Chapter 203 Intraoperative Crisis Management in Spine Surgery
What to Do When Things Go Bad
Anesthetic Crises
Prone Cardiopulmonary Arrest
Most spine operations are carried out in the prone position. However, this position may present some difficulty for the anesthesiologist by interfering with monitoring techniques, altering respiratory function, and limiting airway and vascular access.1 Cardiac arrest in patients who undergo surgery in the prone position is very uncommon. The major preoperative risk factor is understandably cardiac disease, while intraoperative factors include hemorrhage, gas embolism, and diminished venous return.2 The rare occurrence of cardiac arrest during spine surgery and modern anesthetic techniques nearly obviate the need for cardiopulmonary resuscitation (CPR) in the operating room. Nevertheless, it may be necessary in some circumstances. The prone position substantially complicates cardiopulmonary arrest management for several reasons. First, the time necessary to procure a stretcher and turn the patient to the supine position to perform CPR may delay therapy. The wound must be closed quickly, risking infection and costing valuable time. Interruption of the surgical procedure may also leave the spine unstable, risking further injury. Additionally, spinal implants may not be fully attached or may protrude from the wound.
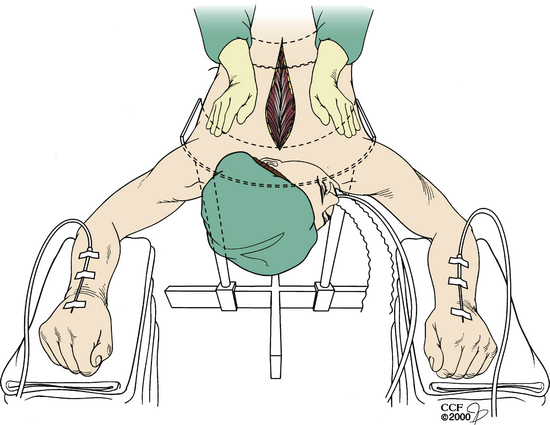
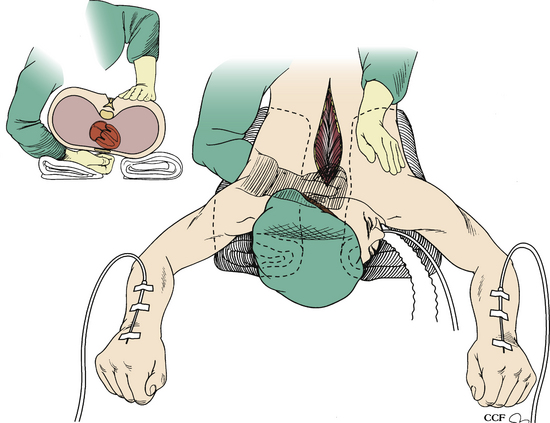
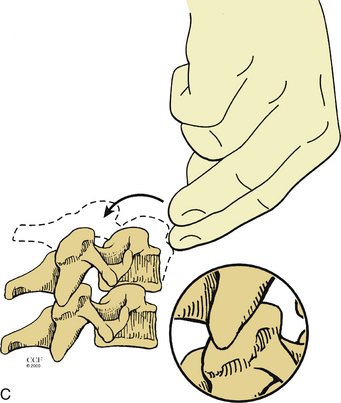
Turning the patient might not be feasible in some situations, owing to the risk of neurologic injury. In these situations, the resuscitation begins with the anesthesiologist, while the wound is being closed. However, the surgeon must initiate CPR. Two options are available in these situations. Closed CPR in the prone position has been described and is typically used first.1–3 If sternal support is present, the surgeon may place his or her hands on either side of the incision at the midthoracic level with the palms placed over the patient’s scapulae1 (Fig. 203-1). Compression is then initiated. The arterial wave form, blood pressure, and end-tidal CO2 should be observed to monitor cardiac output and adequacy of resuscitation. If there is no sternal support (e.g., the patient is placed on chest rolls), the surgeon may clench a fist and place it under the patient’s chest over the lower third of the sternum3 (Fig. 203-2). This requires a break in sterility. The surgeon’s other hand (or an associate’s) then compresses at the midthoracic level.
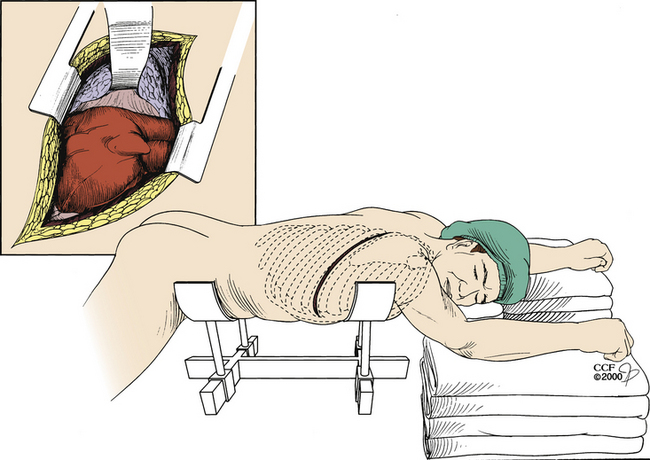
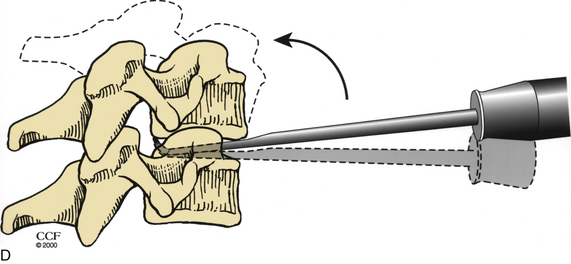
If the aforementioned techniques are not feasible or are unsuccessful owing to lack of adequate cardiac output or in the presence of spine instability, the surgeon has the option to perform a left dorsal thoracotomy.3 This procedure provides direct access to the heart. Following exposure, open internal cardiac massage or defibrillation may be performed (Fig. 203-3).
Air Embolism
Intraoperative venous air embolism (VAE) occurs most commonly when patients are in the sitting position. For this reason, surgeons often avoid the sitting position. Negative pressures are greater in the sitting position, owing to the elevation of the head. However, it should be noted that VAE can occur in any position in which the head is elevated at a higher level than the heart. Depending on the type of monitoring technique that is used, the overall incidence of air embolism has been reported to be anywhere between 25% and 50% in surgeries involving the posterior fossa.4 The incidence during cervical laminectomy has been estimated at 25%.5 In one study in which transesophageal echocardiography was used, 100% of patients in the sitting position demonstrated VAE.6 While the occurrence of VAE may be high, its sequelae remain low as long as appropriate measures to treat the embolism are carried out rapidly. During surgery in the sitting position, appropriate monitoring should be used. This includes the use of the electrocardiograph, arterial catheter, right atrial or pulmonary artery catheter, end-tidal CO2 monitor, and a precordial Doppler (the most sensitive and practical monitor for VAE).
These devices allow for the early detection and treatment of VAE prior to paradoxical air embolism or cardiopulmonary incident. Most frequently, the initial indication of the presence of VAE is the precordial Doppler. A significant VAE elicits audible Doppler indicators and possibly a decrease in end-tidal CO2, ventricular arrhythmias, and hypotension and/or an increase in pulmonary artery pressure. At the first indication of VAE, the surgeon should flood the operative field with saline, wax all bleeding bone edges, and occlude any visual sources of venous bleeding. Bilateral jugular venous compression by the anesthesiologist increases the central venous pressure and may help in identifying the venous source. If only one jugular vein can be compressed, the right one should be chosen. The anesthesiologist should discontinue nitrous oxide infusion, as this has been shown to enlarge the gas volume of air-containing cavities.7,8 Positive end-expiratory pressure should be discontinued (it may alter right-to-left atrial pressure gradients and facilitate the passage of air across an existing patent foramen ovale), and air should be aspirated by using a right atrial catheter if one is available.9,10 These steps should lead to the resolution of VAE-related symptoms. The patient may also be placed in a head-down position with the patient’s right side up in order to trap air in the right atrium to aid in aspiration of the air bolus and minimize pulmonary and cerebral ischemic complications, but this is rarely required.11
Surgical Crises
Vascular Injuries
Cervical Approaches
Vertebral artery injuries in the cervical spine are uncommon but potentially catastrophic. The ventral approach places both the carotid and vertebral arteries at risk for injury, but the carotid artery is fully visualized during ventral exposure and therefore is rarely injured.12 If injury does occur, it can be readily identified and controlled with direct pressure, with or without primary vascular repair. Shunting or temporary clamping of the carotid artery may be performed while a primary or patch repair of the artery is performed.
The ventral and dorsal cervical approaches both place the vertebral artery at risk for injury. In the anterior approach, the rate of injury has been estimated to be between 0.3% and 0.5%.13–17 Injury during the ventral approach can occur because of excessive lateral bone–disc removal, drill use too far off midline into the uncinate processes, subperiosteal elevation of the longus colli muscles, vertebral artery anatomic anomalies, or the softening of bone of the lateral part of the spinal cord owing to pathologic processes.17 Injury to the vertebral arteries during posterior cervical screw placement in the subaxial spine is considered to be rare and generally due to improper drilling and poor trajectories during placement.18
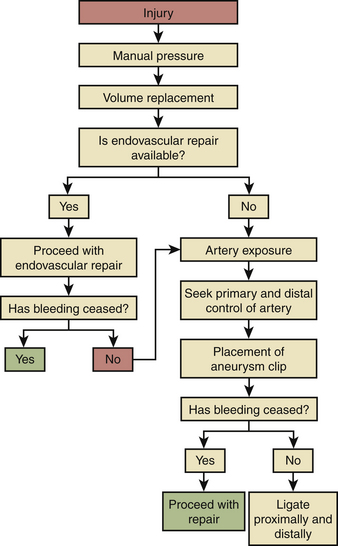
The vertebral artery is hidden within the foramen transversarium, and initial injury to the artery is usually announced by the presence of brisk, bright red arterial bleeding. Occasionally, darker venous bleeding may be seen if damage has been done to the neighboring venous plexus. The management of vertebral artery damage in the subaxial spine is diagrammed in Fig. 203-4. First, manual pressure and the use of a hemostatic agent should be employed at the site of suspected injury. When bleeding is under control, time is available for the anesthesiologist to “catch up” with regard to volume replacement and, if necessary, blood transfusion. Proximal and distal control of the artery should be attempted. In either a ventral or a dorsal approach, the transverse process should be dissected to expose the artery one level above and below the injury. A high-speed drill with a diamond tip bur may be used to open the foramen transversarium and expose the artery. An aneurysm clip may be placed to achieve temporary control of bleeding during injury repair.14 Primary repair of the artery should be considered. If reconstruction of the artery cannot be achieved, it should be ligated proximally and distally.
Intraoperative angiography may be considered.17 Once primary control of the bleeding has been attained, the patient can be taken to the endovascular suite and the vertebral artery can be stented or occluded. Perioperative management remains controversial, with some surgeons choosing immediate postoperative angiography while others reserve further intervention to be based on the patient’s observed clinical course.19
Injury during C1-2 transarticular screw placement has been reported to be as high as 2.2% per screw.19 As in the subaxial spine, injury to the vertebral artery is often heralded by brisk arterial bleeding seen during drilling or K-wire placement. Control of the hemorrhage can be obtained by placing the transarticular screw into the drill hole or by packing the hole with bone wax. Placing the screw into the drill hole is preferred, as it both plugs the hole and provides stability. The opposite screw should not be drilled or placed. Doing so invites a high risk of disastrous sequelae associated with bilateral vertebral artery injury.
Dorsal Thoracic and Lumbar Approaches
Vascular injury during dorsal thoracic surgery is rare. Therefore, the following discussion is limited to dorsal lumbar approaches. Lumbar disc surgery is one of the most commonly performed spine operations. The incidence of vascular complication has been recently estimated to be 0.14% at the most.20–23 The most commonly injured vessels are the common iliac arteries.24 Other potentially injured vessels are the left common iliac vein, median sacral artery, aorta, and inferior vena cava.
Vascular injury is typically caused by aggressive use of a pituitary rongeur that penetrates the ventral anulus fibrosus and causes breach of the vessel wall.25 Neither surgeon experience26 nor amount of disc material removed25 has been demonstrated to affect the incidence of this injury. Brisk bleeding into the interspace is observed in only 25% of these injuries, and so may not be immediately evident to the surgeon.25 When brisk bleeding is observed, it may actually be due to damage to extradural veins and not an arterial injury.26
The injury is usually recognized in the recovery room, although with venous injuries, the symptoms and signs of injury can be delayed. The patient is most often hypotensive and tachycardic and has abdominal distention and pain. Pallor and weak pulses are also usually present. The patient should immediately be taken back to the operating room for laparotomy. Cross-clamping of the aorta can facilitate resuscitation.25 All vessels should be carefully inspected. A tear in a vein may be primarily repaired with suture. The iliac artery may be divided to gain access to an injured vein and then must be reanastomosed. Arterial injuries may be repaired primarily or with an interposition graft. Damage to an internal iliac vessel (artery or vein) may be repaired by ligation if primary repair is not possible.
Ventral Thoracic and Lumbar Approaches
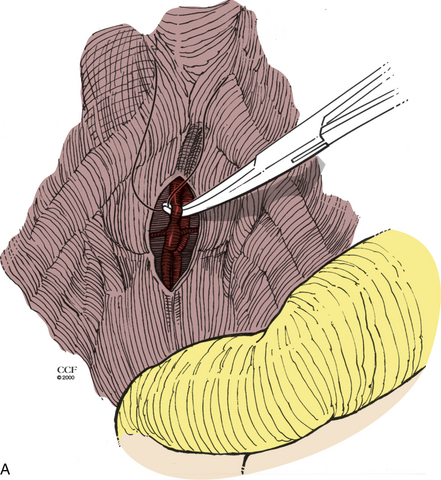
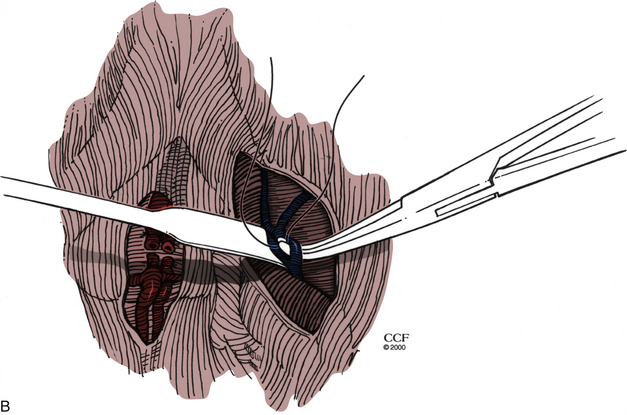
The incidence of vascular injury during the ventral approach to the lumbar spine has recently been estimated to be between 11% and 12%.27,28 However, the majority of injuries are venous and require only simple suture or clip repair. Major crises occur at a rate of only 2%.27 Injury usually occurs during exposure and is more likely to occur with exposure of L4-5.27 Chances of injury increase with use of preoperative radiation therapy or if osteomyelitis is present. Vessels that potentially can be injured include the left iliac vein, the iliolumbar vein, the middle sacral vessels, and the left iliac artery. During ventral exposure and dissection, the middle sacral vessels (Fig. 203-5A) and iliolumbar vein (Fig. 203-5B) should be identified and ligated. If sudden hemorrhage occurs, direct pressure is applied to the vena cava, and identification of the injured vessel is attempted. A torn lumbar or iliolumbar vein may be clamped and ligated. If an iliac vein injury is suspected, direct pressure should be applied, and then proximal and distal control of the vein should be achieved. The perforation can then be repaired primarily.29 If an artery is injured, proximal and distal control must be attained, and the injury is repaired either primarily or with an interposition graft.
Injury to the aorta is rare in the upper lumbar and thoracic region. This injury should be treated immediately with thoracotomy and/or laparotomy to repair the vessel. If injury is suspected in the recovery room and the patient is stable, angiography may be performed to identify the injury prior to attempted repair.11
Iliac Crest Graft Harvest
Injury to the superior gluteal artery is a reported but uncommon occurrence during iliac graft harvest.30,31 Injury usually occurs during exposure of the iliac crest with inadvertent placement of a sharp self-retaining retractor into the sciatic notch. If the vessel is encountered and identified, it should be ligated. This is usually not possible, owing to vessel retraction; in this case, the operative site should be packed, and the patient should be taken to the angiography suite for embolization. The primary operation may be completed the following day. While direct surgical approach and ligation are possible, they require a retroperitoneal approach because the vessel usually retracts to an intrapelvic location.30
Visceral Injuries
The majority of visceral injuries (such as ureteral and intestinal) are not considered crises to the spine surgeon; they are discovered and dealt with postoperatively. The exception is esophageal injury. Estimates of esophageal injury have been placed as high as 3.4% in some series, but it is thought that the true incidence is underreported.32,33 These injuries can be divided into those that are noticed intraoperatively, those that are discovered in the immediate postoperative period, and delayed cases that are brought to light only days, weeks, or even months after discharge from the hospital. The last type is not discussed here. Should an injury be identified intraoperatively, the tear should be primarily repaired with interrupted resorbable sutures in two layers. It may be advantageous to reinforce the repair with muscle (strap) as well. Timing of antibiotics and nasogastric tube drainage will be variable depending on surgeon and institutional preferences.32 An esophagram should be obtained at or around day 7; if it is normal, oral feeding may be started.
Identification of esophageal perforation in the postoperative period has been shown to reduce mortality from nearly 50% to 20% if the perforation is noticed within 24 hours of surgery.33 Therefore, vigilance should be maintained. Warning signs may include dyspnea, dysphagia, dysphonia, swelling, fever, leukocytosis, or consistent unexplained tachycardia.32,33 Plain radiographs may show subcutaneous emphysema, widening of the retropharyngeal space, or displacement or migration of hardware. Imaging and endoscopic tests can have a false-negative rate of 10% to 46%.32 If there is high clinical suspicion of an esophageal perforation, the patient should be taken back to the operating room immediately for exploration. Repair of the perforation may be performed as previously described for intraoperative perforations. Because of contamination, the wound may be left open, and consideration should be given to placing a tissue layer between the fresh suture line and the bone graft. The sternal head of the sternocleidomastoid muscle may be reflected and sutured to the contralateral paravertebral muscles to accomplish this.34 If a ventral spinal implant was placed, the surgeon should strongly consider its removal. Prolonged diversion of the salivary flow and oral intake through the use of gastric feeding or parenteral nutrition should also be considered, should there be any concern about the esophageal repair.
Durotomy
Unintended durotomy is one of the most common complications of spine surgery. While unintended durotomy occurs in anywhere between 1% and 17% of surgeries, the vast majority can be repaired with simple primary repair.35–39 Controversy surrounds the management of dural tears. Differing opinions can be found regarding whether or not a subfascial drain should be placed and the number of days of postoperative bedrest necessary to facilitate healing.39 It is important to note that unintended durotomy, if recognized and repaired, has repeatedly been shown not to influence the final clinical outcome of procedures on the lumbar spine.36,38
As has already been stated, unintended durotomy should be repaired primarily. The type of suture and suture technique are the surgeon’s choice. We favor #4-0 Neurolon (Ethicon, Summerville, NJ) suture, using a running locking technique. Adequate exposure of the tear must be achieved prior to suturing. Proper illumination and often magnification (loupe/microscope) can assist in repair. After exposure, a surgical patty is used over the tear, and a smaller sucker tip is used. If the surgeon believes that the primary closure may lead to excessive tension on the nerve roots, a patch may be used. Many patch varieties are available, including synthetic, cadaveric, and autologous (we prefer autologous tissue). In cases of small defects, the lumbar fascia may be secured to the dura mater with interrupted sutures. A large defect may necessitate the use of fascia lata. Very large dural defects may require patch placement and the use of fibrin glue.
After repair, a Valsalva maneuver should be performed to check for leaks. Any leak that is present should be oversewn. A dry piece of hemostatic gelatin (Gelfoam) or DuraGen (Integra, Plainsboro, NJ) may be placed over the suture line. The more superficial tissues should then be tightly and meticulously closed in layers. A few muscle sutures using nonabsorbable O-gauge braided suture may be placed. The fascia is then closed with the same suture in a watertight manner by using an interrupted stitch that is reinforced with a running locking stitch. Next, #2-0 absorbable suture may be used to close as many layers as possible, including Scarpa’s fascia and dermis. The skin should be carefully closed, usually with sutures. Interrupted vertical mattress closure should be considered because of its excellent skin approximation characteristics. The use of a subfascial drain is controversial because of concerns about cerebrospinal fluid fistula formation.35 Significant recent evidence now exists showing that drain placement can be used with very low rates of complication.36 Khan et al. demonstrated that removal of a subfascial drain the morning following surgery resulted in no cases of fistula in any of their 388 patients who experienced unintended durotomy.38 This is the second large study to report no increased incidence of myelocutaneous fistula with this type of drain.36 The use of a subarachnoid drain during surgery may be considered as well, but no conclusive evidence exists regarding its efficacy.
Facet Reduction
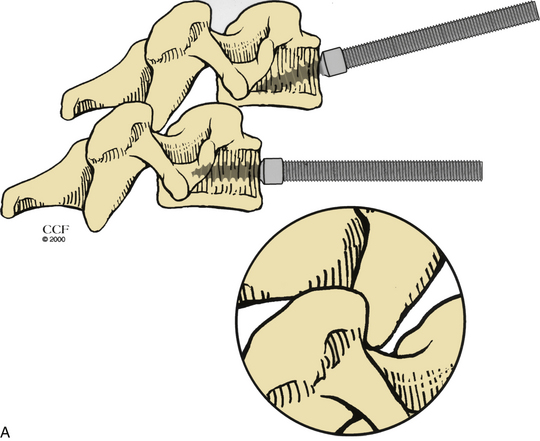
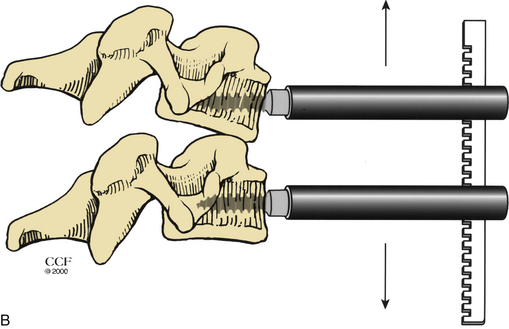
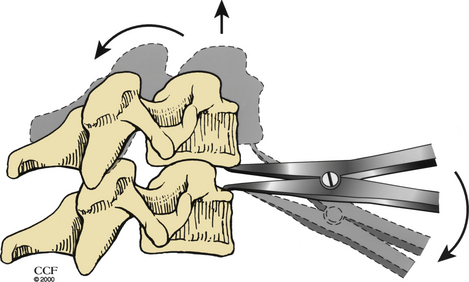
For a ventral approach to reduction and stabilization, the patient is positioned in the supine position, and a standard approach for anterior cervical discectomy is performed. After discectomy is complete, vertebral body posts are placed at 10- to 20-degree diverging angles to each other (Fig. 203-6A). Distraction is applied, and the locked facets are disengaged (Fig. 203-6B). A dorsally directed force is applied to the rostral body by manual pressure (Fig. 203-6C) or curet (Fig. 203-6D), facilitating reduction. Interbody disc spreaders that are placed at an angle and then rotated rostrally may also be used for the reduction (Fig. 203-7). A plain radiograph may then be taken to visualize reduction. If reduction is not accomplished, the procedure should be repeated. In cases such as those involving commuted facet fractures, reduction might not be possible using the aforementioned technique. Instead, the patient should undergo dorsal reduction and arthrodesis followed by ventral arthrodesis. After reduction has been obtained, standard iliac crest harvesting and strut placement are performed. Finally, a ventral cervical fixation device is placed.
Level Identification
Level localization in spine surgery can be challenging. However, it is of obvious importance. Wrong-level spine surgery is an adverse but preventable event. A recent anonymous survey of 415 spine surgeons reported that half had performed at least one wrong-level surgery during their careers.40 It is likely that the incidence of wrong-level surgery is underreported; studies have estimated a rate of 0.4% to 4.3%.41–43 Despite poor reporting, wrong-level surgery is widely recognized as a preventable cause of patient morbidity.
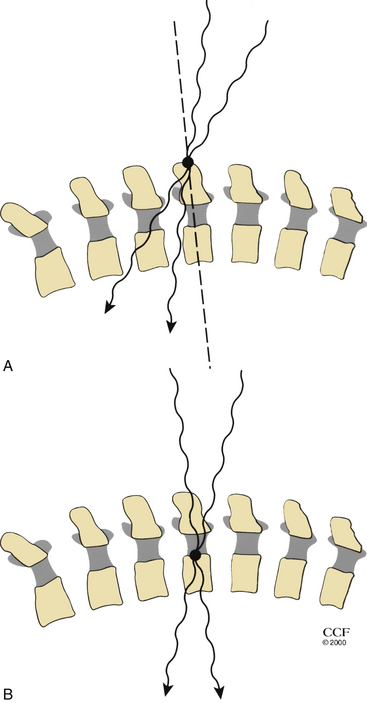
Radiographs can help to identify pathologic levels, but they are helpful only if the radiographs can be correlated with preoperatively acquired images that identify the pathology. Fluoroscopy may assist in expediting localization, as the time required to develop the radiograph plates will be eliminated. In relying on an MRI to demonstrate thoracic pathology, it is essential that the radiographic localization correspond to the MRI localization, as the number of cervical, thoracic, and lumbar levels can be variable. For example, suppose the MRI demonstrates that there is a thoracic vertebra pathology that is 14 vertebral bodies rostral to the sacrum. If the patient has the normal 5 lumbar vertebrae and 12 thoracic vertebrae, then the thoracic vertebral pathology would be at T3. However, if the patient has 4 or 6 lumbar vertebrae and 12 thoracic vertebrae, then the pathology would be at T2 or T4, respectively. If the surgeon then uses a radiologic localization using the C7 spinous process to count down to the pathology relying solely on the MRI that did not include the corresponding cervical levels, then the identification of the pathologic level could be erroneous if the patient had anomalous segmentation. If the surgeon used a radiologic localization corresponding to the MRI localization and counted from the sacrum up, then this error in identifying that pathologic level could be avoided. In some cases, myelography may be helpful in the thoracic spine by providing a radiographic correlate that “relates anatomically” to the pathology. Ribs may then be used as markers with confidence, as this eliminates errors related to discrepancies between radiographs and MRI. Wide anteroposterior myelographic views should be obtained so that ribs can be visualized (particularly the lowest rib) and counted.
Beam trajectory should be considered during interpretation of intraoperative radiographs. Beam trajectory error can be minimized by placing the marker as close to the pathology in the sagittal plane as possible (Fig. 203-8).
Electrophysiologic Spinal Cord Monitoring
Intraoperative spinal cord monitoring has become popular, especially for deformity correction. The goal of monitoring in these cases is to ensure that neural element injury has not occurred secondary to deformity correction maneuvers. Multiple techniques are available, and each is measured against the gold standard of the wake-up test (performed by lessening the level of anesthesia until the patient is able to follow commands, allowing for a gross assessment of motor function). These monitoring techniques include somatosensory-evoked potential (SSEP), motor-evoked potential, spinal-evoked potential, and electromyographic monitoring. SSEP and spinal-evoked potential are intended to monitor dorsal column integrity, while motor-evoked potential is intended for ventral column integrity. Electromyography monitors nerve root integrity and may be used continuously45 or be stimulus evoked.39 It is also possible to utilize multimodality monitoring, which includes the use of ventral and dorsal spinal cord interrogation as well as the nerve roots.
These techniques have not been demonstrated to be without error. False-positive and false-negative test findings for each technique have been documented.40 False-negative results can be due to the failure to detect minor injury, the temporal difference between the actual injury and the observed change on the monitor, and the discrepancy between the area of the spinal cord injured and the region assessed by the monitoring technique.39 Of even greater concern is the lack of a recognized standard for the assessment of injury during electrophysiologic monitoring.39
Neurologic Injuries
New and unexpected neurologic deficits that appear following surgery are indeed a crisis. They are most commonly observed in the recovery room immediately following emergence from anesthesia. Early assessment of these injuries is imperative.
Complete pharmacologic reversal of anesthesia is imperative in situations in which a new neurologic deficit is suspected. Naloxone should be administered in sufficient doses to ensure reversal of opioids given during surgery. As an aside, narcotic antagonists have been shown to reverse ischemic neurologic deficits.46,47 Therefore, the administration of naloxone should perhaps be considered more liberally under such circumstances.
Bingol H., Cingoz F., Yilmaz A.T., et al. Vascular complications related to lumbar disc surgery. J Neurosurg. 2004;100:249-253.
Golfinos J.G., Dickman C.A., Zabramski J.M., et al. Repair of vertebral artery injury during anterior cervical decompression. Spine (Phila Pa 1976). 1994;19:2552-2556.
Mody M.G., Nourbakhsh A., Stahl D.L., et al. The prevalence of wrong level surgery among spine surgeons. Spine (Phila Pa 1976). 2008;33:194-198.
Orlando E.R., Caroli E., Ferrante L. Management of the cervical esophagus and hypofarinx perforations complicating anterior cervical spine surgery. Spine (Phila Pa 1976). 2003;28:E290-E295.
Porter J.M., Pidgeon C., Cunningham A.J. The sitting position in neurosurgery: a critical appraisal. Br J Anaesth. 1999;82:117-128.
Smith M.D., Emery S.E., Dudley A., et al. Vertebral artery injury during anterior decompression of the cervical spine: a retrospective review of ten patients. J Bone Joint Surg [Br]. 1993;75:410-415.
1. Tobias J.D., Mencio G.A., Atwood R., Gurwitz G.S. Intraoperative cardiopulmonary resuscitation in the prone position. J Pediatr Surg. 1994;29:1537-1538.
2. Brown J., Rogers J., Soar J. Cardiac arrest during surgery and ventilation in the prone position: a case report and systematic review. Resuscitation. 2001;50:233-238.
3. Sun W.Z., Huang F.Y., Kung K.L., et al. Successful cardiopulmonary resuscitation of two patients in the prone position using reversed precordial compression. Anesthesiology. 1992;77:202-204.
4. Porter J.M., Pidgeon C., Cunningham A.J. The sitting position in neurosurgery: a critical appraisal. Br J Anaesth. 1999;82:117-128.
5. Papadopoulos G., Kuhly P., Brock M., et al. Venous and paradoxical air embolism in the sitting position: a prospective study with transoesophageal echocardiography. Acta Neurochir (Wien). 1994;126:140-143.
6. Mammoto T., Hayashi Y., Ohnishi Y., Kuro M. Incidence of venous and paradoxical air embolism in neurosurgical patients in the sitting position: detection by transesophageal echocardiography. Acta Anaesthesiol Scand. 1998;42:643-647.
7. Munson E.S., Merrick H.C. Effect of nitrous oxide on venous air embolism. Anesthesiology. 1966;27:783-787.
8. Munson E.S. Transfer of nitrous oxide into body air cavities. Br J Anaesth. 1974;46:202-209.
9. Perkins N.A., Bedford R.F. Hemodynamic consequences of PEEP in seated neurological patients: implications for paradoxical air embolism. Anesth Analg. 1984;63:429-432.
10. Grady M.S., Bedford R.F., Park T.S. Changes in superior sagittal sinus pressure in children with head elevation, jugular venous compression, and PEEP. J Neurosurg. 1986;65:199-202.
11. Frost E.A. Some inquiries in neuroanesthesia and neurological supportive care. J Neurosurg. 1984;60:673-686.
12. Hoh D.J., Maya M., Jung A., et al. Anatomical relationship of the internal carotid artery to C-1: clinical implications for screw fixation of the atlas. J Neurosurg Spine. 2008;8:335-340.
13. Daentzer D., Deinsberger W., Boker D.K. Vertebral artery complications in anterior approaches to the cervical spine: report of two cases and review of literature. Surg Neurol. 2003;59:300-309. discussion 309
14. Golfinos J.G., Dickman C.A., Zabramski J.M., et al. Repair of vertebral artery injury during anterior cervical decompression. Spine (Phila Pa 1976). 1994;19:2552-2556.
15. Smith M.D., Emery S.E., Dudley A., et al. Vertebral artery injury during anterior decompression of the cervical spine: a retrospective review of ten patients. J Bone Joint Surg [Br]. 1993;75:410-415.
16. Burke J.P., Gerszten P.C., Welch W.C. Iatrogenic vertebral artery injury during anterior cervical spine surgery. Spine J. 2005;5:508-514. discussion 514
17. Peng C.W., Chou B.T., Bendo J.A., Spivak J.M. Vertebral artery injury in cervical spine surgery: anatomical considerations, management, and preventive measures. Spine J. 2009;9:70-76.
18. Sekhon L.H. Posterior cervical lateral mass screw fixation: analysis of 1026 consecutive screws in 143 patients. J Spinal Disord Tech. 2005;18:297-303.
19. Wright N.M., Lauryssen C. Vertebral artery injury in C1-2 transarticular screw fixation: results of a survey of the AANS/CNS section on disorders of the spine and peripheral nerves: American Association of Neurological Surgeons/Congress of Neurological Surgeons. J Neurosurg. 1998;88:634-640.
20. Bingol H., Cingoz F., Yilmaz A.T., et al. Vascular complications related to lumbar disc surgery. J Neurosurg. 2004;100:249-253.
21. Goodkin R., Laska L.L. Vascular and visceral injuries associated with lumbar disc surgery: medicolegal implications. Surg Neurol. 1998;49:358-370. discussion 370–372
22. Inamasu J., Guiot B.H. Vascular injury and complication in neurosurgical spine surgery. Acta Neurochir (Wien). 2006;148:375-387.
23. Papadoulas S., Konstantinou D., Kourea H.P., et al. Vascular injury complicating lumbar disc surgery: a systematic review. Eur J Vasc Endovasc Surg. 2002;24:189-195.
24. Erkut B., Unlu Y., Kaygin M.A., et al. Iatrogenic vascular injury during to lumbar disc surgery. Acta Neurochir (Wien). 2007;149:511-515. discussion 516
25. Raptis S., Quigley F., Barker S. Vascular complications of elective lower lumbar disc surgery. Aust N Z J Surg. 1994;64:216-219.
26. Szolar D.H., Preidler K.W., Steiner H., et al. Vascular complications in lumbar disk surgery: report of four cases. Neuroradiology. 1996;38:521-525.
27. Hamdan A.D., Malek J.Y., Schermerhorn M.L., et al. Vascular injury during anterior exposure of the spine. J Vasc Surg. 2008;48:650-654.
28. Kang B.U., Choi W.C., Lee S.H., et al. An analysis of general surgery-related complications in a series of 412 minilaparotomic anterior lumbosacral procedures. J Neurosurg Spine. 2009;10:60-65.
29. Watkins R. Anterior lumbar interbody fusion surgical complications. Clin Orthop Relat Res. 1992;284:47-53.
30. Lim E.V., Lavadia W.T., Roberts J.M. Superior gluteal artery injury during iliac bone grafting for spinal fusion: a case report and literature review. Spine (Phila Pa 1976). 1996;21:2376-2378.
31. Kahn B. Superior gluteal artery laceration, a complication of iliac bone graft surgery. Clin Orthop Relat Res. 1979;140:204-207.
32. Ardon H., Van Calenbergh F., Van Raemdonck D., et al. Oesophageal perforation after anterior cervical surgery: management in four patients. Acta Neurochir (Wien). 2009;151:297-302. discussion 302
33. Orlando E.R., Caroli E., Ferrante L. Management of the cervical esophagus and hypofarinx perforations complicating anterior cervical spine surgery. Spine (Phila Pa 1976). 2003;28:E290-E295.
34. van Berge Henegouwen D.P., Roukema J.A., de Nie J.C., vd Werken C. Esophageal perforation during surgery on the cervical spine. Neurosurgery. 1991;29:766-768.
35. Eismont F.J., Wiesel S.W., Rothman R.H. Treatment of dural tears associated with spinal surgery. J Bone Joint Surg [Am]. 1981;63:1132-1136.
36. Wang J.C., Bohlman H.H., Riew K.D. Dural tears secondary to operations on the lumbar spine: management and results after a two-year-minimum follow-up of eighty-eight patients. J Bone Joint Surg [Am]. 1998;80:1728-1732.
37. Goodkin R., Laska L.L. Unintended “incidental” durotomy during surgery of the lumbar spine: medicolegal implications. Surg Neurol. 1995;43:4-12. discussion 12–14
38. Khan M.H., Rihn J., Steele G., et al. Postoperative management protocol for incidental dural tears during degenerative lumbar spine surgery: a review of 3,183 consecutive degenerative lumbar cases. Spine (Phila Pa 1976). 2006;31:2609-2613.
39. Ben-David B. Spinal cord monitoring. Orthop Clin North Am. 1988;19:427-448.
40. Gonzalez A.A., Jeyanandarajan D., Hansen C., et al. Intraoperative neurophysiological monitoring during spine surgery: a review. Neurosurg Focus. 2009;27:E6.
41. Mody M.G., Nourbakhsh A., Stahl D.L., et al. The prevalence of wrong level surgery among spine surgeons. Spine (Phila Pa 1976). 2008;33:194-198.
42. Ammerman J.M., Ammerman M.D., Dambrosia J., et al. A prospective evaluation of the role for intraoperative x-ray in lumbar discectomy: predictors of incorrect level exposure. Surg Neurol. 2006;66:470-473. discussion 473–474
43. Jhawar B.S., Mitsis D., Duggal N. Wrong-sided and wrong-level neurosurgery: a national survey. J Neurosurg Spine. 2007;7:467-472.
44. Goodkin R., Laska L.L. Wrong disc space level surgery: medicolegal implications. Surg Neurol. 2004;61:323-341. discussion 341–342
45. Holland N.R., Kostuik J.P. Continuous electromyographic monitoring to detect nerve root injury during thoracolumbar scoliosis surgery. Spine (Phila Pa 1976). 1997;22:2547-2550.
46. Baskin D.S., Hosobuchi Y. Naloxone reversal of ischaemic neurological deficits in man. Lancet. 1981;2:272-275.
47. Benzel E.C., Khare V., Fowler M.R. Effects of naloxone and nalmefene in rat spinal cord injury induced by the ventral compression technique. J Spinal Disord. 1992;5:75-77.