Chapter 101 Intramedullary Spinal Cord Lesions
Surgery, once used for the diagnosis of intramedullary spinal cord tumors alone, now represents the most effective treatment of benign well-circumscribed tumors (which constitute the majority of intramedullary neoplasms).1–9 Long-term tumor control or cure, with preservation of neurologic function, can be achieved in most patients with microsurgical removal alone.2,5,10 The benign nature of most intramedullary neoplasms, advances in microsurgical techniques, early clinical diagnosis with MRI, and the ineffectual or inconsistent treatment response of most intramedullary tumors to radiation therapy largely account for the expanded role of surgery in the management of these lesions.4,11,12 Therefore, optimization of surgical treatment is the key to successful management of patients with intramedullary masses. This includes early diagnosis and aggressive primary treatment, the avoidance of technical and judgmental errors and their associated complications, and a strict adherence to contemporary microsurgical technique.
Patient Evaluation
The predominant benefit of surgery for an intramedullary tumor is prophylactic. Preservation, rather than restoration, of neurologic function is the most likely outcome after successful surgical treatment. In fact, significant improvement of a severe or long-standing preoperative neurologic deficit rarely occurs after a technically successful surgical excision. Surgical morbidity is also greater in patients with more significant preoperative deficits. This creates a therapeutic irony in which the risk of surgery is actually less in patients with minimal or no objective neurologic deficit. Thus, early clinical diagnosis and, if possible, definitive initial treatment are critical to successful clinical management of most intramedullary tumors. A therapeutic dilemma arises, however, in the asymptomatic patient in whom an incidental intramedullary spinal cord lesion has been discovered. A posterior column deficit is a common consequence of a dorsal median myelotomy; thus, some degree of morbidity often accompanies even the most successful surgical removal.13 In completely asymptomatic patients, therefore, observation with serial clinical and radiologic follow-up may be an appropriate management strategy. This is also true for patients with conditions such as neurofibromatosis or von Hippel-Lindau disease.
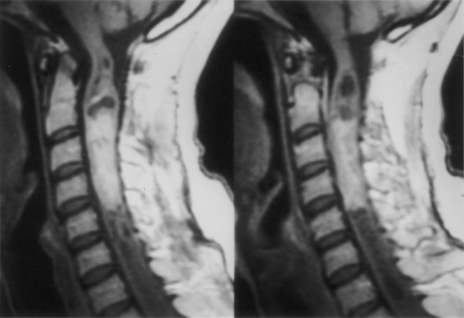
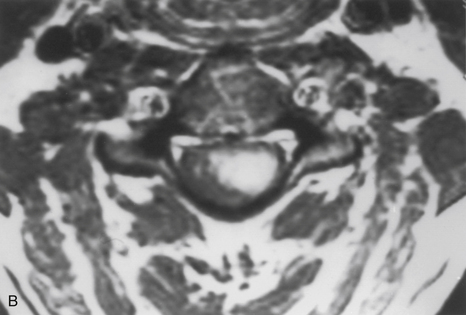
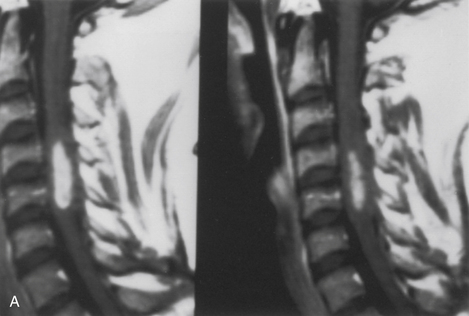
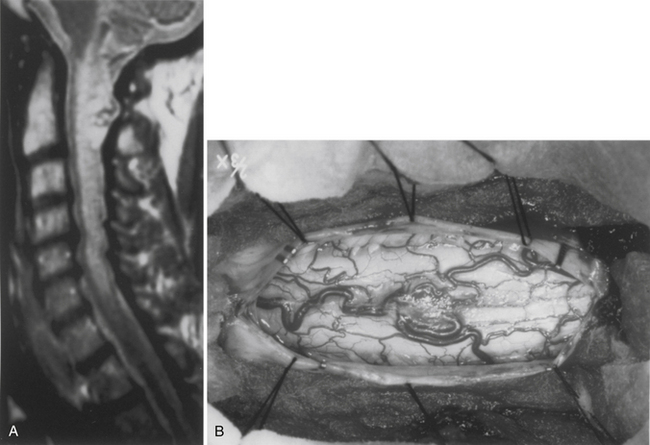
Because of the slow growth rate of benign tumors and the availability of MRI, most patients with intramedullary tumors are diagnosed before the onset of significant neurologic deficit. Gadolinium-enhanced MRI is the procedure of choice for imaging and preoperative evaluation of an intramedullary tumor. Spinal cord enlargement and tumor enhancement are the characteristic findings (Fig. 101-1). Polar cysts are often present. Ependymomas are usually symmetrically located and exhibit uniform tumor enhancement, whereas astrocytomas are associated with a more variable appearance with respect to tumor margins and enhancement patterns (Fig. 101-2). Prediction of these tumor types on MRI appearance is often inaccurate, predominantly because of the variability of presentation on MRI scans, and is therefore avoided because it may unfairly influence the surgical objective. Hemangioblastomas usually appear as intensely enhancing eccentric masses or nodules. There is often diffuse spinal cord enlargement that may extend a considerable distance from the tumor (Fig. 101-3). The cause of this tumor enlargement is most likely vasogenic edema.14
Patient Selection
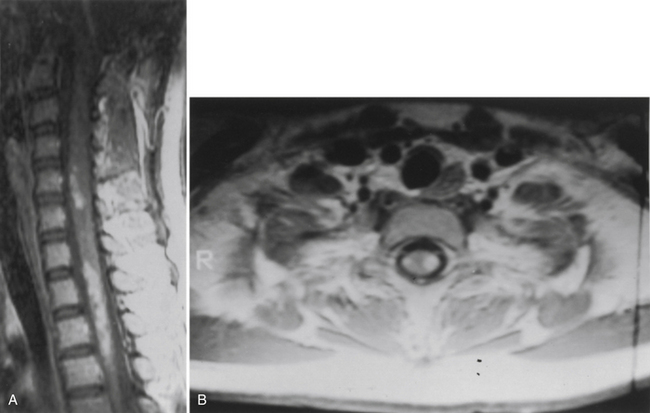
Whereas early diagnosis is routinely achieved with gadolinium-enhanced MRI, the sensitivity of MRI has far exceeded its specificity. Acute inflammatory conditions or demyelinating conditions such as multiple sclerosis or transverse myelitis are exquisitely imaged with MRI. These are not surgical lesions, and biopsy for diagnosis usually reveals only a nonspecific inflammatory response. This rarely provides a specific diagnosis, a prognosis, or treatment options. In most cases, patients with these conditions can be distinguished on the basis of clinical presentation and MRI appearance. These patients usually have symptoms of either acute or subacute onset of significant neurologic deficit. MRI typically shows patchy or focal gadolinium enhancement that may be confined to the white matter (Fig. 101-4). Spinal cord enlargement is subtle or, more likely, absent. In contrast, patients with benign intramedullary tumors usually experience a significant spinal cord enlargement with minimal, if any, objective neurologic deficit. Thus, a patient who shows symptoms of an acute or subacute onset of a significant neurologic deficit in the absence of obvious spinal cord enlargement usually harbors a nonsurgical inflammatory lesion.
Surgical Objectives
The most important factor influencing the surgical objective is the nature of the tumor–spinal cord interface. This interface can be assessed accurately only through an adequate myelotomy, which extends over the entire rostrocaudal extent of the tumor. Benign tumors, such as ependymomas and hemangioblastomas, although unencapsulated, are noninfiltrative lesions that typically exhibit a distinct tumor–spinal cord interface. Gross total removal is the treatment of choice in these cases. Astrocytomas are more variable. Unlike the consistently benign histology, circumscribed nature, and natural history of ependymoma and hemangioblastoma, astrocytomas are much more variable with respect to histology, physical characteristics, and natural history. Although some benign astrocytomas are well circumscribed and allow gross total resection, most exhibit variable infiltration into the surrounding spinal cord. This is often reflected in a gradual transition zone between the tumor and spinal cord. There is rarely a definitive dissection plane. Thus, whereas gross total resection may be achieved in some cases, the extent of removal is uncertain and poorly defined in most cases. Furthermore, more peripheral dissection beyond what is clearly tumor tissue risks loss of neurologic function from the resection of infiltrated, yet functionally viable, spinal cord parenchyma. The surgical objective for spinal cord astrocytomas remains unclear. Specifically, a correlation between the extent of resection and tumor control has not been definitively established.15,16 Because preservation of neurologic function, rather than complete tumor resection, is the more prudent treatment objective in these cases, tumor removal is limited to tissue that is clearly distinguishable from the surrounding spinal cord. Therefore, the extent of tumor removal varies. Diffusely infiltrative tumors without a definite mass are biopsied, whereas gross total resection may be possible in well-circumscribed examples. Variable degrees of resection account for the remainder of astrocytomas.
Cavernous angiomas are congenital vascular malformations that may appear as intramedullary mass lesions. Most of these lesions are well circumscribed and can be completely excised.17
Biopsy results may be particularly helpful in some instances. Identification of a histologically malignant tumor, for example, independently signals an end to the procedure because aggressive surgery is of no benefit for malignant intramedullary neoplasms.18 In other cases in which the tumor–spinal cord juncture may not be apparent, the confident histologic identification of an ependymoma reassures the surgeon that a plane must exist and that surgical removal should continue.
Surgical Technique
After intubation and administration of perioperative steroids and antibiotics, the patient is turned to the prone position. A Mayfield skull clamp is used for cervical and upper thoracic lesions above the T6 level. Neck flexion and head elevation (i.e., military prone position) reduce the spinal curvature at these levels. Sensory and motor evoked potential monitoring may be used throughout the procedure. The acquired data, however, rarely influence the surgical technique or the surgical objective.19
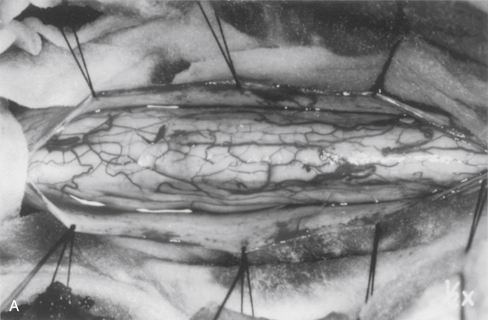
Strict hemostasis must be secured before the dura is opened to prevent ongoing contamination into the dependent microsurgical field. Wide, moist, cottonoid “wall-offs” cover the exposed muscles. Oxidized cellulose (Surgicel) is generously spread over the lateral gutters to prevent contamination of the operative field with blood. The dura mater is opened in the midline and tented laterally to the muscles with sutures (Fig. 101-5A).
Rarely, an exophytic component of a benign glial tumor may extend into the subarachnoid space through a nerve root entry zone. Malignant neoplasms may replace surface spinal cord tissue or fungate through the pia into the subarachnoid space. Most hemangioblastomas arise from the dorsal half of the spinal cord with a visible pial attachment (see Fig. 101-3B).3,7 The size of the pial attachment may bear no relationship to the underlying embedded portion of the tumor.
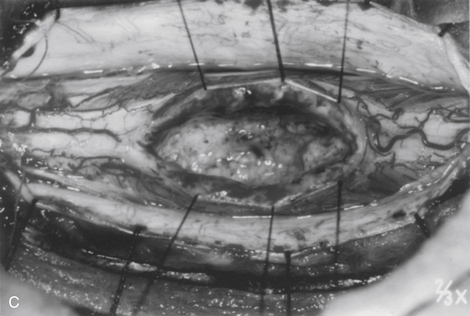
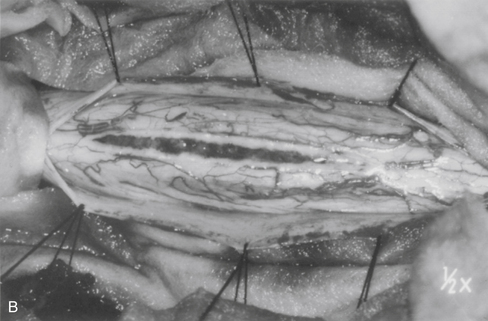
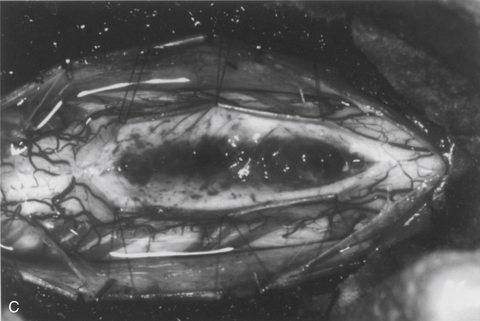
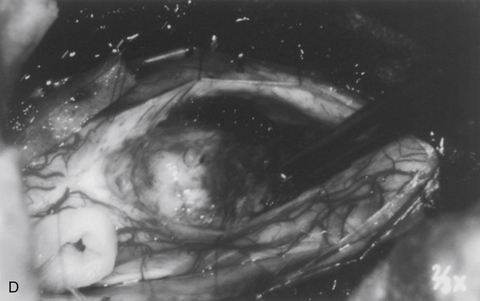
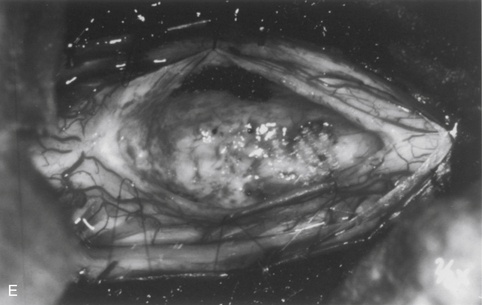
The dorsal midline septum is identified as the midpoint between corresponding dorsal root entry zones. Bipolar cautery marks the dorsal midline over the extent of the intended myelotomy. The myelotomy is begun with a microknife in an avascular pial segment at the point of maximum spinal cord enlargement. The pia is a white, glistening fibrocartilaginous membrane that is tightly applied to the outer glial limiting membrane of the spinal cord. The pia is sharply incised over the entire extent of the tumor. Midline crossing epipial vessels are sequentially cauterized and divided. The myelotomy is deepened by gentle spreading with blunt microforceps and dissectors. Fibrous gliosis at the polar margins of the tumor may require sharp dissection with a microknife. The myelotomy continues until the entire rostrocaudal extent of the dorsal tumor surface has been identified (see Fig. 101-5B). Although the myelotomy must extend a few millimeters beyond the solid portion of the tumor, it is not necessary to completely expose polar cysts. Sizes #6-0 pial sutures are placed and clipped laterally to the dura to maintain gentle traction (see Fig. 101-5C). Evaluation of the tumor–spinal cord interface and frozen-section biopsy examination (to a lesser extent) determine the appropriate treatment objective. Ependymomas are usually characterized by a glistening reddish or brownish-red surface that may be slightly lobulated (see Fig. 101-5C). Blood vessels often course over the tumor surface. These tumors are clearly distinguishable from the surrounding spinal cord on the basis of color and texture. Although unencapsulated, these tumors do not infiltrate and can be easily distinguished and separated from the surrounding spinal cord. Astrocytomas are more heterogeneous with respect to physical characteristics and abut the spinal cord. Intratumoral cysts are quite common, but tumor color and consistency are variable. In adults, most astrocytomas appear as a definable intramedullary mass with a gradual and indistinct transition between the tumor mass and surrounding spinal cord (see Fig. 101-2C). This reflects the infiltrative nature of these neoplasms.
The technique of tumor removal depends on its juncture with the spinal cord and its size. Development of the tumor–spinal cord juncture is preferred for circumscribed tumors with a well-defined plane. The dorsal tumor surface is exposed with pial sutures and gentle, blunt lateral displacement of the overlying dorsal hemicords with dissectors. Fibrous and vascular attachments that tether the spinal cord to the tumor surface are systematically cauterized and divided. The development of the lateral and polar tumor margins is facilitated by forceps traction on the tumor and gentle pial suture and manual dissector countertraction on the spinal cord (see Fig. 101-5D). Larger tumors require internal decompression with an ultrasonic aspirator or laser to allow better visualization and mobilization of the lateral and ventral tumor margins. Infiltrating tumors are removed using an “inside-out” technique. Internal decompression is continued peripherally until the clear distinction of the tumor and spinal cord is no longer obvious (see Fig. 101-2C).
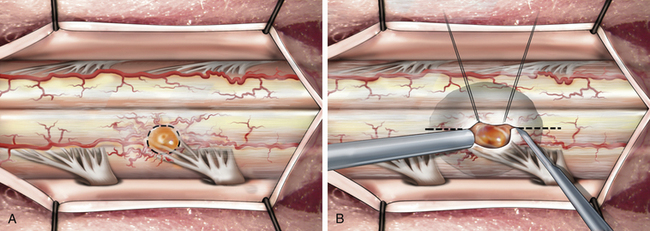
The technique of hemangioblastoma removal differs because of its vascularity and pial attachment. Internal decompression is not an option (because of the vascularity). Instead, the pial attachment should be circumferentially incised (Fig. 101-6A). Systematic cautery on the tumor surface shrinks the tumor bulk to allow adequate mobilization and dissection from the surrounding spinal cord. Small polar longitudinal myelotomies may improve visualization of large tumors embedded in the spinal cord, with only a small exposed pial surface attachment (Fig. 101-6B). After completion of tumor resection, the pial sutures are removed. No attempt is made to suture the dorsal hemicords. The subarachnoid space is copiously irrigated with warm saline. Meticulous multilayer closure is then performed to prevent cerebrospinal fluid (CSF) leakage. This is particularly important in patients who have undergone previous surgery and radiation therapy. There is a high risk of CSF fistula in these patients. An autologous thoracodorsal fascia dural patch graft may be used after biopsy of infiltrative or malignant tumors.
Postoperative Management
Despite confident gross total resection, benign intramedullary tumors present a continued risk of recurrence. Long-term clinical and radiographic follow-up is warranted in these patients. An early postoperative MRI (6–8 weeks after surgery) establishes the completeness of resection and serves as a baseline against which further studies can be compared. Serial gadolinium-enhanced MRI scans are obtained yearly because radiographic evidence of tumor recurrence usually precedes clinical symptoms. Surgical reexploration, if clinically appropriate, can then be performed with minimal surgical morbidity. Exposure of the spinal cord at reoperation can be technically difficult. There is often tethering of the spinal cord to the dural suture line at the previous myelotomy site. If the dura was not closed at the previous surgery, then the spinal cord may be densely adherent to the thick epidural scar. In either case, normal dura should be exposed above and below the tethered segment, even if additional bone removal is required. The dura mater or scar is opened as an ellipse around the tether. Meticulous sharp dissection is carried centrally to free the dorsal surface of the spinal cord.
Aghakhani N., David P., Parker F. Intramedullary spinal ependymomas: analysis of a consecutive series of 82 adult cases with particular attention to patients with no preoperative neurological deficit. Neurosurgery. 2008;62:1279-1285.
Gomez D.R., Missett B.T., Wara W.M., et al. High failure rate in spinal ependymomas with long-term follow-up. Neuro-oncology. 2005;7:254-259.
Lee J., Parsa A.T., Ames C.P., et al. Clinical management of intramedullary spinal ependymomas in adults. Neurosurg Clin North Am. 2006;17(1):21-28.
Raco A., Esposito V., Lenzi J., et al. Long-term follow-up of intramedullary spinal cord tumors: a series of 202 cases. Neurosurgery. 2005;56:972-981.
Woodworth G.F., Chaichana K.L., McGirt M.J., et al. Predictors of ambulatory function after surgical resection of intramedullary spinal cord tumors. Neurosurgery. 2007;61:99-105.
1. Lee J., Parsa A.T., Ames C.P., McCormick P.C. Clinical management of intramedullary spinal ependymomas in adults. Neurosurg Clin North Am. 2006;17(1):21-28.
2. McCormick P.C., Stein B.M. Intramedullary tumors in adults. Neurosurg Clin North Am. 1990;1:609-630.
3. McCormick P.C., Stein B.M. Spinal cord tumors in adults. In: Youmans J.R., editor. Neurological surgery. ed 4. Philadelphia: WB Saunders; 1996:3102-3122.
4. McCormick P.C., Torres R., Post K.D., et al. Intramedullary ependymoma of the spinal cord. J Neurosurg. 1990;72:523-533.
5. Raco A., Esposito V., Lenzi J., et al. Long-term follow-up of intramedullary spinal cord tumors: a series of 202 cases. Neurosurgery. 2005;56:972-981.
6. Sloof J.L., Kernohan J.W., MacCarthy C.S. Primary intramedullary tumors of the spinal cord and filum terminale. Philadelphia: WB Saunders; 1964.
7. Stein B.M., McCormick P.C. Intramedullary neoplasms and vascular malformations. Clin Neurosurg. 1992;39:361-387.
8. Wood E.H., Berne A.S., Taveras J.M. The value of radiation therapy in the management of intrinsic tumors of the spinal cord. Radiology. 1954;63:11-24.
9. Woodworth G.F., Chaichana K.L., McGirt M.J., et al. Predictors of ambulatory function after surgical resection of intramedullary spinal cord tumors. Neurosurgery. 2007;61:99-105.
10. McCormick P.C. Anatomic principles of the intradural spinal surgery. Clin Neurosurg. 1994;41:204-223.
11. Gomez D.R., Missett B.T., Wara W.M., et al. High failure rate in spinal ependymomas with long-term follow-up. Neuro-oncology. 2005;7:254-259.
12. Whitaker S.J., Bessell E.M., Ashley S.E., et al. Post-operative radiotherapy in the management of spinal cord ependymoma. J Neurosurg. 1991;74:720-728.
13. Aghakhani N., David P., Parker F. Intramedullary spinal ependymomas: analysis of a consecutive series of 82 adult cases with particular attention to patients with no preoperative neurological deficit. Neurosurgery. 2008;62:1279-1285.
14. Solomon R.A., Stein B.M. Unusual spinal cord enlargement related to intramedullary hemangioblastoma. J Neurosurg. 1988;68:550-553.
15. Rossitch E., Zeidman S., Burger P.C., et al. Clinical and pathological analysis of spinal and astrocytomas in children. Neurosurgery. 1990;27:193-196.
16. Sandler H.M., Papadopoulos S.M., Thuntan A.F., et al. Spinal cord astrocytoma: results of therapy. Neurosurgery. 1992;30:490-493.
17. McCormick P.C., Michelson W.J., Post K.D., et al. Cavernous malformations of the spinal cord. Neurosurgery. 1988;23:459-463.
18. Cohen A.R., Wisoff J.H., Allen J.C., et al. Malignant astrocytomas of the spinal cord. J Neurosurg. 1989;70:50-54.
19. Adams D.C., Emerson R.G., Heyer E.J., et al. Intraoperative evoked potential monitoring with controlled neuromuscular blockade. Anesth Analg. 1993;77:913-918.