Chapter 187 Intradural Extramedullary Tumors
Introduction
Tumors arising from the intradural extramedullary spinal canal reflect a wide variety of histopathologies. With few exceptions, however, these tumors are histologically benign and amenable to complete surgical resection. Long-term tumor control or cure with preservation or improvement in neurologic function can be achieved with surgery alone for most patients.1–8 This chapter focuses on direct surgical techniques and strategies for these largely benign tumors.
Surgical Considerations
Planning for resection of intradural spinal tumors includes numerous considerations such as tumor type, sagittal and axial location, and surgeon preference. Tumor-specific considerations such as the presence of extradural tumor extension, function of the root of origin for nerve sheath tumors, resection of the dural attachment in the case of meningiomas, or en bloc resection for myxopapillary ependymomas are also relevant. Despite these numerous factors, the vast majority of these lesions can be safely removed through a standard posterior exposure with a nondestabilizing laminectomy or osteoplastic laminoplasty. Modifications of the standard posterior exposures may be required to adequately access ventral and/or paraspinal tumor extension.9 More formalized posterolateral or even anterior exposures may occasionally need to be considered in some patients, particularly for midline ventral intradural tumors.10,11 Minimally invasive exposures for resection of intradural extramedullary spinal tumors have also been described. In experienced hands, safe resection may be achieved by using these techiques.12
Nerve Sheath Tumors
Nerve sheath tumors are the most common intradural extramedullary spinal tumors in adults. Most are sporadically occurring solitary schwannomas. Men and women are equally affected, with a peak occurrence in the fourth and fifth decades of life. They are evenly distributed throughout the spinal canal, although involvement of the dorsal root is more common. Solitary spinal nerve sheath tumors represent a heterogeneous group of neoplasms with respect to size, location, and nerve root of origin. Surgical considerations can be complex and include adequate exposure for safe tumor resection, spinal stability, and function of the nerve root of origin. The techniques of surgical removal of intradural spinal schwannomas are well-established. After routine endotracheal intubation, perioperative steroids and intravenous antibiotics are administered. Intraoperative motor- and sensory-evoked potential monitoring is often utilized. Nerve root stimulation is recommended in patients with tumors arising from critical cervical (C5-T1) or lumbosacral (L2-S1) levels.
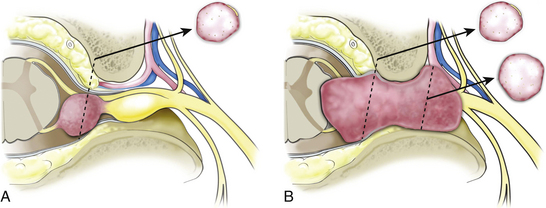
A midline or paramedian longitudinal dural opening is performed. The dural opening should extend just beyond the polar margins of the tumor to facilitate tumor removal and precise identification of the afferent and efferent nerve origin attachments. The dural edges are everted laterally and sutured to the paraspinal muscles to maximize intradural exposure and prevent the introduction of blood from the epidural space or paraspinal muscles into the dependent intradural surgical field. The intermediate arachnoid layer is sharply opened over the dorsal tumor surface. A second arachnoid layer is usually tightly applied to the tumor surface. This layer effectively compartmentalizes and ensheaths individual dorsal and ventral roots. Although the proximal portions of corresponding segmental dorsal and ventral nerve roots remain separate, they become compartmentalized within a common arachnoid sheath as they course toward the dural root sleeve. Identification and opening of the arachnoid nerve sheath is important for two reasons. First, it allows the dissection to take place directly on the tumor surface. This layer is ultimately reflected off the tumor surface at its margins and can make mobilization and visualization of tumor margins difficult if the dissection is performed outside this layer. This is particularly important with regard to nonvisualized tumor margins that abut the spinal cord. Second, the corresponding nerve root is usually tightly applied to the tumor capsule within this arachnoid layer (Fig. 187-1A). Upon initial inspection, this nerve root may appear to be the nonfunctional nerve root of origin because of its tight attachment to the tumor surface. Upon opening this layer, however, it becomes clear that this root may be dissected off the tumor capsule and preserved. The same is not the case for the actual nerve of origin. Although a portion of the afferent and efferent components of the nerve of origin may be dissected and separated from the tumor capsule, eventually this dissection plane disappears as the nerve root becomes incorporated into the tumor capsule.
Once the tumor surface is identified, the polar margins are defined. Direct orthogonal visualization of both rostral and caudal tumor poles facilitates tumor removal. For large tumors, the dorsal tumor capsule is entered, and internal decompression with an ultrasonic aspirator or laser is performed. Sufficient internal decompression allows progressive delivery of initially nonvisualized tumor into the resection bed. Division of the lateral dentate ligament attachment facilitates ventral access. Ultimately, the afferent and efferent tumor attachments need to be divided to achieve removal. Identification of these attachments depends on tumor size, origin, and location. In some cases, the afferent and efferent components may be immediately apparent on the dorsal surface of the tumor. Early division of these attachments allows easy removal of the tumor, particularly at the thoracic levels. More commonly, however, the afferent and efferent tumor attachments are not visualized on initial tumor exposure. The afferent root is often identified by its enlarged, congested, and hypervascular appearance (Fig. 187-2C). In contrast, the efferent root component usually appears normal. Progressive internal decompression allows delivery of tumor margins into the resection bed until the attachments are visualized. The dorsal and ventral nerve roots may already be contained within a common arachnoid sheath at the proximal origin of cauda equina tumors. At these levels, the functional corresponding nerve root may appear to be part of the afferent root of origin. However, fascicles from the corresponding root will be reflected onto the tumor surface and can be dissected and preserved. Occasionally, some of the fascicles from the actual nerve root of origin may also be reflected onto the tumor surface and may be separable from the tumor capsule over much of, or occasionally the entire, tumor surface. Unless these fascicles arise from critical cervical or lumbosacral levels and demonstrate intraoperative stimulation, they need not be preserved, as such futile dissection unnecessarily prolongs the resection.
Tumors that arise from the very proximal portion of the nerve root may not have a definable afferent nerve root attachment. Instead these tumors may abut and be adherent to the spinal cord at the root entry zone. In some cases, proximal tumor growth may actually elevate the pia, where it is reflected at root entry zones, and occupy a subpial location. Great care must be taken to safely remove this subpial tumor component to avoid injury to the spinal cord or fragile epipial vascular network. In these cases, microsurgical dissection directly on the tumor surface usually allows safe, complete removal. This dissection can be difficult for removal of ventral root tumors, even with gentle retraction of a detached dentate ligament. Some portions of the pia may have to be incised to follow the subpial tumor component. If uncertainty regarding the margin of the tumor remains, then further dissection may need to cease. Conversely, tumors arising more distally along the root of origin may have their distal margin near or just beyond the dural nerve root sleeve. In these cases, internal decompression, as well as early identification and division of the afferent attachment, allows adequate visualization and mobilization of the distal tumor component to allow preservation of a critical corresponding functional nerve root (Fig. 187-2D). Nerve root stimulation can be useful during this part of the dissection.
Dumbbell Tumors
Dumbbell spinal schwannomas arise from the intradural segment of a dorsal or ventral nerve root. Thus many of the intradural anatomic features and surgical considerations for purely intradural schwannomas and dumbbell schwannomas are similar. The main difference is the extended lateral growth of dumbbell tumors through the dural root sleeve and neural foramen, beyond the dorsal root ganglion, and into the paraspinal region (Fig. 187-1B). The presentation of dumbbell tumors with respect to the spinal level, as well as to the size of the spinal and paraspinal tumor components, vary considerably. Additional surgical considerations for dumbbell tumors include adequate exposure for removal of the intraspinal and paraspinal tumor components; the logistics and sequencing of tumor removal, particularly if separate surgical exposures are utilized; and possible spinal instability.
The sequence and techniques of dumbbell tumor removal once the exposure has been achieved vary according to a number of factors, including surgeon preference as well as tumor characteristics. Initial removal of the intradural tumor component through a midline longitudinal dural opening allows early, direct decompression of the spinal cord or cauda equina, identification and division of the afferent nerve of origin, and direct nerve stimulation for verification of a nonfunctioning corresponding motor root for sensory root tumors at critical cervical and lumbosacral levels. This sequence is preferred for most tumors, but especially for tumors at these important levels. The intradural tumor is ultimately amputated at the distal dural root sleeve margin. Following intradural tumor resection, the laterally everted dural edge is retracted medially to expose the paraspinal component. For large tumors, the tumor capsule may be incised along the axis of the spinal nerve and internally decompressed with a Cavitron device (Dentsply International Inc., York, PA, USA). Ultimately, the margins of the tumor are identified. A robust epidural and foraminal venous plexus is typically tightly applied and compressed on the tumor surface, particularly in the foramen. It is important to stay directly on the tumor capsule to avoid problematic epidural and foraminal bleeding and to maintain the correct plane as the dissection progresses ventrally. This is particularly important at cervical levels where the tumor may abut the vertebral artery and perivertebral venous plexus. The experience at my institution has been that benign nerve sheath tumors displace, rather than invade or engulf, the vertebral artery. Similarly, in the thoracic spine, the tumor capsule often abuts the pleural membrane. If the dissection is not carried out precisely on the tumor capsule, then the pleural cavity may be inadvertently opened, often necessitating the need for a chest tube and increasing the risk of a postoperative CSF pleural fistula. A well-developed plane between the tumor capsule and the perivertebral plexus serves as an effective dissection plane as long as the dissection is carried out directly on the tumor capsule. Often several layers of epidural vessels are compressed on the tumor surface. These pseudocapsule venous layers must be cauterized and sequentially peeled away to reach the true tumor capsule. The medial aspect of the tumor should be freed of its medial dural root sleeve attachment. This may require a circumferential incision around the dural root sleeve. This allows the medial paraspinal tumor margin to be elevated laterally to facilitate circumferential access. This maneuver also allows the distal or most lateral tumor component to be pulled medially into the surgical field. More internal decompression may be required until the efferent attachment can be identified and divided. Following resection, the midline dura is closed first with a running 4-0 silk or 5-0 PROLENE suture. The dural root sleeve usually requires a muscle or fascial patch. A Valsalva maneuver to 35 mm Hg is used to test the integrity of the dural closure. DuraGen is typically placed over both suture lines. In the experience at my institution, postoperative CSF leaks usually occur at the dural root sleeve.
Meningiomas
Meningiomas make up 13% to 19% of intracranial tumors, and spinal meningiomas account for 12% of all meningiomas.1 Within the spinal canal, these lesions show a preference for the thoracic region. They occur predominantly in the cervical (28%) and thoracic cord (64%) and only rarely in the lumbar spine (8%). Overall, meningiomas are found to be evenly distributed around the circumference of the spinal cord. Anterior meningiomas have been observed to be more common in the cervical spine (up to 48% of cervical meningiomas), lateral meningiomas are the most common in the thoracic spine, and posterior lesions are the most common in the lumbar region. In one study, extradural tumor extension was identified in 6.3% of patients.2
The peak incidence for spinal meningiomas is between the sixth and eighth decades of life. Patients younger than age 50 years have a higher rate of tumors located in the cervical spine (39%) and a greater number related to a predisposing factor, such as neurofibromatosis 2, radiation, or trauma. These patients are also more likely to require reoperation for residual tumor (22% vs. 5% in one case series).5 As with intracranial meningiomas, there is a higher incidence of spinal meningiomas in women, with a rate ranging from 3:1 to 4.2:1.1 This rate is even higher for meningiomas in the thoracic spine, with women presenting with significantly more spinal meningiomas than their male counterparts.
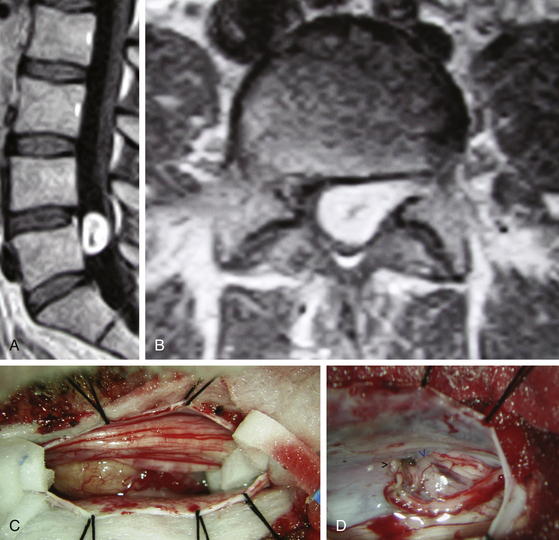
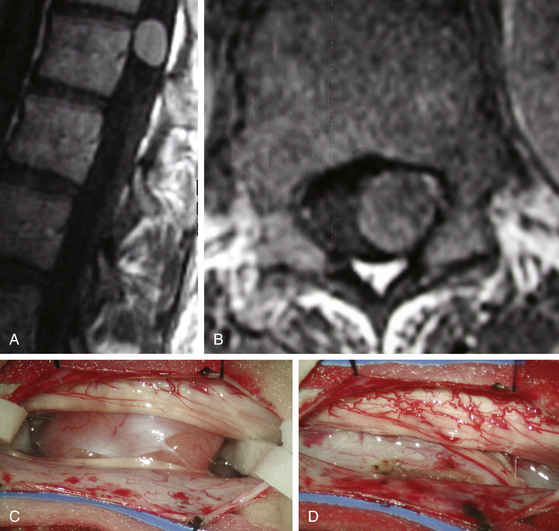
Spinal meningiomas are usually isointense to the spinal cord on both T1- and T2-weighted sequences and demonstrate uniform enhancement following gadolinium administration (Fig. 187-3A, B). A dural attachment is also usually identified, although a dural “tail” is less common. Significant intratumoral mineralization or calcification alters MRI characteristics. The differential diagnosis of spinal meningiomas includes predominantly nerve sheath tumors, which more commonly show cystic regions or heterogeneous enhancement, and ependymomas.
The appropriate approach varies, depending on the level of the tumor, the location of the tumor in relation to the spinal cord, and surgeon preference. Tumor vascularity and consistency can also influence the choice of operative exposure, particularly for ventrally located lesions, although these characteristics are difficult to determine pre-operatively. Anterior or anterolateral tumors may require a more complicated anterior approach, which could be contraindicated in a medically compromised patient. Approaches described in the literature for the cervical region include posterior, posterolateral, and anterior cervical corpectomy and fusion.11 Approaches described for the thoracic region are more varied and carry a higher risk of complications due to the associated difficulty of accessing the anterior spinal cord in this region. Posterior approaches for this area include posterior, posterolateral, and the posterolateral endoscopic techniques.10,11,13 Anterior and anterolateral approaches should be divided on the basis of upper thoracic versus lower thoracic locations. Upper thoracic lateral and anterior approaches include the trap-door approach and the parascapular extrapleural approach. The lower thoracic spine is accessible through the lateral extracavitary approach and retropleural exposures. The lumbar spine is reached predominantly by using the posterior, posterolateral, and lateral retroperitoneal approaches.
Fortunately, most meningiomas of the spinal cord arise posterior, posterolateral, or lateral to the cord and therefore can be accessed by using a posterior approach. Patient positioning and the initial techniques of intradural exposure are similar to those utilized for nerve sheath tumors. The exposed tumor surface should be visualized, including the rostral and caudal tumor poles. A small cottonoid is placed in each lateral gutter above and below the tumor margins. Division of one or more of the dentate ligament attachments to the lateral dura may improve access to ventral tumor extension (Fig. 187-3C). A well-defined arachnoid plane typically exists between the spinal cord and tumor capsule. Depending on the size and consistency of the tumor, internal debulking with an ultrasonic aspirator or laser facilitates visualization and development of the tumor margins. All traction on the tumor should be away from the spinal cord. The tumor surface may be quite friable and of variable vascularity. Cauterization of the dural base may reduce bleeding from highly vascular and/or friable lesions. Fortunately, due to a well-developed spinal epidural space, bony involvement does not typically occur. Management of the dural attachment depends on practical considerations. For dorsal tumors, resection of the dural origin facilitates resection. For more lateral and ventral tumors, cauterization of the tumor origin is preferred because of the difficulty of dural reconstruction in these locations (Fig. 187-3D). In either case, the rate of recurrence is quite low.
Filum Terminale Tumors
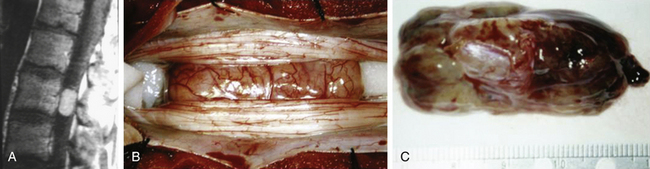
The vast majority of filum terminale tumors are myxopapillary ependymomas. Paragangliomas are less common, whereas astrocytomas, hemangioblastomas, and cavernous malformations are rare. The basic principles of intradural surgical exposure also apply to filum terminale tumors. Ependymomas and paragangliomas should ideally be resected en bloc, as there is some evidence that piecemeal resection of these tumors increases recurrence rates.8 Paragangliomas can be particularly problematic because of their high vascularity. The tumor is carefully freed from adjacent nerve roots, and the filum is identified visually and tested with a neurostimulator. The filum is cauterized above and below the tumor and divided, and the tumor is carefully rotated out of the canal. En bloc resection is often not possible to achieve safely in larger tumors for various reasons (Fig. 187-4). For instance, the tumor may lack sufficient internal integrity and fall apart with even gentle manipulation. The tumor may also be too large to tease out without putting unacceptable amounts of traction on overlying nerve roots. Large tumors may exhibit sheetlike growth along arachnoid fenestrations. Functional roots may appear to course directly through the substance of the tumor. Safe resection can be impossible in these cases because the lack of supportive connective tissue matrix (i.e., epineurium) in the cauda equina nerve roots does not allow safe dissection of tumor off the involved roots. Only subtotal resection may be possible in these patients.
Angevine P.D., Kellner C., Haque R.M., McCormick P.C. Surgical management of ventral intradural spinal lesions. J Neurosurg Spine. 2011;15:28-37.
Barami K., Dagnew E. Endoscope-assisted posterior approach for the resection of ventral intradural spinal cord tumors: report of two cases. Minim Invasive Neurosurg. 2007;50:370-373.
Cohen-Gadol A.A., Zikel O.M., Koch C.A., et al. Spinal meningiomas in patients younger than 50 years of age: a 21-year experience. J Neurosurg. 2003;98:258-263.
Gezen F., Kahraman S., Canakci Z., Beduk A. Review of 36 cases of spinal cord meningioma. Spine (Phila Pa 1976). 2000;25:727-731.
Gottfried O.N., Gluf W., Quiñones-Hinojosa A., et al. Spinal meningiomas: surgical management and outcome. Neurosurg Focus. 2003;14:e2.
Kim P., Ebersold M.J., Onofrio B.M., Quast L.M.. Surgery of spinal nerve schwannoma: risk of neurological deficit after resection of involved root. J Neurosurg, 1989;71:810-814
Klekamp J., Samii M. Surgical results for spinal meningiomas. Surg Neurol. 1999;52:552-562.
McCormick P.C. Retropleural approach to the thoracic and thoracolumbar spine. Neurosurgery. 1995;37:908-914.
McCormick P.C. Surgical management of dumbbell and paraspinal tumors of the thoracic and lumbar spine. Neurosurgery. 1996;38:67-75.
O’Toole J.E., McCormick P.C. Midline ventral intradural schwannoma of the cervical spinal cord resected via anterior corpectomy with reconstruction: technical case report and review of the literature. Neurosurgery. 2003;52:1482-1486.
Seppälä M.T., Haltia M.J., Sankila R.J., et al. Long-term outcome after removal of spinal schwannoma: a clinicopathological study of 187 cases. J Neurosurg. 1995;83:621-626.
Setzer M., Vatter H., Marquardt G., et al. Management of spinal meningiomas: surgical results and a review of the literature. Neurosurg Focus. 2007;23:E14.
Sonneland P.R., Scheithauer B.W., Onofrio B.M. Myxopapillary ependymoma: a clinicopathologic and immunocytochemical study of 77 cases. Cancer. 1985;56:883-893.
1. Gottfried O.N., Gluf W., Quiñones-Hinojosa A., et al. Spinal meningiomas: surgical management and outcome. Neurosurg Focus. 2003;14:e2.
2. Setzer M., Vatter H., Marquardt G., et al. Management of spinal meningiomas: surgical results and a review of the literature. Neurosurg Focus. 2007;23:E14.
3. Gezen F., Kahraman S., Canakci Z., Beduk A. Review of 36 cases of spinal cord meningioma. Spine (Phila Pa 1976). 2000;25:727-731.
4. Klekamp J., Samii M. Surgical results for spinal meningiomas. Surg Neurol. 1999;52:552-562.
5. Cohen-Gadol A.A., Zikel O.M., Koch C.A., et al. Spinal meningiomas in patients younger than 50 years of age: a 21-year experience. J Neurosurg. 2003;98:258-263.
6. Kim P., Ebersold M.J., Onofrio B.M., Quast L.M. Surgery of spinal nerve schwannoma: risk of neurological deficit after resection of involved root. J Neurosurg. 1989;71:810-814.
7. Seppälä M.T., Haltia M.J., Sankila R.J., et al. Long-term outcome after removal of spinal schwannoma: a clinicopathological study of 187 cases. J Neurosurg. 1995;83:621-626.
8. Sonneland P.R., Scheithauer B.W., Onofrio B.M. Myxopapillary ependymoma: a clinicopathologic and immunocytochemical study of 77 cases. Cancer. 1985;56:883-893.
9. McCormick P.C. Surgical management of dumbbell and paraspinal tumors of the thoracic and lumbar spine. Neurosurgery. 1996;38:67-75.
10. O’Toole J.E., McCormick P.C. Midline ventral intradural schwannoma of the cervical spinal cord resected via anterior corpectomy with reconstruction: technical case report and review of the literature. Neurosurgery. 2003;52:1482-1486.
11. Angevine P.D., Kellner C., Haque R.M., McCormick P.C. Surgical management of ventral intradural spinal lesions. J Neurosurg Spine. 2011;15:28-37.
12. Barami K., Dagnew E. Endoscope-assisted posterior approach for the resection of ventral intradural spinal cord tumors: report of two cases. Minim Invasive Neurosurg. 2007;50:370-373.
13. McCormick P.C. Retropleural approach to the thoracic and thoracolumbar spine. Neurosurgery. 1995;37:908-914.