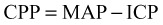103 Intracranial Hemorrhages
• “Head bleed” is an oversimplified term for intracranial hemorrhage because different types of hemorrhages have different causes, signs and symptoms, diagnostic strategies, and therapies.
• Descriptions of intracranial hemorrhage should include the anatomic location, estimation of size, presence of midline shift, and whether the hemorrhage is thought to be spontaneous or secondary to another process.
• Treatment recommendations regarding blood pressure management and anticonvulsant therapy remain controversial and lack strong evidence-based support.
Pathophysiology
intracranial hemorrhage is the umbrella term used to encompass the many types of bleeding within the cranial vault (Figs. 103.1 to 103.5 and Table 103.1). Intraparenchymal hemorrhage implies blood within the substance of the brain. When the hemorrhage is not clearly secondary to a detectable cause, it is deemed a spontaneous hemorrhage. This term is often used synonymously with the terms hypertensive hemorrhage and, when anatomically appropriate, intracerebral hemorrhage. Intraparenchymal hemorrhages may also occur in the brainstem or cerebellum. SAH literally describes blood in the subarachnoid space, and if nontraumatic, a vascular lesion such as an aneurysm is the implied cause of the bleeding. SAH and intraparenchymal hemorrhage may coexist. Intraventricular hemorrhage means that blood is visualized within the ventricles by cranial CT, and it is most often present with other types of intracranial hemorrhage. intracranial hemorrhages outside the brain substance are referred to as extraaxial hemorrhages and include both subdural hemorrhages and epidural hemorrhages. Although uncommon exceptions exist, extraaxial hemorrhages almost always have a traumatic etiology. The term hemorrhagic stroke might literally describe abrupt symptoms with any of the previously mentioned hemorrhages, but it is sometimes used in a more restrictive sense to describe hemorrhagic changes in an area of ischemic stroke to the point of being visible on cranial CT; this is termed hemorrhagic transformation of ischemic stroke.
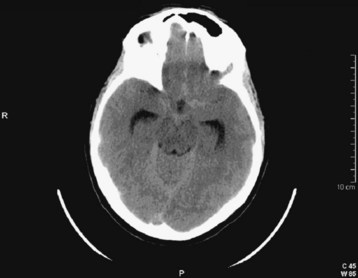
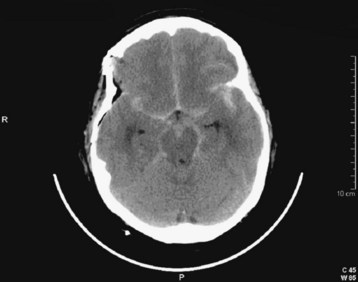
Fig. 103.2 Computed tomography scan: acute subarachnoid hemorrhage.
Blood density is not as dramatic as in Figure 103.13. Hydrocephalus with enlarged temporal horns of the lateral ventricle is present.
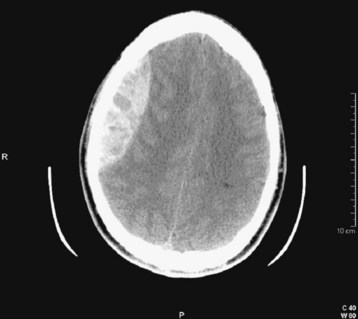
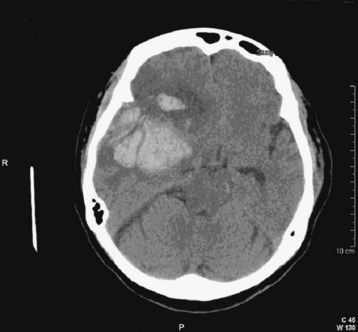
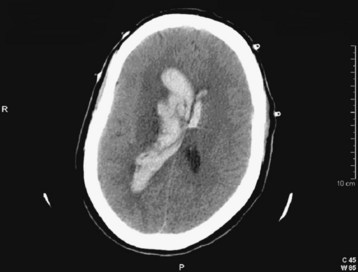
Fig. 103.4 Computed tomography (CT) scan: acute epidural hematoma with the “swirl” sign.
This is a slice from a repeated CT scan of the patient in Figure 103.10 taken approximately 1 hour later. The marbled density of the clot is consistent with ongoing hemorrhage. Note the lens shape of the hematoma.
See Table 103.1, Types of Intracranial Hemorrhage, online at www.expertconsult.com
| TYPE OF HEMORRHAGE | CORRESPONDING FIGURES IN TEXT | FINDINGS IN FIGURES |
|---|---|---|
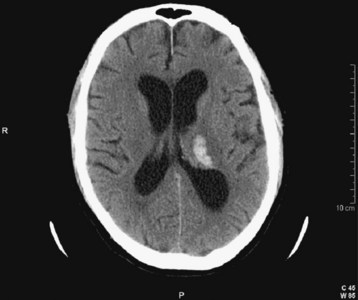
| Intraparenchymal (synonyms: intracerebral, lobar, hypertensive) | Fig. 103.1 | Blood within the substance of the brain; thalamic hemorrhage |
| Subarachnoid | Fig. 103.2 | Acute hemorrhage with blood in the subarachnoid spaces |
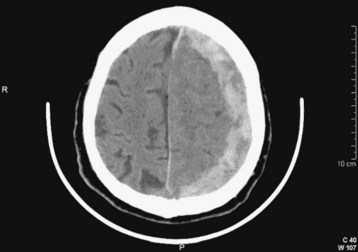
| Subdural | Fig. 103.3 | Blood external to the brain; crescent-shaped hemorrhage |
| Epidural | Fig. 103.4 | Blood external to the brain; lens-shaped hemorrhage |
| Hemorrhagic transformation of ischemic stroke | Fig. 103.5 | Hemorrhage within a wedge-shaped area of ischemia |
Spontaneous intraparenchymal hemorrhages (e.g., intracerebral hemorrhages, lobar hemorrhages, hypertensive hemorrhages) are most often associated with chronic hypertension. Cerebral amyloid angiopathy is increasingly being recognized as a contributing process in the elderly. Chronic excessive alcohol use is also a risk factor. Hemorrhage usually originates from rupture of small penetrating branch arteries of the vessels at the base of the brain1 (Fig. 103.6).
Serial cranial CT demonstrates that many intracerebral hemorrhages expand over the course of several hours.2 The initial hemorrhage may infiltrate the white matter with little direct destruction, but continued hematoma expansion, white matter edema, additional hemorrhage from surrounding vessels, and the development of hydrocephalus may all contribute to increased ICP and secondary neuronal injury. The frequency of anticoagulant-associated intracerebral hemorrhage is increasing.3 Warfarin therapy does not appear to increase hematoma volume initially, but it does increase the risk for later hematoma expansion.4
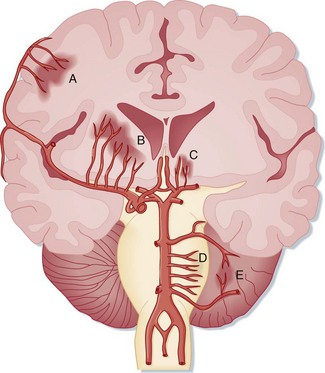
SAH literally means “blood in the subarachnoid space.” Trauma is the most common cause. Spontaneous, or nontraumatic, SAH has an entirely different differential diagnosis. About 80% of spontaneous SAHs are caused by rupture of saccular (berry) aneurysms of the intracranial vessels, which are commonly located near intracranial arterial bifurcations of the circle of Willis5,6 (Fig. 103.7). Aneurysms are often named after the vascular site of origin, such as the anterior communicating artery or middle cerebral artery. Aneurysms that develop following vascular infection from endocarditis are termed mycotic aneurysms. Some aneurysms also cause symptoms without rupture from a mass effect or from emboli originating within the aneurysm. Rupture of an intracranial aneurysm abruptly raises ICP and leads to the onset of symptoms. The bleeding may be confined to the subarachnoid space, or a hematoma may extend into the brain substance and create an intraparenchymal hemorrhage, which in turn may rupture into the ventricles. Vasospasm of the vascular tree related to the aneurysm typically takes hours to develop and may worsen regional ischemia.
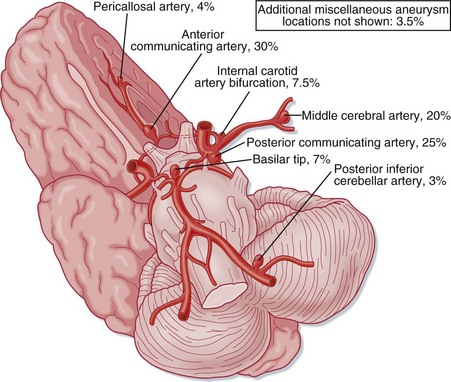
Fig. 103.7 The intracranial vasculature showing the most frequent locations of intracranial aneurysms.
Arteriovenous malformations are another cause of intracranial hemorrhage of both the subarachnoid and intraparenchymal anatomic subtypes. These arteriovenous shunts vary in their anatomy, and many patients have saccular aneurysms as well. Lesions with deep venous drainage and high pressure in the feeding vessels are at increased risk for bleeding.7 Cavernous angiomas are low-pressure vascular lesions associated with small hemorrhages.
The anatomic terminology regarding intracranial hemorrhage historically comes from postmortem neuropathology descriptions. In the ED, intracranial hemorrhages are most often diagnosed by cranial CT, which remains the initial diagnostic modality of choice. The appearance of acute hemorrhage usually contrasts vividly with that of the other intracranial contents. The types of intracranial hemorrhage are anatomically classified primarily by their relationship to the substance of the brain and the meninges.8 Simplistically, hemorrhages may be thought to be located in the brain substance (intraparenchymal, intracerebral), within the ventricles (intraventricular), in the subarachnoid space between the meninges and the brain, or outside the brain and meninges (subdural, epidural) (Figs. 103.8 and 103.9).
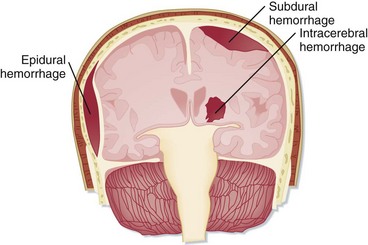
Fig. 103.8 Varieties of intracranial hemorrhage.
(From Snell RS, Smith MS. Clinical anatomy for emergency medicine. St. Louis: Mosby; 1993.)
Presenting Signs and Symptoms
Patients with a large intracranial hemorrhage typically have a diminished level of consciousness with or without a focal neurologic deficit. Intracerebral hemorrhages are responsible for about 20% of acute strokes. It is not possible at the bedside to reliably distinguish between an ischemic stroke and an intracerebral hemorrhage.9 Patients with a diminished level of consciousness often have a larger hemorrhage and increased ICP or distortion of the thalamic and brainstem reticular activating system.1
SAH is typically manifested as the “worst headache of my life.” Abrupt onset of a severe headache that quickly attains maximal intensity is the classic finding. This manifestation—abrupt severe headache reaching maximal intensity within seconds or moments of onset, perhaps occurring with exertion—often leads to the diagnosis. Syncope may be present at the onset of a hemorrhage. Meningismus may also be present. However, initial misdiagnosis of SAH occurs in up to 50% of cases.10,11 The high-risk clinical characteristics of SAH have led to the derivation of preliminary decision rules, which remain under study.12
With SAH and increased ICP, cardiac arrhythmias and changes on the electrocardiogram (ECG) consistent with myocardial ischemia may at times confound the diagnosis.10 Usually, these patients have severe neurologic symptoms, but cases in which the arrhythmia overshadows the clinical findings are reported as well.
intracerebral hemorrhage is the most common manifestation of arteriovenous malformations and accounts for roughly half the presentations. Other manifestations include seizures and focal neurologic deficits.7
Differential Diagnosis and Medical Decision Making
If altered mental status is the initial finding, all the causes of altered mental status should be included in the differential diagnosis (see Chapter 104). The expanded differential diagnosis for hemorrhages without focal lesions or altered mental status includes the universe of headache types. Functional headaches or thunderclap headaches are at times related to different types of exertion; however, they must be part of a diagnosis of exclusion for the EP.
In patients with suspected SAH, CT is very sensitive in detecting acute hemorrhage, with estimates of 95% or better.11,13 Sensitivity starts to diminish as time from the hemorrhage increases, and CT sensitivity is estimated to be less than 50% 7 days after the event.
A suggested approach to analyzing CT scans (see Chapter 74) and a useful structure for communicating with consultants can be determined by asking the following series of simple questions:
Question 1—Is Blood Present?
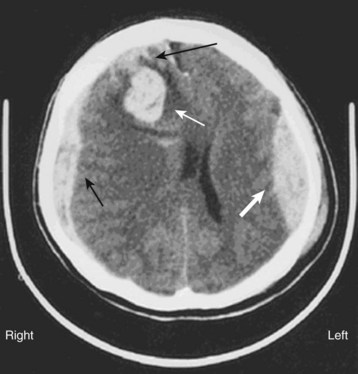
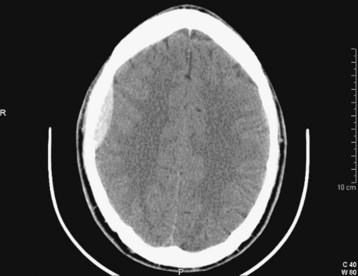
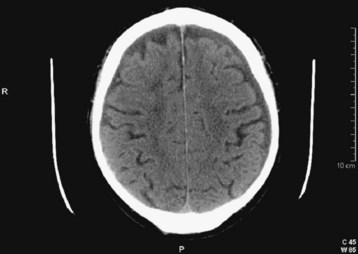
Acute blood appears white or hyperdense on non–contrast-enhanced CT scans (Fig. 103.10). Some intracranial structures such as the dura or choroid plexus may calcify and at times simulate hemorrhage. As blood ages, it becomes increasingly low density or dark (Fig. 103.11). There is a time during this evolution when blood is nearly the same CT density as brain parenchyma and is therefore termed isodense. Rarely, the existence of an isodense hematoma must be inferred from cortical sulcus markings that do not reach the cranium. Clinically, the terms acute, subacute, and chronic are used to reflect the change in appearance on the CT scan, from hyperdense to isodense and finally hypodense. Nonhomogeneous density may be observed in some cases and is an indication of acute or acute on chronic bleeding. The possibility of hyperacute bleeding should always be kept in mind, with the areas of rapid bleeding appearing relatively hypodense within a larger, more dense area on CT (see Fig. 103.4).
Question 2—Where is the Hemorrhage?
If hemorrhage is present, it is then described as external to the brain substance (extraaxial), within the substance of the brain (intraaxial or intraparenchymal), or visible in the intraventricular and subarachnoid or cisternal spaces. Extraaxial hematomas have two basic types of appearance. Subdural hematomas are most often crescentic (see Fig. 103.3), whereas epidural hematomas have a typical lens-shaped pattern (see Fig. 103.4). An intraparenchymal hemorrhage may be located in the cerebrum (intracerebral hemorrhage) or in subcortical or brainstem structures. Intracerebral hemorrhages of hypertensive origin tend to be situated in deep white matter or the basal ganglia or are confined to one lobe of the brain (lobar) (see Fig. 103.1). These hemorrhages tend to have a stereotypic pattern, and deviation from these patterns may suggest an uncommon cause of the hemorrhage.
Cerebellar hemorrhages may be midline or hemispheric (Fig. 103.12) and may cause brainstem compression. SAH may be detected by high density in the suprasellar or perimesencephalic cistern or by blood in the cortical sulci, where ordinarily there should be low-density images from the cerebrospinal fluid signal. Depending on the degree of hemorrhage, SAH may be obvious (Fig. 103.13) or relatively subtle (see Fig. 103.2). Intraventricular hemorrhage (literally “blood within the ventricles”) may result from rupture of an intracerebral hemorrhage into the ventricular system, from trauma, or from SAH (Fig. 103.14).
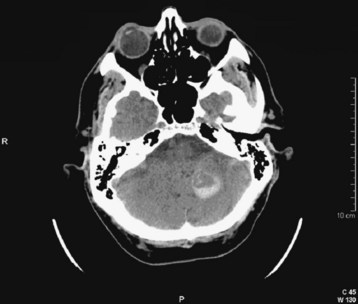
Fig. 103.12 Computed tomography scan: acute hemispheric cerebellar hemorrhage.
The expanding mass in the posterior fossa places the brainstem at risk for compression.
Question 3—How Much Blood is Present, and What is the Effect?
Some quantification of the hemorrhage should follow. For extraaxial hemorrhage, the greatest thickness of the hematoma is easily estimated from the ruler on the CT scan. Volumetric estimation of intraparenchymal hematomas may be estimated from information present on the cranial CT scan, although this is not usually done frequently by EPs. Because most hemorrhages are ellipsoid, the formula ABC/2 may be used to estimate the volume, where A is the greatest hemorrhage diameter on CT, B is the diameter 90 degrees to A, and C is the approximate number of CT slices with hemorrhage multiplied by slice thickness.14 Of more importance is any effect that the hematoma is having on adjacent structures. This may be estimated qualitatively by noting any compression on the ventricular system and the amount of shift of midline structure, as well as by CT signs of herniation (subfalcine, uncal, tonsillar herniations).
Treatment
Supportive care including appropriate management of the ABCs—airway, breathing, and circulation—is of course important. The decision to perform endotracheal intubation is based on the judgment of the physician who assesses the patient’s ability to protect the airway. It is recommended that certain steps be taken for rapid-sequence induction in patients with intracranial hemorrhage or other conditions with suspected increased ICP, including the use of lidocaine and a defasciculating dose of a paralytic agent, although rigorous proof of efficacy is lacking. In the past, hyperventilation was recommended with the goal of reducing abnormally increased ICP. Again, evidence is lacking, but the consensus is that hyperventilation beyond that needed to reduce PaCO2 to only a small degree (PaCO2 of 30 to 35 mm Hg) is not indicated.15
Blood pressure management in the setting of intracranial hemorrhage is controversial. In multiple-trauma patients with central nervous system injury, hypotension is associated with a poor outcome. In patients with intracerebral hemorrhage, the risk of expanding a hematoma associated with sustained hypertension must be weighed against the risk of impairing cerebral perfusion if blood pressure is reduced. A 2010 study suggested a trend toward more favorable outcomes in patients with aggressive blood pressure reduction but admitted that the study was underpowered and called for further investigation.16 In patients with established intracerebral hematoma and hypertension, consensus at this time is to use intravenous agents that can be titrated, such as nitroprusside, labetalol, esmolol, or carvedilol, if needed, to maintain blood pressure with an MAP of less than 130 mm Hg. Systolic blood pressure greater than 180 mm Hg or diastolic blood pressure higher than 105 mm Hg on two readings taken 5 minutes apart are the criteria recommended for intervention.15
In patients with SAH, there is also no clear evidence-supported management strategy. Hypertension should be avoided in patients with a ruptured aneurysm by administering intravenous titratable agents, as described previously. Some experts argue that relative hypotension should be induced based on the theory that a ruptured aneurysm is at risk for rebleeding in the presence of hypertension. Once the aneurysm is secured by interventional techniques, blood pressure is allowed to return to normal levels. The calcium channel antagonist nimodipine is recommended to reduce the chance of ischemia from vasospasm.5,6
Cerebellar hemorrhage is an emergency requiring removal of the hematoma and relief of brainstem compression, which offers the possibility of good recovery in selected cases.1 Surgery for removal of supratentorial intracerebral hematomas is controversial and not generally recommended.17 Other inpatient supportive measures for intracerebral hemorrhage might include prophylaxis for thromboembolic events.
Patients with an acute intracerebral hematoma who are taking warfarin should receive fresh frozen plasma (FFP) and vitamin K as soon as possible to correct the coagulopathy. The best dosing regimens are not known, but for patients with a prolonged international normalized ratio, a reasonable recommendation is 5-10 mg of vitamin K (administered intravenously over a 10-minute period) plus 10 mL/kg of FFP administered as soon as possible. Type AB FFP is available in some blood banks and can speed administration time by eliminating the time needed for cross-matching. Institutional recommendation many vary. Time to treatment of warfarin-associated coagulopathy in patients with intracerebral hemorrhage has been found to be the most important determinant of 14-hour reversal of anticoagulation.18 Antiplatelet therapy has been noted to be associated with clinical deterioration.
Acute administration of recombinant activated factor VII limits expansion of hematomas, but it has limited use for thromboembolic complications.19,20 Other procoagulant pharmaceutical preparations are under study.
In recent years the trend has continued for early surgical intervention for SAH—that is, intervention to isolate the aneurysm or occlude it within 1 to 2 days of bleeding.5,6 Until the aneurysm is secured, the consensus is that blood pressure should be lowered with parenteral medications if necessary. A recent study reported that interventional endovascular coiling may lead to better outcomes in selected patients with ruptured aneurysms.21
Brisman JL, Song JK, Newell DW. Cerebral aneurysms. N Engl J Med. 2006;355:928–939.
Broderick JP, Connolly S, Feldman E, et al. Guidelines for the management of spontaneous intracerebral hemorrhage in adults. Stroke. 2007;38:2001–2023.
Edlow JA, Caplan LR. Avoiding pitfalls in the diagnosis of subarachnoid hemorrhage. N Engl J Med. 2000;342:29–36.
Kothari RU, Brott T, Broderick JP, et al. The ABC’s of measuring intracerebral hemorrhage volumes. Stroke. 1996;27:1304–1305.
Runchey S, McGee S. Does this patient have a hemorrhagic stroke? Clinical findings distinguishing hemorrhagic stroke from ischemic stroke. JAMA. 2010;308:2280–2286.
1 Qureshi AI, Tuhrim S, Broderick JP, et al. Spontaneous intracerebral hemorrhage. N Engl J Med. 2001;344:1450–1460.
2 Brott T, Broderick J, Kothari R, et al. Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke. 1997;28:1–5.
3 Flaherty ML, Kissela B, Woo D, et al. The increasing incidence of anti-coagulant associated intracerebral hemorrhage. Neurology. 2007;68:116–121.
4 Flibotte JJ, Hagan N, O’Donnell J, et al. Warfarin, hematoma expansion, and outcome of intracerebral hemorrhage. Neurology. 2004;63:1059–1064.
5 Suarez JI, Tarr RW, Selman WR. Aneurysmal subarachnoid hemorrhage. N Engl J Med. 2006;354:387–396.
6 Brisman JL, Song JK, Newell DW. Cerebral aneurysms. N Engl J Med. 2006;355:928–939.
7 The Arteriovenous Malformation Study Group. Arteriovenous malformations of the brain in adults. N Engl J Med. 1999;340:1812–1818.
8 Snell RS, Smith MS. The skull, the meninges, and the blood supply of the brain relative to trauma and intracranial hemorrhage. In: Clinical Anatomy for Emergency Medicine. St. Louis: Mosby; 1993:284.
9 Runchey S, McGee S. Does this patient have a hemorrhagic stroke? Clinical findings distinguishing hemorrhagic stroke from ischemic stroke. JAMA. 2010;308:2280–2286.
10 Kowalski RG, Claasen J, Kreiter KT, et al. Initial misdiagnosis and outcome after subarachnoid hemorrhage. JAMA. 2004;291:866–869.
11 Edlow JA, Caplan LR. Avoiding pitfalls in the diagnosis of subarachnoid hemorrhage. N Engl J Med. 2000;342:29–36.
12 Perry JJ, Steill IG, Sivilotti ML, et al. High risk clinical characteristics for subarachnoid hemorrhage in patients with acute headache; prospective cohort study. BMJ. 2010;341:c5204.
13 Mark DG, Pines JM. The detection of nontraumatic subarachnoid hemorrhage: still a diagnostic challenge. Am J Emerg Med.. 2006;24:859–863.
14 Kothari RU, Brott T, Broderick JP, et al. The ABC’s of measuring intracerebral hemorrhage volumes. Stroke. 1996;27:1304–1305.
15 Broderick JP, Connolly S, Feldman E, et al. Guidelines for the management of spontaneous intracerebral hemorrhage in adults. Stroke. 2007;38:2001–2023.
16 Quesshi AI, Palesch YY, Martin R, et al. Effect of systolic blood pressure reduction on hematoma expansion, perihematomal edema, and 3-month outcome among patients with intracerebral hemorrhage: results from the Antihypertensive Treatment of Acute Cerebral Hemorrhage Study. Arch Neurol. 2010;67:570–576.
17 Mendelow AD, Gregson BA, Fernandes HM, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral hematomas in the International Surgical Trial in Intracerebral Hemorrhage (STITCH): a randomized trial. Lancet. 2005;365:387–397.
18 Goldstein JN, Thomas SH, Frontiero V, et al. Timing of fresh frozen plasma administration and rapid correction of coagulopathy in warfarin-related intracerebral hemorrhage. Stroke. 2006;37:151–155.
19 Mayer SA, Brun NC, Begtrup K, et al. Recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2005;352:777–785.
20 O’Connell KA, Wood JJ, Wise RP, et al. Thromboembolic adverse events after use of recombinant human coagulation factor VIIa. JAMA. 2006;295:293–298.
21 Molyneux AJ, Kerr RS, Yu LM, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005;366:809–817.