Chapter 13 Intracranial Aneurysms
• The incidence of intracranial aneurysms is variable throughout the world and is approximately 6% in the international population, with rates being higher in Asian/Finnish populations and those with a high-risk profile. In those patients without any risk factors, the incidence is approximately 2%. Once the aneurysm has ruptured, one third die and two thirds survive, with 50% of survivors leading independent lives.
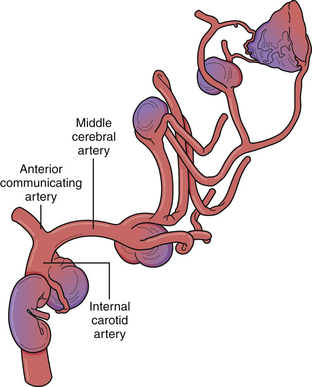
• The majority of intracranial aneurysms arise at branching points of large arteries. Hemodynamic stress likely contributes to both the initial development and subsequent growth. Aneurysm formation can have many other associated conditions, including autosomal dominant polycystic kidney disease, Ehlers-Danlos syndrome, Loeys-Dietz syndrome, Marfan syndrome, tuberous sclerosis, fibromuscular dysplasia, and other genetic and structural predispositions.
• Approximately 25% to 50% of all patients will have warning symptoms that herald the onset of a major subarachnoid hemorrhage (SAH). The patient’s description of the headache is very important, as most involve “thunderclap” onset or the “worst headache of my life.” Computed tomography (CT) scans detect SAH with high sensitivity. If the scan is negative but suspicion remains high, a lumbar puncture may be performed. Although CT angiography is good for imaging aneurysms greater than 2 mm, cerebral angiography remains the gold standard for intracranial vascular imaging.
• The risk of rebleeding after SAH is greatest on the first day (4.1%). By day 14, the cumulative rebleed incidence is 19%. Once an aneurysm is secure, the most significant cause of morbidity and death is cerebral vasospasm. The onset of cerebral vasospasm begins on day 3 and peaks between 7 and 14 days after SAH. Seventy percent of patients will develop radiographic signs of vasospasm and approximately 30% will develop clinical vasospasm requiring adjunctive therapy.
• The treatment goal of intracranial aneurysms is to exclude them from the parent circulation. In ruptured aneurysms, this must be done so early and safely so that maximum treatment of cerebral vasospasm can be administered if necessary. Symptomatic vasospasm can be treated with triple H therapy (involving hypertension, hypervolemia, and hemodilution) and endovascular means, namely intra-arterial injection of vasodilators or angioplasty.
• To achieve the goal of multidisciplinary cerebrovascular care, aneurysm patients are ideally treated at centers of excellence that employ expertise in all areas of neurovascular care, including endovascular, microvascular, and neurocritical care, and neuroanesthesia.
Intracranial aneurysms are potentially life threatening or disabling vascular lesions, which can pose formidable treatment challenges. Morphologically and pathologically they encompass diverse and occasionally overlapping entities including saccular, fusiform, and dissecting aneurysms with a wide variety of etiological origins, including hemodynamic, traumatic, infectious, and tumorogenic. Although intervention in most cases of ruptured intracranial aneurysms is indicated, the management of unruptured intracranial aneurysms remains controversial. On the other hand, as medicine shifts more toward prevention, greater interest has arisen in defining screening and risk reduction strategies for high-risk populations. Over the last several decades, insight into the pathobiology and pathophysiology has significantly advanced, leading to improvements in the areas of natural history, epidemiology, biology, genetics, therapeutic intervention, and clinical outcomes. This chapter will provide an overview of intracranial aneurysms, clinical presentations, current therapeutic options, and technical aspects of surgical treatment. Although intracranial aneurysms encompass a diverse disease group, as stated earlier, this chapter will focus on the most common type, the so-called “saccular berry aneurysm.”
Epidemiology
The incidence and prevalence of intracranial aneurysms in the general population are very difficult to estimate owing to clustering in various high-risk groups, large numbers of deaths without autopsy or neuroimaging, and the presence of asymptomatic lesions. Furthermore, autopsy studies have demonstrated disparate results that do not appear to link with the prevalence of subarachnoid hemorrhages (SAHs) and rupture risk incidence. Fox1 reviewed the autopsy literature, which spanned from 1926 to 1973, and found the rate of occurrence to be 0.8% when studies included more than 5000 cases and 2% when studies included 2 to 5000 patients. Stehbens2 conducted an autopsy study between 1952 and 1954 and found at least one aneurysm in 5.6% of cadavers. In his review of the pathological literature between 1890 and 1966, the prevalence of aneurysms at autopsy was found to be approximately 2.4% with a range of 0.2% to 9%.3 Intracranial aneurysms in children are rare but are thought to occur in approximately 0.5% to 4.6%.4 Currently, it is thought that approximately 6% of the international population harbor intracranial aneurysms with a higher prevalence in the Asian and Finnish populations (4-9%) and those who harbor significant risk factors, such as environmental, systemic, or genetic.5 Risk factors associated with the presence and rupture of intracranial aneurysms have included high blood pressure, tobacco usage, and genetic- and ethnic-related factors; however, the prevalence of lesions is still thought to be approximately 2% in those without any known risk factors.5 Although the most prevalent risk factors are those that are modifiable, the strongest risk factors are thought to occur through genetic and familial predisposition.
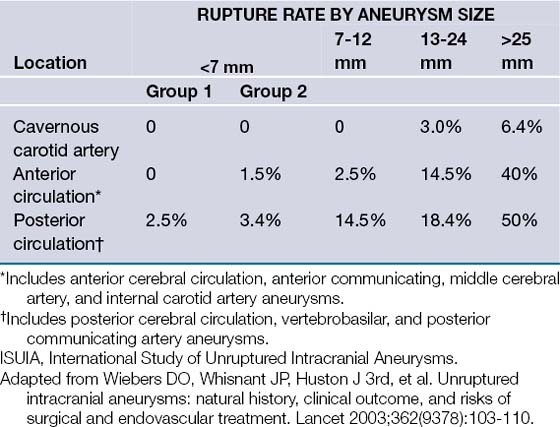
The risk of intracranial aneurysm rupture has also been a controversial topic. Although the annual risk of rupture has been reported to be between 0.1 and 8%,5 depending on variable lesional characteristics, recent prospective data have challenged this view. The International Study of Unruptured Intracranial Aneurysms (ISUIA) reported in two recent publications6,7 that the risk of aneurysm rupture was substantially lower than previously thought, that is, approximately 0% in anterior circulation aneurysms in those less than 7 mm, the most commonly encountered entities (Table 13.1). Although these studies represented the largest prospective collection of data regarding unruptured aneurysms, they have also been met with intense scrutiny and controversy, especially in light of a multitude of studies reporting an order of magnitude greater rupture risk.8 Common problems with estimating the annual rupture risk are the inclusion of all patients with aneurysms, regardless of presentation, proper lesion classification and grouping, taking into account specific lesional characteristics, and proper classification of patients into various risk groups.8 Given the broad spectrum of intracranial aneurysms, a balance of prospective data and clinical experience is necessary to properly and individually assess an individual patient’s rupture risk.
Of those patients who experience (SAH) after aneurysmal rupture, approximately one third return to a functional life, one third live a dependent life, and the last third do not survive.1,5,7,9 As advances in neurocritical care have increased survivability, neurological morbidity rate remains about the same. The greatest risk after the initial aneurysmal rupture is for re-rupture. Once the aneurysm is secure, the greatest risk is of cerebral vasospasm and ischemic complications from such events, thereby underlying the importance of early intervention and close monitoring in the postrupture period.
Pathogenesis
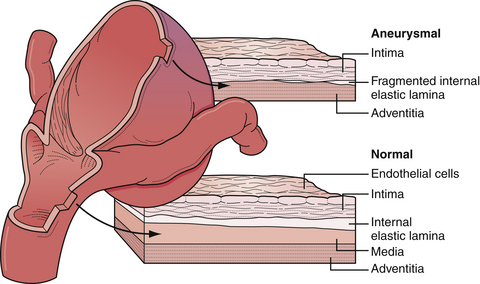
Intracranial arteries are unique to the systemic vascular system in that they lack the external elastic lamina present in extracranial vessels (Fig. 13.1). The pathogenesis of intracranial aneurysms has been thought to be due to a number of congenital factors such as vascular wall defects (i.e., media and elastic lamina), hemodynamic load on tortuous segments and bends with or without branch formation in the appropriate distribution, and discontinuity of the tunica media at origins of small vessels from larger parent vessels. Certainly, a multitude of systemic factors are thought to contribute to aneurysmal pathogenesis, such as degenerative changes, hypertension, atherosclerosis, connective tissue disease, and hemodynamic stress.
Genetic predisposition through various systemic and syndromic conditions has also been associated with the presence of intracranial aneurysms and is thought to represent approximately 10% to 12% of all aneurysms. Autosomal dominant polycystic kidney disease, Ehlers-Danlos syndrome, Loeys-Dietz syndrome, Marfan syndrome, tuberous sclerosis, and fibromuscular dysplasia have all been associated with the presence of intracranial aneurysms.10–15 In recent years, many genetic loci have been proposed to be associated with familial linkage. Ruigrok and associates14 recently reviewed the genetics of intracranial aneurysms and reported that probable loci included chromosomes 5q and 17cen, from which the strongest associations code for versican, an extracellular matrix protein, and TNFRSF13B, a transmembrane activator and a calcium modulator ligand interactor, respectively. Other suggestive loci are 1p34.3-p36.13, 7q11, 19q13.3, and Xp22.9,14,16,17
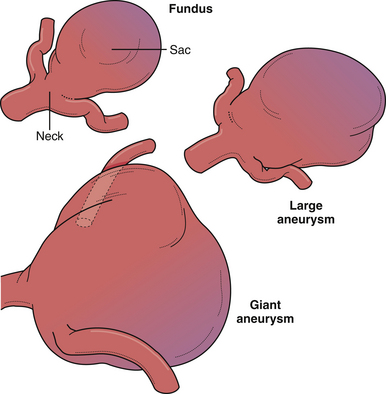
Despite the numerous factors likely to be related to intracranial aneurysm pathogenesis, it is most likely a complex, multifactorial set of circumstances involving anatomical, environmental, and genetic factors that lead to arterial wall weakening and aneurysm formation (Fig. 13.2). In fact, multiple aneurysms are present in up to 30% of patients. However, the majority of lesions are located at branching points along the proximal arterial tree, suggesting that hemodynamic factors play a significant role in aneurysm formation. In addition, formation of de novo aneurysms has been shown to occur at communicating arteries, and fatal rupture of aneurysms has occurred after recruitment of collateral blood flow through communicating arteries after the occlusion of carotid arteries. Furthermore, the incidence of aneurysms associated with high-flow states (e.g., arteriovenous malformations) is higher than in the general population, and some of these lesions regress after treatment of the vascular malformation. Finally, recurrent aneurysms can develop after incomplete treatment, whether by endovascular or microsurgical means.
Natural History
For patients with unruptured intracranial aneurysms, the natural history is much more difficult to resolve. Prior to 1998, the estimated rupture rate for aneurysms was thought to be 1% to 2.5% per year cumulatively. For young patients, the data suggested that intracranial aneurysms should be treated, as the cumulative risk of rupture over many years is very significant. However, the largest and most debated prospective database on unruptured intracranial aneurysms was published in two parts by the International Study of Unruptured Intracranial Aneurysms (ISUIA) and suggested that the rupture risk of unruptured asymptomatic aneurysms was much more benign than once thought (see Table 13.1). The 5-year cumulative rupture rate without a prior history of SAH of anterior circulation aneurysms (not including cavernous carotid or posterior communicating artery aneurysms) was 0%, 2.6%, and 14.5% for aneurysms less than 7 mm, 7 to 12 mm, and 13 to 24 mm, respectively, and it was 2.5%, 14.5%, and 18.4%, respectively, for the same three size groups of aneurysms in the posterior circulation (including posterior communicating artery aneurysms). Patients with a history of SAH with aneurysms less than 7 mm had a 1.5% rupture rate over 5 years with further increases in rates with increasing aneurysmal size. Many limitations of the ISUIA study have been reported. Selection bias likely played a substantial role because patients with aneurysms that were thought to be at a high risk of rupture, namely, those with multiple lobulations, hemodynamic factors, and so on, were treated and therefore were not included as part of the study. Further, cavernous carotid aneurysms were included in these studies, but these aneurysmal lesions rarely result in SAH. Lastly, the annual incidence of SAH in the United States is approximately 30,000, which when calculated against the incidence of aneurysms, results in a rupture rate of at least 1% per year, significantly higher than that calculated in the ISUIA study.18,19
Other morphological factors of aneurysms, besides the size of the aneurysmal dome, have been thought to contribute to the annual rupture risk. More specifically, neck width, dome width, aneurysm shape, aspect ratio (height/neck width), and bottleneck factor (dome width/neck width) have been examined.8 Among these, a higher aspect ratio (>1.6) is thought to be associated with an increased rupture risk. Other morphological characteristics, such as lobulations, daughter sacs, and surface irregularity, have long been associated with higher rupture risk8 (Box 13.1). Finally, the hemodynamics of the surrounding vasculature to an unruptured aneurysm may put a particular lesion at a greater risk of rupture, for example, dominant A1 feeding into an anterior communicating artery aneurysm.20
Clinical Presentation
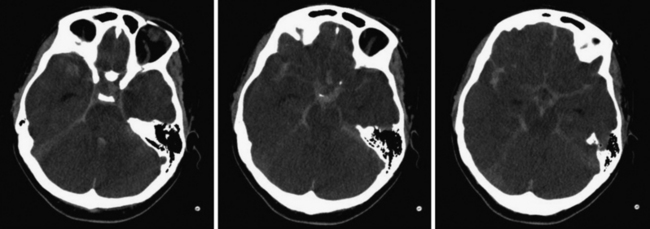
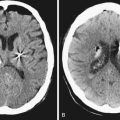
For patients who present with frank aneurysm rupture, the most common clinical symptoms and signs are the sudden onset of the worst headache of their life, severe nausea and vomiting, altered mental status, nuchal rigidity, and loss of consciousness. Most emergency departments automatically order a head computed tomography (CT) scan without contrast to evaluate these patients for possible SAH, although misdiagnosis remains a major problem (Fig. 13.3). It is of paramount importance to identify these patients because aneurysmal re-rupture has devastating consequences. With the technological advancements of CT scanners, the sensitivity/specificity of SAH detection is greater than 98% in the first 2 days.21 As such, lumbar puncture is not necessarily indicated unless the insinuating event happened at least 3 days prior or if head CT image is negative despite strong clinical suspicion. A lumbar puncture is used to detect evidence of red blood cells and xanthochromia. After confirmation of SAH on CT scan, a CT angiogram (CTA) is performed, especially if the clinical history is highly suggestive of aneurymal SAH. CTA detection of intracranial aneurysms is now on the order of 99% for aneurysms larger than 2 mm.22,23 Digital subtraction angiography (DSA) is now often reserved at our center for the absence of or questionable intracranial lesions seen on CTA or when the clinical picture favors endovascular intervention.
Approximately 25% of patients who present with SAH have had sentinel headaches (same symptoms as those related to SAH) in the weeks prior to presentation. The diagnosis of “sentinel headache” is only made in retrospect if the patient did not seek medical attention or was misdiagnosed recently when evaluated. These “sentinel headaches” are thought to be due to “minor leaks” or acute aneurysm expansion. Education of both the public and the medical community (especially physicians from primary care, neurology and emergency medicine) could go a long way to earlier diagnosis and improvement of outcomes. Any sudden onset thunderclap headache should be assumed related to an intracranial aneurysm until proved otherwise.
Various neurological deficits can be detected in aneurysm patients due to local mass effect, increased intracranial pressure, or thromboembolic complications from progressive thrombosis of a large or giant aneurysm. Common neurological findings include third cranial nerve palsy, visual loss (afferent pupillary defect), trigeminal symptoms, sixth cranial nerve palsy, nystagmus, vertigo, persistent nausea, and altered mental status (Fig. 13.4). These symptoms can occur from progressive enlargement of an unruptured aneurysm (Fig. 13.5). Focal neurological deficits can occur with embolization of clot from a thrombotic aneurysm. Magnetic resonance imaging (MRI) may be indicated in the workup of neurological findings but should not overlook the possibility of either aneurysms or the presence of subarachnoid blood.
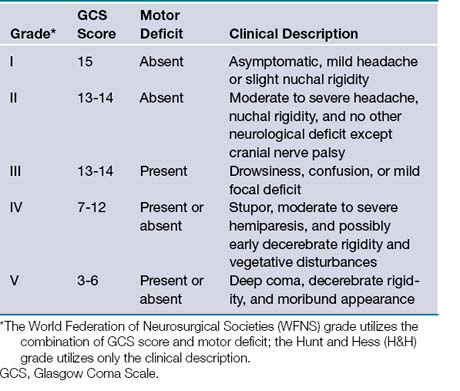
Once aneurysmal SAH has been confirmed, the initial neurological examination is important in determining prognosis. A variety of grading scales have been developed, most commonly the World Federation of Neurosurgical Societies (WFNS) and Hunt-Hess grading scales (Table 13.2). Morbidity and mortality rates increase with a higher grade in each scale.
Initial treatment for SAH involves a complete medical evaluation. Some patients can present with acute mental status changes that are the result of hydrocephalus. In these cases, an extraventricular drain should be placed not only to relieve the hydrocephalus but also to monitor the intracranial pressure. Permanent cerebrospinal fluid (CSF) diversion is necessary in approximately 10% of patients, owing to the hematoma breakdown products affecting the functioning of the arachnoid granulations. However, the most common medical complication after SAH is cerebral ischemia secondary to cerebral vasospasm. Vasospasm usually starts by the fifth posthemorrhage day and can be a threat for up to 14 days after hemorrhage. Angiographic vasospasm has been shown in up to 70% of patients, whereas symptomatic clinical vasospasm occurs in only about 30% of patients.24–26 Medications often administered to SAH patients, namely nimodipine, a calcium channel blocker, and pravachol, an HMG CoA (3-hydroxy-3-methylglutaryl coenzyme A) reductase inhibitor, are thought to limit, although not prevent, the onset of symptomatic vasospasm, thereby improving outcomes, not incidence.27 However, controversy still exists regarding the absolute effects of these medications.
Subarachnoid Hemorrhage
Medical Complications with Subarachnoid Hemorrhage
Subarachnoid hemorrhage has potentially devastating effects not only on the brain but also on the other organ systems of the body, both in the acute and chronic phases after aneurysmal rupture. Although it is beyond the scope of this chapter to detail every medical complication that can occur, brief mention will be made of the most commonly encountered medical complications (Box 13.2).
Soon after the event of SAH, a generalized sympathetic response is often encountered, which has profound effects on the cardiopulmonary system as well as the systemic vasculature. The massive surge of catecholamines after SAH likely induces various degrees of cardiac injury, as evidenced by increased serum troponin T levels, electrocardiogram (ECG) changes, and sometimes severe cardiac wall motion abnormalities.28–31 Pulmonary complications are also anticipated after SAH. Pulmonary edema can occur after SAH and is usually secondary to cardiac failure from cardiogenic shock.28,30 However, acute respiratory distress syndrome (ARDS) can result from primary pulmonary tissue damage from the initial sympathetic response. Further, in patients who lose consciousness after SAH, evidence of aspiration pneumonitis has been reported, which can develop into pneumonia or ARDS.32 When patients are dependent on mechanical ventilators after SAH, an increased incidence of pneumonia occurs.32
Electrolyte disturbances are anticipated after aneurysmal SAH. The most common electrolyte abnormality after SAH is hyponatremia, which occurs in approximately one third of patients.33 Owing to the endocrine disturbances of sodium and intravascular volume regulation, excessive renal excretion of sodium and dehydration lead to a condition known as cerebral salt wasting. In patients with lethal hemorrhages or aneurysms associated with hypothalamic tissue or vascular supply to such tissue, diabetes insipidus can occur, and leads to progressive hypernatremia and increasing urine output. In rare cases, SAH can lead to acute renal failure through posterior hypothalamic dysregulation, which may trigger renal vasoconstriction by activation of the renin-angiotensin system and thereby can reduce renal blood flow.34
Infectious complications also can accompany SAH patients in the chronic phases. Patients who are on mechanical ventilators, arterial and venous access lines, bladder catheters, and ventricular drains are always at an increased risk of infection. Appropriate changing of intravascular lines, tracheostomy procedures, timely removal of ventricular catheters, and changing of bladder catheters is thought to reduce the overall risk of infections.35
Finally, neurosurgical patients are known to experience a higher risk of developing deep venous thrombosis (DVT), potentially leading to an increased incidence of venous thrombosis and even thromboemboli.36 Most medical centers currently use compression stockings and pneumatic compression devices to reduce the incidence of DVT and pulmonary embolism (PE). In patients who develop DVTs and are not able to be anticoagulated due to recent SAH, vena cava filters are often placed. Early administration of prophylactic low-molecular-weight heparins is often used in SAH patients 48 to 72 hours after admission to decrease the risks of DVT/PEs (after aneurysm is secured).
Treatment of Subarachnoid Hemorrhage
Systemic circumstances (e.g., cardiopulmonary instability) may prevent a patient from being ready for aneurysm treatment. Therefore, antifibrinolytic therapy has been suggested to aid in reducing the risk of re-bleeding. The Cooperative Aneurysm Study found that the re-bleeding rate at 14 days was 11.7% if antifibrinolytics were used and 19.4% if no antifibrinolytics were used.37 However, in the face of vasospasm, the risk of ischemic events is higher in patients in whom antifibrinolytics were used, thus equalizing the mortality rates between the two groups. As such, we tend to use antifibrinolytics in patients who present in poor grade or who are medically unstable for treatment with the knowledge that ischemic complications are possible and CSF diversion may be necessary.
Symptomatic vasospasm is the leading cause of morbidity and death following aneurysmal SAH.25,26 Treatment for vasospasm has evolved over the past decade but still lacks definitive treatment (Fig. 13.6). Patients require diligent monitoring and treatment for up to 14 days after hemorrhage through invasive blood pressure monitoring, cerebral blood flow monitoring (transcranial ultrasound, CT/MRI perfusion studies), and often aggressive medical therapy including hypertensive and hypervolemic therapy and hemodilution (triple H therapy). In symptomatic patients, endovascular therapy can also be employed, which may involve intra-arterial injection of vasodilators or balloon angioplasty. In addition to spasm in the large conducting vessels, the phenomenon of SAH also triggers more complex events, which can lead to patchy infarcts in areas apparently unaffected by angiographically visible vasospasm.
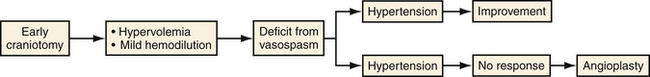
FIGURE 13.6 Prophylactic and therapeutic strategy for vasopasm at Northwestern University Medical School.
Much study has been invested in pharmacological prevention of arterial narrowing but research has not demonstrated changes in the long-term outcome of patients treated with such agents. Calcium channel blockers (e.g., nimodipine) have been used since the 1980s but have produced modest results in the literature.38–41 More specific pharmacological agents, namely, selective endothelin 1a receptor antagonists, have shown promise in effectively preventing and reducing arterial narrowing but failed to demonstrate any effect in long-term outcomes.25,26 Lastly, it has been suggested that statin therapy in conjunction with calcium channel blockers may lower the incidence of delayed cerebral ischemic events and improve patient outcomes; this was demonstrated in a phase II study.42 However, a recent meta-analysis of the literature claims that no statistical benefit could be gleaned from the literature. Nevertheless, many institutions now initiate acute therapy with both calcium channel blockers and statins.43
Approximately 70% of patients demonstrated some form of radiographic arterial narrowing, which does not necessarily warrant immediate treatment. However, up to 30% of these individuals will progress to have clinically symptomatic vasospasm, warranting immediate evaluation and treatment, medical or otherwise. Monitoring vasospasm in comatose patients becomes problematic because of the absence of a clinical examination to follow, thereby placing emphasis on indirect evidence such as transcranial Doppler or CT/MRI perfusion studies on progressively worsening cerebral blood flow changes to indicate time of potential treatment. Medical management of vasospasm has traditionally been through “triple H” therapy, involving hemodilution, hypertension, and hypervolemia.26,30,38,41 Maximizing the rheological properties of blood (i.e., maintaining a hematocrit level from 30 to 34) improves tissue perfusion. Should a focal neurological deficit develop, we immediately raise the systolic blood pressure up to the 175 to 200 mm Hg range or until the deficit reverses. In addition, we will volume expand and raise the central venous pressure (CVP) of the patient to the 10 to 15 mm Hg range. If these medical maneuvers do not result in an improvement of the patient’s condition and a surveillance CT scan does not demonstrate any areas of obvious infarction, we then proceed immediately to the angiography suite for a diagnostic angiogram and potential intra-arterial therapy.
Hydrocephalus, either communicating or noncommunicating, frequently develops in the first few days after SAH and partly explains the appearance of a globally edematous brain. Upon recognition that symptomatic hydrocephalus exists, it is suggested that CSF diversion be performed, usually by placement of an extraventricular drain (EVD). Careful placement of an EVD and conservative drainage of CSF must be maintained to avoid the possibility of an acute increase in the transmural pressure across the aneurysm or ventricular shift leading to the destabilization of the tamponading aneurysmal clot. In about 10% of patients, permanent CSF diversion is necessary.44 The risk of permanent hydrocephalus is higher in patients with ruptured upper basilar trunk aneurysms.
Treatment of Intracranial Aneurysms
Adjunctive Techniques in Aneurysm Surgery
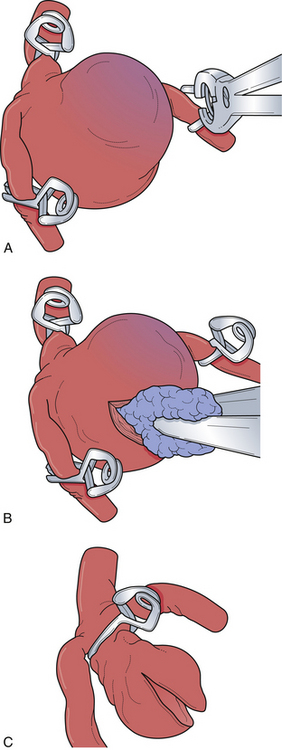
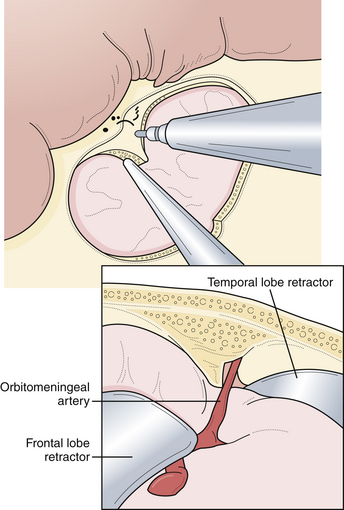
Several advancements have been made in adjunctive techniques and equipment that have significantly improved the surgical evaluation and management of aneurysms. Although the surgical microscope has been utilized in surgery for decades, its impact on aneurysm surgery continues to improve. With the reduction in the size of the microscope head as well as improvement in optics, neurosurgeons can work more comfortably and with improved visualization. The addition of using intraoperative fluorescence techniques such indocyanine green (ICG) video angiography allows the evaluation of aneurysm clip reconstruction in a fast and safe manner without the added risks of intraoperative catheter angiography.45–47 This imaging modality can be repeated multiple times during the procedure. Although catheter angiography remains the gold standard of intracranial vascular imaging, this new modality of intraoperative imaging allows quick and repeated evaluation of aneurysm clipping. Pharmacological agents have also been used more recently to aid in the clipping of complex intracranial aneurysms. Adenosine-induced cardiac arrest provides a safe method to reduce cerebral blood flow and softening of the aneurysmal dome, allowing manipulation and improved clip placement and reconstruction.48–50 With adequate cerebral protection, adenosine obviates the need for temporary clip occlusion in carefully selected cases. However, in most cases, patients can tolerate temporary occlusion without issue (Fig. 13.7). These measures are only a few of the recent and important developments to aid in aneurysm surgery. With the ever-advancing technology of intraoperative navigation and imaging, aneurysm surgery will become safer and more efficient.
Surgical Management of Anterior Circulation Artery Aneurysms
Pterional “Frontotemporal” Craniotomy
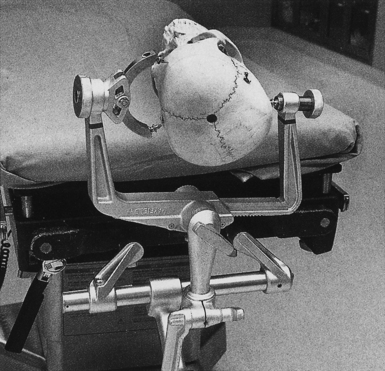
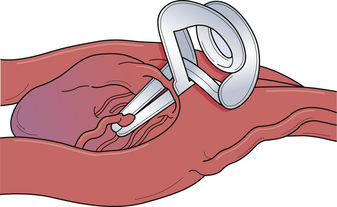
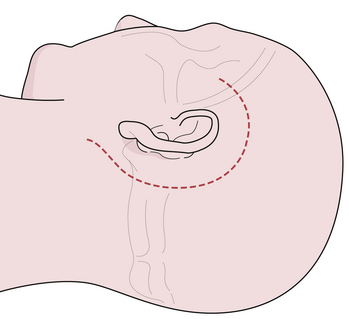
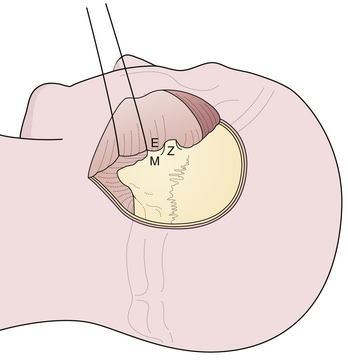
The pterional craniotomy has been the workhorse surgical approach for a variety of anterior circulation artery aneurysms because of its versatility and many modifications. A key element of this approach is the patient’s head position. Careful attention to this basic first step facilitates exposure and allows complex procedures to be carried out through small, tailored craniotomies, limited dural openings, and often without the need of brain retractors. The most frequent means of skull fixation in aneurysm surgery is the Mayfield-Keys three-point device, which provides almost absolute head stability (Fig. 13.8). Positioning of the three points varies for a pterional craniotomy and is at the discretion of the operating surgeon. Once the head is secured in the fixation device, the neurosurgeon performs three distinct maneuvers to position the head optimally for the necessary intracranial exposure.51
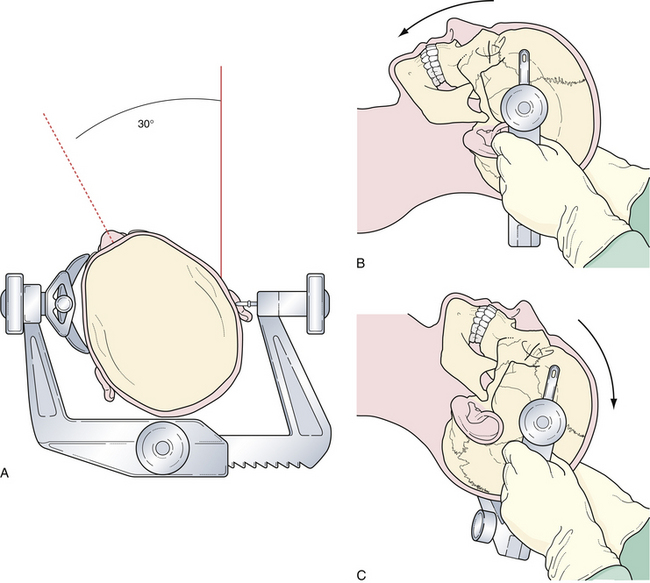
Rotation of the head is the first maneuver and is usually toward the contralateral or nonoperated side to an angle determined by the target anastomotic site (Fig. 13.9A). When the target site is the internal carotid or the distal basilar artery, minimal head rotation is required, usually approximately 20 degrees. Further angulation will allow the temporal lobe to migrate via gravity posteriorly and medially into the incisura and thus potentially obstructing the operative window. Aneurysms of the middle cerebral artery bifurcation require more angulation, approximately 40 to 45 degrees, to expose the sylvian fissure maximally, allowing both the temporal and frontal lobes to fall posteriorly once released from the fissure. For aneurysms of the anterior communicating artery, even more rotation is needed, approximately 45 to 60 degrees, to simplify the exposure of the medial gyrus rectus and interhemispheric fissure.
Flexion of the head is designed to maintain a perpendicular relationship between the floor of the anterior cranial fossa and the long axis of the patient’s body (Fig. 13.9B). After positioning the head in the appropriate rotation, the neck is flexed gently, bringing the chin toward the contralateral clavicle without compromising cervical venous return. This maneuver brings the area of operation away from the ipsilateral shoulder.
Extension or “tilting” of the head allows the frontal lobe to fall away from the floor of the anterior cranial fossa (Fig. 13.9C). To minimize retraction of the brain and to optimally display the vascular structures at the skull base, the vertex of the skull is tilted inferiorly so the maxillary eminence rises superior to the brow ridge.
Surgical Technique
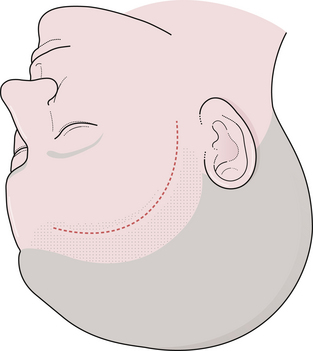
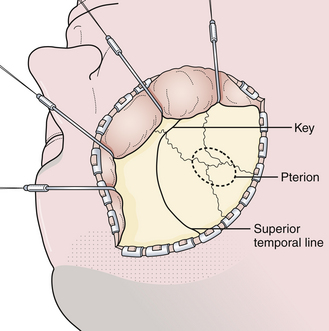
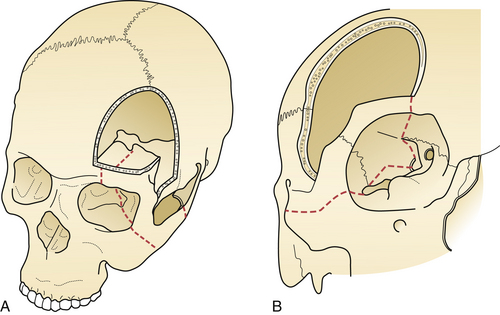
The surgical incision is first drawn on the skin beginning at the level of the zygoma, 1 cm anterior to the tragus, and extends superiorly immediately behind the hairline and gently curves anteriorly to the midline (Fig. 13.10). A simple technique is to put the thenar eminence of the hand onto the brow ridge and rotate the hand starting at the zygoma. A curvilinear marking will result that just approaches the midline. Every attempt is made to keep the incision behind the hairline for cosmesis, although this can be difficult in a balding patient. Numerous options are available for reflecting the scalp. The most common scalp flap is the myocutaneous flap, which involves reflecting the scalp and temporalis muscle together anteriorly (Fig. 13.11). An alternative technique involves the interfacial dissection where the bulk of the temporalis muscle is reflected inferiorly and the intrafascial fat pad, containing the frontalis branch of the facial nerve, and the scalp are reflected anteriorly. When reflecting the scalp and temporalis muscle, the key target of exposure with respect to the skull is the anatomical keyhole. A variable amount of temporal squama is exposed as well, depending on the vascular target. Bur hole placement normally consists of one bur hole in the anatomical key, one at the floor of the middle fossa, and one just inferior to the superior temporal line posteriorly (Fig. 13.12). There are some aneurysmal lesions (e.g., carotid wall, anterior communicating, and basilar apex aneurysms) that may require additional bony removal in addition to the standard pterional craniotomy. The addition of an orbital or orbitozygomatic (OZ) osteotomy may add 10 to 15 degrees of exposure, which is especially helpful in situations in which a swollen, tense brain exists (Fig. 13.13). To prepare for the additional craniotomy, the dura overlying the orbital roof is stripped posteriorly toward the orbital apex, medial to the cribriform, and lateral to the dura of the superior orbital fissure. If exposed, the periorbita is stripped from the superior and lateral walls of the orbit to a depth of approximately 3 cm. If the zygomatic process is to be included in the osteotomy, the full extent of the zygomatic arch is exposed prior to the reflexion of the temporalis muscle. Resection of the orbital zygomatic segment is best accomplished with a combination of reciprocating saw and osteotomes. The initial bone cuts are made with a reciprocating saw through the supraorbital ridge and frontal zygomatic process with the former being just lateral to the supraorbital foramen. Narrow osteotomes complete the resection across the orbital roof and the greater and lesser wings of the sphenoid bone. Once the masseter has been released from the inferior surface of the zygomatic arch, the piece may be removed en bloc. It is important to preserve at least 3 cm of the orbital roof and lateral wall so as to minimize the possibility of a postoperative pulsatile enophthalmos.
Depending on the adherence of the dura or in older patients, a fourth bur hole may be placed in the frontal bone just superior to the midline of the superior temporal line. It is important to try to separate the dura from the bone flap through the bur holes with a Penfield 2 or 3. This maneuver will allow easier removal of the bone flap. A craniotome is used to make cuts between the bur holes. After the bone flap is removed, the anterior temporal bone is extracted with rongeurs, as is the lateral third of the sphenoid ridge. Often it is necessary to remove the deepest portion of the sphenoid ridge with a power drill (Fig. 13.14). The inner table of the frontal bone is also removed with careful drilling. This maneuver allows exposure of the parasellar region with minimal frontal retraction. The dural opening is centralized around the sylvian fissure and has a semilunar shape (Fig. 13.15).
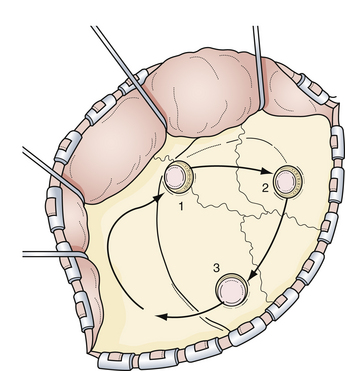
After the surgical microscope is sterilely draped, it is positioned into place so that the parasylvian region can be easily visualized. Following the sphenoid ridge along the inferior frontal lobe will lead to the carotid cistern where the optic nerve and the carotid artery should be easily visualized. The surrounding arachnoid membranes, including the medial sylvian fissure, are opened sharply to separate the medial frontal and temporal lobes from the underlying carotid cisternal structures (Fig. 13.16). After this maneuver, the entire subarachnoid course of the carotid artery, including the bifurcation into the middle and anterior cerebral arteries, should be seen. From this point, the specific aneurysmal site governs the principles of further dissection.
Aneurysms of the Proximal (Paraclinoidal) and Posterior Carotid Artery Wall
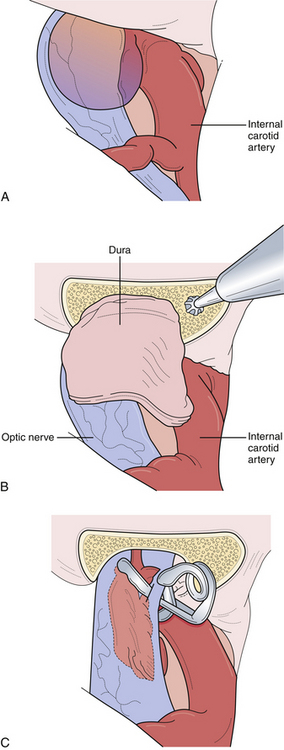
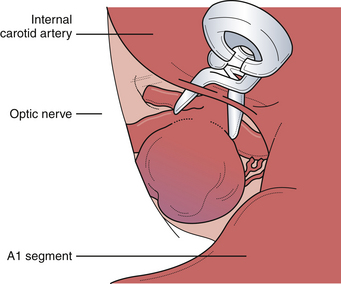
This unique group of aneurysms arises from the most proximal intracranial segment of the internal carotid artery. Most of the aneurysms in this area can be separated into three subgroups, namely, carotid-ophthalmic, superior hypophyseal, and proximal posterior carotid wall. Each of these aneurysms presents several unique technical problems to the neurosurgeon. Although their proximity to the skull base was once a formidable problem in regard to obtaining any significant proximal vascular control, endovascular access and proximal control have reduced this difficulty. When these aneurysms reach a significant size, they can compress and distort the optic nerve, resulting in visual neurological deficits. The aneurysm must then be decompressed to relieve the mass effect. Further, large and giant aneurysms are often found to have thick, calcified walls, which must be refashioned with creativity, requiring multiple aneurysm clips because of the rigidity and differential wall thickness. The lateral aspect of paraclinoidal, especially carotid-ophthalmic, aneurysms can be partially covered by the anterior clinoid process as it emerges from the roof of the cavernous sinus (Fig. 13.17). The optic strut, the inferior bony aspect of the optic canal, can also obscure the proximal neck of the aneurysm. Our experience suggests that although large aneurysm domes can distort various neurovascular structures, the proximal portion of the normal carotid artery is always located at the lateral aspect of the optic canal. As such, this proximal portion of the carotid can normally be exposed via an incision in the falciform ligament, which will uncover the proximal carotid and ophthalmic artery origin, and the proximal neck of the aneurysm, particularly in carotid ophthalmic and superior hypophyseal variants. If the aneurysm projects laterally or arises from within the carotid cave, then aggressive bony resection of the anterior clinoid process or the optic strut will be required to demonstrate the proximal neck. In ruptured proximal carotid aneurysms, it is important for proximal control to be established early either through an open cervical exposure to the internal carotid artery or through endovascular means with a proximal internal carotid balloon. Either way, it is important to guarantee early proximal control should difficulties arise and require temporary arterial occlusion, aneurysm decompression, and intraoperative angiography.
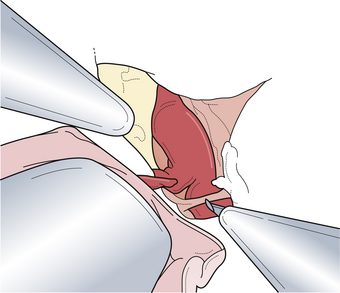
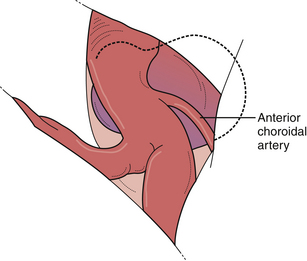
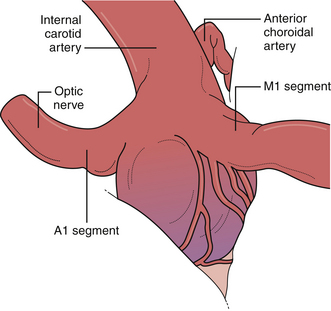
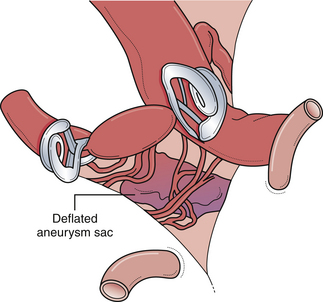
Posterior carotid wall aneurysms include lesions arising immediately distal to the posterior communicating artery as well as those arising immediately distal to the origin of the anterior choroidal artery (Fig. 13.18). These lesions are some of the most common aneurysmal lesions seen in neurosurgical practice, and intimate knowledge of the microanatomy is paramount in order to treat these lesions successfully. Neurological signs of an enlarging aneurysm in this area are pain, from compression of the tentorial incisura, and third cranial nerve deficits. The intimate association between the distal neck of the internal carotid–posterior communicating artery aneurysm to the fragile anterior choroidal artery place it at risk when treating these aneurysms. Injuring the anterior choroidal artery, which supplies the posterior limb of the internal capsule, the lentiform nucleus, the optic tract, the amygdala, and the choroid plexus of the temporal horn, can result in profound and devastating neurological deficits, including hemiplegia and hemianopia. Maneuvers that can result in injury include aggressive dissection of the aneurysm dome and the placement of Gelfoam or cotton to control minor intraoperative bleeding. It should be emphasized that the distal course of the anterior choroidal artery often runs close to the aneurysmal dome and should be carefully dissected away so that the distal clip blade does not compromise its blood flow or the vessel becomes “accordianed” by clip closure (Fig. 13.19). The posterior communicating artery usually arises just proximal to the aneurysmal neck, and every attempt should be made to preserve this vessel because of important anterior thalamoperforating branches that ramify from its proximal portion. The neurosurgeon should also know if the posterior communicating artery is of fetal origin, therefore lacking an ipsilateral P1 segment. In this situation, it is paramount to not sacrifice this artery. However, in patients with a normal circle of Willis, it is sometimes acceptable to include the posterior communicating artery in the clip, assuming the anterior thalamoperforators will fill from retrograde flow from the P1 segment off the basilar artery. This maneuver should be reserved for extreme situations because we have seen major deficits arise from it. Nevertheless, when possible, all components of the circle of Willis should be preserved, particularly in the setting of potential vasospasm after SAH and reliance on available collateral pathways. The most common clip arrangement for a posterior communicating artery aneurysm is perpendicular to the carotid artery. In smaller aneurysms, the carotid artery may narrow a bit after clipping but is usually trivial. In larger broad-based aneurysms, clipping perpendicular to the carotid artery can substantially narrow, even wrinkle, the carotid artery and impose shear forces on the fragile neck of the aneurysm during the closure of the clip. The angled fenestrated aneurysm clip allows clip placement so that the blades close parallel to the carotid artery, minimizing the shear forces. Regardless of the clip chosen, it is critical to inspect the course of the anterior choroidal and the posterior communicating arteries to ensure that these vessels have not been compromised.
Aneurysms of the Carotid Bifurcation
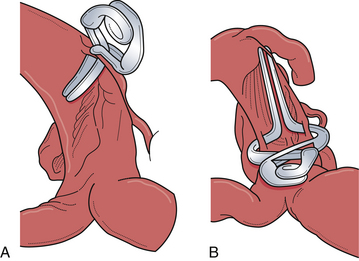
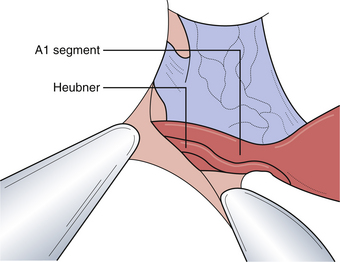
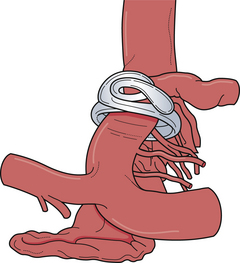
Appropriate operative exposure of the carotid terminus/bifurcation requires judicious drilling of the sphenoid ridge and dissection of the opercular-insular cortex as well as the sphenoidal portions of the sylvian fissure. We prefer a lateral to medial approach followed by dissection of the carotid cistern to allow “unfolding” of the anatomy at the carotid terminus. The aneurysmal dome and neck can often be intimately associated with local vascular structures, namely, the anterior choroidal artery, anterior cerebral artery, the recurrent artery of Heubner, and sometimes medial lenticulostriate branches from the middle cerebral artery (Fig. 13.20). These vessels typically hide posterior to the aneurysmal dome. It is paramount that the operating surgeon identify all of these important vessels so that the clip blades do not include them during closure of the aneurysm clip.
The two most important aspects of the exposure are gaining proximal control of the internal carotid artery and a wide opening of the carotid cistern. In addition, the sylvian fissure should be opened completely to expose the M1 segment, which can be followed proximally to the carotid terminus/bifurcation. Full exposure of the carotid terminus/bifurcation ensures both afferent and efferent circulation control. When treating difficult large and giant carotid terminus aneurysms, it is paramount for the neurosurgeon to know if there is a patent anterior communicating artery, which, if present, allows the potential sacrifice of the ipsilateral A1 segment in these cases and in the event of tears involving the medial aspect of the aneurysmal neck (Fig. 13.21).
Aneurysms of the Middle Cerebral Artery Bifurcation
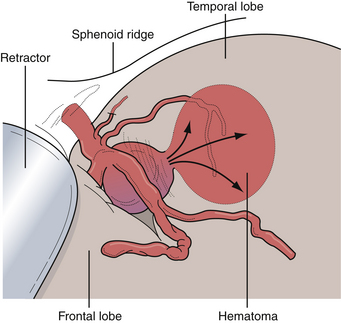
Aneurysms of the middle cerebral artery, in particular at the site of the bifurcation, are quite common and hemorrhage from these lesions can produce several unique features. As such, diagnosis can be difficult in terms of the etiology of either an intraparenchymal or subdural hemorrhage in this location. Aneurysms at this location can also become very large or giant due to growth into the substance of the temporal lobe or invagination into the pia-arachnoid of the medial temporal lobe. When rupture occurs at the distal aspect of the aneurysmal dome, the resultant hematoma is in the temporal lobe parenchyma. Patients can present after a middle cerebral artery (MCA) aneurysm rupture with profound mass effect from the hematoma that necessitates surgical evacuation (Fig. 13.22). Modern CT angiography allows the immediate evaluation of the cerebral vasculature in cases such as these so that definitive treatment of the aneurysm can be considered during the evacuation of the hematoma.
Aneurysms of the Anterior Communicating Artery
Treatment of anterior communicating artery aneurysms can present the surgeon with unique challenges. To adequately expose the surgical target, the bony craniotomy should be carried somewhat more medially and inferiorly than the craniotomy designed for other lesions because of the more anterior location of the anterior communicating artery complex. This is done by enlarging the craniotomy to the midpupillary line and carrying the bony incision as close to the brow as possible, without infiltrating the frontal sinuses. To augment this approach, a significant amount of bone, namely the sphenoid ridge to the superior orbital fissure and the inner tables of the anterior and middle fossa, must be removed. An alternative surgical approach is the fronto-orbito-zygomatic, which involves the lateral superior and lateral orbital wall and zygomatic arch. Both of these approaches can lead to minimal visual obstruction by surrounding bone without significant cosmetic effects.
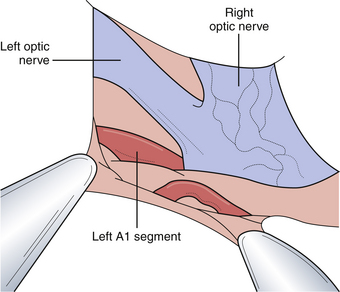
The microsurgical procedure for anterior communicating artery aneurysms focuses on the wide opening of the carotid cistern. One approach is to locate the proximal portion of the A1 segment at the carotid bifurcation and follow it to the anterior communicating complex. Alternatively, we advocate a direct dissection over the optic nerve toward the midline. The A1 segment swings anteriorly as it approaches the anterior communicating complex. The contralateral A1 segment should then be visualized by crossing the midline and working superior to the contralateral ophthalmic nerve (Fig. 13.23). This cistern may be filled with hematoma if done in a patient with SAH and, therefore, careful dissection in this area should be done to avoid inadvertently injuring the many important vessels traversing this region. The gyrus rectus, just inferior to the olfactory tract, can be aggressively resected with cautery and suction (Fig. 13.24). The overlying veil of pia can be manipulated to facilitate gyral resection. Once the gyrus resection is completed, the ipsilateral and contralateral A2 segments should be identified for distal control. Once both of the A1 and A2 segments are dissected and localized, the dissection can focus on the anterior communicating artery and the associated aneurysm. The relationship of the aneurysm to the surrounding vasculature as well as the proximal and distal aneurysmal necks needs to be understood completely before definite clipping can occur. It is paramount to identify the small perforating arteries that emanate from the posterior aspect of the anterior communicating artery (Fig. 13.25). Laceration or inclusion of these perforators in the clip blades can lead to serious and irreversible cognitive and hypothalamic sequelae.
Aneurysms of the Distal Basilar Artery
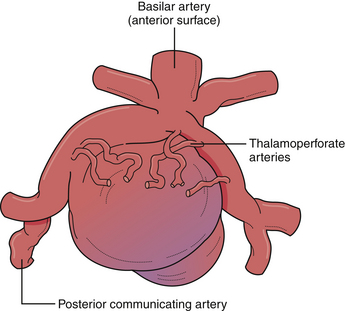
Aneurysms arising from the basilar apex or immediately distal to the origin of the superior cerebellar arteries are the most common form of posterior circulation aneurysms. Access to the distal basilar artery is complicated by the myriad of vital perforating arteries arising from the posterior aspect of the distal basilar artery and both P1 segments (Fig. 13.26). As such, endovascular strategies are often employed as an initial treatment. However, some situations, such as efferent segments off the aneurysmal dome, acute angle of aneurysm neck from parent artery, or failed endovascular therapy make surgery more desirable. To ensure preservation of the vital perforating vessels, clear surgical strategies for clip placement must be made (Fig. 13.27). Pharmacological agents to induce systemic hypotension and asystole (i.e., adenosine) are especially helpful in the narrow surgical corridors through which these aneurysms are treated. Several surgical approaches are used to expose the distal basilar trunk, and the advantages, disadvantages, and limitations are discussed here.
Pterional Transsylvian Approach
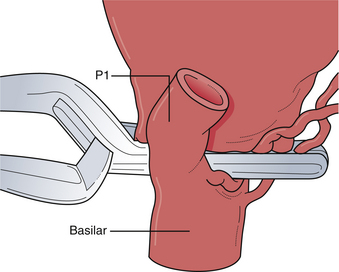
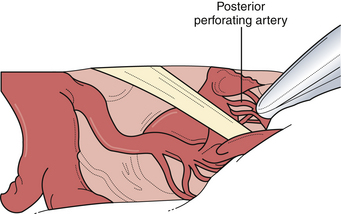
The pterional approach, particularly with wide sylvian fissure dissection, is extremely versatile and used for many aneurysms of the distal basilar complex. Wide dissection of the parasellar cisterns enables access to the interpeduncular cistern by one or multiple routes (Fig. 13.28). The dissection plane and the approach can be developed lateral to the internal carotid artery wherein the posterior communicating artery is followed to the junction of the P1 and P2 segments of the posterior cerebral artery. The P1 can be followed proximally to the basilar artery to gain proximal control. In a similar fashion, a dissection plane can be developed medial to the carotid artery that separates the small perforating arteries to the hypothalamic region and optic tract, opening the membrane of Liliequist, directly exposing the basilar trunk in the interpeduncular cistern. To achieve more superior exposure, the binding arachnoid fibers of the small lenticulostriate vessels can be divided to allow elevation of the optic tract opening up the interpeduncular cistern. To minimize the obstruction of this limited surgical view, the clip applier is often passed lateral to the carotid artery toward the basilar apex. This exposure allows exquisite access to the proximal basilar trunk and both P1 segments for definitive temporary occlusion with aneurysmal decompression, adequate dissection space for large and giant lesions, and, if necessary, control of intraoperative bleeding.
Subtemporal Approach
The lateral view of the interpeduncular cistern offers the ideal view of the vital perforators posterior to the aneurysmal dome (Fig. 13.29). However, visualization of the contralateral P1 segment can be difficult. To improve visualization of the contralateral P1 segment; the space anterior to the aneurysmal neck can usually be gently retracted to expose the initial anterior course of this segment. The initial course of the P1 is superior and anterior. No vessels or neural structures obscure this route. When confusion arises as to whether the identified structure is the P1 or superior cerebellar artery, the P1 is usually white and thicker walled, and if it is followed laterally, the third cranial nerve will be encountered, which clarifies the anatomy. (The P1 is superior to the nerve.) The power of the fenestrated aneurysm clip is especially realized using this approach as the perforating arteries can often be incorporated into the fenestration (Fig. 13.30).
Petrosal Approach
The patient is positioned supine with a shoulder roll ipsilateral to the side of the approach and the head is turned through 90 degrees, then gently extended toward the floor, and, if necessary, the shoulder is taped caudally. A curvilinear incision is made originating 2 cm superior and anterior to the ear and curved along a 2 cm posterior margin from the ear to a point 2 cm inferior to the mastoid tip (Fig. 13.31). The temporalis and occipital frontalis muscles are divided and reflected laterally with the scalp to expose the edge of the external auditory meatus centrally, the temporal zygomatic root rostrally, and the entire mastoid process posterior inferiorly (Fig. 13.32). A mastoidectomy is performed with early identification and skeletonization of the transverse and sigmoid sinuses as far laterally as the jugular bulb. Care is taken to preserve the bony labyrinth in situations in which only a retrolabyrinthine, presigmoid exposure is necessary. With a partial labyrinthectomy, as many as 5 additional millimeters of exposure can be created, providing for several degrees of added maneuverability at the level of the petrous apex. This is accomplished with cautious drilling of the posterior and superior semicircular canals to expose the underlying membranous labyrinth. Once identified, the exposed orifices are immediately waxed off. The horizontal semicircular canal is preserved. A completed mastoidectomy should yield exposure of tegmental and posterior fossa dura. This dural exposure facilitates the addition of a subtemporal and suboccipital craniotomy. The dural opening is typically presigmoid, originating just superior and anterior to the jugular bulb and directed toward the superior petrosal sinus. The sinus is ligated and the dural cut carried rostrally to the dura of the middle fossa, then the dura is tented laterally. The tentorium is coagulated with the bipolar cautery and cut toward the incisura. Care then is exercised to avoid injury to the fourth cranial nerve as it courses adjacent to the midbrain en route to the tentorial edge. The tentorium can be partially excised or coagulated to enhance visualization. The midbrain, clivus, basilar artery, and cranial nerves III to XII are clearly visible with this sigmoid dural exposure with ligation of the nondominant transverse sinus; however, in our experience this maneuver creates an unacceptable risk of venous infarction. We advocate the use of dural grafts and lumbar drainage because watertight dural closures are often very difficult in these cases. We also utilize fat grafts over the dural closure to provide a hydrophobic barrier to leakage and a natural, autologous sealant.
Aneurysms of the Vertebral Confluens and Lower Basilar Trunk
Aneurysms of the vertebral confluens and the lower basilar trunk are some of the most difficult aneurysms to treat due to the depth of exposure, the requirement for optimal exposure ventral to the brainstem, and the frequent need for innovative cranial base exposures. As a result, these aneurysms are more commonly treated with endovascular strategies if possible; however, in some circumstances, endovascular strategies are not possible. The vertebral confluens, or the vertebrobasilar junction, is a unique arterial structure where two large vessels join to form a larger artery. This configuration is often very tortuous, involves different calibers and lengths of vertebral arteries, and potentially involves fenestrations of the confluens and lower basilar trunk. As such, these configurations allow complex hemodynamics that predisposes this area to aneurysm development. Aneurysms of the lower basilar trunk are usually associated with the origin of the anterior inferior cerebellar artery (AICA) and the sixth cranial nerve.
Bederson J.B., Connolly E.S.Jr., Batjer H.H., et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 2009;40(3):994-1025.
Lall R.R., Eddleman C.S., Bendok B.R., Batjer H.H. Unruptured intracranial aneurysms and the assessment of rupture risk based on anatomical and morphological factors: sifting through the sands of data. Neurosurg Focus. 2009;26(5):E2.
Pluta R.M., Hansen-Schwartz J., Dreier J., et al. Cerebral vasospasm following subarachnoid hemorrhage: time for a new world of thought. Neurol Res. 2009;31(2):151-158.
Rabinstein A.A. The AHA guidelines for the management of SAH: what we know and so much we need to learn. Neurocrit Care. 2009;10(3):414-417.
Wiebers D.O., Whisnant J.P., Huston J.3rd, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362(9378):103-110.
Please go to expertconsult.com to view complete list of references
1. Fox J. Intracranial Aneurysms. New York: Springer-Verlag; 1983.
2. Stehbens W. Aneurysmal and anatomical variation of cerebral arteries. Arch Pathol. 1963;75:45-64.
3. Stehbens W. Pathology of Cerebral Blood Vessels. St. Louis: Mosby; 1972.
4. Hetts S.W., Narvid J., Sanai N., et al. Intracranial aneurysms in childhood: 27-year single-institution experience. AJNR Am J Neuroradiol. 2009;30(7):1315-1324.
5. Weir B. Unruptured intracranial aneurysms: a review. J Neurosurg. 2002;96(1):3-42.
6. International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured intracranial aneurysms—risk of rupture and risks of surgical intervention. N Engl J Med. 1998;339(24):1725-1733.
7. Wiebers D.O., Whisnant J.P., Huston J.3rd, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362(9378):103-110.
8. Lall R.R., Eddleman C.S., Bendok B.R., Batjer H.H. Unruptured intracranial aneurysms and the assessment of rupture risk based on anatomical and morphological factors: sifting through the sands of data. Neurosurg Focus. 2009;26(5):E2.
9. Woo D., Khoury J., Haverbusch M.M., et al. Smoking and family history and risk of aneurysmal subarachnoid hemorrhage. Neurology. 2009;72(1):69-72.
10. Chow C.L., Ong A.C. Autosomal dominant polycystic kidney disease. Clin Med. 2009;9(3):278-283.
11. Foroud T., Sauerbeck L., Brown R., et al. Genome screen in familial intracranial aneurysm. BMC Med Genet. 2009;10:3.
12. Nguyen T.V., Chandrashekar K., Qin Z., et al. Epidemiology of intracranial aneurysms of Mississippi: a 10-year (1997-2007) retrospective study. J Stroke Cerebrovasc Dis. 2009;18(5):374-380.
13. Rodrigues V.J., Elsayed S., Loeys B.L., et al. Neuroradiologic manifestations of Loeys-Dietz syndrome type 1. AJNR Am J Neuroradiol. 2009;30(8):1614-1619.
14. Ruigrok Y.M., Rinkel G.J., Wijmenga C., et al. Association analysis of genes involved in the maintenance of the integrity of the extracellular matrix with intracranial aneurysms in a Japanese cohort. Cerebrovasc Dis. 2009;28(2):131-134.
15. Shi C., Awad I.A., Jafari N., et al. Genomics of human intracranial aneurysm wall. Stroke. 2009;40(4):1252-1261.
16. Woo D., Hornung R., Sauerbeck L., et al. Age at intracranial aneurysm rupture among generations: familial intracranial aneurysm study. Neurology. 2009;72(8):695-698.
17. Ruigrok Y.M., Rinkel G.J. Genetics of intracranial aneurysms. Stroke. 2008;39(3):1049-1055.
18. Weir B. Patients with small, asymptomatic, unruptured intracranial aneurysms and no history of subarachnoid hemorrhage should be treated conservatively: against. Stroke. 2005;36(2):410-411.
19. Clarke M. Systematic review of reviews of risk factors for intracranial aneurysms. Neuroradiology. 2008;50(8):653-664.
20. Sadatomo T., Yuki K., Migita K., et al. Evaluation of relation among aneurysmal neck, parent artery, and daughter arteries in middle cerebral artery aneurysms, by three-dimensional digital subtraction angiography. Neurosurg Rev. 2005;28(3):196-200.
21. Provenzale J.M., Hacein-Bey L. CT evaluation of subarachnoid hemorrhage: a practical review for the radiologist interpreting emergency room studies. Emerg Radiol. 2009;16(6):441-451.
22. Chen W., Wang J., Xing W., et al. Accuracy of 16-row multislice computerized tomography angiography for assessment of intracranial aneurysms. Surg Neurol. 2009;71(1):32-42.
23. Franklin B., Gasco J., Uribe T., et al. Diagnostic accuracy and inter-rater reliability of 64-multislice 3D-CTA compared to intra-arterial DSA for intracranial aneurysms. J Clin Neurosci. 2010;17(5):579-583.
24. Bederson J.B., Connolly E.S.Jr., Batjer H.H., et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 2009;40(3):994-1025.
25. Pearl J.D., Macdonald R.L. Vasospasm after aneurysmal subarachnoid hemorrhage: need for further study. Acta Neurochir Suppl. 2008;105:207-210.
26. Pluta R.M., Hansen-Schwartz J., Dreier J., et al. Cerebral vasospasm following subarachnoid hemorrhage: time for a new world of thought. Neurol Res. 2009;31(2):151-158.
27. McGirt M.J., Garces Ambrossi G.L., Huang J., Tamargo R.J. Simvastatin for the prevention of symptomatic cerebral vasospasm following aneurysmal subarachnoid hemorrhage: a single-institution prospective cohort study. J Neurosurg. 2009;110(5):968-974.
28. Diringer M.N. Management of aneurysmal subarachnoid hemorrhage. Crit Care Med. 2009;37(2):432-440.
29. Lee V.H., Oh J.K., Mulvagh S.L., Wijdicks E.F. Mechanisms in neurogenic stress cardiomyopathy after aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2006;5(3):243-249.
30. Rabinstein A.A. The AHA guidelines for the management of SAH: what we know and so much we need to learn. Neurocrit Care. 2009;10(3):414-417.
31. Urbaniak K., Merchant A.I., Amin-Hanjani S., Roitberg B. Cardiac complications after aneurysmal subarachnoid hemorrhage. Surg Neurol. 2007;67(1):21-28. discussion 28–29
32. Friedman J.A., Pichelmann M.A., Piepgras D.G., et al. Pulmonary complications of aneurysmal subarachnoid hemorrhage. Neurosurgery. 2003;52(5):1025-1031. discussion 1031–1022
33. Sherlock M., O’Sullivan E., Agha A., et al. The incidence and pathophysiology of hyponatraemia after subarachnoid haemorrhage. Clin Endocrinol (Oxf). 2006;64(3):250-254.
34. Bendel S., Koivisto T., Ruokonen E., et al. Pituitary-adrenal function in patients with acute subarachnoid haemorrhage: a prospective cohort study. Crit Care. 2008;12(5):R126.
35. Naidech A.M., Bendok B.R., Tamul P., et al. Medical complications drive length of stay after brain hemorrhage: a cohort study. Neurocrit Care. 2009;10(1):11-19.
36. Ray W.Z., Strom R.G., Blackburn S.L., et al. Incidence of deep venous thrombosis after subarachnoid hemorrhage. J Neurosurg. 2009;110(5):1010-1014.
37. Kassell N.F., Torner J.C., Adams H.P.Jr. Antifibrinolytic therapy in the acute period following aneurysmal subarachnoid hemorrhage. Preliminary observations from the Cooperative Aneurysm Study. J Neurosurg. 1984;61(2):225-230.
38. Keyrouz S.G., Diringer M.N. Clinical review: prevention and therapy of vasospasm in subarachnoid hemorrhage. Crit Care. 2007;11(4):220.
39. Rinkel G.J., Feigin V.L., Algra A., et al. Calcium antagonists for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev. 2005(1):CD000277.
40. Tomassoni D., Lanari A., Silvestrelli G., et al. Nimodipine and its use in cerebrovascular disease: evidence from recent preclinical and controlled clinical studies. Clin Exp Hypertens. 2008;30(8):744-766.
41. Weyer G.W., Nolan C.P., Macdonald R.L. Evidence-based cerebral vasospasm management. Neurosurg Focus. 2006;21(3):E8.
42. Vergouwen M.D., Meijers J.C., Geskus R.B., et al. Biologic effects of simvastatin in patients with aneurysmal subarachnoid hemorrhage: a double-blind, placebo-controlled randomized trial. J Cereb Blood Flow Metab. 2009;29(8):1444-1453.
43. Kramer A.H., Fletcher J.J. Statins in the management of patients with aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. Neurocrit Care. 2010;12(2):285-296.
44. Sheehan J.P., Polin R.S., Sheehan J.M., et al. Factors associated with hydrocephalus after aneurysmal subarachnoid hemorrhage. Neurosurgery. 1999;45(5):1120-1127. discussion 1127–1128
45. Dashti R., Laakso A., Niemela M., et al. Microscope-integrated near-infrared indocyanine green videoangiography during surgery of intracranial aneurysms: the Helsinki experience. Surg Neurol. 2009;71(5):543-550. discussion 550
46. Raabe A., Nakaji P., Beck J., et al. Prospective evaluation of surgical microscope-integrated intraoperative near-infrared indocyanine green videoangiography during aneurysm surgery. J Neurosurg. 2005;103(6):982-989.
47. Woitzik J., Horn P., Vajkoczy P., Schmiedek P. Intraoperative control of extracranial-intracranial bypass patency by near-infrared indocyanine green videoangiography. J Neurosurg. 2005;102(4):692-698.
48. Groff M.W., Adams D.C., Kahn R.A., et al. Adenosine-induced transient asystole for management of a basilar artery aneurysm. Case report. J Neurosurg. 1999;91(4):687-690.
49. Heppner P.A., Ellegala D.B., Robertson N., et al. Basilar tip aneurysm—adenosine induced asystole for the treatment of a basilar tip aneurysm following failure of temporary clipping. Acta Neurochir (Wien). 2007;149(5):517-520. discussion 520–511
50. Luostarinen T., Takala R.S., Niemi T.T., et al. Adenosine-induced cardiac arrest during intraoperative cerebral aneurysm rupture. World Neurosurg. 2010;73(2):79-83.
51. Samson D., Batjer H. Intracranial Aneurysm Surgery: Techniques. Mount Kisco, NY: Futura Publishing; 1990.
52. Hassan T., Timofeev E.V., Saito T., et al. A proposed parent vessel geometry-based categorization of saccular intracranial aneurysms: computational flow dynamics analysis of the risk factors for lesion rupture. J Neurosurg. 2005;103(4):662-680.
53. Hoi Y., Meng H., Woodward S.H., et al. Effects of arterial geometry on aneurysm growth: three-dimensional computational fluid dynamics study. J Neurosurg. 2004;101(4):676-681.
54. Hoh B.L., Sistrom C.L., Firment C.S., et al. Bottleneck factor and height-width ratio: association with ruptured aneurysms in patients with multiple cerebral aneurysms. Neurosurgery. 2007;61(4):716-722. discussion 722–713
55. Ujiie H., Tamano Y., Sasaki K., Hori T. Is the aspect ratio a reliable index for predicting the rupture of a saccular aneurysm? Neurosurgery. 2001;48(3):495-502. discussion 492–493
56. Weir B., Amidei C., Kongable G., et al. The aspect ratio (dome/neck) of ruptured and unruptured aneurysms. J Neurosurg. 2003;99(3):447-451.
57. Beck J., Rohde S., el Beltagy M., et al. Difference in configuration of ruptured and unruptured intracranial aneurysms determined by biplanar digital subtraction angiography. Acta Neurochir (Wien). 2003;145(10):861-865. discussion 865
58. Hademenos G.J., Massoud T.F., Turjman F., Sayre J.W. Anatomical and morphological factors correlating with rupture of intracranial aneurysms in patients referred for endovascular treatment. Neuroradiology. 1998;40(11):755-760.
59. Ma B., Harbaugh R.E., Raghavan M.L. Three-dimensional geometrical characterization of cerebral aneurysms. Ann Biomed Eng. 2004;32(2):264-273.
60. Sadatomo T., Yuki K., Migita K., et al. Morphological differences between ruptured and unruptured cases in middle cerebral artery aneurysms. Neurosurgery. 2008;62(3):602-609. discussion 602–609
61. Sampei T., Mizuno M., Nakajima S., et al. [Clinical study of growing up aneurysms: report of 25 cases.]. No Shinkei Geka. 1991;19(9):825-830.
62. Dhar S., Tremmel M., Mocco J., et al. Morphology parameters for intracranial aneurysm rupture risk assessment. Neurosurgery. 2008;63(2):185-196. discussion 196–187