 to 20 Gy with 15 to 23 MeV). After recovery from surgery, the patient received post-op EBRT for an additional 35 to 39.6 Gy (4-field technique to clipped tumor with or without lymph nodes [LN]) in conjunction with intravenous (IV) 5-FU (500 mg/m2 for 3 days during week 1 of EBRT).
Misonidazole, a hypoxic-cell sensitizer, was combined with IOERT in a series of 41 MGH patients in an attempt to improve local tumor control; patients also received EBRT plus concurrent 5-FU.43 Outcomes were compared with those in 22 IOERT patients who did not receive misonidazole (1-year LC of 67% [versus 55%] and 2-year 45% [versus 31%], favoring misonidazole patients; P > .05). One-year overall survival (OS) was 50% (versus 77%), 2-year OS was 20% (versus 33%), and median survival was 12 months (versus 16.5 months; favoring non–misonidazole patients; P > .05). Median survival in the entire group of 63 patients was 14 months. There was a bias toward larger tumors in patients treated with misonidazole (15% of misonidazole/IOERT patients with small tumors ≤4.5 cm versus 50% in the control group of 22 patients).
In the most recent update of MGH results, 150 patients with locally unresectable pancreas cancer received IOERT as a component of treatment from 1978 to 2001 in conjunction with EBRT and 5-FU based chemotherapy.44 Long-term survival was seen in 8 patients and 5 were alive at or beyond the 5-year interval. Actuarial 1-, 2-, 3- and 5-year survival for the 150 patients was 54%, 15%, 7%, and 4%, respectively and median survival was 13 months. Survival was significantly related to the diameter of the IOERT treatment applicator (which is a surrogate for tumor size). In the 26 patients treated with a 5- or 6-cm applicator, 2- and 3-year survival were 27% and 17%, respectively; 0 of 11 patients treated with a 9-cm diameter applicator survived beyond 18 months and those treated with a 7- or 8-cm applicator had intermediate survival (P < .05).
Mayo Clinic
In the initial MCR series, IOERT usually preceded EBRT.37 When results were compared with EBRT with or without 5-FU, LC at 1 year was 82% for EBRT plus IORT with or without 5-FU (versus 48% for EBRT with or without 5-FU); at 2 years, LC for EBRT plus IORT with or without 5-FU was 66% (versus 20% for EBRT with or without 5-FU; P = .0005). This did not translate into a difference in either median or 2-year SR (median SR: 13.4 months with IOERT versus 12.6 months without; 2-year SR: 12% with IOERT versus 16.5% without). A higher percentage of patients in the non-IOERT group received concurrent 5-FU during EBRT. The lack of survival improvement was related to a high incidence of abdominal relapse in both groups (20 of 37 IOERT patients [54%] developed liver or peritoneal metastases versus 68 of 122 [56%] in non-IOERT patients).
In an attempt to improve patient selection and survival, investigators from MCR delivered the EBRT plus chemotherapy before restaging and exploration.41 In 27 patients who received IOERT after EBRT, LC was achieved in 21 of 27 (78%) with actuarial rates of 86% and 68% at 1 and 2 years, respectively. Median survival was 14.9 months with this sequence and 2 and 5 year survival were, respectively 27% and 7%. These findings were compared with results in 56 patients who had IOERT before receiving the high-dose external component at Mayo or elsewhere (median SR: 10.5 months; 2-year SR: 6%; P = .001). In an earlier analysis of 37 patients treated solely at Mayo with the latter sequence, median and 2-year survival were, respectively, 13.6 months and 12%. Although 2-year SR appeared to improve with the altered sequence of pre-op treatment followed by IOERT, this was likely due to altered patient selection, because the rate of liver plus peritoneal failure did not change (14 of 27 at risk [52%]).
Investigators from Mayo Clinic Arizona have used only the sequence of pre-op chemoradiation (chemoRT) followed by restaging, surgical exploration with resection/IOERT, as indicated, for select patients with borderline resectable or unresectable pancreas cancer.45 A series of 26 patients with no previous treatment have received IOERT after pre-op chemoRT; resection was performed in 12 of 26 patients before IOERT (R0 or R1 = 9; R2 = 3). Median SR for the total group was 19 months; 2-year OS was 27%; 3-year OS was 20% (see Table 16-5). Survival outcomes appeared to be improved in patients with resection after pre-op chemoRT versus those without resection (median survival: 23 months versus 10 months; 2-year OS: 40% versus 17%; 3-year OS: 40% versus 0%; P = .011, log-rank). Liver or peritoneal relapse has been documented in 13 of 26 patients (50%).
European Pooled Analysis
A pooled analysis of 270 patients from five European Institutions was presented at International Society of Intraoperative Radiation Therapy (ISIORT) 2008 by Valentini et al.46 Radical surgery was performed in 247 cases (91.5%; R0 resection: 53.4%; R1: 27.4%; R2: 19.2%) and exploratory laparotomy in 8.5%. Surgery was preceded by EBRT in 63 patients (concurrent chemotherapy in 38%) and 106 received post-op ERBT (concurrent chemotherapy in only 7.5%). Median OS was 19 months for the total group of patients and 5-year OS was 17.7%. Survival and LC appeared better in patients treated with pre-op EBRT/chemoRT compared to post-op EBRT (or chemoRT) or IORT alone (median OS: 30 months, 22 months, and 13 months, respectively; median LC: not reached, 28 months, and 8 months, respectively). On multivariate analysis, nodal status and timing of EBRT significantly affected survival. In the subset of patients who remained free from local relapse for more than 2 years, 3-year and 5-year OS were 32% and 28% (versus 12% and 0% in patients with local relapse within 2 years).
Gastric IOERT
EBRT Alone or Plus Chemotherapy
When EBRT with or without chemotherapy is utilized for patients with residual disease after resection or unresected lesions, most trials show an advantage to combined versus single modality treatment (EBRT plus chemotherapy versus EBRT or chemotherapy alone). Long-term survival is 10% to 15%.47,48
IORT Alone or Plus EBRT
For partially resected gastric cancer, the use of IOERT alone or in conjunction with EBRT has yielded a 5-year SR of 15% to 20%.47–58 Abe and Takahashi reported a Kyoto trial of surgery with or without IOERT in 211 patients in which subset analyses suggested SR advantages with IOERT for Japanese stages II to IV.49–51 The 5-year SR for stage IV disease in 27 IOERT patients was 15% (versus 0% for 18 patients treated with surgery alone; three of four IOERT survivors had residual disease). Five-year results with stages II and III were 84% (versus 62% for patients treated with surgery alone) and 62% (versus 37%). In a Pamplona pilot study, EBRT with or without chemotherapy has been combined with IOERT in 48 patients (EBRT doses of 46 Gy in 1.8- to 2.0-Gy fractions; IOERT dose usually 15 Gy).52 In 13 patients with stage IV disease, in-field relapse occurred in only three patients. Two of eight patients with known residual disease after maximal resection were long-term disease-free survivors (>22 and >65 months).
Separate randomized trials have been reported from Beijing53 and the NCI.54 In the Beijing series, patients with stage III (serosal involvement or node positive tumors) or IV disease (unresectable metastases or adjacent organ involvement) were randomized to surgery with or without IOERT (25 to 40 Gy). In a report of 200 patients,37 a SR advantage with IOERT was shown for stage III patients (5-year SR: 65% versus 30%; 8-year SR: 52% versus 22%; P < .01). At NCI, Sindelar et al. performed a small randomized trial of IOERT versus EBRT after complete resection, which demonstrated improved LC with IOERT but no SR benefit.54
A series of 24 patients with esophageal or gastric cancer received IOERT as a component of treatment at MCR in conjunction with pre- or post-op EBRT and maximal resection.58 Local and regional control of disease was excellent (85% at 5-year) but survival was less than optimal in view of the ∼80% rate of distant metastases.
MCR analyses demonstrated that even patients with local or regional relapse of gastric cancer may be candidates for salvage with aggressive approaches that includes IOERT as a component of treatment. For patients who received preoperative EBRT plus concurrent chemotherapy followed by maximal resection and IOERT, 4-year survival was ∼20%57 and 5-year survival exceeded 10%.58
Future Possibilities
For patients with locally advanced gastric cancer (resection but residual disease or unresectable), it seems reasonable to build on three positive segments of treatment data (EBRT plus chemotherapy, IOERT, neoadjuvant chemotherapy) and patterns of failure data.48 For patients with residual disease after resection, EBRT plus chemotherapy or IOERT with or without EBRT has produced long-term SR in 10% to 20% of patients in most single-institution analyses and randomized trials. Neoadjuvant chemotherapy for locally advanced disease has resulted in subsequent total resection of disease is ≥50% of patients in several European trials; however, the incidence of subsequent local regional failure (LRF) is ≥50% even after total resection. For patients with unresectable or borderline resectable disease on the basis of pre-op imaging, further evaluation of “neoadjuvant” chemotherapy with or without EBRT is reasonable. In patients with subsequent resection but residual disease or resection but high risk factors (beyond gastric wall, node positive, or both), IOERT or EBRT or both could be evaluated in conjunction with further chemotherapy.
Colorectal IORT (IOERT, HDR-IORT)
EBRT Alone or Plus Chemotherapy/Resection
EBRT has been combined with resection and chemotherapy for locally advanced colorectal cancers. In separate series from Princess Margaret Hospital (PMH) and Mayo Clinic, using EBRT alone (PMH, Mayo) or combined with systemic therapy (Mayo), the local relapse rate was more than 90% in evaluable patients.59Although a combination of EBRT (with or without 5-FU) with surgery for residual disease after subtotal resection or initially unresectable disease produces better LC than no resection, local relapse is too high at 30% to 50%. For locally recurrent rectal cancers, EBRT with or without chemotherapy results in excellent short-term palliation (6 to 12 months), but LC and long-term survival are infrequent (0% to 5%; 5 years).60
EBRT Plus IORT: Primary Unresectable Cancers
In an attempt to decrease local recurrence and improve survival, institutions in the United States, Europe, Japan, and Scandinavia have combined an IORT boost with fractionated EBRT (45 to 50 Gy in 1.8-Gy fractions) and resection.32,59–67 In US studies, the IOERT dose varies from 10 to 20 Gy, depending on the amount of residual disease after maximal resection (microscopic: 10 to 12.5 Gy; gross <2 cm: 15 Gy; gross ≥2 cm or unresectable: 17.5 to 20 Gy. The HDR-IORT dose at Memorial Sloan-Kettering Cancer Center (MSKCC) has been 12 or 15 Gy as calculated at a 0.5 cm distance from the Harrison-Anderson-Mick (HAM) applicator surface.62,63
MGH Results: EBRT Plus Resection With or Without IOERT
EBRT Plus Resection
The incidence of local relapse as a function of disease extent after preoperative EBRT alone or plus concomitant 5-FU (no IOERT) has been evaluated in three separate MGH analyses.59,60 For patients with locally unresectable T4 rectal cancers treated with preoperative EBRT and curative resection in the original MGH series, five of eight patients (62.5%) with persistent gross tumor extension beyond the rectal wall relapsed in the pelvis versus 0 of 3 patients with tumor confined to the wall or only microscopic extrarectal extension.59,60 In an analysis of 28 patients with tethered (T3) rectal cancers treated with preoperative EBRT and resection at MGH, 5-year actuarial local relapse and disease-free survival were 24% and 66%, respectively.59 No correlation between LC and posttreatment extent of tumor extension into or beyond the rectal wall and/or lymph node involvement was observed.
In another MGH analysis, 47 patients with T4 rectal cancers received 45 to 50.4 Gy pre-op EBRT and R0 resections.59 Patients did not receive IOERT, either because it was not felt to be indicated because of a favorable response to preoperative EBRT, or IOERT was not technically feasible. For 24 patients with no residual tumor or tumor confined to the rectal wall after preoperative EBRT, the 5-year actuarial local relapse rate was only 13% versus 68% for 27 patients with persistent transmural tumor and/or lymph node metastases and no clearly defined indication for IOERT.
EBRT Plus Resection, IOERT
In the initial MGH report, 16 patients with locally unresectable primary lesions received EBRT before resection and IOERT.60 When results were compared with historical controls treated only with EBRT and resection, 1- and 2-year SR was statistically better in the IOERT patients and disease relapse within irradiation fields was 0% versus 43%, respectively, (IOERT vs non-IOERT).
Sixty-four patients with locally unresectable T4 rectal cancer had full-dose pre-op EBRT (alone or plus 5-FU) followed by resection and IOERT at MGH.59,61 The 5-year LC and DSS for 40 patients undergoing complete resection plus IOERT were 91% and 63%, respectively (Table 16-6). For 24 patients undergoing partial resection, LC and DSS correlated with the extent of residual cancer (microscopic residual disease: 65% and 47%, respectively; gross residual disease: 57% and 14%, respectively).
Mayo Clinic Results: EBRT With or Without 5-FU, Resection, IOERT
In a MCR comparison of 17 non-IOERT and 56 IOERT-plus-EBRT patients with locally advanced primary rectal or colon cancers,32 LC was 24% (vs 87%), median survival was 18 months (vs 40 mo), and 3-year survival was 24% (vs 55%; Table 16-7). All relapses in non-IORT patients occurred before 18 months. Prognostic factors that had a statistically positive impact on disease control and survival in IOERT patients included EBRT with 5-FU versus EBRT alone, treatment sequence of preoperative EBRT with 5-FU versus postoperative EBRT with 5-FU, colon versus rectal primary and microscopic residual disease or less after maximal resection (R0 or R1).32
Table 16-7 Locally Advanced Primary and Recurrent Colorectal: EBRT With or Without IOERT, Mayo Clinic Rochester
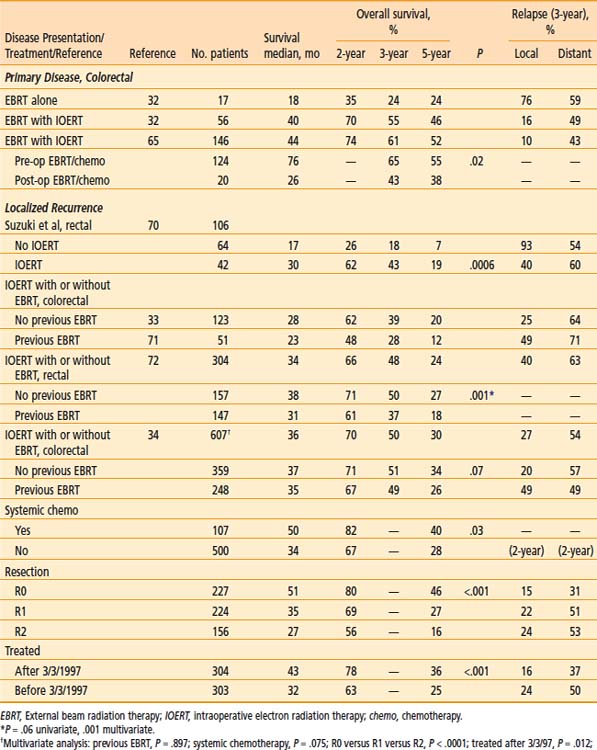
The impact of degree of resection and amount of residual disease on disease control and survival in the Mayo IOERT series was evaluated (see Table 16-6).32 The 5-year survival for the entire group of 56 colorectal IOERT with EBRT patients was 46%. Patients with microscopic residual disease or less fared better than those with gross residual disease with a 5-year actuarial OS of 59% (versus 21%; P = .0005). Relapse within an irradiation field occurred in 4 of 16 patients (25%) with gross residual disease after partial resection versus 2 of 39 (5%) with microscopic residual disease or less after gross total resection (P = .01).
A separate Mayo Clinic analysis was done regarding the use of EBRT (alone or plus chemotherapy) alone or plus IOERT as a supplement to maximal resection for 103 patients with locally advanced colon cancers.64 The 5-year actuarial local relapse rate was 10% for patients with no residual disease, 54% for patients with microscopic residual disease, and 79% for patients with gross residual disease (P < .0001). For patients with residual disease, local relapse occurred in only 11% of patients receiving IOERT plus EBRT (versus 82% of patients receiving only EBRT; P = .02). The 5-year actuarial survival rate was 66% for patients with no residual disease, 47% for patients with microscopic residual disease, and 23% for patients with gross residual disease (P = .0009). The 5-year survival rate for patients with residual disease was 76% for patients receiving IOERT plus EBRT (versus 26% for patients with EBRT alone; P = .04).
In the most recent MCR analysis, 146 patients with locally unresectable primary colorectal cancer had IOERT in addition to pre or post-op chemoRT and resection.65 Median and OS were slightly better than in the earlier series of rectal cancer patients (see Table 16-7). The sequence of pre-op chemoRT appeared to have a survival advantage over post-op chemoRT (median: 76 months versus 26 months; 5-year OS: 55% versus 38%; P = .02).
European Results
Madrid Series
In a Madrid series of 558 patients with T3 to T4 rectal cancer,66 281 received pre-op chemoRT plus IOERT (50.5%) and 277 (49.5%) received post-op chemoRT with no IOERT. Outcomes appeared better in patients who received pre-op chemoRT plus IOERT in spite of having higher stage disease at presentation (pelvic control: 91.5 versus 83.7%, P = .03; DFS: 65 versus 56%, P = .05; OS: 68 versus 58%, P = .016).
Pooled Analysis
A pooled analysis of 651 IOERT patients from 4 major European centers67 was presented at ISIORT 2008 by Rutten et al. Five-year OS was 67% and 5-year LC was 88%. Positive circumferential resection margins were a strong predictor for both OS (P < .0001) and local relapse (P < .01). Pre-op CRT seemed to improve OS (5-year OS: 70% versus 64%; P < .05).
IOERT With or Without EBRT: Locally Recurrent Colorectal
MGH Results
In MGH IOERT analyses, 5-year actuarial DFS was 16% and OS was 30% in 41 patients with locally recurrent lesions68,69 (exceeds expected long-term survival of ≤5% when treated with standard techniques). Patients with gross residual disease had 5-year LC and DFS of 21% and 7%, respectively (versus 47% and 21%, respectively, for patients with clear or microscopic positive resection margins). Patients with R1 resection (microscopic residual disease) had better in-field disease control than those with unresected or gross residual disease.
Mayo Clinic Results: IOERT With or Without EBRT
Additional data supporting the use of IOERT for locally recurrent disease is found in a MCR analysis of 106 patients with palliative resection of locally recurrent rectal cancers from 1981 to 1988 (see Table 16-7).70 None had evidence of extrapelvic disease and 42 received IOERT as a component of treatment. The IOERT dose was 15 to 20 Gy in 40 of 42 patients. In 34 of the 42 patients, gross residual disease remained after maximal resection and microscopic residual disease remained in nine. EBRT was given in 41 patients (≥45 Gy in 38). Significant factors with regard to 3- and 5-year survival included amount of residual disease (microscopic versus gross: 3-year, 44% versus 26%; 5-year, 33% versus 9%; P = .032), IOERT versus no IOERT (3-year: 43% versus 18%; 5-year: 19% versus 7%; P = .0006), type of symptoms (asymptomatic versus symptomatic without or with pain: 3-year 49%, 28%, and 7%, respectively, P = .0075), type of fixation (none or 1 versus 2 or ≥3 sites: 3-year 45%, 29%, and 0%, respectively; P ≤ .0001), and pre-op ECOG status (P = .03). For IOERT patients, 3-year SR with gross residual disease or presentation with pain was 44% and 43%, respectively. The 3-year cumulative probability of distant metastasis was 60%.
Updated Mayo Clinic analyses on locally recurrent colorectal cancer patients without previous EBRT (n = 123)33,68 or with previous EBRT (n = 51)68,71 are seen in Table 16-7. Local relapse rates at 3 years appear higher in patients with previous EBRT. For the 123 patients without previous EBRT, survival results parallel those seen in the earlier Suzuki analysis, with 2-year survival of 62% and 5-year survival of 20%. In those who presented with prior EBRT, median survival was 23 months, and 2- and 5-year SR were 48% and 12%, respectively (5-year SR was 17% in those with less than microscopic residual disease).
A series of 304 patients with locally recurrent rectal cancer receiving IOERT as a component of treatment was presented at ISIORT 2002.72 In the 159 patients without previous EBRT, 5-year survival was 27%; in the 145 with previous EBRT, 5-year survival was 20%. Improvements in long-term survival appeared to be related to more intensive treatment in recent years (patients usually receive protracted venous infusion [PVI 5]-FU during EBRT in both groups; previous EBRT patients often receive reirradiation EBRT doses of 25.2 to 30.6 Gy plus PVI 5-FU), including the use of maintenance systemic chemotherapy. LC appeared to improve in previous EBRT patients retreated with ≥25.2 Gy (P = .06).
The most recent MCR analysis presented at ISIORT 2008 included 607 recurrent colorectal cancer patients receiving IOERT as a component of treatment.34 Survival at 5 years was 30%; on multivariate analysis, (1) complete resection, (2) no previous chemotherapy, and (3) treatment after 1996 were associated with improved survival (see Table 16-7). In patients with an R0 resection, 5-year survival was 46%. Previous in-field radiation was associated with increased risk of local relapse (3-year: 39% versus 20%; P ≤ .0001) but was not associated with survival.
European Results: IORT With or Without EBRT
The current philosophy in Europe is closely related to the US concept, which utilizes IORT as a segment of a multidisciplinary approach in cancer management.68,73–76 A component of EBRT with or without 5-FU-based chemotherapy is always attempted, either before or after surgery, if no previous EBRT has been delivered.
Pamplona Experience
In a Pamplona series73 of 37 patients treated with IOERT for locally recurrent colorectal cancer, 12 were treated with IOERT alone, because they had received previous EBRT for their primary disease. The remaining 25 were treated with EBRT with IOERT (11 with postoperative EBRT and 14 with preoperative chemoradiation therapy; 40 to 50 Gy in 1.8- to 2.0-Gy fractions). In the preoperative approach, carboplatin (55 mg/m2) plus 5-FU (1 gm/m2, maximum tolerated dose of 1.5 gm) were given as a continuous infusion for 3 to 5 days concurrently with the initiation and end of the EBRT course.
French IORT Group
Similar findings have been observed by investigators from the French IORT group.74 In 30 patients treated with IORT alone, no long-term survivors were found after 42 months (versus 70% OS for the 16 patients treated with IOERT plus EBRT). LC was 0% with IORT alone (versus 60% for EBRT plus IORT).
Eindhoven Results
From 1994 to 1998, 37 patients with locally recurrent rectal cancer (without distant metastasis) received IOERT as a component of treatment in Eindhoven.75,76 Patients with no previous irradiation (n = 17) received preoperative EBRT of 16 to 60 Gy (16 Gy = one patient; 50.4 Gy = 15; 60 Gy = one) alone or with concomitant 5-FU based chemotherapy (n = 2). Those who had received previous EBRT (n = 20) either had no further EBRT (n = 15) or in the latter part of the study were given 30 Gy in 2.0-Gy fractions (n = 5; reported as well tolerated). Surgical resection was attempted 4 to 6 weeks following full dose preoperative EBRT. Negative resection margins (R0) were achieved in 15 patients, gross total resection but microscopic residual disease (R1) in eight, and unresectable or gross residual disease (R2) in 14 patients.
Eindhoven results were updated in a series of 147 consecutive IOERT patients at ISIORT 2008.76 Median OS was 28 months; 5-year OS, DFS, metastasis-free survival (MFS), and LC were 31.5, 34.1, 49.5, and 54.1%, respectively. R0 resections were achieved in 84 patients (57.2%), R1 in 34 (23.1%), and R2 in 29 (19.7%). After R0 resections, 5-year OS, DFS, MFS, and LC were 48.4, 52.3, 65.5, and 68.9%, respectively (P < .001 for OS, DFS, and LC compared with R1 or R2 resection outcomes). Patients treated with IOERT alone had worse outcomes than those who were reirradiated or treated with full-dose pre-op EBRT, with regard to 5-year OS (P = .043), LC (P = .038), and MFS (P < .001).
IOERT for Nodal Presentation or Relapse
A MCR analysis evaluated IOERT as a component of treatment in a series of 48 colorectal cancer patients with bulky mesenteric or para-aortic nodal disease at time of presentation or nodal relapse.77 5-year survival was ∼35% for all patients at risk and ∼40% in those with colon cancer. These results strongly support the use of CT abdomen as a component of follow-up in resected high-risk colon cancer patients.
Colorectal HDR-IORT, Memorial Sloan-Kettering Cancer Center
From November 1992 to December 1996, 112 patients with rectal cancer were explored; 68 received HDR-IORT (primary unresectable = 22; locally recurrent = 46) and results were reported in the 42 with adequate follow-up.62,63 Patients with primary unresectable disease received preoperative EBRT (45 to 50 Gy for 5 to 5.5 weeks) alone (n = 2) or plus concomitant 5-FU based chemotherapy (n = 18, usually 5-FU and leucovorin). Four to 6 weeks later, they underwent resection plus HDR-IORT to a dose of 10 to 20 Gy (median: 12 Gy). Patients with pelvic recurrence of a previously treated colorectal cancer (n = 26) had resection plus HDR-IORT (10 to 20 Gy; median 15 Gy). If there was no previous EBRT (n = 16), preoperative chemoRT was given followed by maximal resection and HDR-IORT (range: 10 to 20 Gy; median: 12 Gy).
Results have been updated in two series of patients who were given HDR-IORT for recurrent rectal cancer.78,79 In the initial series of 74 patients, all had complete resection (R0 = 53, R1 = 21).78 Additional EBRT was given to 29 patients and 5-FU based chemotherapy to 33 patients. The HDR-IORT dose ranged from 10 to 18 Gy. With median follow-up of 22 months, 2-year LC was 55%, 5-year LC was 39% (R0 = 43%, R1 = 26%; P = .02), and 5-year OS was 23% (R0 versus R1 = 36 versus 11%, P = .04; IORT with EBRT = 39 versus 22%, P = .04; adjuvant chemotherapy versus none = 45 versus 19%, P = .11). In a subsequent series of 111 patients who received HDR-IORT with curative intent, 100 were evaluable for predictors of survival after resection and HDR-IORT.79 R0 resection and the absence of vascular invasion in the resected specimen were the only predictors of improved LC (P < .05 and < .01) and improved disease-free and DSS (P < .01 for both).
Gynecologic IORT
EBRT With or Without Chemotherapy/Resection
A number of centers have treated metastatic para-aortic nodal disease from primary cervical cancers with EBRT in the hope of obtaining cure.80 Although 15% to 30% of such patients appear to be cured, if an EBRT dose of 55 to 60 Gy is employed, complication rates are high. For aggressive treatment to this location, tolerable doses of EBRT could be supplemented by IORT.80–90
For patients with relapse in the pelvic sidewalls or para-aortic nodes, salvage therapy results in overall 5-year SR of 0% to 5% for endometrial and 2% to 30% for cervical cancer.80 In previously irradiated patients, retreatment with meaningful doses of EBRT is compromised, and utilization of IORT (IOERT or HDR-IORT) as a supplement to low-dose EBRT with or without multidrug chemotherapy becomes one of the available options to salvage patients with tumor bed or nodal relapse.
IOERT With or Without External Irradiation and Resection
Mayo Clinic Rochester
From January 1983 through January 2005, 148 women with gynecologic cancers received IOERT at MCR after maximal surgical resection;80,82,86,87125 had local or regionally recurrent tumors (site of origin: cervix = 66 patients, endometrium = 31, corpus = six, vagina = six, ovary = 16) and 23 had locally advanced primary cancers (site of origin: cervix = 11, endometrium = one, corpus = four, vagina = three, genitourinary = four). At the time of IOERT and after maximum surgical debulking, 115 patients had microscopic residual disease or less and 33 (22%) had gross residual disease. Of the 148 IOERT patients, 113 also received EBRT as a component of treatment (median: 48.6 Gy; range: 0.9 to 75.4 Gy; 85 of 148 patients had previous EBRT). Concurrent chemotherapy during EBRT was given in 31 of 148 (22%). The median IOERT dose was 20 Gy in previously irradiated patients and 15 Gy in previously unirradiated patients. Systemic chemotherapy was given to 42 patients (28%) before or after resection (methotrexate vinblastine adriamycin [doxorubicin] cisplatin [MVAC] [n = 29] or platinum-based [n = 39]).
The 5-year actuarial OS for the total group was 27%. Five-year actuarial central (i.e., within IOERT field), local, and distant relapse rates were 28%, 40%, and 51%, respectively (Table 16-8). Patients with microscopic residual disease or less after maximum resection at the time of IOERT (n = 115) had a significantly higher SR than patients with gross residual disease (median SR: 21 months versus 15 months; 5-year OS: 31% versus 13%; P = .01). Patients with no previous in-field EBRT (n = 63) had improved survival rates compared to patients with previous in-field EBRT (median SR: 22 months versus 15 mo; 5-year OS: 35% versus 15%; P = .01). Recurrent disease patients with a disease-free interval (DFI) of >2 years (n = 81) had improved survival relative to those with DFI of ≤2 years (n = 44; median SR: 29 months versus 15 months; 5-year OS: 35% versus 14%; P = .002).
University of Washington
Similar results were achieved in a University of Washington series in which 21 patients received IOERT as a component of salvage therapy for locally recurrent or persistent cervical cancer.83 In all cases, surgery alone was inadequate to address tumor extent. From the time of IOERT, 5-year SR was 32% with a median SR of 22 months. Five-year LC was 46% with a median time to local failure of 20 months. Patients receiving IOERT for gross versus microscopic residual disease appeared to have decreased LC (5-year LC: 36% vs 55%, respectively).
University of Navarre, IOERT With or Without EBRT
Investigators at the University of Navarre in Pamplona used IOERT as a component of treatment in 67 patients with locally advanced primary or locally recurrent cervical cancer.84 Previously unirradiated patients were treated with pre-op CRT before resection and IOERT, but those with previous EBRT either proceeded directly to resection and IOERT or received pre-op chemotherapy. As seen in Table 16-8, the best outcomes were achieved in patients who presented with locally advanced primary lesions (5-year OS: 67%; 21% local relapse) or previously unirradiated recurrent disease.
French Group Series
The French IORT Group reported results in a series of 70 patients with recurrent cervix cancer who received IOERT (dose of 18 to 19 Gy) alone or combined with EBRT and chemotherapy.85 OS at 1, 2, and 3 years was 47%, 17%, and 8%, respectively. LC was achieved in only 21% of patients.
Orthovoltage-IORT, Stanford University
Investigators at Stanford University Medical Center used orthovoltage IORT as a component of treatment in 36 patients from September 1986 to November 2005 (previous EBRT = 72%; recurrent disease presentation = 89%).88 The primary sites were cervix (47%), endometrium (31%), vulva (14%), vagina (6%), and fallopian tubes (3%). Maximum cytoreductive surgery was accomplished in 84% of patients, including 18% exenterations, and mean IORT dose was 11.5 Gy (range: 6 to 17.5 Gy). EBRT (mean dose: 44 Gy, range: 10 to 79 Gy) and chemotherapy were given to 53% and 24% of the patients, respectively, after IORT.
The 5-year local regional control, distant metastasis–free (DM-free) survival, and DSS probabilities for the entire group were 44%, 51%, and 47%, respectively (see Table 16-8), and for those with cervical cancers, 45%, 60%, and 46%, respectively. On multivariate analysis, the prognostic factor that predicted local regional control was disease-free interval; for DM-free survival, it was tumor size; and for DSS, cervical primary, previous surgery, and local regional relapse. The actuarial 5-year grade 3 to 4 complication-free survival rate was 72%; exenterative surgery was an independent predictor of grade 3 to 4 complications (P ≤ .05) on multivariate analysis. The post-IORT treatments including EBRT and/or chemotherapy did not statistically significantly affect clinical outcomes. The series included three patients with positive margins after pelvic exenteration for recurrent cancers who remain alive after IORT (198, 187, and 14 months at time of analysis).
HDR-IORT, Memorial Sloan-Kettering Cancer Center
From November 1993 to June 1998, 17 patients with recurrent gynecologic cancer had radical resection and HDR-IORT at MSKCC.89 The primary lesion was treated with definitive irradiation in three of 17 patients; 14 had surgical resection alone (n = 3) or resection plus adjuvant irradiation (n = 11). The site of the primary was cervix in nine (53%), uterus in seven (41%) and vagina in one. Surgery for local relapse was exenterative in 10 (59%) and tumor resection in seven (41%). Gross total resection (R0 or R1) was achieved in 13 of 17 (76%). The mean HDR-IORT dose was 14 Gy (range: 12 to 15 Gy). I-125 implants were performed in three of four patients with an R2 resection as a supplement to HDR-IORT. No patient received EBRT at time of local relapse. With median follow-up of 20 months (range: 3 to 65 months), 3-year actuarial results demonstrate LC for the entire group of 67% (R0/R1 resection 83% versus R2 resection 25%; P < .01), distant control of 54%, and OS of 54%.
IORT for Recurrent Ovarian Cancer
Excellent results have been found with the use of IORT as a component of treatment for select patients with recurrent ovarian cancer at both MCR86,87 and Stanford University90 (see Table 16-8). The MCR series of 148 patients included 16 with recurrent ovarian cancer.87,88 In this select group of patients, median survival was 78 months, 2-year OS was 61%, and 5-year OS was 54%.
Twenty-four patients had maximal resection of recurrent disease followed by orthovoltage IORT at Stanford University; 22 were evaluable for analysis.90 Most had undergone cytoreductive surgery plus chemotherapy at the time of initial diagnosis; mean DFI was 48.2 months before resection and/or IORT. After maximal resection, only microscopic residual disease or <0.5 cm residual disease remained. The median IORT dose was 12 Gy (range: 9 to 14 Gy). Postoperative EBRT (n = 14) or chemotherapy treatment (n = 6) was given to 20 of 22 patients and was individualized according to both site of relapse and previous therapy (EBRT: abdominal/pelvic = nine, pelvic = four, inguinal = one). With median follow-up of 24 months, 5 patients remained free of disease. The 5-year OS was 22% with a median survival of 26 months from the time of IORT. Five patients recurred within the radiation fields (local-regional relapse rate of 32%) and 12 relapsed at distant sites. Nine patients (41%) experienced grade 3 treatment-related toxicities.
IORT: Retroperitoneal Soft Tissue Sarcomas
EBRT With or Without Chemotherapy/Resection
When surgery is the sole treatment modality for retroperitoneal sarcomas, subsequent local relapse rates are often 70 to 90%.91–93 If EBRT is combined with resection, the dose of EBRT that can be delivered safely (45 to 50 Gy in 1.8- to 2.0-Gy fractions) is much lower than with extremity sarcomas in view of dose-limiting structures (small intestine, stomach, liver, kidney, spinal cord). In a randomized NCI trial,94 patients with primary sarcomas randomized to EBRT alone after marginal resection had a local relapse rate of 80% and excessive acute and chronic small bowel morbidity. The use of IORT supplements is therefore reasonable and practical.91–105
NCI Randomized Trial: EBRT and Resection With or Without IOERT
NCI conducted a randomized trial in which 35 patients with retroperitoneal sarcomas were randomized to receive EBRT with or without IOERT.94 All had primary lesions; none had previous EBRT or chemotherapy. All had gross total resection; most had microscopic residual disease. Patients randomized to EBRT alone (n = 20) received 35 to 40 Gy to an extended field over 4 to 5 weeks and an additional 15 Gy over 2 weeks to a reduced field. The IOERT group (n = 15) received 35 to 40 Gy EBRT in 4 to 5 weeks to an extended field and an IOERT dose of 20 Gy to abutting fields.
IOERT With or Without EBRT, Resection
Mayo Clinic Rochester
Between March 1981, and September 1995, 87 patients with retroperitoneal or pelvic sarcomas had resection plus IOERT at MCR91,92,96,101 and ≥1 year follow-up (median: >3 years). More tumors were high grade (62%). EBRT was given in 77 patients (43 of 43 primary, 34 of 44 recurrent).
Forty-nine patients had documented disease relapse. Twenty (23%) had local or central failure (central in seven of 87 [8%], local in 16 [18%]; three of 20 patients had both local and central relapse). Local or central failure occurred in only three of 43 patients with primary lesions (7%), versus 17 of 44 (39%) who presented with recurrent disease. With median follow-up of >3.5 years, 46 of 87 patients (53%) were alive, with 5-year OS of 47%. Five-year SR was unaffected by primary versus recurrent status (52% versus 42%, respectively), and low- versus high-grade lesions (2-year: 97% versus 75%; 5-year: 45% versus 47%, respectively). Patients with gross total resection had a trend towards improved survival compared with those with gross residual disease (median: 4.7 versus 3.2 years; 5-year SR: 49% versus 36%; P = .08). Prognostic factor analyses for patients with primary disease are shown in Table 16-9. Severe GI intolerance was uncommon in primary disease patients (two of 43 [5%]), but seven of 44 recurrent-disease patients developed grade 3 to 5 GI fistulae (16%), with one fatality. Grade 3 peripheral neuropathy developed in four (9%) of 43 patients with primary disease and five (11%) of 44 with recurrent lesions.
The most recent analysis of MCR results was presented at ISIORT 2008 from a series of 226 patients who received IOERT as a component of treatment for retroperitoneal or pelvic sarcoma.103 The series was almost equally divided between primary (52%) and recurrent-disease (48%) patients. An R0 resection was achieved in 90 patients (40%) and R1 resection in 116 (51%). Five-year OS was 50% for the total group, and patients with a gross total resection appeared to do better (R0 resection: 52%; R1: 55%; R2: 28%; P = .08). Five-year local relapse rates also varied by degree of resection (R0: 18%; R1: 31%; R2: 61%). Central relapse in the IOERT field occurred in 10% of patients and distant metastases were documented in 42% of patients.
MGH Results
Results were analyzed in a group of 37 MGH patients with retroperitoneal sarcoma (EBRT = 17; EBRT with IOERT = 20).92,95,100 In patients with microscopic residual disease or less after maximal resection, survival trends favored EBRT with IORT (16 patients) over EBRT alone (n = 13), with a 5-year LC of 83% (versus 61%) and a 5-year OS of 74% (versus 30%).
European Pooled Analysis
Results of a pooled European analysis were presented at ISIORT 2008 by Krempien et al.104 In a series of 122 patients with retroperitoneal sarcoma who received IOERT as a component of treatment from 1991 to 2007 (recurrent = 81, primary = 41), post-op EBRT was given in 75 patients; 40 patients had previously been irradiated. Five-year actuarial OS, DFS, LC, and overall DM-free survival were 64%, 28%, 40%, and 50%, respectively. Central relapse within the IOERT field was related to degree of resection (R0: 5%; R1: 23%; R2: 75%). Grade 2 or higher late complications occurred in 21% of patients, but only 5% of patients required surgical intervention.
HDR-IORT, Memorial Sloan-Kettering Cancer Center
Between November 1992 and December 1996, 32 patients with retroperitoneal sarcoma received HDR-IORT as a component of treatment at MSKCC93,99 following resection (R0/R1 = 30; R2 = 2). Twelve had primary lesions and 20 had recurrent tumors (previous radiation and/or surgery). Mean HDR-IORT dose was 15 Gy (range: 12 to 15), mean HDR-IORT procedure time was 124 minutes (range: 32 to 250 minutes) and mean HDR-IORT delivery time was 60 minutes (range: 17 to 165 minutes). When post-op EBRT was delivered, the dose was 45 to 50.4 Gy in 25 to 28 fractions of 1.8 Gy for 5 to 5.5 weeks.
Orthovoltage-IORT, Stanford
Investigators at Stanford University used orthovoltage IORT for treatment of 62 sites of disease in 50 adult patients with locally advanced primary (30%) or recurrent soft tissue sarcomas (70%).105 The primary sites included the retroperitoneum-pelvis (78%), extremities (8%), and others (14%). Previous EBRT had been given in 32% of patients and previous systemic chemotherapy in 24%. Orthovoltage IORT was delivered after maximal resection with a mean dose of 11.6 Gy (range: 6 to 16 Gy). Postoperative EBRT or chemotherapy was administered to 32% of patients.
Conclusions and Future Possibilities
IORT combined with resection and EBRT offers an effective means of improving LC with retroperitoneal sarcomas. On the basis of the NCI randomized trial, use of adjuvant EBRT without IORT after marginal resection could be questioned, since the rate of tumor bed relapse with adjuvant EBRT in that trial was 80%, which is equivalent to results from surgery alone. A more practical approach is to give preoperative EBRT (with or without concomitant or neoadjuvant chemotherapy) after skinny needle biopsy and perform the resection at an institution which has the capability of giving an IORT supplement with IOERT or HDR-IORT. Local, regional, and distant failures are still common, however, emphasizing the need for further improvement in local therapy and an effective systemic treatment. Increased use of IMRT and IGRT to deliver the EBRT component of treatment may be useful in improving tolerance to the aggressive combined modality treatment approaches that include EBRT/IOERT and maximal resection, and may allow some dose escalation of the EBRT component of treatment. A phase II RTOG study was performed to evaluate neoadjuvant adriamycin and ifosfamide plus preoperative EBRT, resection, and IORT for moderate and high-grade retroperitoneal sarcomas,102 but the study was closed prematurely because of inadequate accrual.
IOERT: Renal/Other Genitourinary
Mayo Clinic Rochester
IOERT was combined with EBRT with or without resection at MCR in 49 patients with genitourinary cancers (renal = 28, bladder = eight, prostate = seven, ureter = two, miscellaneous = four).106–108 The IOERT dose was 7.5 to 30 Gy (median 15 Gy) and EBRT was given pre- or post-op in 42 patients (median 49.9 Gy). Survival was best for patients with a renal versus nonrenal primary (5-year: 37% versus 16%; P = .05) and patients with gross total R0/R1 versus R2 resection preceding IOERT (5-year OS: 41% versus 0%; P < .0001). Central failure was higher for patients with gross residual disease at time of IOERT (3-year: 28% versus 7%; P = .03).
University of Heidelberg
Eble et al. reported results in a series of 11 renal cancer patients (primary = three, locally recurrent = eight) treated at the University of Heidelberg109 with maximal resection, IOERT (15 to 20 Gy) and postoperative EBRT (40 Gy in 2.0-Gy fractions 5 days per week). With mean follow-up of 23 months, all patients were controlled locally, but distant metastases had occurred in 5 of 11 patients. Overall and disease-free SR at 3 years were 54% and 26%, respectively.
University of Navarre
Pamplona investigators also reported a series of 11 patients in which IOERT was used as a component of treatment for locally advanced primary (n = 8) or locally recurrent (n = 3) renal cancer.110 Fractionated EBRT was also given in seven of the 11 patients. Distant metastases were subsequently diagnosed in three of 11 and local relapse in one patient. Three of 11 were no evidence of disease (NED) with more than 3 years of follow-up.
University of California, San Francisco (UCSF)
At UCSF, 14 patients underwent resection of recurrent renal cancer and 10 also received IOERT.111 Mean time to relapse was 40 months (range: 5 to 180 months). Nine of 14 died of disease (mean: 17 months; range 1 to 56 months) and 5 are alive (mean: 66 months; range: 14 to 86). Survival was 40% at 2 years and 30% at 5 years from surgery.
Conclusions and Future Possibilities
Aggressive IOERT containing approaches appear reasonable for locally advanced renal cancers on the basis of small series from Mayo Clinic,106–108 the University of Heidelberg,109 Pamplona,110 and UCSF.111 The best candidates are previously untreated patients (four of six Mayo Clinic patients with progression after IOERT had received EBRT (n = 3) or chemotherapy (n = 2) before referral for IOERT). Development of novel and effective systemic therapy is needed in high-risk patients with renal cancer. For high grade transitional cell cancer (TCC) or SCC patients (bladder, ureter), chemotherapy should be combined with locally aggressive approaches because of systemic risks and potentially effective multidrug therapy.106
Breast IOERT
There is increasing interest in the use of IORT as a supplement or alternative to EBRT in selected cases.112–120 This includes single-institution, multi-institution, and multination series and multi-institution pooled analyses, in addition to phase II and phase III studies.
IOERT Alone
Investigators from Milan have the most experience in the use of IOERT as the only component of irradiation for early breast cancers;113,114 the only US experience with this approach is at the University of North Carolina (UNC).118 Veronesi et al. reported early results in a series of 237 patients with primary tumors ≤2 cm who had wide excision plus either sentinel lymph node biopsy and/or axillary lymph node dissection.113 Patients received IOERT doses of 17 to 21 Gy (more than 90% received 21 Gy as the prescribed dose) with 3 to 9 MeV IOERT. At a median follow-up of 19 months, the rate of posttreatment complications was low, with 1.7% developing breast fibrosis. A review of seven published IORT-alone series for patients with early breast cancer suggested that short-term LC, DFS, and OS were similar to reported EBRT series.114 The UNC approach differs from other institutions in that IOERT was given before resection of the breast cancer in a series of 71 patients. Cosmetic outcomes and tumor radiation response were reported at ISIORT 2008.118
Preliminary findings in the phase III Italian trial comparing IOERT alone with standard EBRT for early stage breast cancers was presented at ISIORT 2008.120 A total of 452 patients have been randomized from January 2003 to December 2007 (IOERT alone = 227; standard EBRT = 225), and accrual is ongoing. The primary endpoint is local relapse. With median follow-up of 31 months in 314 evaluable patients, no local relapses have occurred to date.
IOERT Combined With EBRT
IOERT has been combined with local excision/axillary dissection and EBRT in single and multi-institution series in the US and Europe.112,115–117,119 In a combined series from the Medical College of Ohio and Montpelier (France), 72 patients with early-stage breast cancer underwent local excision/axillary node dissection followed by 10 to 15 Gy IOERT using 6 to 20 MeV electrons.112 Patients later received EBRT doses of 45 to 50 Gy at 1.8- to 2.0-Gy per fraction. No significant complications were observed and cosmetic results were described as excellent. With minimum 2-year follow-up in all patients, no patients had experienced local relapse.
The University of Salzburg used IOERT combined with EBRT in 351 consecutive patients from October 1998 to April 2002 and reported their results in the initial 170 patients treated through December 2000.115 LC results were compared to patients treated with EBRT alone. At the time of publication, 3-year LC was 100% with an IOERT boost (versus ∼97% with EBRT boost).
Sedlmayer reported an ISIORT Europe pooled analysis of 1200 patients who received linear accelerator–based IOERT boosts from October 1998 to December 2005, combined with whole-breast EBRT doses of 50 to 54 Gy.119 As of February 2008, only 8 in-breast relapses were observed in 1121 patients with median follow-up of 59.6 months (LC: 99.3%). Seven-year OS, DFS, and DSS were 91.5%, 88.8%, and 94.8%, respectively.
IORT: Head and Neck Cancer
IORT has been used for both primary and recurrent head and neck cancers.121–133 In most settings the use of IOERT is associated with either high risk or previously irradiated patients. LC rates are impressive, given that many series report patients with previous radiation therapy and/or locally advanced or unresectable tumors.
IOERT for Primary or Locally Recurrent Disease
UCSF
In the largest series to date, 137 patients were treated at UCSF with gross total resection and IOERT for persistence or recurrence of local-regional cancer of the head and neck between 1991 and 2004.122 One hundred thirteen patients (83%) had previously received EBRT as a component of definitive therapy. Final surgical margins were positive in 56 patients (41%). The median IOERT dose was 15 Gy (range: 10 to 18 Gy). The median follow-up among surviving patients was 41 months. The 3-year in-field LC was 61%, and positive margins predicted for an in-field relapse (3-year in-field LC: 82% versus 48% for patients with negative versus positive margins. respectively). The 3-year LRC, DM-free survival, and OS were 51%, 46%, and 36%, respectively. Overall, results of resection with IOERT were effective in disease control with acceptable toxicity (wound infections = four patients; fistula = two; flap necrosis = one; trismus = one; and neuropathy = one).
For recurrent salivary gland tumors, IOERT at the time of resection significantly improves disease control.123 At UCSF, 95 patients underwent salvage surgery for locally recurrent salivary gland carcinomas; 81 (82%) had received previous radiation. IOERT was given in 37 of 95 while the remaining 58 patients had resection alone. The median IOERT dose was 15 Gy (range: 10 to 18 Gy). Five-year LC and OS were 69% and 34%, respectively. Both margin status and use of IOERT were associated with improved LC. Five-year LC was 77% for patients with negative margins versus 63% for patients with positive margins and 82% for patients receiving IOERT versus 60% for patients receiving no IOERT. The distant metastasis rate was 42%, emphasizing the need for improved systemic therapies.
Methodist Hospital of Indiana
Forty-seven patients with recurrent head and neck cancer (SCC = 42; adenoid cystic = five) in a previously irradiated field were treated with surgical resection (R1: 41; R2: 6) and IOERT at Methodist Hospital of Indiana.124 All patients received IOERT with a median of 20 Gy. Two-year actuarial survival was 54.9%; 15 patients were alive and disease free with a median survival of 29 months. Two-year LC was 61.5%. A trend toward increased survival and local recurrence control was noticed when treating microscopic (R1 resection) versus gross (R2 resection) residual disease. Perioperative mortality was seen in 8.5%, with no increase in morbidity due to IOERT.
Freeman et al. treated 104 patients with IOERT for mixed histology; the average IOERT dose was 15 to 20 Gy.125 Most cases represented recurrent, previously irradiated disease. LC with a minimum of 2 years follow-up was 40% (SCC histology: 30% for close margins, 44% for microscopic residual disease, 43% for gross residual disease; salivary gland tumors had similar results). This series included autopsy findings in 25 patients with local recurrence; 22 of these had relapse outside the IOERT field edge.
Eighty-two patients with locally advanced cervical node metastasis were approached with aggressive resection and IOERT with or without EBRT.126 Forty-nine (60%) patients had received previous irradiation. All metastatic nodes were greater than 3 cm; 65% were fixed on clinical examination and 35% involved the carotid artery. Fifteen patients required extended neck dissections with carotid resections and grafting. The IOERT dose was 15 Gy (n = 31) or 20 Gy (n = 49). EBRT was given in 77 of 82 patients either as previous primary treatment (n = 14; median 65 Gy) or in conjunction with resection and IOERT (n = 63 patients; pre-op = 42 patients, median: 60 Gy; post-op = 21 patients, median: 50 Gy). At 2 years, the LC rate within the IOERT field was 68% and the absolute survival rate was 45%. LRC rates for patients with close or microscopic margins (LRC: 85%; n = 46, 30 patients, respectively) were significantly better than with gross residual disease (20%). The combination of extended neck dissection, including carotid artery resection if necessary, and IOERT appeared to offer improved nodal control with no apparent additional morbidity.
Skull-based lesions of mixed histology were treated with 15 to 20 Gy IOERT with or without EBRT.127 Fifty-six percent of the patients were treated for recurrence, with 79% having received previous EBRT. LC was 64% at 12 months. Control rates were 86% for microscopically positive, 54% for close, and 50% for gross margins. The lack of difference in LC by margin status may be related to the large percentage of patients who had previous EBRT.
Mayo Clinic in Rochester
From 1991 to 1996, 44 patients received IOERT as a component of treatment at Mayo Clinic in Rochester for either SCC (n = 34) or non-SCC (n = 10).128 Most had relapsed after previous surgery (n = 27), EBRT (n = 28), or chemotherapy (n = 5); 13 had received no previous treatment. The site of relapse/IOERT (50 sites in 44 patients) were most commonly the neck (44%) and skull base (56%). Outcomes in the SCC versus non-SCC patients are as follows: 2-year OS: 32% versus 50%; 2-year DFS: 21% versus 40%; 2-year central control (in IOERT field): 46% versus 52%, respectively. Patients with R0 or R1 resections did better than those with R2 resections, with improved DFS (P = .03) and a trend for improved OS (P = .09).
University of the Ryukyus School of Medicine
Toita et al. treated 25 patients at 30 sites with locally advanced primary or recurrent lesions with IOERT doses of 10 to 30 Gy (median: 20 Gy).129 EBRT was given to 20 of 30 sites to a dose of 10 to 70 Gy (mean: 41.2 Gy). The in-field control rate was 54% with a 2-year OS of 45%. The 2-year OS for patients with close margins was 70% (n = 11), for patients with microscopic residual disease 33% (n = 12), and for patients with gross residual disease 0% (n = 7; P < .01). Margin status also predicted LC: 81.8% for close margins, 54.5% for microscopically positive, and 0% for grossly positive margins (P < .01).
IOERT or HDR-IORT
Ohio State University
Ohio State University used IOERT or HDR-IORT in patients with either primary high-risk disease or local-regional recurrence.130 In 3 prospective phase II clinical trials, 123 patients with previously untreated, resectable, advanced SCCs of the oral cavity, oropharynx, or hypopharynx were treated with perioperative cisplatin chemoRT, surgical resection with IORT (7.5 Gy for negative margins and 10 Gy for positive margins), and postoperative paclitaxel and cisplatin chemoRT. Compliance with all 3 intensification regimens averaged 61% (75 of 123 patients). Patient-directed noncompliance occurred in 16 patients (13%). With median follow-up of 62 months, the average locoregional (112 of 123 patients [91%]) and systemic (106 of 123 patients [86%]) disease control rates were excellent. Overall long-term DSS was 73%. The intensification regimens result in excellent disease control rates and long-term survival in this particular patient population. Future evolution of these regimens will include some modifications to further decrease toxic effects followed by phase II multi-institutional trials to determine whether the single-institutional experience can be duplicated.
IOERT for Palliation of Gross Disease
The Technical University in Aachen, Germany
IOERT has been used for the palliative treatment of unresectable tumors in Aachen for 95 patients, 71% of whom had gross residual disease at surgery.131 Only 11 patients had microscopically negative margins. LC was 17% in patients with gross residual disease and 64% in those with negative margins. In a separate series, 84 previously-irradiated patients received IOERT after maximal resection of recurrent disease.132 Median survival was 6.8 months, and median time to recurrence was 3.7 months. Local recurrence was 82% (51 of 62 patients) after R2 resection/IOERT versus 54% after R0 or R1 resection/IOERT (R0: six of 12 patients; R1: seven of 12 patients). The Aachen data suggests that gross residual disease is not adequately treated with IOERT alone.
Similar findings were seen in an IORT series from the University of Navarra in Pamplona.133 Local failure was 71.4% for patients with gross residual disease versus 57.1% for an R0 or R1 resection.
Pediatric IORT
IOERT Results
Denver Children’s Hospital
A pediatric IOERT series from Denver Children’s Hospital (Haase et al.) is of clear interest, because the series size of 59 patients far surpasses any other published single-institution experience.134,135 Equally impressive are the LC rates achieved in 75% of the 48 patients with malignancy and 91% of the 11 with benign but locally aggressive lesions that had recurred after previous resection. Survival of patients with malignancy was 63% (30 of 48).
Mayo Clinic
A Mayo Clinic pediatric IOERT series included 13 patients who were ≤18 years of age (Mayo Clinic uses 18 years as the cutoff age for pediatric patients; Denver Children’s uses 21 years).134,136 Eleven were at risk ≥20 months at the time of data analysis and presentation (median: 79 months; range: 20 to 113 months). The histologies were variable: neuroblastoma = four; soft tissue sarcoma = four; desmoid = two; paraganglioma = one. Eight of 11 (73%) were alive NED at the time of analysis, and LC was achieved in 10 of 11 patients (91%).
UCSF
At UCSF, 23 children received IOERT for pediatric neuroblastoma. IOERT energies ranged from 4 to 16 MeV and median dose was 10 Gy (range: 7 to 16 Gy).137 Twenty-one of 23 patients were classified as high risk. A gross total resection (R0 or R1) was achieved in 18 patients before IOERT, and 14 of 18 (77.7%) are disease-free survivors. Disease relapse occurred in six of 18 patients, and two of six had a local-regional component. Five patients had an R2 resection, with gross residual disease after surgery. All five recurred locally and died of their disease. For high-risk neuroblastoma patients, IOERT alone produced excellent LC after a R0 or R1 resection. However, IOERT as the sole radiotherapy to the primary was inadequate for patients with extensive adenopathy or an R2 resection.
HDR-IORT
HDR-IORT has been used as a component of treatment in 66 pediatric patients at MSKCC from February 1993 to December 2002.138,139 Eligible patients included those with primary (n = 31) or recurrent (n = 35) solid pediatric tumors with microscopic or minimal gross residual disease after maximal resection or with narrow margins after maximal resection. Histologies included rhabdomyosarcoma (n = 20), neuroblastoma (n = 16), extraosseous Ewing’s sarcoma (n = 9), Wilms’ tumor (n = 3), synovial sarcoma (n = 3), undifferentiated sarcoma (n = 3), desmoids (n = 2), and other (n = 10). The median HDR-IORT dose to the tumor bed was 12 Gy (range: 4 to 15 Gy) prescribed 0.5 cm from the applicator. Supplemental EBRT was used in 29 cases (44%; pre-op = 2, post-op −27; median: 35 Gy; range: 10 to 53 Gy). With median follow-up of 12 months, 22 of 66 (33%) developed relapse in the HDR-IORT field. Two-year LC was 56%, which was negatively affected by a number of factors: previous EBRT to the HDR-IORT site (39% versus 67%; P = .02), recurrent versus primary disease (43% versus 74%; P = .19), no adjuvant EBRT (29% versus 83%; P = .002), and positive resection margin (56% versus 61%; P = .09). On multivariate analysis, only lack of adjuvant EBRT was significant (P = .001) for 2-year LC.
Future Possibilities
Experience has demonstrated that variable combinations of EBRT, IORT, and surgical resection are feasible and practical in settings where close interdisciplinary cooperation exists. These aggressive approaches appear to affect LC with or without survival both in the disease sites covered in this chapter and in other disease sites.140–143
Attempts to complete phase III IORT clinical trials in a single country have been largely unsuccessful. The ISIORT organization attempted to develop a clinical trials program in which phase II to III studies would be performed in an international setting.144 This did not occur, because of an inability to secure funding for a centralized statistics and data-management center.
New technologies have improved the availability of IORT from the perspective of cost-effective alternatives. These technologies include mobile HDR-IORT units such as those in use at MSKCC, Beth Israel (NYC), MCR, and other institutions, and the mobile IOERT machines—Mobetron145 and NOVAC-7. The initial Mobetron unit was evaluated at UCSF and subsequent units were placed in other US institutions, including Mayo Clinic Arizona and Stanford University, as well as European and Japanese institutions. For the mobile HDR-IORT machine, a shielded facility is necessary in either the operating room area or in the radiation oncology department. Instead of shielding an entire operating room, however, technology now exists to create a shielded box (a room within a room) into which the patient can be placed for the HDR-IORT component of treatment after surgical resection and placement of the HAM applicator have been accomplished. The Mobetron has built-in shielding in a C-arm design and could theoretically be moved from one operating room to another, if indicated.
The existence of dedicated facilities and new technologies increases the likelihood of evaluating IORT in combination with “curative resection” and reduced dose EBRT with or without chemotherapy in adjuvant disease settings, where adjuvant EBRT doses necessary to achieve LC approach or exceed an acceptable level of normal tissue tolerance. An excellent example of this philosophy was the randomized NCI abdominal sarcoma trial in which adjuvant-level doses of EBRT resulted in excessive small bowel morbidity in addition to poor LC.91,92,94 For lesions of various histologies, the use of moderate dose EBRT (45 Gy in 25 fractions of 1.8 Gy over 5 weeks) plus IORT of 10 to 12.5 Gy may be preferable to high EBRT doses of 60 to 65 Gy when a marginal resection has been performed. The EBRT plus IORT option may improve both local tumor control and normal tissue tolerance.
IORT could also be used to replace a component or majority of EBRT in select node-negative patients. European pilot studies have been performed in both breast (T1 to T2, N0) and rectal cancer patients (T3N0) that demonstrate acceptable tolerance and local tumor control.112–117,119,120 Investigators at both Mayo Clinic Arizona and UNC118 have performed phase II breast cancer studies in which IOERT either replaces 1 to 1.5 weeks of EBRT boost-dose irradiation (Mayo Clinic Arizona) or serves as the total treatment (UNC).
Methodological comparison of IOERT, HDR-IORT, and perioperative brachytherapy have been discussed in detail elsewhere4 and have not been reviewed in detail here. The relative advantages or disadvantages of each depend on the amount of residual disease after maximal resection. A comprehensive IORT program would preferably have combinations of IOERT, HDR-IORT, and perioperative brachytherapy available to treat all disease sites and situations. For some institutions, this will mean having or obtaining both IOERT and HDR-IORT; at others, it may mean having expertise in both HDR-IORT and perioperative brachytherapy; a few institutions may have expertise in all three options. These modalities are not competitive but rather complement one other.
1 Gunderson LL, Tepper JE, Biggs DJ, et al. Intraoperative +/− external beam irradiation. Curr probl cancer. 1983;7:1-69.
2 Johnstone PA, Sindelar WF, Kinsella TJ. Experimental and clinical studies of intraoperative radiation therapy. Curr Probl Cancer. 1994;18:249-292.
3 Gunderson LL, Willett CG, Harrison LB, Calvo F. Intraoperative irradiation: techniques and results. Totowa, NJ: Humana Press; 1999. pp 1–550
4 Nag S, Gunderson LL, Willett CG, Harrison LB, Calvo FC. Intraoperative irradiation with electron-beam or high- dose-rate brachytherapy: Methodological comparisons. In: Gunderson LL, Willett CG, Harrison LB, Calvo FA, editors. Intraoperative irradiation: techniques and results. Totowa, NJ: Humana Press; 1999:111-130.
5 Suit HD. Radiation biology: a basis for radiotherapy. In: Fletcher GH, editor. Textbook of radiotherapy. ed 2. Philadelphia: Lea & Febiger; 1973:75-121.
6 Fletcher GH, Shukovsky LJ. The interplay of radiocurability and tolerance in irradiation of human cancers. J Radiol Electrol. 1975;56:383-400.
7 Griscom NT, Wang CC. Radiation therapy of inoperable breast carcinoma. Radiol. 1962;79:18-23.
8 Tepper J. Clonogenic potential of human tumors: a hypothesis. Acta Radiol Oncol. 1981;20:283-288.
9 Gunderson LL, Martenson JA, Gastrointestinal tract radiation tolerance. In Radiation Tolerance of Normal Tissues, (Ed) Vaeth JM, Meyer JE; Front Radiat Ther Oncol; 23; 1989:277-298.
10 Suit HD. Local control and patient survival. Int J Radiat Oncol Biol Phys. 1992;23:653-660.
11 Ramsay J, Suit HD, Sedlacek R. Experimental studies on the incidence of metastases after failure of radiation treatment and the effect of salvage surgery. Int J Radiat Oncol Biol Phys. 1988;14:1165-1168.
12 Suit HD, Sedlacek RS, Gillette EL. Examination for a correlation between probabilities of development of distant metastasis and of local recurrence. Radiol. 1970;95:189-194.
13 Suit HD. Potential for improving survival rates for the cancer patient by increasing the efficacy of treatment of the primary lesion. Cancer. 1982;50:1227-1234.
14 Fuks Z, Leibel SA, Wallner KE, et al. The effect of local control on metastatic dissemination in carcinoma of the prostate: long-term results in patients treated with I-125 implantation. Int J Radiat Oncol Biol Phys. 1991;21:537-547.
15 Leibel SA, Scott CB, Mohiuddin M, et al. The effect of local-regional control on distant metastatic dissemination in carcinoma of the head and neck: Results of an analysis for RTOG head and neck database. Int J Radiat Oncol Biol Phys. 1991;21:549-556.
16 Kapp DS, et al. Prognostic factors in patients with carcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys. 1998;47:531-540.
17 Okunieff P, Sundararaman S, Chen Y. Biology of large dose per fraction radiation therapy. Totowa, NJ: Humana Press; 1999. pp 25–46
18 Tepper JE, Gunderson LL, Orlow E. Complications of intraoperative radiation therapy. Int J Radiat Oncol Biol Phys. 1984;10:1831-1839.
19 Tepper JE, Gunderson LL, Goldson AL, et al. Quality control parameters of intraoperative irradiation. Int J Radiat Oncol Biol Phys. 1986;12:1687-1695.
20 Sindelar WE, Johnston PA, Hoekstra H, Kinsella TJ. Normal tissue tolerance to intraoperative irradiation: The National Cancer Institute experimental studies. In: Gunderson LL, Willett CG, Harrison LB, Calvo FC, editors. Intraoperative irradiation: techniques and results. Totowa NJ: Humana Press; 1999:131-146.
21 Gillette EL, Gillette SM, Powers BE. Studies at Colorado State University of normal tissue tolerance of beagles to IOERT, EBRT or a combination. In: Gunderson LL, Willett CG, Harrison LB, Calvo FC, editors. Intraoperative irradiation: techniques and results. Totowa NJ: Humana Press; 1999:147-164.
22 Shaw EG, Gunderson LL, Martin JK, et al. Peripheral nerve and ureteral tolerance of intraoperative radiation therapy: Clinical and dose response analysis. Radiother Oncol. 1990;18:247-255.
23 Kinsella TJ, DeLuca AM, Barnes M, et al. Threshold dose for peripheral neuropathy following intraoperative radiotherapy (IOERT) in a large animal model. Int J Radiat Oncol Biol Phys. 1991;20:697-701.
24 LeCouteur RA, Gillette EL, Powers EL, et al. Periopheral neuropathies following experimental intraoperative radiation (IORT). Int J Radiat Oncol Biol Phys. 1989;17:583-590.
25 Gillette EL, Powers BE, Gillette SM, Gunderson LL, Willett C. Peripheral nerve tolerance: Experimental and clinical. In: Gunderson LL, Willett CG, Harrison LB, Calvo FC, editors. Intraoperative irradiation: techniques and results. Totowa NJ: Humana Press; 1999:165-174.
26 Gillette EL, Gillette SM, Vujaskovic Z, et al. Influence of volume on canine ureters and peripheral nerves irradiated intraoperatively. In: Schildberg FW, Willich N, Kramling H, editors. Intraoperative Radiation Therapy–Proceedings 4th International IORT Symposium, Munich, 1992. Verlag Die Blaue Eule; 1993:61-63.
27 Vujaskovic Z, Gillette SM, Powers BE, et al. Effects of intraoperative irradiation and intraoperative hyperthermia on canine sciatic nerve: neurologic and electrophysiologic study. Int J Radiat Oncol Biol Phys. 1996;34:125-131.
28 Kinsella TJ, Sindelar WF, DeLuca AM, et al. Tolerance of the canine bladder to intraoperative radiation therapy: an experimental study. Int J Radiat Oncol Biol Phys. 1988;14:939-946.
29 Sindelar WF, Tepper JE, Travis EL. Tolerance of bile duct to intraoperative irradiation. Surgery. 1982;92:533-546.
30 Sindelar WF, Tepper JE, Kinsella TJ. Late effects of intraoperative radiation therapy on retroperitoneal structures, intestine and bile duct in a large animal model. Int J Radiat Oncol Biol Phys. 1994;29:781-788.
31 Miller RC, Haddock MG, Petersen IA, Gunderson LL, Furth AF. Intraoperative electron-beam radiotherapy and ureteral obstruction. Int J Radiat Oncol Biol Phys. 2006;64:792-798.
32 Gunderson LL, Nelson H, Martenson J, et al. Locally advanced primary colorectal cancer: Intraoperative electron and external beam irradiation ± 5-FU. Int J Radiat Oncol Biol Phys. 1997;37:601-614.
33 Gunderson LL, Nelson H, Martenson J, et al. Intraoperative electron and external beam irradiation with or without 5-fluorouracil and maximum surgical resection for previously unirradiated, locally recurrent colorectal cancer. Dis Colon Rectum. 1996;39:1379-1395.
34 Haddock MG, Miller RC, Nelson H, Gunderson LL. Intraoperative electron irradiation for locally recurrent colorectal cancer. ISIORT 2008 Proceedings. Rev Cancer. 2008;22:50.
35 Whittington R, Solin L, Mohiuddin M, et al. Multimodality therapy of unresectable pancreatic adenocarcinoma. Cancer. 1984;54:1991-1998.
36 Mohiuddin M, Cantor RJ, Bierman W, et al. Combined modality treatment of localized unresectable adenocarcinoma of the pancreas. Int J Radiat Oncol Biol Phys. 1988;14:79-84.
37 Roldan GE, Gunderson LL, Nagorney DM, et al. External beam vs external beam and intraoperative irradiation for locally advanced pancreatic cancer. Cancer. 1988;61:1110-1116.
38 Termuhlen P, Evans D, Willett CG. IORT in pancreatic carcinoma. In: Gunderson LL, Willett CG, Harrison LB, Calvo FC, editors. Intraoperative Irradiation: Techniques and Results. Totowa NJ: Humana Press; 1999:201-222.
39 Foo ML, Gunderson LL, Urrutia R. Pancreatic cancer. In: Gunderson LL, Tepper JE, editors. Clinical radiation oncology. New York: Churchill Livingstone/Harcourt Health Sciences Co; 2000:686-706.
40 Shipley WU, Wood WC, Tepper JE, et al. Intraoperative electron beam irradiation for patients with unresectable pancreatic carcinoma. Ann Surg. 1984;200:25-32.
41 Garton GR, Gunderson LL, Nagorney DM, et al. High dose preoperative external beam and intraoperative irradiation for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 1993;27:1153-1157.
42 Mohiuddin M, Regine WF, Stevens J, et al. Combined intraoperative radiation in perioperative chemotherapy for unresectable cancers of the pancreas. J Clin Oncol. 1995;13:2764-2768.
43 Tepper JE, Shipley WU, Warshaw AL, et al. The role of misonidazole combined with intraoperative radiation therapy in the treatment of pancreatic carcinoma. J Clin Onc. 1987;5:579-584.
44 Willett CG, Del Castillo CF, Shih HA, et al. Long-term results of intraoperative electron beam irradiation (IOERT) for patients with unresectable pancreatic cancer. Ann Surg. 2005;241:295-299.
45 Gunderson LL, Moss A, Callister MG, et al. Preoperative chemoradiation and IOERT for unresectable or borderline resectable pancreas cancer. ISIORT 2008 Proceedings. Rev Cancer. 2008;22:32-33.
46 Valentini V, D’Agostino G, Mattiucci GC, et al. IORT in pancreatic cancer: a joint analysis on 270 patients. ISIORT 2008 Proceedings. Rev Cancer. 2008;22:34-35.
47 Martinez-Monge R, Gerard JP, Kramling HJ, et al. Gastric IORT with or without EBRT. In: Gunderson LL, Willett CG, Harrison LB, Calvo FC, editors. Intraoperative irradiation: techniques and results. Totowa NJ: Humana Press; 1999:175-200.
48 Calvo FA, Martinez-Monge R, Ortiz de Urbina D, Gunderson LL. Stomach Cancer. In: Gunderson LL, Tepper JE, editors. Clinical radiation oncology. New York: Churchill Livingstone/Harcourt Health Sciences Co; 2000:652-685.
49 Abe M, Takahashi M, Ono K, et al. Japan gastric trials in intraoperative radiation therapy. Int J Radiat Oncol Biol Phys. 1988;15:1431-1433.
50 Abe M, Shibamoto Y, Ono K, Takahaski M. Intraoperative radiation therapy for carcinoma of the stomach and pancreas. Front Radiat Ther Oncol. 1991;25:258-269.
51 Abe M, Nishimura Y, Shibamoto Y. Intraoperative radiation therapy for gastric cancer. World J Surg. 1995;19:544-547.
52 Calvo FA, Aristu JJ, Azinovic I, et al. Intraoperative and external radiotherapy in resected gastric cancer: updated report of a phase II trial. Int J Radiat Oncol Biol Phys. 1992;24:729-736.
53 Chen G, Song S. Evaluation of intraoperative radiotherapy for gastic carcinoma: analysis of 247 patients. In: Abe M, Takahaski M, editors. Proceedings of Third International IORT Symposium, Kyoto, Japan. New York: Pergamon Press, Inc; 1991:190-191.
54 Sindelar WF, Kinsella TJ, Tepper JE, et al. Randomized trial of intraoperative radiotherapy in carcinoma of the stomach. Am J Surg. 1993;165:178-187.
55 Coquard R, Azyac L, Gilly FN, et al. IORT in gastric adenocarcinoma: The Lyon experience. Front Radiat Ther Oncol. 1997;31:162-164.
56 Gilly FN, Gerard JP, Braillon G, et al: Surgery, IORT and post-operative radiotherapy in the treatment of gastric cancer: the long-term experience in Lyon. ISIORT 2002 Proceedings; Abstract 4.4; Aachen, Germany.
57 Henning GT, Schild SF, Stafford SL, Gunderson LL. Results of irradiation or chemoradiation for primary unresectable, locally recurrent or grossly incomplete resection of gastric adenocarcinomas. Int J Radiat Oncol Biol Phys. 2000;46:109-118.
58 Miller RC, Haddock MG, Gunderson LL, et al. Intraoperative radiotherapy for treatment of locally advanced and recurrent esophageal and gastric adenocarcinoma. Diseases of the Esophagus. 2007;19:487-495.
59 Willett CG, Shellito PC, Gunderson LL. Primary colorectal EBRT and IOERT. In: Gunderson LL, Willett CG, Harrison LB, Calvo FC, editors. Intraoperative irradiation: techniques and results. Totowa NJ: Humana Press; 1999:249-272.
60 Gunderson LL, Cohen AM, Dosoretz DE, et al. Residual, unresectable or recurrent colorectal cancer: external beam irradiation and intraoperative electron beam boost +/− resection. Int J Radiat Oncol Biol Phys. 1983;9:1597-1606.
61 Willett CG, Shellito PC, Tepper JE, et al. Intraoperative electron beam radiation therapy for primary locally advanced rectal and rectosigmoid carcinoma. J Clin Oncol. 1991;9:843-849.
62 Harrison L, Enker W, Anderson L. High-dose rate intraoperative radiation therapy for colorectal cancer. Oncol. 1995;9:679-683. 737–741
63 Harrison L, Minsky BD, White C, et al. HDR-IORT for colorectal cancer. In: Gunderson LL, Willett CG, Harrison LB, Calvo FC, editors. Intraoperative Irradiation: Techniques and Results. Totowa NJ: Humana Press; 1999:307-314.
64 Schild SE, Gunderson LL, Haddock MG, et al. Radiotherapy as a Component of Treatment for Locally Advanced Colon Cancer. Int J Radiat Oncol Biol Phys. 1997;37:51-58.
65 Mathis KL, Nelson H, Pemberton JH, Haddock MG, Gunderson LL. Unresectable colorectal cancer can be cured with multi-modality therapy. Ann Surg. 2008;248:592-598.
66 Gomez Espi M, Calvo FA, Gonzalez C, et al. Timing and intensity of neoadjuvant treatment in rectal cancer: results of pre (plus IOERT) vs post (no IOERT) chemoradiation. ISIORT 2008 Proceedings. Rev Cancer. 2008;22:45.
67 Rutten H, Valentini V, Krempien R, Calvo FA for European Working Party of ISIORT. ISIORT 2008 Proceedings. Rev Cancer. 2008;22:45-46.
68 Gunderson LL, Willett CG, Haddock MG, et al. Recurrent colorectal: EBRT with or without IOERT or HDR-IORT. In: Gunderson LL, Willett CG, Harrison LB, Calvo FC, editors. Intraoperative irradiation: techniques and results. Totowa NJ: Humana Press; 1999:273-306.
69 Wallace HJ, Willett CG, Shellito PC, et al. Intraoperative radiation therapy for locally advanced recurrent rectal or rectosigmoid cancer. J Surg Onc. 1995;60:122-127.
70 Suzuki K, Gunderson LL, Devine RM, et al. Intraoperative irradiation after palliative surgery for locally recurrent rectal cancer. Cancer. 1995;75:939-952.
71 Haddock M, Gunderson L, Nelson H, et al. Intraoperative irradiation for locally recurrent colorectal cancer in previously irradiated patients. Int J Radiat Oncol Biol Phys. 2001;49:1267-1274.
72 Haddock MG, Miller RC, Nelson H, Devine RM, Gunderson LL: Intraoperative electron irradiation for locally recurrent rectal cancer. ISIORT 2002 Proceedings, Abstract 8.3, Aachen.
73 Abuchaibe O, Calvo FA, Tangeo E, et al. Intraoperative irradiation in locally advanced recurrent colorectal cancer. Int J Radiat Oncol Biol Phys. 1993;26:859-867.
74 Bussieres E, Gilly FN, Rouanet P, et al. Recurrences of rectal cancers: results of a multimodal approach with intraoperative radiation therapy. Int J Radiat Oncol Biol Phys. 1996;34:49-56.
75 Mannaerts GHH, Martijn H, Crommelin MA, et al. Intraoperative electron beam radiation therapy for locally recurrent rectal carcinoma. Int J Radiat Oncol Biol Phys. 1999;45:297-308.
76 Dresen RC, Gosens MJ, Martijn H, et al. Radical resection after IORT containing multimodality treatment is important determinant for outcome in patients treated for locally recurrent rectal cancer. ISIORT 2008 Proceedings. Rev Cancer. 2008;22:45-46.
77 Haddock MG, Nelson H, Donahue J, et al. IORT as a component of salvage therapy for colo-rectal cancer patients with advanced nodal metastases. Int J Rad Oncol Biol Phys. 2003;56:966-973.
78 Alektiar KM, Zelefsky MJ, Paty PB, et al. High-dose-rate intraoperative brachytherapy for recurrent colorectal cancer. Int J Radiat Oncol Biol Phys. 2000;48:219-226.
79 Shoup M, Guillem JG, Alektiar KM, et al. Predictors of survival in recurrent rectal cancer after resection and intraoperative radiotherapy. Dis Colon Rectum. 2002;45:585-592.
80 Haddock MG, Martinez-Monge R, Petersen IA, Wilson TO. Locally advanced primary and recurrent gynecologic malignancies: EBRT with or without IOERT or HDR-IORT. In: Gunderson LL, Willett CG, Harrison LB, Calvo FC, editors. Intraoperative irradiation: techniques and results. Totowa NJ: Humana Press; 1999:397-420.
81 Delgado G, Goldson AL, Ashayeri E, et al. Intraoperative radiation in the treatment of advanced cervical cancer. Obstet Gynecol. 1984;63:246-252.
82 Garton GR, Gunderson LL, Webb MJ, et al. Intraoperative irradiation in gynecologic cancer: The Mayo Clinic experience. Gyn Oncol. 1993;48:328-332.
83 Stelzer K, Koh W, Greer B, et al. The use of intraoperative radiation therapy in radical salvage for recurrent cervical cancer: outcome and toxicity. Am J Obstet Gyn. 1995;172:1881-1888.
84 Martinez-Monge R, Jurado M, Aristu JJ, et al. Intraoperative electron beam radiotherapy during radical surgery for locally advanced and recurrent cervical cancer. Gynecol Oncol. 2001;82:538-543.
85 Mahe M, Gerard JP, Dubois JB, et al. Intraoperative radiation therapy in recurrent carcinoma of the uterine cervix: report of the French Intraoperative Group on 70 patients. Int J Radiat Oncol Biol Phys. 1995;34:21-26.
86 Haddock MG, Petersen IA, Webb MJ, et al: Intraoperative radiation therapy for locally advanced gynecological malignancies. ISIORT 2002 Proceedings, Abstract 5.5, Aachen.
87 Haddock MG: Intraoperative radiation therapy for locally advanced gynecologic malignancies. ISIORT 2005; personal communication.
88 Tran PT, Kapp D, et al. Long-term survivors using intraoperative radiotherapy for recurrent gynecologic malignancies. Int J Radiat Oncol Biol Phys. 2007;69:504-511.
89 Gemignani ML, Alektiar KM, Leitao M, et al. Radical surgical resection and high-dose intraoperative radiation therapy (HDR-IORT) in patients with recurrent gynecologic cancers. Int J Radiat Oncol Biol Phys. 2001;50:687-694.
90 Yap OW, Kapp D, et al. Intraoperative radiation therapy in recurrent ovarian cancer. Int J Radiat Oncol Biol Phys. 2005;63:1114-1121.
91 Gunderson LL, Peterson I, Pritchard D, Haddock M, et al. Role and methods of irradiation as a component of treatment for extremity and retroperitoneal soft tissue sarcomas. Problems in General Surgery. Prob Gen Surg. 1999;16:43-61.
92 Gieschen HL, Willett CG, Donohue J, Petersen IA, et al. Electron or orthovoltage IORT for retroperitoneal sarcomas. In: Gunderson LL, Willett CG, Harrison LB, Calvo FC, editors. Intraoperative irradiation: techniques and results. Totowa NJ: Humana Press; 1999:329-350.
93 Harrison LB, Anderson L, White C, Brennan MF. HDR-IORT for retroperitoneal sarcomas. In: Gunderson LL, Willett CG, Harrison LB, Calvo FC, editors. Intraoperative Irradiation: Techniques and Results. Totowa NJ: Humana Press; 1999:351-358.
94 Sindelar WF, Kinsella TJ, Chen PW, et al. Intraoperative radiotherapy and retroperitoneal sarcomas: final results of a prospective, randomized, clinical trial. Archives of Surgery. 1993;128:402-410.
95 Willett CG, Suit HD, Tepper JE, et al. Intraoperative electron beam radiation therapy for retroperitoneal soft tissue sarcoma. Cancer. 1991;68:278-283.
96 Gunderson LL, Nagorney DM, McIlrath DC, et al. External beam and intraoperative electron irradiation for locally advanced soft tissue sarcomas. Int J Radiat Oncol Biol Phys. 1993;25:647-656.
97 Calvo FA, Azinovic I, Martinez R, et al. Intraoperative radiotherapy for the treatment of soft tissue sarcomas of central anatomic sites. 5th International IORT Symposium Abstracts. Hepato-Gastroenterol. 41(4), 1994.
98 Dubois JB, Hay MH, Gely S, et al. Intraoperative radiation therapy (IORT) in soft tissue sarcomas. 5th International IORT Symposium Abstracts. Hepato-Gastroenterol. 41(3), 1994.
99 Alektiar KM, Hu K, Anderson L, Brennan MP, Harrison LB. High-dose-rate intraoperative radiation therapy (HDR-IORT) for retroperitoneal sarcomas. Int J Radiat Oncol Biol Phys. 2000;47:157-163.
100 Gieschen HL, Spiro IJ, Suit HD, et al. Long-term results of intraoperative electron beam radiotherapy for primary and recurrent retroperitoneal soft tissue sarcoma. Int J Radiat Oncol Bio Phys. 2001;50:127-131.
101 Petersen I, Haddock M, Donohue J, et al. Use of intraoperative electron beam radiotherapy in the management of retroperitoneal soft tissue sarcomas. Int J Radiat Oncol Bio Phys. 2002;52:469-475.
102 Pisters PWT, Ballo MT, Fenstermacher MJ, et al. Phase I trial of preoperative concurrent doxorubicin and radiation therapy, surgical resection and intraoperative electron-beam radiation therapy for patients with localized retroperitoneal sarcoma. J Clin Oncol. 2003;21:3092-3097.
103 Petersen I, Haddock M, Stafford SL, et al. Use of intraoperative radiation therapy in retroperitoneal sarcomas: Update of the Mayo Clinic Rochester Experience. ISIORT 2008 Proceedings. Rev Cancer. 2008;22:57.
104 Krempien R, Roeder F, Buchler MW, et al. for European Working Party of ISIORT. Intraoperative radiation therapy (IORT) for primary and recurrent retroperitoneal soft tissue sarcoma: first results of a pooled analysis. ISIORT 2008 Proceedings. Rev Cancer. 2008;22:56-57.
105 Tran PT, Hara W, Su Z, et al. Intraoperative radiation therapy for locally advanced and recurrent soft-tissue sarcomas in adults. Int J Radiat Oncol Biol Phys. 2008;72:1146-1153.
106 Calvo FA, Zincke H, Gunderson LL, et al. Genitourinary IORT. In: Gunderson LL, Willett CG, Harrison LB, Calvo FC, editors. Intraoperative irradiation: techniques and results. Totowa NJ: Humana Press; 1999:421-436.
107 Frydenberg M, Gunderson LL, Hahn G, et al. Preoperative external beam radiotherapy followed by cytoreductive surgery and intraoperative radiotherapy for locally advanced primary or recurrent renal malignancies. J Urol. 1995;152:15-21.
108 Haddock MG, Miller RC, Zincke J, Gunderson LL: Intraoperative electron irradiation (IOERT) for locally advanced genitourinary malignancies. ISIORT 2002 Proceedings, Abstract 5.1, Aachen.
109 Eble MJ, Stähler G, Wannemacher M. IORT for locally advanced or recurrent renal carcinoma. Front Rad Ther Oncol. 1997;31:253-255.
110 Santos M, Ucas A, Ramos H, et al. Radiotherapia intraoperatoria en el carcinoma renal localmente avanzado: experiencia inicial. Actas Urol Esp. 1989;13:36-40.
111 Master VA, Gottschalk AR, Kane C, Carroll PR. Management of isolated renal fossa recurrence following radical nephrectomy. J Urol. 2005;174:473-477.
112 Battle JA, DuBois JB, Merrick HW, Dobelbower RR. IORT for breast cancer. In: Gunderson LL, Willett CG, Harrison LB, Calvo FA, editors. Intraoperative Irradiation: Techniques and Results. Totowa, NJ: Humana Press; 1999:521-526.
113 Veronesi U, Gatti G, Luini A, et al. Full-dose intraoperative radiotherapy with electrons during breast-conserving surgery. Arch Surg. 2003;138:1253-1256.
114 Cuncins-Harn A, Saunders C, Walsh D. A systematic review of intraoperative radiotherapy in early breast cancer. Breast Cancer Res Treat. 2004;85:271-280.
115 Reitsamer R, Peintinger F, Kopp M, et al. Local recurrence rated in breast cancer patients treated with intraoperative electron-boost radiotherapy versus post-operative external beam electron boost irradiation. Strahlenther Onkol. 2004;1:38-44.
116 Ciabattoni A, Mirri MA, Checcaglini F, et al. Italian report on IORT as anticipated boost in I and II stage breast cancer. ISIORT 2008 Proceedings. Rev Cancer. 2008;22:14.
117 Ivaldi GB, Leonardi MC, Orecchia R, et al. Preliminary results of electron intraoperative therapy boost and hypofractionated external beam radiotherapy after breast conserving surgery in premenopausal women. ISIORT 2008 Proceedings. Rev Cancer. 2008;22:15.
118 Sartor CI, Kimple RJ, Kuzmiak CM, et al. Cosmetic outcomes and tumor radiation response following single dose intraoperative radiotherapy for early stage breast cancer. ISIORT 2008 Proceedings. Rev Cancer. 2008;22:16.
119 Sedlmayer F, Fastner G, Merz F, et al, on behalf of the ISIORT Europe. ISIORT pooled analysis on linac-based IORT as boost strategy during breast conserving therapy, ISIORT 2008 Proceedings; Rev Cancer; 22; 2008:21-22.
120 Arcangeli G, Arcangeli S, Giordano C, et al, for the collaborative Breast IORT Group of AIRO. Intraoperative (IORT) vs standard radiotherapy (EBRT) in breast cancer: An update of an ongoing Italian multicenter, randomized study, ISIORT 2008 Proceedings; Rev Cancer; 22; 2008:12-13.
121 Foote RL, Garrett P, Rate W, et al. IORT for head and neck cancer. In: Gunderson LL, Willett CG, Harrison LB, Calvo FA, editors. Intraoperative Irradiation: Techniques and Results. Totowa, NJ: Humana Press; 1999:471-498.
122 Chen AM, Bucci MK, Singer M, et al. Intraoperative radiation therapy for recurrent head-and-neck cancer: The UCSF experience. Int J Radiat Oncol Biol Phys. 2007;67:122-129.
123 Chen AM, Garcia J, Bucci MK, et al. Recurrent salivary gland carcinomas treated by surgery with or without intraoperative radiation therapy. Head & Neck. 2008;30:2-9.
124 Rate WR, Garrett P, Hamaker R, et al. Intraoperative radiation therapy for recurrent head and neck cancer. Cancer. 1991;67:2738-2740.
125 Freeman SB, Hamaker RC, Singer MI, et al. Intraoperative radiotherapy of head and neck cancer. Arch Otolaryngol Head Neck Surg. 1990;116:165-168.
126 Freeman SB, Hamaker RC, Rate WE, et al. Management of advanced cervical metastasis using intraoperative radiotherapy. Laryngoscope. 1995;105:575-578.
127 Freeman SB, Hamaker RC, Singer MI, et al. Intraoperative radiotherapy of skull base cancer. Laryngoscope. 1991;101:507-509.
128 Pinheiro AD, Foote RL, McCaffrey TV, et al. Intraoperative radiotherapy for head and neck and skull base cancer. Head & Neck. 2003;25:217-226.
129 Toita T, Nakano M, Takizawa Y, et al. Intraoperative radiation therapy (IOERT) for head and neck cancer. Int J Radiat Oncol Biol Phys. 1994;30:1219-1224.
130 Schuller DE, Ozer E, Agrawal A, et al. Multimodal intensification regimens for advanced, resectable, previously untreated squamouis cell cancer of the oral cavity, oropharynx or hypopharynx, A 12-year experience. Arch Otolaryngol Head Neck Surg. 2007;133:320-326.
131 Spaeth J, Andreopoulos D, Unger T, et al. Intra-operative radiotherapy—5 years of experience in the palliative treatment of recurrent and advanced head and neck cancers. Oncology. 1997;54:208-213.
132 Schleicher UM, Phonias C, Spaeth J, et al. Intraoperative radiotherapy for pre-irradiated head and neck cancer. Radiother Oncol. 2001;58:77-81.
133 Martinez-Monge R, Azinovic I, Alcalde J, et al. IOERT in the management of locally advanced or recurrent head and neck cancer. Front Radiat Ther Oncol. 1997;31:122-125.
134 Schomberg PJ, Merchant TE, Haase G, Aristu J. Pediatric malignancies – IORT alone or with EBRT. In: Gunderson LL, Willett CG, Harrison LB, Calvo FA, editors. Intraoperative irradiation: techniques and results. Totowa, NJ: Humana Press; 1999:455-470.
135 Haase GM, Meagher DP, McNeely LK, et al. Electron beam intraoperative radiation therapy for pediatric neoplasms. Cancer. 1994;73:740-747.
136 Schomberg PJ, Gunderson LL, Moir CR, et al. Intraoperative electron irradiation in the management of pediatric malignancies. Cancer. 1997;79:2251-2256.
137 Haas-Kogan DA, Fisch BM, Wara WM, et al. Intraoperative radiation therapy for high-risk pediatric neuroblastoma. Int J Radiat Oncol Biol Phys. 2000;47:985-992.
138 Merchant TE, Zelefsky MJ, Sheldon JM, et al. High-dose rate intraoperative radiation therapy for pediatric solid tumors. Med Pediat Oncol. 1998;30:34-39.
139 Goodman KA, Wolden SL, LaQuaglia MP, et al. Intraoperative high-dose-rate brachytherapy for pediatric solid tumors: a 10-year experience. Brachytherapy. 2003;2:139-146.
140 Todoroki T, Gunderson LL, Nagorney D. Biliary tract IORT: bile duct and gallbladder. In: Gunderson LL, Willett CG, Harrison LB, Calvo FA, editors. Intraoperative irradiation: techniques and results. Totowa, NJ: Humana Press; 1999:223-248.
141 Peterson IA, Calvo FA, Gunderson LL, et al. Extremity and trunk soft tissue sarcomas: EBRT with or without IORT. In: Gunderson LL, Willett CG, Harrison LB, Calvo FA, editors. Intraoperative irradiation: techniques and results. Totowa, NJ: Humana Press; 1999:359-378.
142 Aristu J, Calvo FA, Martinez A, et al. Lung cancer: EBRT with or without IORT. In: Gunderson LL, Willett CG, Harrison LB, Calvo FA, editors. Intraoperative irradiation: techniques and results. Totowa, NJ: Humana Press; 1999:437-454.
143 Ortiz de Urbina D, Willich N, Dobelbower RR. IORT for CNS tumors. In: Gunderson LL, Willett CG, Harrison LB, Calvo FA, editors. Intraoperative irradiation: techniques and results. Totowa, NJ: Humana Press; 1999:499-520.
144 Gunderson LL, Willett CG, Calvo FA, Harrison LB. Conclusions and future possibilities – IORT. In: Gunderson LL, Willett CG, Harrison LB, Calvo FA, editors. Intraoperative irradiation: techniques and results. Totowa, NJ: Humana Press; 1999:527-536.
145 Meurk ML, Goer DA, Spalek G, Cock T. The Mobetron: a new concept for intraoperative radiotherapy. Front Radiat Ther Oncol. 1997;31:65-70.