CHAPTER 367 Intracranial Internal Carotid Artery Aneurysms
Saccular aneurysms of the internal carotid artery (ICA) trunk and posterior communicating segment represent about 30% to 50% of all intracranial aneurysms. Although in the past these aneurysms were considered relatively easier to approach surgically than other aneurysms, currently most of them are amenable to endovascular coil embolization, which has proved to be less risky in selected cases.1 Consequently, the remaining aneurysms referred for surgical treatment are no longer straightforward cases, are generally large or giant, and incorporate a major artery into their neck. These aneurysms may have a complex anatomy and relationship to surrounding neurovascular structures in the subarachnoid space; thus, an intimate understanding of the relationship of the aneurysm to these structures is necessary and can be achieved by careful assessment using multislice computed tomographic angiography (CTA), three-dimensional CTA,2 and if necessary, four-vessel cerebral angiography.
Diagnostic Evaluation
In the setting of subarachnoid hemorrhage (SAH), computed tomography (CT) scan of the brain is the investigation of choice to detect blood in the subarachnoid space. It is extremely sensitive for detecting subarachnoid blood in the acute phase. It also gives an idea about the possible location of the aneurysm, which may be helpful in determining the aneurysm that has likely ruptured in a patient with multiple intracranial aneurysms. CTA, three-dimensional CTA, and magnetic resonance angiography (MRA) have shown reliable results in detecting aneurysms equal to or grater than 2 to 3 mm in diameter.2–4 Digital subtraction angiography (DSA) remains the “gold standard” when the CTA findings are negative or doubtful and when dynamic studies need to be undertaken. It is superior to other diagnostic modalities in determining certain characteristics of the aneurysm, although the current CTA technology can better detect some features such as intra-aneurysmal calcification and atherosclerotic changes in the parent vessel as well as the relationship with the bony structures intracranially. The possibility of sacrificing the posterior communicating artery during clipping of the aneurysm, which is extremely dangerous in patients with fetal origin of posterior cerebral artery, could be evaluated using dynamic DSA. DSA also demonstrates some of the perforating arteries in and around the aneurysm and the parent vessel. The location of the proximal neck of the aneurysm and the projection of the angiographic pictures are extremely important in deciding the surgical strategy and the need for additional bone removal. It is now routine practice to manage intracranial aneurysms based on CTA5 results, and DSA is requested only in specific situations, such as very large or giant aneurysms or the need for dynamic studies and carotid test occlusion.
Operative Management
Preoperative Care
Based on the presentation of the patient, preoperative preparations vary. Patients with SAH are first checked for airway, breathing, and circulation, and are then assessed neurologically to determine SAH clinical grade using the World Federation of Neurological Surgeons (WFNS) grading system.6 The four major issues to be addressed before planning a strategy to obliterate the aneurysm are rebleeding, hydrocephalus, electrolyte abnormalities, and vasospasm. Rebleeding can be as high as 6% in the first 48 hours and may be associated with devastating results. Hydrocephalus may occur as early as a few hours after the hemorrhage, and when shown on a CT scan of a patient with a poor-grade SAH or a patient whose condition has deteriorated, an external ventricular drain will help return most of these patients back to a better grade. Serum electrolyte disturbance is also seen after SAH and must be corrected before deciding on management plans. Vasospasm starts and peaks at day 3 through day 14 and kills or severely disables about 14% of patients.7
Specific Aneurysm Location
Posterior Communicating Artery Aneurysms
Anatomy
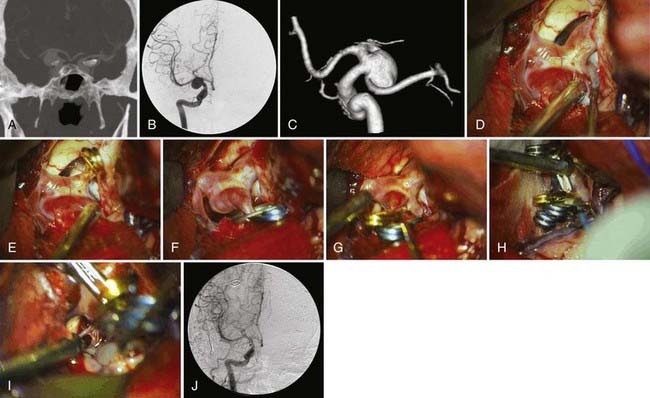
The communicating segment of the ICA (C7 segment)8 begins just below the posterior communicating artery and ends at the bifurcation. Two major arterial branches—the posterior communicating artery and the anterior choroidal artery—arise from this segment. The posterior communicating artery arises from the posteromedial surface of the ICA and courses medially and inferiorly, through the membrane of Liliequist, above and medial to the oculomotor nerve, to join the posterior cerebral artery at the junction of the P1 and P2 segments of the latter. Multiple perforators arise from the posterior communicating artery and are named the anterior thalamic perforators. These can be stuck to the aneurysm and should not be clipped with the aneurysm. In about 20% of patients, the P1 segment of the posterior cerebral artery is hypoplastic, and the posterior cerebral artery arises directly from the posterior communicating artery.9 This is called fetal origin of posterior cerebral artery, and in these patients, the posterior communicating artery cannot be sacrificed, and the aneurysm must be clipped in a way to guarantee patency of the parent vessel.
The typical posterior communicating artery aneurysm arises just distal to the origin of the artery from the wall of the ICA and hence is classified as an ICA aneurysm. It projects posteriorly, laterally, and slightly inferiorly and may pinch the oculomotor nerve as it enters the dural fold of cavernous sinus, and hence the third nerve palsy, with an acutely expanded posterior communicating aneurysm. It does not usually point medially and so does not bleed into the sella because its pushed out by the curve of the internal carotid laterally. However, some posterior communicating artery aneurysms arise just proximal to the posterior communicating artery origin and might have a slightly less lateral or even medial projection.10
Presentation
Aneurysms of this segment of the ICA are the most common type of ICA aneurysms, representing about 50%,11 and are more common in females. They usually cause symptoms when smaller than 10 mm in patients with SAH, with a lateral suprasellar and ambient cistern pattern, intraparenchymal hemorrhage into the uncus of the temporal lobe, intraventricular hemorrhage into the temporal horn, or hemorrhage into the subdural space, or they could expand and compress the third cranial nerve, causing painful non–pupil-sparing oculomotor nerve palsy. SAH could also irritate the dura and cause retro-orbital pain,12 The environmental conflict of this location has been suggested as a risk factor for rupture of these aneurysms with smaller size.13 The International Study of Unruptured Intracranial Aneurysms (ISUIA) has shown a likelihood of rupture for this location, similar to that associated with posterior circulation aneurysms.14
Operative Technique
The large bur of the drill is used to make a bur hole in the posterior temporal region under the muscle, and the dura is stripped away from the bone to allow placement of the foot plate epidurally. The drill is used to carry out the craniotomy, and the keyhole region is drilled down to the internal sphenoid ridge. The dura is then separated from the sphenoid wing medially, and the wing is either drilled or rongeured to enter the lateral exposure of the superior orbital fissure. The frontal inner table is then beveled with the drill. If the frontal air sinus is opened, it is exenterated and packed with the muscle piece and covered with the vascularized pericranial flap and fibrin adhesive at the end of the procedure. The dura at the edge of the craniotomy is then tacked up to the bone through tangential holes. A curvilinear incision is made in the dura, and the dural flap is reflected anteriorly. If the brain is still full despite mannitol and hyperventilation to PCO2 of 25 to 30 mmol/L, especially if the patient has hydrocephalus, a catheter is passed into the frontal horn of the lateral ventricle 2.5 cm above the base of the frontal lobe and 2.5 cm anterior to the sylvian fissure.15 Wide splitting of the fissure should be performed for all aneurysms in the anterior circulation to minimize brain retraction. The aneurysm can be exposed without brain retractors because the surgeon can use microsurgical bipolar forceps and the microsuction simultaneously to keep the fissure open and work around the aneurysm. The use of retractors is recommended for ruptured aneurysms and when the splitting of the fissure is completed.
Dissection on the ICA should be done on the anterosuperior surface until proximal and distal control is achieved. The optical-carotid triangle is opened, and dissection is continued on the medial aspect of the ICA unless the aneurysm is pointing medially on the preoperative angiogram. The clot on the base of the aneurysm is swiped away from the neck to visualize it better. The posterior communicating artery and its anterior thalamic perforators and the anterior choroidal artery are identified. Anteromedial retraction on the ICA is dangerous because it may pull on the dome of the aneurysm and tear it; occasionally, the dome may be stuck to the third nerve, and traction may cause permanent damage to this nerve. After identifying the proximal and distal ends of the neck, a straight clip can usually occlude the neck completely. Occasionally, a right-angle fenestrated clip that incorporates more than 180 degrees of the carotid circumference, keeping the ICA in the fenestration and the blades parallel to it, may be necessary for broad-based neck and more medially projecting aneurysms. After applying the clip, the tips are inspected to ensure complete closure around the aneurysm and patency of the posterior communicating artery, thalamoperforator, and most important, anterior choroidal artery. A small residual neck might be left to maintain the caliber of the parent vessel (ICA). Many of the current surgical cases have the proximal posterior communicating artery incorporated into the aneurysm, which is the reason for failure of a safe endovascular treatment (Fig. 367-1). The general rule is to preserve the posterior communicating artery and the fetal posterior communicating artery (see Fig. 367-1); however, if the preoperative angiogram ensures the absence of ipsilateral fetal origin of the posterior cerebral artery and confirms filling of the artery from the posterior circulation, the origin of the posterior communicating artery may be included in the clip (if there is no other alternative). This is followed by a second clip applied between the aneurysm and the first thalamoperforator. Temporary clipping of the parent artery should be used in large aneurysms to reduce the flow in order to reconstruct the parent vessel under low pressure. This will help to avoid tearing the aneurysm neck. Temporary clipping is done for no longer than 3 minutes at a time, while allowing at least 5 minutes between temporary clips. After clipping the aneurysm, the dome may be pulled and punctured with a 25-gauge needle to ensure obliteration. Patency of the carotid is confirmed with intraoperative Doppler. Blood clots in the underlying cistern are then washed out, although this has not shown to be effective against vasospasm development.16,17
Specific Consideration: Selective Intradural Anterior Clinoidectomy
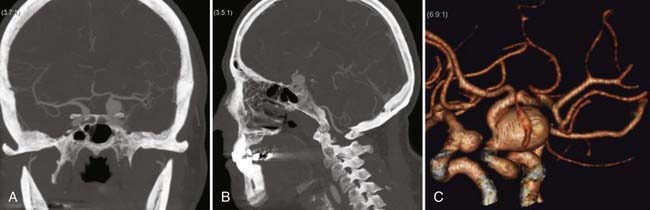
In some particular posterior communicating artery aneurysms (or proximal carotid aneurysms) with a very proximal neck, the intraoperative exposure is not satisfactory unless the anterior clinoid process can be removed. Thorough analysis of diagnostic imaging is essential to identify this subgroup in which the proximal control should be obtained at the cervical carotid, especially in the setting of ruptured aneurysms (Fig. 367-2). After exposure of the distal neck of the aneurysm, a selective intradural anterior clinoidectomy is performed, with separation of the clinoid dura laterally and drilling of the process with a 3-mm diamond drill. The direction of the drilling is from medial to lateral, from the optic nerve toward the superior orbital fissure, and finally the optic strut is drilled and the clinoid process freed from adjacent bony structures. If the proximal neck of the aneurysm is found to be under the distal dural ring of the carotid artery, the duraring and the falciform ligaments are opened to access the proximal neck. Venous bleeding from the lateral cavernous sinus wall is common during this part of the procedure and generally responds to tamponade and pressure.
Anterior Choroidal Artery Aneurysms
Anatomy
According to the new classification of the carotid artery segments,8 aneurysms of the anterior choroidal artery arise from the C7 segment, which is also called the posterior communicating segment; whereas in the commonly used classification, it arises from the choroidal segment, which begins at the take-off of the anterior choroidal artery and ends at the carotid bifurcation. This artery is the only named branch that arises from this segment, and it arises distal and lateral to the posterior communicating artery. It has a characteristic course, swinging initially laterally and then posteriorly, following the optic tract and supplying a branch to the mesial temporal structures. The main trunk then continues posteriorly, inferior to the optic tract, to enter the choroid fissure.18 The size of this artery is variable, and duplication occurs in as many as 30% of normal autopsy specimens.18
Internal Carotid Artery Bifurcation Aneurysms
Anatomy
The bifurcation of the ICA into an anterior and middle cerebral artery takes place beneath the basal forebrain and the anterior perforated substance. The anterior cerebral artery (ACA) passes forward and medially over the optic nerve to meet its counterpart in the midline through the anterior communicating artery. It sends perforating branches to the basal forebrain and gives rise to the recurrent artery of Heubner, which passes medial to the carotid bifurcation and its lenticulostriate perforators.19 The middle cerebral artery (MCA) passes laterally and then posteriorly, dividing under cover of the frontal and parietal opercula. Aneurysms of the ICA bifurcation therefore tend to point up in the direction of the jet of blood inside the vessel toward the anterior perforated substance. The aneurysm usually points anterosuperiorly, straight superiorly, or posterosuperiorly, but in most cases, the lenticulostriate perforators of the ICA are displaced posteriorly and may be adherent to the aneurysm. These perforators usually supply the basal ganglia but may also supply the optic apparatus, hypothalamus, and mesial temporal lobe.
Operative Technique
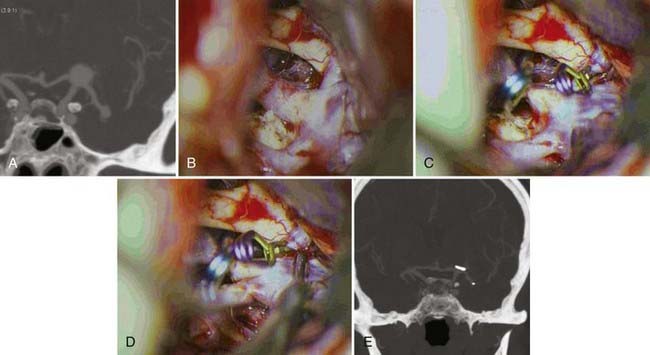
The positioning and standard pterional craniotomy are done in the same fashion described for posterior communicating aneurysms. With the aid of the microscope, the sylvian fissure should be widely split. Before approaching the aneurysm, proximal control must be obtained; after which the inferior aspects of both the ACA and MCA should be exposed. Only then can the arachnoid membrane around the bifurcation be rolled up to expose the neck of the aneurysm and the perforating vessels. The dome of the aneurysm, which is usually buried into the substance of the basal forebrain, should not be disturbed. A small frontal corticotomy may be performed to facilitate visualization of the lenticulostriate and the recurrent artery of Heubner. In the setting of SAH, the lamina terminalis is opened for CSF drainage and better visualization of the anterior communicating complex and its perforators. This has been shown to decrease the rate of shunt-dependent hydrocephalus.20 The aneurysm neck is dissected from the superior aspect of the ACA anteriorly and the MCA posteriorly; the recurrent artery of Heubner is then identified running medial to and behind the bifurcation. The type and direction of the clip to be used are dictated by the configuration of the aneurysm (Fig. 367-3). Usually, a straight clip or laterally angled straight clip is applied perpendicular to the direction of ACA and MCA. The clip should not exceed the size of the aneurysm to avoid clipping the lenticulostriate perforators, recurrent artery of Heubner, basal vein of Rosenthal, or deep sylvian vein. After clip placement, the vessels are inspected and checked with Doppler, and the aneurysm is punctured with a 25-gauge needle to ensure obliteration. Meticulous hemostasis is secured, followed by dural closure and then closure of the craniotomy and the soft tissues.
Blood Blister–like Aneurysms at Nonbranching Sites of the Internal Carotid Artery
Aneurysms have also been located in the dorsal, distal medial, superior, and anterior walls of the ICA. These lesions are classified into two types: saccular aneurysms and blood blister–like aneurysms. The latter are thin-walled, broad-based aneurysms that lack an identifiable neck. They are fragile and can rupture during microsurgery, causing postoperative rebleeding more frequently than saccular aneurysms. The diagnosis of these rare aneurysms is crucial before surgery because the strategy for clipping or other treatment is different than that for saccular aneurysms. They mostly present with SAH and necessitate treatment. Three-dimensional CTA can easily detect these aneurysms, but rotational DSA may be helpful in some particular instances, mainly because of small size and anterior location of these aneurysms. Magnetic resonance studies of the aneurysm wall may also help determine the presence of a dissection process because the true physiopathology of these rare aneurysms is not clearly known. Endovascular embolization is not generally recommended for this kind of aneurysm because of their large base and the very loose fibrinous tissue of the dome. The competency of collateral flow should be tested during the preoperative evaluation by DSA because these aneurysms have a high likelihood of neck laceration during exploration and consequently carotid sacrifice. Therefore, safe proximal control is mandatory, and manipulation, dissection, and clipping of the aneurysm should be performed under low intra-aneurysmal pressure, temporary proximal clipping, or trapping of the ICA.13 One safe alternative is to clip onto wrapping materials around the aneurysms. Application of an encircling clip is another method that has limits because of possible sacrifice of the perforating vessel. If clipping is not successful, carotid sacrifice by trapping with or without revascularization should be done. Wrapping the aneurysm is another alternative; however, because of the fragility and likelihood of further growth, the success rate after wrapping alone is low. The most important point in the management of these aneurysms is thorough preoperative diagnosis and planning for every possible scenario in order to avoid hemorrhagic and ischemic complications.
Outcome
Surgical outcome for ICA aneurysms is generally good, although straightforward cases are no longer treated by surgery, and more complex aneurysms are referred to vascular neurosurgeons. Poor results are usually related to poor aneurysm grade, atherosclerotic intracranial carotid artery, and severe persistent vasospasm. Third nerve palsy following SAH or rapid expansion of a posterior communicating aneurysm usually resolves completely after 3 months in up to 90% of patients and resolves partially in the remaining 10%.16
Adams WM, Laitt RD, Jackson A. The role of MR angiography in the pretreatment assessment of intracranial aneurysms: a comparative study. AJNR Am J Neuroradiol. 2000;21:1618-1628.
Bouthillier A, van Loveren HR, Keller JT. Segments of the internal carotid artery: a new classification. Neurosurgery. 1996;38:425-432.
Dehdashti AR, Mermillod B, Rufenacht DA, et al. Does treatment modality of intracranial ruptured aneurysms influence the incidence of cerebral vasospasm and clinical outcome? Cerebrovasc Dis. 2004;17:53-60.
Dehdashti AR, Rilliet B, Rufenacht DA, de Tribolet N. Shunt-dependent hydrocephalus after rupture of intracranial aneurysms: a prospective study of the influence of treatment modality. J Neurosurg. 2004;101:402-407.
Dehdashti AR, Rufenacht DA, Delavelle J, et al. Therapeutic decision and management of aneurysmal subarachnoid haemorrhage based on computed tomographic angiography. Br J Neurosurg. 2003;17:46-53.
Lanzino G, Andreoli A, Tognetti F, et al. Orbital pain and unruptured carotid-posterior communicating artery aneurysms: the role of sensory fibers of the third cranial nerve. Acta Neurochir (Wien). 1993;120:7-11.
Macdonald RL, Weir B, editors. Cerebral Vasospasm. San Diego: Academic Press, 2001.
Molyneux A, Kerr R, Stratton I, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002;360:1267-1274.
Nael K, Villablanca JP, Saleh R, et al. Contrast-enhanced MR angiography at 3T in the evaluation of intracranial aneurysms: a comparison with time-of-flight MR angiography. AJNR Am J Neuroradiol. 2006;27:2118-2121.
Paine JT, Batjer HH, Samson D. Intraoperative ventricular puncture. Neurosurgery. 1988;22:1107-1109.
Rabinstein AA, Pichelmann MA, Friedman JA, et al. Symptomatic vasospasm and outcomes following aneurysmal subarachnoid hemorrhage: a comparison between surgical repair and endovascular coil occlusion. J Neurosurg. 2003;98:319-325.
Rhoton ALJr, Fujii K, Fradd B. Microsurgical anatomy of the anterior choroidal artery. Surg Neurol. 1979;12:171-187.
Rosner SS, Rhoton ALJr, Ono M, Barry M. Microsurgical anatomy of the anterior perforating arteries. J Neurosurg. 1984;61:468-485.
San Millan Ruiz D, Yilmaz H, Dehdashti AR, et al. The perianeurysmal environment: influence on saccular aneurysm shape and rupture. AJNR Am J Neuroradiol. 2006;27:504-512.
Teasdale GM, Drake CG, Hunt W, et al. A universal subarachnoid hemorrhage scale: report of a committee of the World Federation of Neurosurgical Societies. J Neurol Neurosurg Psychiatry. 1988;51:1457.
Weir B. Surgery: Specific Sites and Results of Series in Aneurysms Affecting the Nervous System. Baltimore: Williams & Wilkins; 1987.
Wiebers DO, Whisnant JP, Huston J3rd, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362:103-110.
Wintermark M, Uske A, Chalaron M, et al. Multislice computerized tomography angiography in the evaluation of intracranial aneurysms: a comparison with intraarterial digital subtraction angiography. J Neurosurg. 2003;98:828-836.
Wollschlaeger G, Wollschlaeger PB, Lucas FV, Lopez VF. Experience and result with postmortem cerebral angiography performed as routine procedure of the autopsy. AJR Am J Roentgenol Radium Ther Nucl Med. 1967;101:68-87.
Yasargil M. Microneurosurgery, vols I and II. New York: Thieme-Stratton; 1987.
1 Molyneux A, Kerr R, Stratton I, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002;360:1267-1274.
2 Wintermark M, Uske A, Chalaron M, et al. Multislice computerized tomography angiography in the evaluation of intracranial aneurysms: a comparison with intraarterial digital subtraction angiography. J Neurosurg. 2003;98:828-836.
3 Adams WM, Laitt RD, Jackson A. The role of MR angiography in the pretreatment assessment of intracranial aneurysms: a comparative study. AJNR Am J Neuroradiol. 2000;21:1618-1628.
4 Nael K, Villablanca JP, Saleh R, et al. Contrast-enhanced MR angiography at 3T in the evaluation of intracranial aneurysms: a comparison with time-of-flight MR angiography. AJNR Am J Neuroradiol. 2006;27:2118-2121.
5 Dehdashti AR, Rufenacht DA, Delavelle J, et al. Therapeutic decision and management of aneurysmal subarachnoid haemorrhage based on computed tomographic angiography. Br J Neurosurg. 2003;17:46-53.
6 Teasdale GM, Drake CG, Hunt W, et al. A universal subarachnoid hemorrhage scale: report of a committee of the World Federation of Neurosurgical Societies. J Neurol Neurosurg Psychiatry. 1988;51:1457.
7 Macdonald RL, Weir B, editors. Cerebral Vasospasm. San Diego: Academic Press, 2001.
8 Bouthillier A, van Loveren HR, Keller JT. Segments of the internal carotid artery: a new classification. Neurosurgery. 1996;38:425-432.
9 Weir B. Surgery: Specific Sites and Results of Series in Aneurysms Affecting the Nervous System. Baltimore: Williams & Wilkins; 1987.
10 Yasargil M. Microneurosurgery, vols I and II. New York: Thieme-Stratton; 1987.
11 Wollschlaeger G, Wollschlaeger PB, Lucas FV, Lopez VF. Experience and result with postmortem cerebral angiography performed as routine procedure of the autopsy. AJR Am J Roentgenol Radium Ther Nucl Med. 1967;101:68-87.
12 Lanzino G, Andreoli A, Tognetti F, et al. Orbital pain and unruptured carotid-posterior communicating artery aneurysms: the role of sensory fibers of the third cranial nerve. Acta Neurochir (Wien). 1993;120:7-11.
13 San Millan Ruiz D, Yilmaz H, Dehdashti AR, et al. The perianeurysmal environment: influence on saccular aneurysm shape and rupture. AJNR Am J Neuroradiol. 2006;27:504-512.
14 Wiebers DO, Whisnant JP, Huston J3rd, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362:103-110.
15 Paine JT, Batjer HH, Samson D. Intraoperative ventricular puncture. Neurosurgery. 1988;22:1107-1109.
16 Dehdashti AR, Mermillod B, Rufenacht DA, et al. Does treatment modality of intracranial ruptured aneurysms influence the incidence of cerebral vasospasm and clinical outcome? Cerebrovasc Dis. 2004;17:53-60.
17 Rabinstein AA, Pichelmann MA, Friedman JA, et al. Symptomatic vasospasm and outcomes following aneurysmal subarachnoid hemorrhage: a comparison between surgical repair and endovascular coil occlusion. J Neurosurg. 2003;98:319-325.
18 Rhoton ALJr, Fujii K, Fradd B. Microsurgical anatomy of the anterior choroidal artery. Surg Neurol. 1979;12:171-187.
19 Rosner SS, Rhoton ALJr, Ono M, Barry M. Microsurgical anatomy of the anterior perforating arteries. J Neurosurg. 1984;61:468-485.
20 Dehdashti AR, Rilliet B, Rufenacht DA, de Tribolet N. Shunt-dependent hydrocephalus after rupture of intracranial aneurysms: a prospective study of the influence of treatment modality. J Neurosurg. 2004;101:402-407.