CHAPTER 120 Intraoperative Magnetic Resonance Imaging
The field of neurosurgery has always been one in which the accuracy of its procedures is closely linked to the advances in neuroimaging that accompany it. The introduction of the surgical microscope was paramount in the evolution of neurosurgical technique because it increased the precision of interventions beyond the limitations of the naked eye.1 Later, frame-based and frameless stereotactic systems using preoperative computed tomography (CT) and magnetic resonance imaging (MRI) provided three-dimensional maps for neuronavigation, aiding greatly in the exploration of the brain.2 MRI in particular was appealing in the imaging of cortical structures because it had unparalleled soft tissue resolution, multiple contrast mechanisms, and the ability to acquire tomographic images in multiple planes.3,4 Irrespective of these benefits, however, the use of preoperatively obtained images to guide stereotactic navigation results in discrepancies between the virtually mapped space and actual anatomy in real time.
These inconsistencies in neuronavigation are largely a result of “brain shift,” or the deformation of cortical and subcortical structures secondary to loss of cerebrospinal fluid (CSF), tissue resection, patient position, edema, and other factors that alter the intraoperative brain environment from its previous state during preoperative scanning.4–8 Although such changes resulting from dural opening are minimal, they slowly progress with time, leading to increased stereotactic errors as the operation continues.5 To keep up with these dynamic shifts in cortical anatomy throughout surgery, a high-resolution, intraoperative imaging solution that could appreciate these changes and redirect navigation was needed. Intraoperative MRI (iMRI) was developed for this purpose.
iMRI has been used for only a little more than a decade. As with all new technologies, the beginning years of iMRI consisted of working out the setup and protocols to perform MRI-guided procedures within the operating theater.9,10 In their infancy, iMRI systems were able to improve the efficacy of surgical resection and monitoring for intraoperative complications such as ischemia, acute hemorrhage, and diffuse brain edema.1 As they became increasingly present in the neurosurgical operating room (OR), it was necessary to ask if these results translated into better patient outcomes and, if so, for which procedures was this benefit significant.11 The answer to this question is ever changing as the technology of iMRI continues to evolve. What may have been a small or undetectable benefit with the first generation of iMRI systems, owing to limitations in magnet field strength, system design, or the reporting of results, may not be applicable in the future. With the transition from low-field iMRI systems with poorer imaging quality and acquisition techniques to systems with high-field magnets, the degree of precision and the indications for which iMRI appears to be useful may continue to increase. As iMRI systems continue to improve in their ease of use and resolution, further studies will be needed to determine the extent to which iMRI is necessary in the neurosurgical OR.
Development of Intraoperative Magnetic Resonance Imaging
Since the development of the first iMRI system in 1995,9 various arrangements have emergedwith differing permutations in magnet strength, patient access, and OR suite setupbecause no one design has proved superior to all others. Currently, iMRI systems consist of magnets that range in field strength from 0.12 to 3.0 tesla (T) and are often subdivided accordingly into low-field (0.12 to 0.5 T) and high-field (1.5 and 3.0 T) systems. Although the high-field configurations enjoy superior image quality and advanced imaging capabilities, such as functional MRI (fMRI) and diffusion tensor imaging (DTI), in exchange, they sometimes sacrifice surgical access to the patient or time by requiring patient transport within the OR or to an adjacent but separate MRI suite.12 Conversely, low-field systems emphasize patient access and real-time imaging in the truest sense because many of these configurations do not require patient movement; however, their imaging resolution and capabilities are somewhat limited.9
Custom Intraoperative Magnet Designs
The first iMRI system was developed through a collaborative effort between physicians at Brigham and Women’s Hospital in Boston and engineers at General Electric Medical Systems. The design for the new magnet was paramount in the evolution of iMRI because it strayed from the traditional closed-coil configurations that prohibited patient access during scanning.9 Two superconducting magnets with bores 60 cm in diameter were vertically oriented in parallel, creating a 56-cm-wide gap between the magnets that provided nearly full access to the patient on either side of the OR table. The Signa SP design (General Electric Medical Systems, Milwaukee, WI), more commonly known as the “double doughnut,” positioned the patient’s head within the 0.5-T imaging isocenter located both between the two magnets and between the two surgical access sites, allowing simultaneous imaging and surgery without the need to move the patient (Fig. 120-1). The scanner also had a three-dimensional navigational system (Flashpoint, Integrated Technologies, Boulder, CO) that provided tracking capabilities within the imaging area. Because surgery took place within the magnetic field, MRI-compatible surgical and anesthesia equipment was required. Also of note was a specially constructed MRI-compatible microscope that provided the magnification necessary for neurosurgery but did not have many of the features offered on other neurosurgical microscopes, such as autofocus or autozoom.3 As the first of its kind, this iMRI system offered the unprecedented ability of producing near real-time intraoperative magnetic resonance images without the need to move the patient or magnet, while still providing adequate surgical access. The disadvantages included lower image quality relative to systems with high-field magnets, the need for more costly magnetic resonance–compatible equipment, and the somewhat limited surgical maneuverability given the confines of the magnets.1
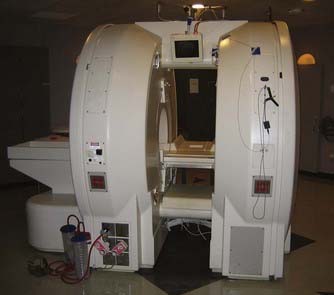
After the debut of the Signa SP into the neurosurgical world, other iMRI systems emerged that used novel magnet designs to optimize patient access with intraoperative imaging. The PoleStar magnet (Odin Medical Technologies, Yokneam, Israel) was one such design, which consisted of two vertical parallel disks containing ceramic magnets placed 25 cm apart and connected by a U-shaped arm that wrapped around the patient’s head from below (Fig. 120-2). The portability of the magnet limited its field intensity (0.12 to 0.15 T) but granted it great compatibility within the traditional OR. Given its low field strength, traditional surgical tools and equipment could be used and, if needed, the magnet could be placed inside its iron storage cabinet to virtually eliminate the magnetic field within the OR. Its small size allowed it to be wheeled to and placed beneath the patient’s head when needed for scanning, without any additional floor reinforcement. An optional radiofrequency (RF) shield that extended around the patient and magnet like a tent during imaging was available for ORs that were not RF shielded. Moreover, the surgical navigation system and the scanner could be operated by the neurosurgeon or OR staff, obviating the need for a neuroradiologist or a specific MRI technician.3,13
The physicians and scientists from Toronto Western Hospital developed a vertical gap system that took the first step toward a mobile patient model in the evolution of iMRI. This system consisted of a traditional 0.2-T horizontal gap permanent magnet system that was rotated 90 degrees to create a vertical gap. While the patient was positioned within the magnet’s isocenter, simple procedures such as catheter and shunt insertions could be performed by the single surgeon who stood within the vertical gap. By sliding the patient, head first, 1 to 1.5 meters away from the scanning position, a surgical position with 270 degrees of access could be achieved. Throughout the movement of the patient, overhead cameras maintained patient registration, allowing shifts between surgery and scanning without the need for reregistering anatomic coordinates to magnetic resonance images.3 Despite its decommissioning in 2003, the 5-year trial with this vertical gap system demonstrated that moving the patient in and out of the scanner was not the serious hindrance it was once believed to be. From this experience, others began to explore the possibility of combining a high-field closed-bore MRI system, which would provide superior image quality, with this efficient means of switching between scanning and performing surgery.
Adaptations of Traditional Magnetic Resonance Imaging System Magnets
In addition to the custom MRI system magnets specially designed for intraoperative use, some iMRI systems incorporated preexisting low-field or high-field scanners into a modified OR environment. Horizontal gap systems, such as the Hitachi AIRIS I and II (Hitachi Medical Systems, Twinsburg, OH, and Kashiba, Chiba, Japan) and the Magnetom Open Viva (Siemens Medical Solutions, Erlangen, Germany), consisted of traditional low-field scanners with a single, integrated MRI-compatible OR table (Fig. 120-3). The table was modified so that it could rotate the patient’s head away from the magnetic isocenter and beyond the 5-Gauss (5G) line, where standard surgical instruments could be used. These iMRI systems were an extension of work initially done at the University of California, Los Angeles (UCLA), which first suggested that in addition to brain biopsies10 and transsphenoidal surgeries14 performed beyond the magnet’s 5G line, full craniotomies and surgeries could be safely conducted there as well.15 Monitoring and surgical devices that were needed within the isocenter zone (e.g., anesthesia equipment, electrocardiogram leads, and tools for interventional procedures) needed to be magnetic resonance compatible, whereas routine surgical instruments could be used in the outer zone. The patient transfer between imaging and surgery took only a few minutes, and sometimes the magnet was shared as a diagnostic MRI unit as well, thereby reducing overall costs. Despite the low-field nature of these systems (0.3 T), many investigators who have used them have reported their definite utility in the resection of gliomas and pituitary tumors and have even argued that they may be comparable to their high-field counterparts.16,17
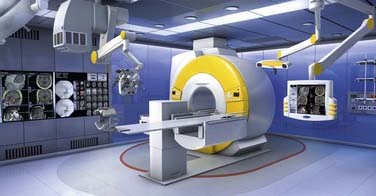
High-field systems such as the BrainSUITE (BrainLAB, Feldkirchen, Germany) use the same rotating MRI system OR table but with conventional 60- or 70-cm closed-bore magnets, Magnetom Sonata Maestro Class and Magnetom Espree (Siemens Medical Systems), respectively (Fig. 120-4). These 1.5-T systems afford the same degree of patient access and ease of transition between the magnetic and surgical fields while providing superior imaging quality and advanced acquisition techniques, such as fMRI, diffusion-weighted imaging (DWI), DTI, magnetic resonance angiography (MRA), magnetic resonance venography (MRV), and magnetic resonance spectroscopy (MRS).12
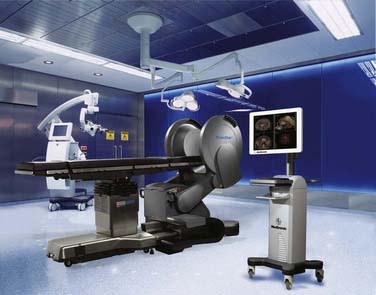
Other innovations in modifying the OR suite to accommodate intraoperative imaging included the rail-mounted system (Fig. 120-5), which was first implemented in Calgary, Canada (IMRIS, Calgary, AB, Canada). This system emphasized the preservation of the traditional neurosurgical operating theater, arguing that many compromises were made to accommodate preexisting iMRI systems, including the need for custom magnetic resonance–compatible surgical equipment, restricted patient access, and the need to transport the patient within the OR or to an adjacent imaging room. Storing the magnet in a separate room not only permits use of the OR as a traditional neurosurgical OR but also allows the MRI unit to be used in other surgical suites or for diagnostic purposes. The 1.5-T closed-bore magnet can be moved from its resting site outside the OR into position and ready to image in less than 90 seconds. All magnetic resonance–incompatible surgical tools and equipment are moved outside the 5G line and powered down during imaging. This transition has been completed in as short a time as 10 to 12 minutes.3
Aside from the original concept of conducting intraoperative imaging in the same time and space as conventional MRI, there have been many twin operating theater models in which the MRI system is located in an adjacent but otherwise separate room. This setup was first described by Tronnier and colleagues10 and was actually developed soon after the original double-doughnut model. The clear advantages of having a specialized MRI suite apart from the operating theater include being able to use MRI-incompatible surgical tools and equipment, having full patient access in a traditional surgical environment, and being able to use the MRI equipment in multiple ORs and departments, thereby greatly reducing overall costs.3,16 In one such 3-T system, the patient is manually slid onto a modified MRI docking table, using a custom tabletop transfer system, and is subsequently moved down a sterile corridor into the MRI suite. The table docks with the MRI scanner, and imaging can then proceed. The portable anesthesia system that maintains sedation while the patient is away from the OR and the physiologic monitoring equipment are both MRI compatible.3
Indications for Intraoperative Magnetic Resonance Imaging
iMRI has been used in many different neurosurgical procedures since its advent over a decade ago. Because the technology is relatively new, investigators are still trying to determine the types of interventions in which the benefit gained from it will justify the increased cost and operating time. Although there have been many surgical improvements, given the aid of iMRI, it is uncertain whether these will affect patient outcomes in the long run. Some commonly accepted indications for iMRI guidance include glioma resection (especially for larger tumors and low-grade gliomas), transsphenoidal pituitary adenoma resection, resection of seizure foci in refractory epilepsy, brain biopsies, and monitoring of intraoperative complications such as hyperacute hemorrhage.4,6,11,18
Glioma Resection
Much of the work with iMRI has focused on its ability to facilitate glioma resection. As was previously discussed, traditional stereotactic neuronavigation relies on fixed preoperative MRI scans of the lesion and its surrounding anatomy. The reliability of these images slowly diminishes throughout the surgery as CSF loss, surgical maneuvers, patient positioning, edema, and tumor resection all progressively alter the environment of the brain, producing increasing image inaccuracy with time (Fig. 120-6).4–811 Neurosurgeons have been cognizant of this phenomenon of “brain shift” during volumetric stereotaxis since it was first reported by Kelly and associates in 1986.19 Although shifts of the surface cortex could be compensated for through the visualization of superficial structures and were appreciable by previously available intraoperative navigation, subcortical shift was poorly understood.8
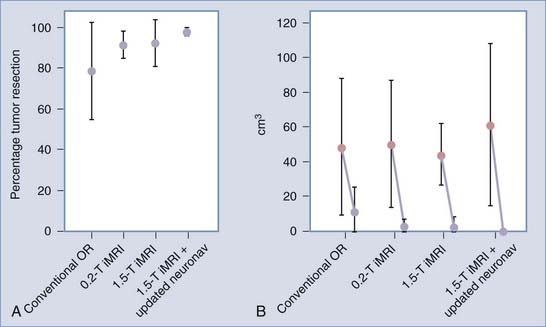
More recently, however, with the advent of iMRI and the resultant ability to better assess shifts of cortical and subcortical matter in patients within the OR, investigators have begun to quantify the magnitude and direction of brain deformation under surgical conditions. Shifts of up to 5 cm have been reported on the cortical surface and of up to 3 cm within the deep brain tissue during glioma resection, with great interpatient variability.5–8 The inaccuracy of stereotaxis is made apparent during glioma removal when the surgeon appreciates the discordance between the neuronavigation information and the appearance of the actual resection cavity. The surgeon’s inability to ascertain precisely the extent to which subcortical structures are shifted results in the overresection or, more often, underresection of lesions. With the use of iMRI, however, the neuronavigation system can be updated to agree with the intraoperative anatomy with an accuracy to within a couple of millimeters, even when using low-field systems.8,11 Reregistering the patient’s data with the navigation system using high-field iMRI has been shown to improve the consistency of intended gross-total resections (GTRs) significantly, especially with gliomas larger than 40 cm3 (Fig. 120-7).11 Although other surgical adjuncts, such as intraoperative ultrasonography, intraoperative CT, and mathematical models, have all been used to assess and even predict brain deformation, these methods still lack the resolution and compensatory ability to illustrate the changing brain structures adequately during neurosurgery.4,7
The benefit of rapidly updatable, near real-time anatomic imaging went hand in hand with the ability of iMRI to detect residual tumor, its boundaries, and its proximity to eloquent brain cortex in the surgical treatment of glioma. During the first decade of implementation, iMRI has been used most frequently after the neurosurgeon has determined that maximal tumor resection has been achieved. Although the results have varied greatly among groups, probably related to patient selection bias and different surgical philosophies at various institutions, evidence of residual resectable glioma on intraoperative scans at this point has been reported in 33% to 92% of cases.1,11,16,20–25 There are several possibilities for why such a large percentage of surgeries end with residual tumor present. For instance, the visual and textural similarity between common brain tumors, such as low-grade gliomas, and normal brain makes it difficult to distinguish pathologic tissue from healthy cortex during resection.22 In addition, the more malignant tumors are often characterized by microinvasion of the surrounding brain parenchyma, including eloquent areas, making complete resection without subsequent neurological sequelae very difficult, if not impossible. The inability of stereotactic navigation to reliably delineate tumor-brain margins because of dynamic brain shift throughout surgery can cause neurosurgeons to perform less aggressive resections to avoid postoperative deficits, especially if preoperative imaging indicates tumor proximity to brain regions controlling eloquent function. However, iMRI allows the neurosurgeon to better evaluate the progress of the resection, identify critical structures, and maximize more confidently the safe removal of tumor without inadvertently compromising neurological function.12
In the published literature, evidence of glioma remnants visualized on intraoperative images influenced surgical decision making most of the time, with additional resection being advocated in 50% to 92% of cases based on iMRI findings.23 Nimsky and associates4 published a synopsis of patients treated during a 5-year period within their low-field 0.5-T iMRI suite. An analysis of the 95 patients with gliomas in this study revealed that the authors achieved initial GTR in 37% of their patients. In 38% of the remaining patients who had undergone incomplete tumor removal, resection was expanded based on iMRI findings, increasing the rate of total resection from 87% to 100% of patients with World Health Organization (WHO) grade I astrocytomas (n = 15) and from 25% to 56% in those who had grade II astrocytomas (n = 32). There were very few patients with high-grade gliomas whose resections were extended based on iMRI findings (n = 5), and this produced only a minimal benefit, increasing the rate of GTR from 42% to 47% in patients with grade III tumors and from 21% to 24% in those with grade IV tumors. Overall, complete tumor resection was improved from 37% to 51% in this study. The large benefit realized with iMRI in low-grade gliomas was further corroborated by Schneider and colleagues26 who found that the use of a 0.5-T iMRI system increased the percentage of resected low-grade gliomas having less than 10% residual tumor volume from 33% to 92%. These findings suggest a significant benefit for iMRI in low-grade glioma surgeries, with only marginal benefit in resection of high-grade gliomas.
Other studies, however, have shown a significant utility for intraoperative imaging in patients with malignant (WHO grade III and grade IV) neoplasms, reporting that the use of low-field iMRI expanded resections of high-grade gliomas in 60% to 63% of patients.16,24 Some groups have reported that the rate of GTR improved from between 6.5% and 36.6% to between 35.5% and 75.6% of cases when iMRI was used.25,27 The inconsistency of data supporting use of iMRI in the case of high-grade gliomas may be the result of the varying degrees to which these more microinvasive tumors infiltrate surrounding eloquent brain tissue. The involvement of tumor in brain areas controlling eloquent function, which would result in neurological deficits if violated, frequently prohibits the aggressive resection of these neoplasms. Yet, the rate and degree in which eloquent cortex is involved may vary from one patient group to another, and this information is not regularly reported. Further randomized prospective studies may help to indicate the tumor conditions in which iMRI will be beneficial in the removal of high-grade gliomas.
Since the initial success in improving glioma resection through the use of low-field iMRI, the enhanced imaging quality and advanced acquisition techniques of high-field iMRI have marked these more sophisticated systems as the future generation of intraoperative imaging. In addition to the obvious benefits of faster scanning and superior image quality, the high-field magnet provides the ability to invoke other intraoperative imaging modalities such as fMRI, DTI, and MRS. Nimsky and colleagues28 reported on their experience of using DTI intraoperatively with a high-field 1.5-T iMRI system to track pyramidal white matter fibers during resection of gliomas near motor areas. After obtaining routine preoperative images, including T1- and T2-weighted sequences for standard anatomic visualization, they included DTI and fMRI for pyramidal tractography and isolation of motor regions. The functional information was subsequently integrated into a three-dimensional magnetization-prepared rapid acquisition gradient-echo (3D MP RAGE) data set for intraoperative neuronavigation.18 The precentral gyrus, as identified by fMRI, was used as one of the seeds for fiber tracking in their knowledge-based dual region-of-interest (ROI) approach. The second arbitrary seed they used to determine the path of the pyramidal tract was located in the posterior parts of the internal capsule and the genu. From these two ROIs, tracking took place in both retrograde and orthogonal directions relative to the principal diffusion vector until the fiber tract was delineated. This process took only about 1 to 2 minutes and could thus be performed intraoperatively without too much concern for increased operating time. Just by employing the updated DTI and anatomic images alone, resection was extended in 6 of their 19 patients (32%). Postoperative analysis of preoperative and intraoperative fiber tracking further supported the use of iMRI-DTI in this scenario because they experienced pyramidal fiber shifts of up to 8 mm, with only 3 of 19 patients (15.8%) experiencing no detectable shift. In addition, no new neurological deficits were seen postoperatively.28 Although further multicenter studies are necessary to evaluate the efficacy of this model on a larger scale, having such a system appears to be beneficial in improving resection of gliomas near eloquent cortex and fiber tracts.
Given the costs, increased operating time, and technique modifications needed to accommodate this new technology, it is appropriate to ask whether such measures to maximize glioma removal will actually improve patient outcome. To address the inference that increasing the degree of resection using iMRI may lead to improved patient survival, some groups have directly compared the outcomes of patients undergoing glioma removal with and without the aid of iMRI. Claus and associates29 described their 6-year experience with 156 patients with low-grade gliomas who were operated on using iMRI and found that the age- and histology-adjusted mortality rates of these patients at 1, 2, and 5 years were significantly lower than those reported in the national databases. In addition, this group found that patients who underwent only subtotal resection instead of GTR were at a significantly increased risk for disease recurrence and death.29 Other studies did not adjust for age or histology, but they also reported both significant and nonsignificant increases in survival time in patients who underwent GTR aided by iMRI compared with those who did not.30,31
There is increasing evidence that imaging-documented complete resection of low-grade gliomas may improve patient survival time, despite the relative paucity of similar evidence in the more difficult to resect high-grade tumors. This has caused some to suggest that the potential for cure in patients with some WHO grade I and II gliomas through GTR may warrant the use of iMRI, whereas the need for iMRI in patients having more malignant gliomas may not be as clear.22 Regardless, because the current operative goal in almost all glioma patients is to resect as much of the tumor as possible without causing new postoperative neurological deficits, iMRI may serve as a critical tool, especially when the tumor borders or invades eloquent cortex, irrespective of its definitive effect on patient mortality. As such, with increasing availability, iMRI may someday become part of the standard of care for glioma surgery.32
Brain Biopsy
During the past few decades, brain biopsy techniques have progressed from freehand sampling using CT to stabilized, MRI-based stereotaxis procedures.33 A large literature review revealed that stereotactically guided brain biopsies yield diagnostic tissue about 91% of the time.34 Nondiagnostic biopsies are often the result of inaccurate tissue targeting, small target size, small sample size, and attempting to biopsy areas with an increased T2-weighted magnetic resonance signal.35 Many of these problems can be attributed to the use of static neuronavigation systems reliant on preoperative images that are unable to account for intraoperative brain shifts, as was discussed earlier. Although one can expect to observe a minimal amount of shift during brain biopsy compared with more extensive intracranial procedures such as large tumor resections, even small discrepancies in stereotactic and actual space can become problematic when targeting smaller lesions. Furthermore, after radiation treatment or infectious processes, it is difficult to distinguish the target of interest (e.g., tumor, active infection) from areas of postirradiation necrosis or gliosis in heterogeneous lesions showing contrast enhancement on T1-weighted magnetic resonance images, thereby complicating diagnostic sampling.36 iMRI, with its ability to provide near real-time updates of the biopsy procedure, as well as aid in the functional discrimination of pathologic from normal brain tissue (through MRS or perfusion MRI), can address many of these problems.
In June of 1995, at Brigham and Women’s Hospital in Boston, a stereotactic brain biopsy was the first procedure to be completed in the iMRI environment.9 After positioning the patient within the Signa SP scanner, preoperative images were taken for biopsy planning, and a bur hole was subsequently made. Simply by using a Brookwalter arm, the biopsy cannula was fixed in place as the needle was passed through into the brain under direct real-time image guidance.37 The double-doughnut open-MRI system allowed for repeated imaging of the needle’s trajectory as it continued through to the biopsy site, without needing to move the patient. By maintaining visualization of the needle throughout the procedure, entry into the target lesion and avoidance of critical structures could be confirmed.
Since then, other groups have modified their OR environments to conduct intraoperative imaging for brain biopsies without the use of equipment specifically designed for iMRI.10,38 In these setups, trajectory planning took place within a sterile MRI suite. However, after imaging was performed, the patient was moved away from the magnetic isocenter, beyond the 5G line. In this magnetic “fringe field,” conventional surgical equipment, including drills and electrocautery, could be used. Nevertheless, because biopsy needle insertion took place within the magnet, MRI-compatible instruments necessary for guided tissue sampling were still required.10,38 Although initial studies of iMRI-guided brain biopsies revealed diagnostic tissue in 97% to 100% of cases, the relatively small numbers of patients included made it difficult to compare these results directly with outcome data in series of patients undergoing more conventional frame-based and frameless stereotactic biopsies conducted without the aid of iMRI.37–39
Prominent developments after the first iMRI-guided biopsies have continued to improve the reliability and diagnostic ability of this technology. Needle-stabilizing devices were soon developed because freehand biopsies have often been criticized for being inaccurate and potentially increasing the risk for acute intracerebral hemorrhage.35 Currently, skull-mounted trajectory guides with three-point alignment algorithms help account for any patient head movements, maintain the desired trajectory, and stabilize the needle throughout the procedure.33,40
Another advancement was the addition of MRS in high-field iMRI systems to facilitate the localization of pathologic areas and subsequent biopsy of diagnostic tissue. Stereotactic biopsy has often been used to distinguish recurrent neoplasm from postirradiation necrosis, given the similarity of these two findings on imaging scans.38 In patients who have received external beam radiation for central nervous system tumor treatment, this becomes problematic because imaging studies cannot conclusively distinguish the two, and biopsy may not accurately sample the pathologic tissue within a large lesion that shows heterogeneous contrast enhancement. Through intraoperative MRS techniques, including turbospectroscopic imaging (TSI) and single-voxel spectroscopy (SVS), spectroscopic maps based on levels of cellular metabolites (e.g., choline and N-acetyl aspartate) can be superimposed on images of cortical anatomy, guiding the biopsy needle to pathologic tissue.35,36,41 Diagnostic tissue was obtained in 100% of cases in two preliminary studies using intraoperative MRI or MRS.35,36
Initial studies of iMRI-guided brain biopsies have shown marked reductions in morbidity and superior rates of diagnostic tissue sampling compared with conventional stereotactic navigation systems.39,42 In addition to being able to plan the biopsy path in nearly real time to avoid critical anatomic structures, the ability of iMRI to detect intraoperative complications contributes strongly to reducing patient morbidity. This is especially true for intracranial hemorrhage, which has been reported to occur in 0% to 11.5% of stereotactic brain biopsies.37 Initially, bleeds were assessed by observing changes in serial images that were consistent with blood collection, although more recently, with high-field systems, a combination of T2-weighted half-Fourier acquisition single-shot turbo spin echo (T2-HASTE), gradient echo (GE), and fluid-attenuated inversion recovery (FLAIR) techniques has been shown to sensitively detect hyperacute hemorrhage.43 Through immediate detection and evacuation of bleeds during surgery, rather than later in response to clinical deterioration, neurological sequelae can be avoided.
Transsphenoidal Resection of Pituitary Adenomas
Although the first transsphenoidal approach for pituitary adenoma removal was performed more than a century ago,44 it was relatively recently, with the development of adjuvant technologies, that it became the method of choice in the resection of pituitary tumors.45 Innovations such as intraoperative fluoroscopy, the operating microscope, frameless stereotaxy, and the neuroendoscope have all aided in improving navigation to the tumor bed and visualization of the tumor to maximize resection.45–47 However, these advances were not without their limitations. Although fluoroscopy allows for rapid real-time imaging, its visualization is limited to the bony structures of the sella, and it is unable to aid in tumor detection.48 The operating microscope greatly increases visibility within the surgical field, but it is limited by angles of viewing, and microscopic visualization alone does not permit appreciation of the entire sella and its surrounding regions.45 The phenomenon of brain shift interferes with the accuracy of frameless stereotactic systems of neuronavigation (as described earlier in the case of glioma resection), and these systems cannot be updated intraoperatively to identify remaining tumor, thus decreasing their utility. Finally, despite the drastic improvement that endoscopy has provided relative to the surgical microscope alone, it requires direct visualization to confirm the presence or absence of tumor and does not allow one to see beyond the surface anatomy.45 This becomes especially troublesome in the case of tumors with suprasellar and parasellar extension because focused removal requires appreciation of structures located beneath the surgical field of view. Given these limitations, it is not surprising that complete removal of pituitary adenomas remains a problem, with studies suggesting that this is achieved in only about 65% of macroadenoma cases.49
iMRI brings a distinct advantage to the previous armament of tools for transsphenoidal navigation and neuroimaging. Because it is unhindered by a need for direct visualization of the obscured, ever-changing resection cavity, iMRI can provide images of tissue during an operation that enable assessment of residual tumor. Serial updates allow the neurosurgeon to localize remnant tumor while accounting for brain shift throughout the surgery and to direct resection while preserving critical native structures (e.g., the optic chiasm and cavernous sinuses). Furthermore, dynamic image sequences help distinguish normal pituitary tissue (fast contrast enhancement) from adenoma (slow contrast enhancement) and hemorrhage (heme-sensitive gradient echo).46,48
Transsphenoidal pituitary macroadenoma surgery performed with low-field iMRI systems revealed residual tumor in 20% to 66% of patients after initial resection, with some investigators reporting the ability to achieve complete removal in 75% to 100% of cases with the use of iMRI.4,45–47 In a study using high-field iMRI, Nimsky and colleagues50 found that with intraoperative scanning, they were able to increase their rate of complete pituitary tumor removal from 58% to 82%, despite selecting for patients with macroadenomas and large suprasellar extension. They argued that high-field iMRI is notably better than its low-field equivalent for pituitary adenoma surgery because it can detect tumor remnants as small as 3 to 4 mm in maximal diameter and is not subject to any of the artifact or image quality problems found in the low-field systems. The importance of magnet strength in detecting small tumor pieces is supported by other studies that argue the superiority of 3-T MRI relative to standard 1.0- to 1.5-T magnets in detailing the invasion of pituitary adenomas into adjacent structures, during preoperative planning.51 In addition to the immediate benefits of guiding and improving resection, iMRI provides instantaneous feedback regarding the success of the operation. This allows the surgeon to determine on the day of surgery whether further management is required, rather than waiting the standard 2 to 3 months for artifact-free postoperative scans to decide among surveillance, radiation therapy, or secondary transcranial surgery.4,50
Several other groups reported superior rates of complete tumor removal while using iMRI to guide transsphenoidal resection.4,45–48,50 However, the inclusion criteria among these studies varied greatly, with some choosing nonfunctioning macroadenomas with suprasellar extension, whereas others did not specify the hormonal status or amount of tumor extension into neighboring brain regions. This makes objective comparison of surgical outcomes difficult. Furthermore, a study conducted by Fahlbusch and associates52 assessing the use of iMRI in the transsphenoidal resection of growth hormone (GH)-producing pituitary macroadenomas found that even high-field iMRI could not detect tumor remnants in all cases, as confirmed by persistent endocrine abnormalities in patients, despite negative findings on intraoperative scans. Consequently, it may be too early to conclude whether the reported imaging outcomes translate into truly beneficial clinical outcomes that can be compared with national statistics derived from studies with more than 10 years of follow-up.49 Given the relatively few studies using iMRI specifically for pituitary tumors and their relatively short follow-up periods, the degree to which iMRI guidance will improve the rates of surgical cure in pituitary adenomas has yet to be determined.
Intractable Epilepsy
Although surgery has been a treatment option for pharmacoresistant epilepsy for more than 50 years, it was only recently that a randomized controlled trial53 investigated the efficacy of surgery compared with medical management. The study found that surgical intervention for temporal lobe epilepsy allowed 58% of patients to be seizure free after 1 year, compared with only 8% of the medically managed group. This outcome, in conjunction with other reports of increased seizure-free intervals after surgery, has continued to support this invasive treatment option over the years. Surgical techniques available for intractable epilepsy include focal cortical resection, anatomic lobectomy, lesionectomy, corticectomy, and hemispherectomy and its variants.54 The actual procedure performed depends on the location of the seizure focus and characterization of the epilepsy syndrome involved. For example, patients with epilepsy secondary to a specific seizure focus, including neoplasms and vascular malformations, undergo focal cortical resection to remove the epileptogenic lesion; alternatively, mesiotemporal lobe epilepsy patients with hippocampal sclerosis benefit from amygdalohippocampectomy or en bloc temporal lobe resection.54 A review of previous literature reveals mounting evidence that the moderate to high recurrence rate (20% to 60%) after surgery in seizure syndromes such as temporal lobe epilepsy may be a result of the inadequate resection of implicated structures, such as the amygdala and hippocampus, with freedom from seizures being correlated with more complete and accurate resection.55,56 Because iMRI had proved useful in surgeries in which the extent of resection was critical to patient outcome, it appeared reasonable to extend its application to epilepsy surgery.
The few studies that have investigated the use of iMRI in epilepsy surgery have looked at the treatment of distinct seizure pathologies. Overall, they report the utility of intraoperative imaging at helping navigate through cortex while accommodating for brain shift as well as aiding in the detection and resection of residual epileptogenic structures or lesions.55–58 Kaibara and coworkers56 reported that although iMRI was particularly useful in the more selective amygdalohippocampectomy performed in a small subset of their patients, intraoperative imaging allowed detection of residual hippocampus or amygdala in 50% of all patients, suggesting that even with the more aggressive anterior temporal lobectomy, critical structures implicated in seizure production may be left behind. Among their patients, 93% who underwent surgery with iMRI were seizure free at a mean follow-up interval of 17 months, supporting their surgical outcomes. Schwartz and associates55 published similar results in a small study in which 100% of their patients were seizure free at 10 months. Other studies reported the use of iMRI in resecting neoplasm-related seizure foci but described more moderate benefits of this technology in seizure control.57,58
In addition to the obvious benefit of iMRI in removing seizure-producing lesions, it was also shown to guide the elimination of nonlesional epileptogenic foci, undetectable by traditional imaging modalities. Through iMRI-guided placement of depth electrodes, neurosurgeons are able to customize the resection by mapping out the precise locations of aberrant neuronal firing.57 It also allows for more confident extension of the resection in both lesional and nonlesional cases, if electrocorticography reveals residual ictal activity. This technique has been reported previously with other imaging modalities such as CT and preoperative MRI-based stereotaxis59; however, these earlier systems were incapable of performing real-time anatomic updates to account for brain shift in the operative setting. Thus, iMRI may serve as a quality control enhancement in the removal of both lesion-based and nonlesional seizure foci.
Other Applications
Given the broad range of procedures that require precise localization and near real-time knowledge of intracranial anatomy during neurosurgical procedures, it is not surprising that iMRI has been used for many different indications. Less frequent applications of iMRI include use during intracranial cyst drainage, abscess evacuation, deep brain stimulator placement, and assessment of vascular compromise using MRA in high-field systems.1,4,9,41,60
Feasibility and Cost Considerations
Despite the advancements iMRI has brought to the field of neurosurgery, one of the greatest criticisms levied against this technology is its excessive cost. It was estimated that to establish and maintain an iMRI suite with the appropriate equipment, OR setup, and navigational system for the first 5 years of implementation would cost about $10 million dollars, not including the additional expense for each procedure (estimated to be an added $2000 for each iMRI-guided glioma resection).42 Far from placing a price tag on human life, these critiques stem from the absence of evidence-based data to suggest whether the use of iMRI significantly affects patient outcome. Although preliminary results with various low-field and high-field iMRI systems were able to demonstrate a significant reduction in morbidity for procedures such as brain biopsies,42 the age-old controversy regarding whether extended resection improves outcome in malignant glioma surgeries and the relatively short follow-up of patients who have undergone iMRI-guided pituitary adenoma removal provide limited evidence to support the cost-benefit of this new technology.45–48,50,61,62
Nevertheless, a recent fiscal analysis of brain tumor patients undergoing iMRI-guided resection showed that overall hospital cost and total hospital stay were both significantly reduced in adults and children within the iMRI group.41 Arguably, the money saved on immediate postoperative care may be sufficient to offset the increased cost of each iMRI procedure.42 In addition, the establishment of shared-resource iMRI systems, either between diagnostic and surgical use or among various departments and ORs, has greatly reduced the economic burden placed on any one budget.3,16 Such financial incentives and adaptations may allow iMRI to be more appealing and feasible for widespread neurosurgical adoption in the future.
Albayrak B, Samdani AF, Black PM. Intra-operative magnetic resonance imaging in neurosurgery. Acta Neurochir (Wien). 2004;146:543-557.
Bergsneider M, Liau LM. Intraoperative magnetic resonance imaging. In: Badie B, editor. Neurosurgical Operative Atlas. 2nd ed. New York: Thieme; 2007:104-113.
Bergsneider M, Sehati N, Villablanca P, et al. Extent of glioma resection using low-field (0.2 T) versus high-field (1.5 T). intraoperative MRI and image-guided frameless neuronavigation. Clin Neurosurg. 2005;52:389-399.
Black PM, Alexander E3rd, Martin C, et al. Craniotomy for tumor treatment in an intraoperative magnetic resonance imaging unit. Neurosurgery. 1999;45:423-433.
Black PM, Moriarty T, Alexander E3rd, et al. Development and implementation of intraoperative magnetic resonance imaging and its neurosurgical applications. Neurosurgery. 1997;41:831-845.
Bohinski RJ, Kokkino AK, Warnick RE, et al. Glioma resection in a shared-resource magnetic resonance operating room after optimal image-guided frameless stereotactic resection. Neurosurgery. 2001;48:731-744.
Claus EB, Horlacher A, Hsu L, et al. Survival rates in patients with low-grade glioma after intraoperative magnetic resonance image guidance. Cancer. 2005;103:1227-1233.
Hall WA, Galicich W, Bergman T, et al. 3-Tesla intraoperative MR imaging for neurosurgery. J Neurooncol. 2006;77:297-303.
Hall WA, Truwit CL. Intraoperative MR-guided neurosurgery. J Magn Reson Imaging. 2008;27:368-375.
Hushek SG, Martin AJ, Steckner M, et al. MR systems for MRI-guided interventions. J Magn Reson Imaging. 2008;27:253-266.
Kucharczyk W, Bernstein M. Do the benefits of image guidance in neurosurgery justify the costs? From stereotaxy to intraoperative MR. AJNR Am J Neuroradiol.. 1997;18:1855-1859.
Nimsky C, Ganslandt O, Merhof D, et al. Intraoperative visualization of the pyramidal tract by diffusion-tensor-imaging-based fiber tracking. Neuroimage. 2006;30:1219-1229.
Nimsky C, Ganslandt O, Von Keller B, et al. Intraoperative high-field-strength MR imaging: implementation and experience in 200 patients. Radiology. 2004;233:67-78.
Rubino GJ, Farahani K, McGill D, et al. Magnetic resonance imaging-guided neurosurgery in the magnetic fringe fields: the next step in neuronavigation. Neurosurgery. 2000;46:643-654.
Sanai N, Berger MS. Glioma extent of resection and its impact on patient outcome. Neurosurgery. 2008;62:753-766.
Tronnier VM, Wirtz CR, Knauth M, et al. Intraoperative diagnostic and interventional magnetic resonance imaging in neurosurgery. Neurosurgery. 1997;40:891-902.
1 Albayrak B, Samdani AF, Black PM. Intra-operative magnetic resonance imaging in neurosurgery. Acta Neurochir (Wien). 2004;146:543-557.
2 Willems PW, van der Sprenkel JW, Tulleken CA, et al. Neuronavigation and surgery of intracerebral tumours. J Neurol. 2006;253:1123-1136.
3 Hushek SG, Martin AJ, Steckner M, et al. MR systems for MRI-guided interventions. J Magn Reson Imaging. 2008;27:253-266.
4 Nimsky C, Ganslandt O, Tomandl B, et al. Low-field magnetic resonance imaging for intraoperative use in neurosurgery: a 5-year experience. Eur Radiol. 2002;12:2690-2703.
5 Nimsky C, Ganslandt O, Cerny S, et al. Quantification of, visualization of, and compensation for brain shift using intraoperative magnetic resonance imaging. Neurosurgery. 2000;47:1070-1080.
6 Black PM, Alexander E3rd, Martin C, et al. Craniotomy for tumor treatment in an intraoperative magnetic resonance imaging unit. Neurosurgery. 1999;45:423-433.
7 Nabavi A, Black PM, Gering DT, et al. Serial intraoperative magnetic resonance imaging of brain shift. Neurosurgery. 2001;48:787-798.
8 Nimsky C, Ganslandt O, Hastreiter P, et al. Intraoperative compensation for brain shift. Surg Neurol. 2001;56:357-365.
9 Black PM, Moriarty T, Alexander E3rd, et al. Development and implementation of intraoperative magnetic resonance imaging and its neurosurgical applications. Neurosurgery. 1997;41:831-845.
10 Tronnier VM, Wirtz CR, Knauth M, et al. Intraoperative diagnostic and interventional magnetic resonance imaging in neurosurgery. Neurosurgery. 1997;40:891-902.
11 Bergsneider M, Sehati N, Villablanca P, et al. Extent of glioma resection using low-field (0.2 T) versus high-field (1.5 T) intraoperative MRI and image-guided frameless neuronavigation. Clin Neurosurg. 2005;52:389-399.
12 Bergsneider M, Liau LM. Intraoperative magnetic resonance imaging. In: Badie B, editor. Neurosurgical Operative Atlas. 2nd ed. New York: Thieme; 2007:104-113.
13 Levivier M, Wikler D, De Witte O, et al. PoleStar N-10 low-field compact intraoperative magnetic resonance imaging system with mobile radiofrequency shielding. Neurosurgery. 2003;53:1001-1007.
14 Steinmeier R, Fahlbusch R, Ganslandt O, et al. Intraoperative magnetic resonance imaging with the magnetom open scanner: concepts, neurosurgical indications, and procedures. A preliminary report. Neurosurgery. 1998;43:739-748.
15 Rubino GJ, Farahani K, McGill D, et al. Magnetic resonance imaging-guided neurosurgery in the magnetic fringe fields: the next step in neuronavigation. Neurosurgery. 2000;46:643-654.
16 Bohinski RJ, Kokkino AK, Warnick RE, et al. Glioma resection in a shared-resource magnetic resonance operating room after optimal image-guided frameless stereotactic resection. Neurosurgery. 2001;48:731-744.
17 McPherson CM, Bohinski RJ, Dagnew E, et al. Tumor resection in a shared-resource magnetic resonance operating room: experience at the University of Cincinnati. Acta Neurochir Suppl. 2003;85:39-44.
18 Nimsky C, Ganslandt O, Von Keller B, et al. Intraoperative high-field-strength MR imaging: implementation and experience in 200 patients. Radiology. 2004;233:67-78.
19 Kelly PJ, Kall BA, Goerss S, et al. Computer-assisted stereotaxic laser resection of intra-axial brain neoplasms. J Neurosurg. 1986;64:427-439.
20 Nimsky C, Fujita A, Ganslandt O, et al. Volumetric assessment of glioma removal by intraoperative high-field magnetic resonance imaging. Neurosurgery. 2004;55:358-371.
21 Nimsky C, Ganslandt O, Buchfelder M, et al. Intraoperative visualization for resection of gliomas: the role of functional neuronavigation and intraoperative 1.5 T MRI. Neurol Res. 2006;28:482-487.
22 Bradley WG. Achieving gross total resection of brain tumors: intraoperative MR imaging can make a big difference. AJNR Am J Neuroradiol. 2002;23:348-349.
23 Lewin JS, Nour SG, Meyers ML, et al. Intraoperative MRI with a rotating, tiltable surgical table: a time use study and clinical results in 122 patients. AJR Am J Roentgenol. 2007;189:1096-1103.
24 Hirschberg H, Samset E, Hol PK, et al. Impact of intraoperative MRI on the surgical results for high-grade gliomas. Minim Invasive Neurosurg. 2005;48:77-84.
25 Schneider JP, Trantakis C, Rubach M, et al. Intraoperative MRI to guide the resection of primary supratentorial glioblastoma multiforme—a quantitative radiological analysis. Neuroradiology. 2005;47:489-500.
26 Schneider JP, Schulz T, Schmidt F, et al. Gross-total surgery of supratentorial low-grade gliomas under intraoperative MR guidance. AJNR Am J Neuroradiol. 2001;22:89-98.
27 Knauth M, Wirtz CR, Tronnier VM, et al. Intraoperative MR imaging increases the extent of tumor resection in patients with high-grade gliomas. AJNR Am J Neuroradiol. 1999;20:1642-1646.
28 Nimsky C, Ganslandt O, Merhof D, et al. Intraoperative visualization of the pyramidal tract by diffusion-tensor-imaging-based fiber tracking. Neuroimage. 2006;30:1219-1229.
29 Claus EB, Horlacher A, Hsu L, et al. Survival rates in patients with low-grade glioma after intraoperative magnetic resonance image guidance. Cancer. 2005;103:1227-1233.
30 Wirtz CR, Knauth M, Staubert A, et al. Clinical evaluation and follow-up results for intraoperative magnetic resonance imaging in neurosurgery. Neurosurgery. 2000;46:1112-1122.
31 Trantakis C, Winkler D, Lindner D, et al. Clinical results in MR-guided therapy for malignant gliomas. Acta Neurochir Suppl. 2003;85:65-71.
32 Oh DS, Black PM. A low-field intraoperative MRI system for glioma surgery: is it worthwhile? Neurosurg Clin N Am. 2005;16:135-141.
33 Hall WA, Liu H, Martin AJ, et al. Brain biopsy sampling by using prospective stereotaxis and a trajectory guide. J Neurosurg. 2001;94:67-71.
34 Hall WA. The safety and efficacy of stereotactic biopsy for intracranial lesions. Cancer. 1998;82:1749-1755.
35 Hall WA, Martin A, Liu H, et al. Improving diagnostic yield in brain biopsy: coupling spectroscopic targeting with real-time needle placement. J Magn Reson Imaging. 2001;13:12-15.
36 Martin AJ, Liu H, Hall WA, et al. Preliminary assessment of turbo spectroscopic imaging for targeting in brain biopsy. AJNR Am J Neuroradiol. 2001;22:959-968.
37 Moriarty TM, Quinones-Hinojosa A, Larson PS, et al. Frameless stereotactic neurosurgery using intraoperative magnetic resonance imaging: stereotactic brain biopsy. Neurosurgery. 2000;47:1138-1146.
38 Hall WA, Martin AJ, Liu H, et al. Brain biopsy using high-field strength interventional magnetic resonance imaging. Neurosurgery. 1999;44:807-814.
39 Hall WA, Liu H, Martin AJ, et al. Comparison of stereotactic brain biopsy to interventional magnetic-resonance-imaging-guided brain biopsy. Stereotact Funct Neurosurg. 1999;73:148-153.
40 Bernays RL, Kollias SS, Khan N, et al. Histological yield, complications, and technological considerations in 114 consecutive frameless stereotactic biopsy procedures aided by open intraoperative magnetic resonance imaging. J Neurosurg. 2002;97:354-362.
41 Hall WA, Truwit CL. Intraoperative MR-guided neurosurgery. J Magn Reson Imaging. 2008;27:368-375.
42 Kucharczyk W, Bernstein M. Do the benefits of image guidance in neurosurgery justify the costs? From stereotaxy to intraoperative MR. AJNR Am J Neuroradiol. 1997;18:1855-1859.
43 Hall WA, Galicich W, Bergman T, et al. 3-Tesla intraoperative MR imaging for neurosurgery. J Neurooncol. 2006;77:297-303.
44 Lindholm J. A century of pituitary surgery: Schloffer’s legacy. Neurosurgery. 2007;61:865-868.
45 Bohinski RJ, Warnick RE, Gaskill-Shipley MF, et al. Intraoperative magnetic resonance imaging to determine the extent of resection of pituitary macroadenomas during transsphenoidal microsurgery. Neurosurgery. 2001;49:1133-1144.
46 Pergolizzi RSJr, Nabavi A, Schwartz RB, et al. Intra-operative MR guidance during trans-sphenoidal pituitary resection: preliminary results. J Magn Reson Imaging. 2001;13:136-141.
47 Schwartz TH, Stieg PE, Anand VK. Endoscopic transsphenoidal pituitary surgery with intraoperative magnetic resonance imaging. Neurosurgery. 2006;58:ONS44-ONS51.
48 Martin CH, Schwartz R, Jolesz F, et al. Transsphenoidal resection of pituitary adenomas in an intraoperative MRI unit. Pituitary. 1999;2:155-162.
49 Mortini P, Losa M, Barzaghi R, et al. Results of transsphenoidal surgery in a large series of patients with pituitary adenoma. Neurosurgery. 2005;56:1222-1233.
50 Nimsky C, von Keller B, Ganslandt O, et al. Intraoperative high-field magnetic resonance imaging in transsphenoidal surgery of hormonally inactive pituitary macroadenomas. Neurosurgery. 2006;59:105-114.
51 Pinker K, Ba-Ssalamah A, Wolfsberger S, et al. The value of high-field MRI (3T) in the assessment of sellar lesions. Eur J Radiol. 2005;54:327-334.
52 Fahlbusch R, Keller B, Ganslandt O, et al. Transsphenoidal surgery in acromegaly investigated by intraoperative high-field magnetic resonance imaging. Eur J Endocrinol. 2005;153:239-248.
53 Wiebe S, Blume WT, Girvin JP, et al. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345:311-318.
54 Kuzniecky R, Devinsky O. Surgery Insight: surgical management of epilepsy. Nat Clin Pract Neurol. 2007;3:673-681.
55 Schwartz TH, Marks D, Pak J, et al. Standardization of amygdalohippocampectomy with intraoperative magnetic resonance imaging: preliminary experience. Epilepsia. 2002;43:430-436.
56 Kaibara T, Myles ST, Lee MA, et al. Optimizing epilepsy surgery with intraoperative MR imaging. Epilepsia. 2002;43:425-429.
57 Buchfelder M, Fahlbusch R, Ganslandt O, et al. Use of intraoperative magnetic resonance imaging in tailored temporal lobe surgeries for epilepsy. Epilepsia. 2002;43:864-873.
58 Walker DG, Talos F, Bromfield EB, et al. Intraoperative magnetic resonance for the surgical treatment of lesions producing seizures. J Clin Neurosci. 2002;9:515-520.
59 Van Roost D, Solymosi L, Schramm J, et al. Depth electrode implantation in the length axis of the hippocampus for the presurgical evaluation of medial temporal lobe epilepsy: a computed tomography-based stereotactic insertion technique and its accuracy. Neurosurgery. 1998;43:819-827.
60 Schwartz RB, Hsu L, Wong TZ, et al. Intraoperative MR imaging guidance for intracranial neurosurgery: experience with the first 200 cases. Radiology. 1999;211:477-488.
61 Sanai N, Berger MS. Glioma extent of resection and its impact on patient outcome. Neurosurgery. 2008;62:753-766.
62 Pandin P, Dewitte O. Open low-field intraoperative MRI for transsphenoidal pituitary surgery. Anesth Analg. 2007;105:886.