Chapter 192 Intradiscal Electrothermy
Low back pain affects up to 80% of the U.S. population at least once in their lifetime.1–3 Although many cases of acute low back pain are self-limiting, resolving within 12 weeks, there is a recurrence rate of 20% within 6 months and an overall recurrence rate of 70% to 90%.1,4 In approximately 5% of patients, low back pain becomes chronic and disabling.5 Thus, chronic low back pain is the most common cause of disability between the ages of 45 and 65.3,6
Low back pain presents a diagnostic challenge, as only 10% to 20% of cases are attributable to a precise anatomic cause.7 There is poor correlation between symptoms, imaging findings, and physiologic changes. However, it has been estimated that about 40% of chronic low back pain originates from the intervertebral disc.8 Kuslich et al. demonstrated concordant pain on direct stimulation of the outer anulus of in vivo intervertebral discs, supporting the role of the disc as a “pain generator.”9 With degeneration theorized to be the underlying cause, the mechanism of discogenic pain has been pursued recently in clinical and basic research. Pathologic neoinnervation, nociceptive sensitization, biomechanical alteration, and internal anular fissuring/disruption have been implicated as possible mechanisms.
Treating chronic discogenic low back pain has been difficult. Medication and aggressive nonoperative treatment, such as physical therapy, often fail to improve function or to reduce pain significantly. In the past, individuals with continued pain and disability after initial therapy were treated with conservative pain management therapy or surgical intervention. Discectomy and fusion can have a 40% failure rate, particularly with multiple degenerative disc levels, in addition to the risk of complications, surgical morbidity, and prolonged recovery.10,11 As another option, less invasive intradiscal interventions have been the subject of much clinical and basic research since 1997, when Saal and Saal proposed intradiscal electrothermal therapy (IDET).12
IDET targets the intervertebral disc that is thought to be the pain generator, often aided by provocative discography. Under conscious sedation, a thermal coil is introduced into the dorsal aspect of the anulus fibrosus, and an amount of thermal energy is delivered. Though the mechanism of action is unclear, it has been proposed that the heat applied may alter the collagen organization in the anulus and coagulate nociceptive fibers to improve biomechanical function and reduce pain.13
Discogenic Low Back Pain
Intervertebral discs are bordered by thin hyaline cartilage end plates of the superior and an inferior vertebral body. The nucleus pulposus is the core of the disc, consisting of type II collagen and elastin embedded in the gelatinous substance aggrecan, which is rich in hydrated proteoglycans.3 Encasing the nucleus is a dense ring of type I collagen known as the anulus fibrosus; it is highly organized into lamellae and maintained by fibroblast-like cells. The disc is avascular, though nutrients can diffuse from the marrow cavity of the adjacent vertebrae across the cartilaginous end plates. In the lumbar spine, intervertebral discs are about 40 mm in diameter and 7 to 10 mm in height.3
Degeneration of the intervertebral disc seems to be intimately linked to the loss of nuclear hydrostatic pressure. Fragmentation of proteoglycans in the nucleus leads to reduced hydrostatic pressure, fewer proteoglycans in the nucleus, and fewer proteoglycans adjacent to the cartilaginous end plates. End-plate subchondral sclerosis and calcification will occur and further reduce the nutrient flow to the disc.14 It has been proposed that the anulus delaminates and buckles inward when the nuclear hydrostatic pressure is lost, leading to collapse of the normal anular organization.13 Resultant internal mobility and shear stress can lead to fissuring of the anular wall.15
Fissuring of the anulus can be assessed by CT discography. Although CT discography is not generally the diagnostic test of choice for discogenic low back pain, provoked pain concordant with chronic symptoms and CT evidence of internal disc disruption can be helpful in determining the benefit of possible operative intervention.3 However, no class I studies exist that compare outcomes in patients with and without positive discography for either spine fusion or IDET.
MR imaging demonstrating a high-intensity zone on T2-weighted images correlates with painful fissured discs in 65% to 95% of individuals; however, MR has a sensitivity of less than 50% for predicting the presence of anular fissures.13,16–18
Pain resulting from degeneration may arise from the disc, the mechanical effect of the disc on an adjacent structure, and the inflammatory mediators that are present in the setting of a degenerated disc.3 The anterior aspect of the disc is sparsely innervated by perivascular nerve plexuses joining branches of the sympathetic trunk. The posterior aspect is innervated more densely, with nociceptive nerve endings containing substance P primarily arising from the sinovertebral nerve endings with contributions from the sympathetic trunk.13,19,20 Discogenic pain is primarily axial, though referred leg pain can occur from converging nociceptive fibers from the leg and from the sinovertebral nerve.21
Nociceptive fibers are generally present in the outer third of the anulus fibrosus, the posterior longitudinal ligament, and the cartilaginous end plates.22 In vivo studies have shown that the two most common pain generators are the posterior longitudinal ligament and the dorsal anulus fibrosus.20 However, a degenerated disc may undergo neoinnervation with small, unmyelinated nociceptive fibers growing deep into the anulus fibrosus and even into the nucleus pulposus.23,24 Furthermore, nociceptors undergo presensitization from granulation tissue and inflammatory substances, including phospholipase A2, interleukin-1, nitrous oxide, and metalloproteinase.25–27
The complex interplay between continued mechanical loading and neural properties, including neoinnervation and presensitization, underlies the development of chronic discogenic low back pain.13 This entity is distinct from pain due to nerve compression from protruding or herniated discs; it is the disc that is the pain generator owing to an internal derangement.13 Therefore, the target of intervention is the disc itself.
Intradiscal Electrothermal Therapy
Given the suboptimal results of surgical treatment, the difficulties of chronic pain management, and the widespread prevalence of degenerative disc disease, a minimally invasive treatment for chronic discogenic low back pain is appealing. Saal and Saal developed IDET in 1997, theorizing that the clinical benefits that are observed from heat-induced collagen alteration as performed in joint stabilization procedures could translate to the degenerated, painful intervertebral disc.12
Briefly, the IDET procedure targets a painful disc as identified on provocative discography. IDET can be performed under conscious sedation. With fluoroscopic guidance, a navigable thermal resistive heating coil is introduced into the outer portion of the anulus fibrosus in the region of an anular fissure as noted by imaging, typically in the dorsal anulus. The catheter should lie about 5 mm from the outer edge of the anulus for adequate heating of the inner and outer anulus.28 In practice, the final position of the catheter is generally the contralateral posterolateral outer anulus, then passing anteriorly parallel to the anular lamellae.28 The coil is heated gradually to achieve tissue temperatures that are adequate to disrupt hydrogen cross-linking in the collagen triple helix, 60°C to 65°C, and coagulate nerve endings more than 42°C.5,29 Saal and Saal give 60°C to 75°C as a target tissue temperature.13 Up to 40% of the time, a bilateral approach is necessary to address the entire posterior anulus. Intradiscal antibiotics are given as discitis prophylaxis.13
Course of Recovery
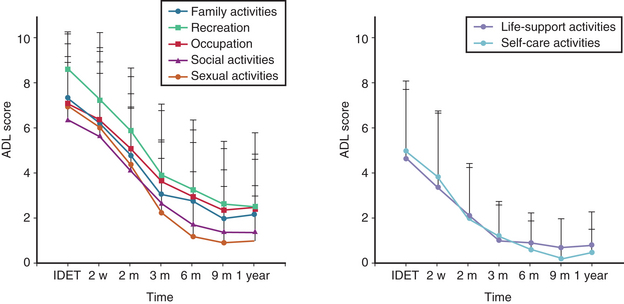
IDET typically causes a transient increase in pain that lasts 1 to 2 weeks. Within 6 to 12 weeks, significant pain improvement and reduced disability are reported (Fig. 192-1). Benefits seem to be enduring, with progressive improvement 1 to 2 years postprocedure.30,31 However, some reports suggest that a significant population that will revert to baseline after initial improvement (Fig. 192-2).32
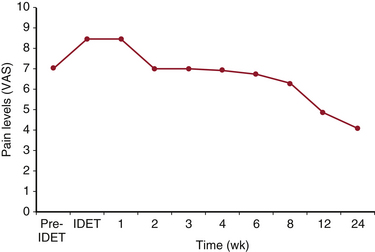
FIGURE 192-1 Typical recovery curve as presented by Saal and Saal, based on their first 100 treated patients.
(Redrawn from Saal JA, Saal JS: Intradiscal electrothermal therapy for the treatment of chronic discogenic low back pain. Clin Sports Med 21[1]:167–187, 2002.)
Postprocedure Rehabilitation
Transient worsening of symptoms is expected after IDET. Avoiding bending, lifting, and prolonged sitting should be recommended initially postprocedure. A corset is recommended for 6 to 8 weeks. Patients can gradually increase activities to include low-intensity stretching and walking over the first 4 weeks. Floor stabilization exercises at 4 weeks can be encouraged. Waiting 6 months postprocedure is recommended for athletic activity. Box 192-1 lists guidelines for patients following the procedure.
BOX 192-1. Guidelines for Patients
Lifting: Limit 5 to 10 lb for the first 6 weeks.
No bending or twisting of the low back.
Housework: No bending or twisting for the first 6 weeks.
You will be wearing a lumbar corset for the first 6 to 8 weeks after your IDET.
Saal JA, Saal JS: Intradiscal electrothermal therapy for the treatment of chronic discogenic low back pain. Clin Sports Med 21(1):167–187, 2002.
Selection Criteria
Pain reduction and functional improvement from IDET are observed in 23% to 86% of individuals, but it is widely accepted that stringent inclusion criteria are necessary, given the large number of nonresponders that has been observed.30,33–35 Studies suggest that individuals with multilevel degenerative disc disease respond poorly to IDET, on the basis of improved study outcomes with selection criteria excluding severe multilevel disease.31,33,36 Another group presents a study demonstrating poor outcomes correlated to obesity, with only 10% benefiting from IDET.37
Kloth et al. recently published a review of IDET selection criteria and indications for use to achieve clinical benefit in 75% of patients.38 Criteria that may be used include the following:
• Persistent axial low back pain with or without leg pain and nonresponsiveness to 6 or more weeks of conservative care
• History consistent with discogenic low back pain (e.g., pain with lumbar motion, decreased sitting duration and tolerance) with a normal lower-extremity neurologic examination
• One to three desiccated discs seen on T2-weighted MRI with or without a high-intensity zone, with or without small, contained disc protrusion, with at least 50% remaining disc height
• Concordant pain with low-pressure provocative discography (<50 psi above opening pressure) without discordant pain at pressures greater than 50 psi
• Posterior anular disruption (radial or concentric fissuring) on postdiscography CT images38
Exclusion Criteria
Contraindications presented by Kloth et al. include the following38:
• Severe disc degeneration at the affected lumbar level(s) as evidenced by greater than 50% disc height loss on plain anteroposterior and lateral lumbar radiographs and/or sectional imaging
• Extruded or sequestered herniated nucleus pulposus at the affected level(s)
• Previous lumbar back surgery (e.g., laminectomy, discectomy, or fusion) at the affected level(s)
• IDET performed within the last 6 months at the same level
• Nerve root impingement and/or compression with chronic lower extremity radiculopathy causing new-onset motor deficit
• Moderate to severe spinal stenosis (i.e., central, lateral, or foraminal) due to osteophyte and/or ligamentous overgrowth as evidenced by MRI or CT, provided that stenosis is the cause of pain
• Moderate to severe end-plate degenerative changes (e.g., spondylosis) at the affected level(s)
• Spondylolisthesis with motion on flexion-extension radiographs or any translational instability at the affected level(s)
Relative contraindications presented in the same review include (1) moderate spinal stenosis (i.e., central, lateral, or foraminal) due to soft disc bulging, protrusion, or herniation with claudication; (2) grade I spondylolisthesis with no or minimal motion on flexion-extension radiographs; (3) previous discectomy at the affected level(s); and (4) thoracic degenerated disc(s).38
Risks
IDET is a minimally invasive procedure, but it is not without risks. Cohen reports a 10% complication rate, though most of the complications in this study were transient and self-limiting.37 Severe complications have been infrequent.28 Cauda equina syndrome after IDET has been reported several times.39–41 Osteonecrosis of the vertebral body and giant herniation of the disc have been associated with IDET.42–44 Derby reports end-plate damage post-IDET leading to disc space collapse in two patients.45 A case of intradural catheter migration has also been reported.46
At the 16th North American Spine Society Meeting, Saal and Saal presented a review of 1675 IDET procedures and 35,000 SpineCATH devices from five centers. Six nerve root injuries, 6 cases of disc herniation (4 resolved nonoperatively), 19 catheter breakages, 8 superficial skin burns, and 1 case of bladder dysfunction post-IDET were reported.47
Proposed Mechanisms
Targeting the disrupted and degenerated anulus fibrosus is the basis of the IDET procedure. The proposed mechanisms underlying observed clinical benefits of IDET are compelling though widely speculative. Collagen denaturation and contraction may increase disc stability, heating-induced fibroblast activation may lead to reduced mobility and pain, and coagulation of granulation tissue may reduce inflammatory mediators responsible for presensitizing nerve fibers. Furthermore, the direct effect of coagulating nociceptive fibers in the anulus may reduce pain; however, the postprocedure pain flare that is observed clinically suggests that neural coagulation is not wholly responsible for any observed benefits. Though neural coagulation often is cited as a primary mechanism for IDET, convincing evidence does not currently exist.48–51
Much of the debate in the literature scrutinizes the thermal distribution in the anulus during IDET. Clearly, temperatures less than 45°C are necessary at the posterior longitudinal ligament and the neural foramina to prevent iatrogenic neurologic impairment. Closer to the catheter, temperatures of 60°C to 65°C are theoretically optimal to disrupt hydrogen cross-linking in the collagen triple helix, causing collagen contraction. Southern et al. describe collagen fibrillar disorganization on histology and evidence of collagen denaturation on quantitative spectroscopy with IDET.50 Despite evidence of functional and histologic changes in cadaver models, attempts to map the thermal distribution of IDET in cadaveric discs have demonstrated contradictory results.29,49,50
Also theorized, heating to temperatures that do not cause irreversible cellular damage will lead to fibroblast activation, anular thickening, and stabilization in the intervertebral disc following IDET.28 In vivo models in sheep, though, have not been encouraging. One such study presented by Bass et al. showed that high-dose thermal treatments led to biomechanical degradation, while low-dose and sham intervention led to little biomechanical change at 180 days post IDET.52
Pollintine et al. found that IDET has a significant but inconsistent effect on compressive stress distribution in 18 cadaveric motion segments of the lumbar spine. Twelve “responders” showed a 78% mean reduction in anular peak pressure; two discs demonstrated increases in anular peak pressure; four discs demonstrated no change. These outcomes did not correspond to degeneration grade or fissuring present in the disc.53 Lee et al. found no significant difference in the stability of five cadaveric motion segments after IDET (P > .05).54 However, another study demonstrated a 10% mean increase in motion at 5 Nm torque.49
Subgroups of Intradiscal Electrothermal Therapy
Variations on the theme of intradiscal thermal anuloplasty-type procedures have been reported. Saal and Saal performed their IDET procedure with the SpineCATH (ORATEC Interventions, Inc., Menlo Park, CA) with a 5-cm active thermal tip. Also available is the Decompression catheter (Smith & Nephew, Memphis, TN), with a 1.5-cm active thermal tip. Differences in the heating protocol may lead to tissue temperature increases of 13°C to 15°C compared to those with the 5-cm catheter. 28 However, any benefit of increased temperature remains unproven, as a retrospective analysis by Derby et al. found that procedures achieving higher temperature via SpineCATH showed no correlation with improved outcomes at 16 months; in fact, a correlation with negative outcomes at 8 months was observed.55
Higher temperatures appear to increase the postprocedure pain flare without significantly affecting outcomes.55 A cadaveric study performed by Southern et al. comparing the SpineCATH and the Decompression catheters demonstrated temperatures sufficient to denature collagen using both instruments, though the clinical importance of collagen alteration has not been established.51
It is important to distinguish intradiscal anuloplasty-type procedures from the broad category of percutaneous intradiscal intervention. For example, radiofrequency intradiscal interventions with either unipolar or bipolar electrodes are commonly employed.28 Barendse et al. performed a randomized, controlled trial demonstrating no effect from radiofrequency thermocoagulation at the center of the disc.56 It is inappropriate to use this and other studies in reference therapies targeting the anular pathology specifically.
Another common variation uses radiofrequency energy targeted at disruptions in the posterior anulus (DiscTRODE, Radionics, Burlington, MA). One study demonstrated benefit compared to conservative therapy only in a subgroup of individuals studied.57 However, a recent randomized, double-blind controlled trial demonstrated no benefit at 12 months and increased pain in some patients following radiofrequency intra-anular thermal therapy.58 Furthermore, Kapural et al. present a comparison between radiofrequency intradiscal anuloplasty and thermal intradiscal anuloplasty. Radiofrequency treatment led to no observed improvement, while thermal treatment showed significant improvement in pain and self-reported function.59 Therefore, generalization between thermal and radiofrequency intradiscal anuloplasty-type procedures may not be possible.
Bipolar radiofrequency therapy, known as biacuplasty, has been performed with the Trans-Discal system (Baylis Medical, Montreal, Canada). This new technology delivers a bipolar current through the posterior anulus. This has been studied with a porcine model, a cadaveric application, and a 12-month, 15-patient prospective pilot study demonstrating sufficient temperatures for neural ablation, safe temperatures near neural structures, and clinical benefits enduring up to one year in about half the patients treated.60–62 More research is needed to comment on the usefulness of biacuplasty in the treatment of chronic discogenic back pain.
Outcomes
Pauza et al. in 2004 conducted the first randomized, placebo-controlled, prospective trial to address IDET. The study presents the 6-month follow-up of 64 patients randomized to either IDET or sham IDET procedure, though eight patients were excluded or lost. The group found more than 50% pain relief in 40% of those who had undergone IDET; however, 50% of those in the IDET group reported no significant pain relief. To achieve 75% pain relief, the number needed to treat was five. The group that had undergone sham treatment did demonstrate improvements, but the IDET group demonstrated significantly less pain on visual analogue scale (VAS) scoring (P = .045) and better function outcome on the Oswestry Disability Scale (P = .050). The authors concluded that the efficacy of IDET cannot be entirely attributed to a placebo effect.63
Freeman et al. in 2005 presented contradictory results from a prospective, randomized, double-blind, placebo-controlled trial of 57 patients followed for 6 months, though 2 patients were excluded or lost. No significant improvement compared to placebo was observed. The authors attributed this difference to methodologic differences as well as to more significant disability in the present study when compared to Pauza et al.64
Though Freeman et al. argue that the statistical significance observed by Pauza et al. does not necessarily represent clinical significance, proponents of IDET point out that the benefits observed by Pauza et al. are the result of more stringent selection criteria.64,65 For example, Freeman et al. included individuals with multilevel disc degeneration, full-thickness anular tears, and up to 50% loss in disc height.65 Freeman et al. state that secondary analysis of patients with single-level disease yielded no statistically significant or clinically important benefits.64
It is difficult to reconcile the data from the two randomized, controlled trials on the matter. The majority of prospective controlled and observational trials have demonstrated significant benefit in highly selected patient groups.66
Saal and Saal presented their own prospective 2-year follow-up data for 58 patients; 27 had undergone single-level treatment, and 28 had undergone multilevel treatment.30 At 24 months, 72% reached at least a two-point reduction in pain based on the VAS score, and 50% reached at least a four-point pain reduction. Similarly, 59% reached at least a 14-point improvement in the SF-36. No significant difference was observed in single-level or multilevel IDET.
In an observational study of 35 IDET-treated individuals with a “convenience control” of 17 individuals who were denied IDET by their insurance provider, Bogduk and Karasek reported the 2-year follow-up, with about 54% experiencing more than 50% improvement in pain and 20% of patients treated with IDET experiencing complete relief; only 1 control patient partially improved.31
Kapural et al. compared a group with multilevel disc degeneration to a group with one- or two-level disc degeneration. The two groups started out with a difference in VAS pain scores of .353 (P = .6673) that was not significant; 12 months post-IDET, a significant difference in VAS pain scores of 2.412 (P = .0037) was observed. Twelve of 17 from the one- or two-level group maintained more than 50% pain improvement at 12 months. Seven of 17 in the multilevel group maintained more than 50% pain improvement at 12 months. Improvement in Pain Disability Index–based assessment of function was also significantly different between the groups at 6 and 12 months (P = .455, P = .410, respectively).36 The results of this comparative study are augmented by a prospective study from the same authors, which includes patients with only mild one- or two-level degenerative disc disease. This study demonstrates an average 78% pain decrease based on VAS at 12 months.33
Because of almost universally poor outcomes among workers’ compensation claimants, a number of studies have investigated the utility of IDET in this population. Among 53 claimants, Nunley et al. found a mean 63% reduction in VAS score and 69% reduction in Oswestry scores at 12 months; 19 patients halted consumption of narcotic pain medications.67 This does correlate with the outcome study by Mekhail and Kapural, which demonstrated a difference in the IDET outcome of workers’ compensation claimants compared to nonclaimants, although both groups did benefit from treatment.33
In a recent review of the literature, Kloth et al. state that with proper inclusion criteria clinicians may expect benefit from IDET in 3 out of 4 patients treated.38 A meta-analysis by Appleby et al. in 2006 concludes that there is compelling evidence for the relative efficacy of IDET.68 Derby et al., in a systematic review, support IDET as treatment more for pain than for disability in a highly selected group.28 However, in a critical appraisal of the evidence, Freeman states that evidence of efficacy has not yet passed the standard of scientific proof.69
A systematic review by Helm et al. and the American Society of Intervention Pain Physicians–Intervention Pain Management guidelines grade the available evidence at level II-2 according to the U.S. Preventive Services Task Force grading of evidence.66,70 A 2A/weak recommendation for IDET was given in both publications, with the conclusion that the benefits are closely balanced by the risks and burdens of the procedure.70
Freeman B.J., Fraser R.D., Cain C.M., et al. A randomized, double-blind, controlled trial: intradiscal electrothermal therapy versus placebo for the treatment of chronic discogenic low back pain. Spine (Phila Pa 1976). 2005;30(21):2369-2377. discussion 2378
Kapural L., Hayek S., Malak O., et al. Intradiscal thermal annuloplasty versus intradiscal radiofrequency ablation for the treatment of discogenic pain: a prospective matched control trial. Pain Med. 2005;6(6):425-431.
Kloth D.S., Fenton D.S., Andersson G.B. Block JE: Intradiscal electrothermal therapy (IDET) for the treatment of discogenic low back pain: patient selection and indications for use. Pain Physician. 2008;11(5):659-668.
Manchikanti L., Boswell M.V., Singh V., et al. Comprehensive evidence-based guidelines for interventional techniques in the management of chronic spinal pain. Pain Physician. 2009;12(4):699-802.
Mekhail N., Kapural L. Intradiscal thermal annuloplasty for discogenic pain: an outcome study. Pain Pract. 2004;4(2):84-90.
Pauza K.J., Howell S., Dreyfull P., et al. A randomized, placebo-controlled trial of intradiscal electrothermal therapy for the treatment of discogenic low back pain. Spine J. 2004;4(1):27-35.
Saal J.A., Saal J.S. Intradiscal electrothermal therapy for the treatment of chronic discogenic low back pain. Clin Sports Med. 2002;21(1):167-187.
1. Carey T.S., Garrett J.M., Jackman A.M. Beyond the good prognosis. Examination of an inception cohort of patients with chronic low back pain. Spine (Phila Pa 1976). 2000;25(1):115-120.
2. Andersson G.B. Epidemiological features of chronic low-back pain. Lancet. 1999;354(9178):581-585.
3. Walker J.3rd, El Abd O., Isaac Z. Muzin S: Discography in practice: a clinical and historical review. Curr Rev Musculoskelet Med. 2008;1(2):69-83.
4. Cassidy J.D., Côté P., Carroll L.J. Kristman V: Incidence and course of low back pain episodes in the general population. Spine (Phila Pa 1976). 2005;30(24):2817-2823.
5. Shah R.V., Lutz G.E., Lee J., et al. Intradiskal electrothermal therapy: a preliminary histologic study. Arch Phys Med Rehabil. 2001;82(9):1230-1237.
6. Frank J.W., Kerr M.S., Brooker A.S., et al. Disability resulting from occupational low back pain. I: What do we know about primary prevention? A review of the scientific evidence on prevention before disability begins. Spine (Phila Pa 1976). 1996;21(24):2908-2917.
7. Margo K. Diagnosis, treatment and prognosis in patients with low back pain. Am Fam Physician. 1994;49(1):171-179. 183–184
8. Schwarzer A.C., Aprill C.N., Derby R., et al. The prevalence and clinical features of internal disc disruption in patients with chronic low back pain. Spine (Phila Pa 1976). 1995;20(17):1878-1883.
9. Kuslich S.D., Ulstrom C.L., Michael C.J. The tissue origin of low back pain and sciatica: a report of pain response to tissue stimulation during operations on the lumbar spine using local anesthesia. Orthop Clin North Am. 1991;22(2):181-187.
10. Wetzel F.T., LaRocca S.H., Lowrey G.L. Aprill CN: The treatment of lumbar spinal pain syndromes diagnosed by discography. Lumbar arthrodesis. Spine (Phila Pa 1976). 1994;19(7):792-800.
11. Thomsen K., Christensen F.B., Eiskjaer S.P., et al. 1997 Volvo Award winner in clinical studies: the effect of pedicle screw instrumentation on functional outcome and fusion rates in posterolateral lumbar spinal fusion: a prospective, randomized clinical study. Spine (Phila Pa 1976). 1997;22(24):2813-2822.
12. Saal J.S., Saal J.A. Management of chronic discogenic low back pain with a thermal intradiscal catheter: a preliminary report. Spine (Phila Pa 1976). 2000;25(3):382-388.
13. Saal J.A., Saal J.S. Intradiscal electrothermal therapy for the treatment of chronic discogenic low back pain. Clin Sports Med. 2002;21(1):167-187.
14. Chung S.A., Khan S.N., Diwan A.D. The molecular basis of intervertebral disk degeneration. Orthop Clin North Am. 2003;34(2):209-219.
15. Moore R.J., Vernon-Roberts B., Fraser R.D., et al. The origin and fate of herniated lumbar intervertebral disc tissue. Spine (Phila Pa 1976). 1996;21(18):2149-2155.
16. Ito M., Incorvaia K.M., Yu S.F., et al. Predictive signs of discogenic lumbar pain on magnetic resonance imaging with discography correlation. Spine (Phila Pa 1976). 1998;23(11):1252-1258. discussion 1259–1260
17. Aprill C., Bogduk N. High-intensity zone: a diagnostic sign of painful lumbar disc on magnetic resonance imaging. Br J Radiol. 1992;65(773):361-369.
18. Peng B., Hou S., Wu W., et al. The pathogenesis and clinical significance of a high-intensity zone (HIZ) of lumbar intervertebral disc on MR imaging in the patient with discogenic low back pain. Eur Spine J. 2006;15(5):583-587.
19. Freemont A.J., Watkins A., Le Maitre C., et al. Nerve growth factor expression and innervation of the painful intervertebral disc. J Pathol. 2002;197(3):286-292.
20. Gilchrist R.V., Isaac Z., Bhat A.L. Innervation of the anterior spinal canal: an update. Pain Physician. 2002;5(2):167-171.
21. O’Neill C.W., Kurgansky M.E., Derby R. Ryan DP: Disc stimulation and patterns of referred pain. Spine (Phila Pa 1976). 2002;27(24):2776-2781.
22. Fagan A., Moore R., Vernon Roberts B., et al. ISSLS prize winner: the innervation of the intervertebral disc: a quantitative analysis. Spine (Phila Pa 1976). 2003;28(23):2570-2576.
23. Coppes M.H., Marani E., Thomeer R.T. Groen GJ: Innervation of “painful” lumbar discs. Spine (Phila Pa 1976). 1997;22(20):2342-2349. discussion 2349–2350
24. Freemont A.J., Peacock T.E., Goupille P., et al. Nerve ingrowth into diseased intervertebral disc in chronic back pain. Lancet. 1997;350(9072):178-181.
25. Kang J.D., Georgescu H.I., McIntyre-Larkin L., et al. Herniated lumbar intervertebral discs spontaneously produce matrix metalloproteinases, nitric oxide, interleukin-6, and prostaglandin E2. Spine (Phila Pa 1976). 1996;21(3):271-277.
26. Franson R.C., Saal J.S., Saal J.A. Human disc phospholipase A2 is inflammatory. Spine (Phila Pa 1976). 1992;17(Suppl 6):S129-S132.
27. Kawakami M., Tamaki T., Weinstein J.N., et al. Pathomechanism of pain-related behavior produced by allografts of intervertebral disc in the rat. Spine (Phila Pa 1976). 1996;21(18):2101-2107.
28. Derby R., Baker R.M., Lee C.H. Anderson PA: Evidence-informed management of chronic low back pain with intradiscal electrothermal therapy. Spine J. 2008;8(1):80-95.
29. Bono C.M., Iki K., Jalota A., et al. Temperatures within the lumbar disc and endplates during intradiscal electrothermal therapy: formulation of a predictive temperature map in relation to distance from the catheter. Spine (Phila Pa 1976). 2004;29(10):1124-1129. discussion 1130–1131
30. Saal J.A., Saal J.S. Intradiscal electrothermal treatment for chronic discogenic low back pain: prospective outcome study with a minimum 2-year follow-up. Spine (Phila Pa 1976). 2002;27(9):966-973. discussion 973–974
31. Bogduk N., Karasek M. Two-year follow-up of a controlled trial of intradiscal electrothermal anuloplasty for chronic low back pain resulting from internal disc disruption. Spine J. 2002;2(5):343-350.
32. Freedman B.A., Cohen S.P., Kuklo T.R., et al. Intradiscal electrothermal therapy (IDET) for chronic low back pain in active-duty soldiers: 2-year follow-up. Spine J. 2003;3(6):502-509.
33. Mekhail N., Kapural L. Intradiscal thermal annuloplasty for discogenic pain: an outcome study. Pain Pract. 2004;4(2):84-90.
34. Saal J.A., Saal J.S. Intradiscal electrothermal treatment for chronic discogenic low back pain: a prospective outcome study with minimum 1-year follow-up. Spine (Phila Pa 1976). 2000;25(20):2622-2627.
35. Maurer P., Block J.E., Squillante D. Intradiscal electrothermal therapy (IDET) provides effective symptom relief in patients with discogenic low back pain. J Spinal Disord Tech. 2008;21(1):55-62.
36. Kapural L., Mekhail N., Korunda Z., Basali A. Intradiscal thermal annuloplasty for the treatment of lumbar discogenic pain in patients with multilevel degenerative disc disease. Anesth Analg. 2004;99(2):472-476. table of contents
37. Cohen S.P., Larkin T., Abdi S., et al. Risk factors for failure and complications of intradiscal electrothermal therapy: a pilot study. Spine (Phila Pa 1976). 2003;28(11):1142-1147.
38. Kloth D.S., Fenton D.S., Andersson G.B. Block JE: Intradiscal electrothermal therapy (IDET) for the treatment of discogenic low back pain: patient selection and indications for use. Pain Physician. 2008;11(5):659-668.
39. Hsia A.W., Isaac K., Katz J.S. Cauda equina syndrome from intradiscal electrothermal therapy. Neurology. 2000;55(2):320.
40. Wetzel F.T. Cauda equina syndrome from intradiscal electrothermal therapy. Neurology. 2001;56(11):1607.
41. Ackerman W.E.3rd. Cauda equina syndrome after intradiscal electrothermal therapy. Reg Anesth Pain Med. 2002;27(6):622.
42. Djurasovic M., Glassman S.D., Dimar J.R.2nd, Johnson J.R. Vertebral osteonecrosis associated with the use of intradiscal electrothermal therapy: a case report. Spine (Phila Pa 1976). 2002;27(13):E325-E328.
43. Scholl B.M., Theiss S.M., Lopez-Ben R., Kraft M. Vertebral osteonecrosis related to intradiscal electrothermal therapy: a case report. Spine (Phila Pa 1976). 2003;28(9):E161-E164.
44. Cohen S.P., Larkin T., Polly D.W.Jr. A giant herniated disc following intradiscal electrothermal therapy. J Spinal Disord Tech. 2002;15(6):537-541.
45. Derby R. Intradiscal electrothermal annuloplasty: current concepts. Pain Physician. 2003;6(3):383-385.
46. Orr R.D., Thomas S.A. Intradural migration of broken IDET catheter causing a radiculopathy. J Spinal Disord Tech. 2005;18(2):185-187.
47. Saal JA, Saal JS, Wetzel FT, et al: IDET related complications: a multi-center study of 1675 treated patients with a review of the FDA MDR data base. Proceedings of the 16th Annual Meeting of the North American Spine Society
48. Freeman B.J., Walters R.M., Moore R.J., Fraser R.D. Does intradiscal electrothermal therapy denervate and repair experimentally induced posterolateral annular tears in an animal model? Spine (Phila Pa 1976). 2003;28(23):2602-2608.
49. Kleinstueck F.S., Diederich C.J., Nau W.H., et al. Acute biomechanical and histological effects of intradiscal electrothermal therapy on human lumbar discs. Spine (Phila Pa 1976). 2001;26(20):2198-2207.
50. Kleinstueck F.S., Diederich C.J., Nau W.H., et al. Temperature and thermal dose distributions during intradiscal electrothermal therapy in the cadaveric lumbar spine. Spine (Phila Pa 1976). 2003;28(15):1700-1708. discussion 1709
51. Southern D., Lutz G., Bracilovic A., et al. Histological and molecular structure characterization of annular collagen after intradiskal electrothermal annuloplasty. HSS J. 2006;2(1):49-54.
52. Bass E.C., Nau W.H., Diederich C.J., et al. Intradiscal thermal therapy does not stimulate biologic remodeling in an in vivo sheep model. Spine (Phila Pa 1976). 2006;31(2):139-145.
53. Pollintine P., Findlay G., Adams M.A. Intradiscal electrothermal therapy can alter compressive stress distributions inside degenerated intervertebral discs. Spine (Phila Pa 1976). 2005;30(6):E134-E139.
54. Lee J., Lutz G.E., Campbell D., et al. Stability of the lumbar spine after intradiscal electrothermal therapy. Arch Phys Med Rehabil. 2001;82(1):120-122.
55. Derby R., Seo K.S., Kazala K., et al. A factor analysis of lumbar intradiscal electrothermal annuloplasty outcomes. Spine J. 2005;5(3):256-261. discussion 262
56. Barendse G.A., van Den Berg S.G., Kessels A.H., et al. Randomized controlled trial of percutaneous intradiscal radiofrequency thermocoagulation for chronic discogenic back pain: lack of effect from a 90-second 70 C lesion. Spine (Phila Pa 1976). 2001;26(3):287-292.
57. Finch P.M., Price L.M., Drummond P.D. Radiofrequency heating of painful annular disruptions: one-year outcomes. J Spinal Disord Tech. 2005;18(1):6-13.
58. Kvarstein G., Måwe L., Indahl A., et al. A randomized double-blind controlled trial of intra-annular radiofrequency thermal disc therapy: a 12-month follow-up. Pain. 2009;145(3):279-286.
59. Kapural L., Hayek S., Malak O., et al. Intradiscal thermal annuloplasty versus intradiscal radiofrequency ablation for the treatment of discogenic pain: a prospective matched control trial. Pain Med. 2005;6(6):425-431.
60. Petersohn J.D., Conquergood L.R., Leung M. Acute histologic effects and thermal distribution profile of disc biacuplasty using a novel water-cooled bipolar electrode system in an in vivo porcine model. Pain Med. 2008;9(1):26-32.
61. Pauza K. Cadaveric intervertebral disc temperature mapping during disc biacuplasty. Pain Physician. 2008;11(5):669-676.
62. Kapural L. Intervertebral disk cooled bipolar radiofrequency (intradiskal biacuplasty) for the treatment of lumbar diskogenic pain: a 12-month follow-up of the pilot study. Pain Med. 2008;9(4):407-408.
63. Pauza K.J., Howell S., Dreyfuss P., et al. A randomized, placebo-controlled trial of intradiscal electrothermal therapy for the treatment of discogenic low back pain. Spine J. 2004;4(1):27-35.
64. Freeman B.J., Fraser R.D., Cain C.M., et al. A randomized, double-blind, controlled trial: intradiscal electrothermal therapy versus placebo for the treatment of chronic discogenic low back pain. Spine (Phila Pa 1976). 2005;30(21):2369-2377. discussion 2378
65. Andersson G.B., Mekhail N.A., Block J.E. A randomized, double-blind, controlled trial: intradiscal electrothermal therapy versus placebo for the treatment of chronic discogenic low back pain. Spine (Phila Pa 1976). 2006;31(14):1637-1638. author reply 1638
66. Helm S., Hayek S.M., Benyamin R.M., Manchikanti L. Systematic review of the effectiveness of thermal annular procedures in treating discogenic low back pain. Pain Physician. 2009;12(1):207-232.
67. Nunley P.D., Jawahar A., Brandao S.M., Wilkinson K.M. Intradiscal electrothermal therapy (IDET) for low back pain in worker’s compensation patients: can it provide a potential answer? Long-term results. J Spinal Disord Tech. 2008;21(1):11-18.
68. Appleby D., Andersson G., Totta M. Meta-analysis of the efficacy and safety of intradiscal electrothermal therapy (IDET). Pain Med. 2006;7(4):308-316.
69. Freeman B.J. IDET: a critical appraisal of the evidence. Eur Spine J. 2006;15(Suppl 3):S448-S457.
70. Manchikanti L., Boswell M.V., Singh V., et al. Comprehensive evidence-based guidelines for interventional techniques in the management of chronic spinal pain. Pain Physician. 2009;12(4):699-802.