58 Intracerebral Hemorrhage
Clinical Vignette
There are two forms of intrinsic cerebral hemorrhage, primary ICH, which has a predilection to affect the striatum, thalamus, midbrain, pons, and cerebellum, and subarachnoid hemorrhage (Chapter 57). ICH comprises approximately 10% of all strokes in the Caucasian population and up to 20% in the Asian population. Over the past several years, improved treatment of hypertension has decreased the number of patients experiencing ICH.
Pathophysiology of Hypertensisve Primary Ich
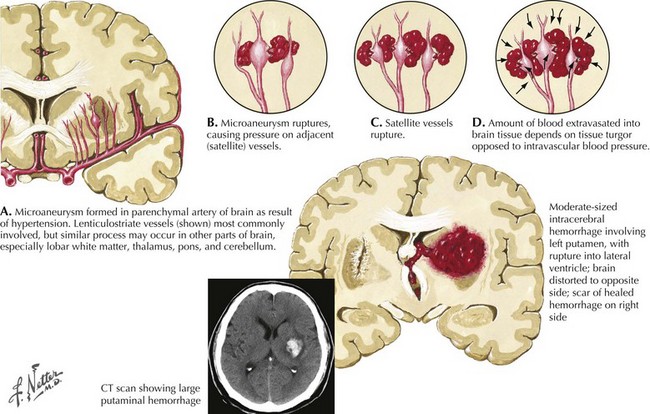
Intracranial hemorrhage is a rapidly evolving process that may progress over hours or days. The pressure effects of the initial hemorrhage lead to mechanical disruption and tearing of surrounding vessels with subsequent gradual expansion of the hematoma out from the original center. Rebleeding is the most feared early complication of ICH and occurs in approximately 40% of patients. Rebleeding usually occurs within the first 24 hours but, on occasion, has been reported up to a week later. The underlying pathological mechanism of primary hypertensive ICH is attributable to either the formation of miliary microaneurysms or primary arteriolar degeneration (lipohyalinosis and weakening of the blood vessel intima and media wall layers) (Fig. 58-1). The presence of miliary aneurysms is directly related to hypertension but is not necessarily the initial site of bleeding, and cases of hypertensive ICH outside the areas of microaneurysms have been noted. This suggests that degeneration of the arteriolar smooth muscle wall is likely an important factor in the evolution of ICH. Hypertensive intracerebral hemorrhages have a predilection to occur in the basal ganglia and the thalamus. The arterioles in these structures are likely more vulnerable to degenerative changes brought on by diffuse, large pressure pulses over time.
Clinical Presentation
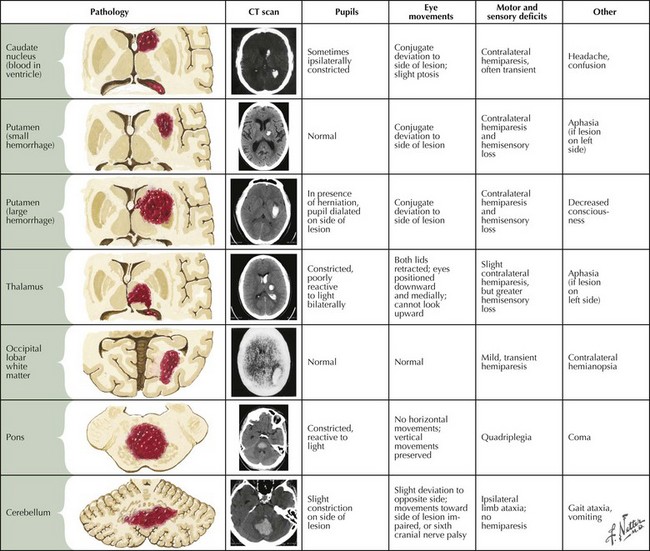
Intraparenchymal hemorrhages vary in presentation depending on the site of the bleeding (Fig. 58-2). In approximately 60% of patients, neurologic symptoms develop gradually or stepwise over a period of hours. To some extent, the location and size of the hematoma predict clinical outcome.
Deep Supratentorial Hemorrhage
Superficial Lobar Hemorrhages
Secondary Intracerebral Hemorrhage
ICH not directly caused by hypertension is encountered with vascular malformations, hemorrhagic transformation of ischemic stroke, anticoagulants, as well as fibrinolytic agents or irreversible antiplatelet therapy (Box 58-1). Primary amyloid angiopathy is often the underlying cause of nonhypertensive lobar hemorrhage. Less common causes include primary and metastatic malignancies, sinus thrombosis with venous infarctions and bleeds, acquired or inherited coagulopathies, induced or autoimmune vasculitides and systemic granulomatous disorders, central infectious processes, and trauma (Box 58-2).
Box 58-2 Uncommon Causes of Intracerebral Hemorrhage*
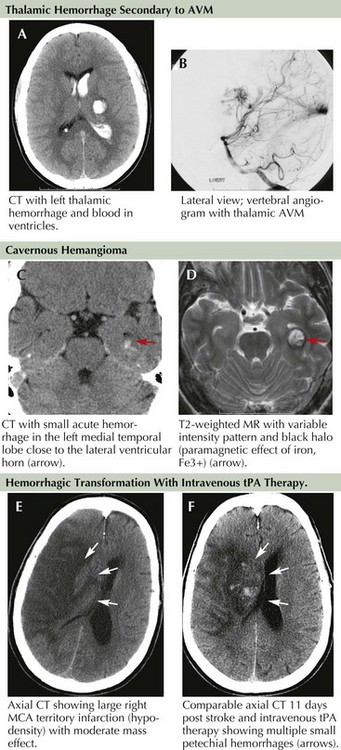
Occult vascular malformations were possibly the most underdiagnosed causes of lobar hemorrhages prior to CT and MRI scanning and were frequently missed by early angiography due to the presence of clot and mass effect on the brain. They were diagnosed only during surgical or pathologic specimen inspection after hematoma removal. The most common occult vascular lesions include small AVMs and cavernous angiomas (Fig. 58-3A–D).
Hemorrhagic brain infarct (HBI), in contrast to primary intracranial hemorrhage, is a secondary phenomenon that occurs as a result of ischemic damage to both the brain parenchyma and the vessel wall distal to the site of occlusion. The vascular wall endothelium and the blood–brain barrier are subsequently damaged and leak with reperfusion as they no longer tolerate normal arterial pressure. Petechial bleeding and, at times, gross hemorrhage through the damaged vessel into the infracted area may be seen (Fig. 58-3E&F). It is estimated that petechial bleeding develops in more than 50% of patients with embolic infarcts. Although it is often implied that cardioembolic strokes are more likely to be associated with development of HBI, some investigators suggest that any large infarct, regardless of mechanism, is predisposed to such bleeding. The use of IV heparin or heparinoid for secondary stroke prevention also promotes the development of HBI. However, the presence of petechial hemorrhage without frank hematoma formation does not seem to worsen neurologic outcome.
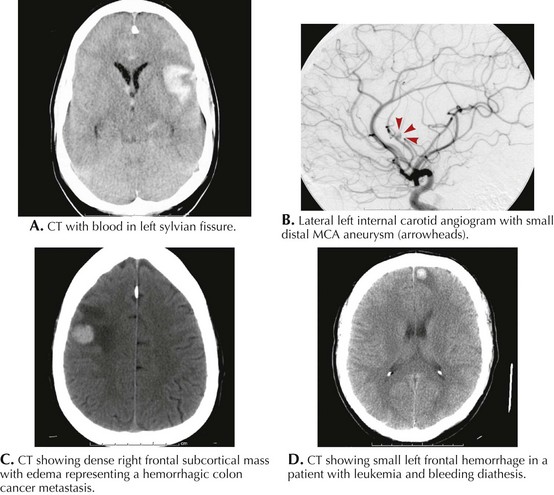
Along with typical subarachnoid hemorrhage, aneurysmal rupture can, at times, cause intraparenchymal hematoma with focal neurologic signs (Fig. 58-4A&B). The location of the blood often hints to the site of the aneurysm. Lateral temporal lobe hematomas suggest MCA aneurysmal rupture while medially located bleeds are associated with carotid artery aneurysms. Frontal hematomas indicate anterior communicating artery aneurysm. Posterior communicating artery aneurysms cause thalamic hemorrhages, often with intraventricular extension.
Primary or metastatic brain tumors can lead to ICH (Box 58-3; Fig. 58-4C&D). A single small hemorrhage from a metastatic lesion, as can be seen with melanomas or hypernephromas, may be difficult to distinguish from primary ICH unless evidence of other lesions is identified. Other clues, such as an atypical cortical or subcortical location of the bleed, irregular margins, and unexpected contrast enhancement of the lesion must prompt further investigations to exclude systemic disorders. A detailed dermatologic examination may reveal irregularly pigmented lesions, suggesting melanoma, and ultrasound or body CT scan may uncover a renal tumor.
Amyloid Angiopathy
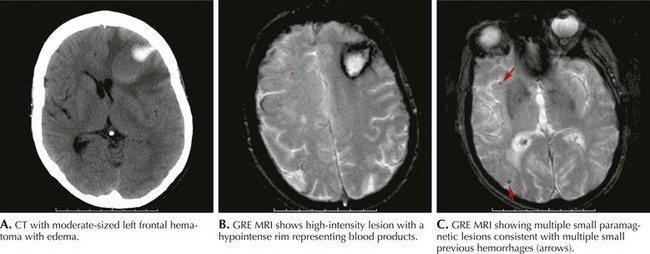
MRI evidence of previous asymptomatic small hemorrhages is encountered in many patients (Fig. 58-5). However, cases of rapidly successive, small intracranial hemorrhages within a short time period with progressive disability and death have been described. The presence of subcortical white matter disease seen on CT or MRI scans may be a reflection of chronic ischemia from amyloid-laden arterioles. These changes may suggest a higher risk for future hemorrhages and therefore a more cautionary approach to anticoagulation or the potential use of thrombolytic agents is advised.
Caplan LR. Caplan’s Stroke: A Clinical Approach, 3rd ed. Woburn, Mass: Butterworth-Heinemann; 2000.
Broderick J, Connolly S, Feldmann E, et al. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke. Stroke. 2007 Jun;38(6):2001-2023. Evidence-based guidelines to help manage intracerebral hemorrhage and its complications
Dennis MS. Outcome after brain haemorrhage. Cerebrovasc Dis. 2003;16(Suppl 1):9-13.
Fisher CM. Pathological observations in hypertensive cerebral hemorrhage. J Neuropathol Exp Neurol. 1971;30:536-550.
Garcia JH, Ho KL. Pathology of hypertensive arteriopathy. Neurosurg Clin North Am. 1992;3:497-507.
Garibi J, Bilbao G, Pomposo I, et al. Prognostic factors in a series of 185 consecutive spontaneous supratentorial intracerebral haematomas. Br J Neurosurg. 2002;16:355-361.
Kaneko M, Tanaka K, Shimada T, et al. Long term evaluation of ultra-early operation for hypertensive intracerebral hemorrhage in 100 cases. J Neurosurg. 1983;58:838-842.
Kase CS. Intracerebral hemorrhage: non-hypertensive causes. Stroke. 1986;17:590-595.
Mayer SA. Intracerebral hemorrhage: natural history and rationale of ultra early hemostatic therapy. Intensive Care Med. 2002;28(Suppl 2):S235-S240.
Mayer SA, Brun NC, Begtrup K, et al. Recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2005;352(8):777-785.
Mayer SA, Brun NC, Begtrup K, et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2008 May 15;358(20):2127-2137. Treatment with rFVIIa within 4 hours of ICH reduced hematoma volume growth but did not improve survival or functional outcome. Also, there were higher rates of arterial thrombotic events in the higher-dose group
Morgenstern LB, Frankowski RF, Shedden P, et al. Surgical treatment for intracerebral hemorrhage (STICH): a single-center, randomized clinical trial. Neurology. 1998;51(5):1359-1363. This clinical study has influenced our approach to surgical evacuation of ICH with most neurosurgeons and neurologists considering it as generally showing lack of benefit for hematoma evacuation. However, a subset of patients with superficial ICH and no intraventricular blood emerged as potentially benefiting from surgical evacuation and are currently being randomized to STICH II
Skidmore CT, Andrefsky J. Spontaneous intracerebral hemorrhage: epidemiology, pathophysiology, and medical management. Neurosurg Clin North Am. 2002;13:281-288.
Woo D, Broderick JP. Spontaneous intracerebral hemorrhage: epidemiology and clinical presentation. Neurosurg Clin North Am. 2002;13:265-279.