Intra-aortic Balloon Counterpulsation
THE INTRA-AORTIC BALLOON PUMP APPARATUS
Mechanical Complications of Acute Myocardial Infarction
Acute Myocardial Infarction Without Shock
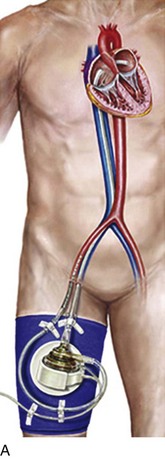
The first and most widely utilized of the percutaneously placed cardiac assist devices, the intra-aortic balloon pump (IABP) displaces blood from the descending aorta during diastole, resulting in altered myocardial mechanics during systole. By raising diastolic perfusion pressure, the IABP has the potential to augment coronary flow. Unlike the majority of more recently developed and generally mechanically more complex devices, the IABP provides auxiliary rather than independent support of cardiac output. Although it uses complex software algorithms, it is the simplest of the invasive devices mechanically, is the lowest in profile, and is associated with relatively low failure rates. Its advantages include the feasibility of allowing insertion in the cardiac catheterization laboratory or operating room or at the bedside, as well as a relatively small footprint allowing placement in the vasculature with less morbidity than other devices in its class. The indications, complications, and relative effectiveness of the IABP have been studied extensively for nearly 4 decades. National Center for Health Statistics data show that at least 37,000 intra-aortic balloons were placed in the United States in 2004,1 whereas some estimates range to more than 130,000 by 2010. There were close to 20,000 used in high-risk percutaneous coronary intervention (PCI) among U.S. centers contributing to the National Cardiovascular Data Registry (NCDR) during a recent 3-year period.2 The primary hospital location where IABPs are placed is highly variable depending on patient acuity and types of procedures performed in individual institutions, but the cardiac catheterization laboratory has become the primary site,3,4 and bedside placement in critical care units has declined to the low single-digit percentages.
History
Initial experiments aimed at altering the timing of phases of the cardiac cycle originated with animal experiments conducted by Adrian Kantrowitz in the early 1950s in the laboratory of Carl Wiggers at Western Reserve Medical School.5 The focus was initially oriented toward improving coronary blood flow rather than augmentation of cardiac output. Appreciating that coronary flow (particularly in the left coronary circulation) occurs primarily in diastole, the concept was to delay the peak systolic pressure pulse to the diastolic phase of the cardiac cycle.6 During the subsequent decade, this was followed by animal experiments attempting to use the diaphragm to provide the power for diastolic augmentation by wrapping it around the distal thoracic aorta.7 Clauss and colleagues effectuated counterpulsation by withdrawing blood from the circulation during systole and restoring it during diastole,8 but it was the work of Moulopoulos and associates9 that introduced counterpulsation with a carbon dioxide–filled tube synchronized to the electrocardiogram (ECG) in canines. Kantrowitz subsequently changed the gas to helium, used in modern IABPs because it has only 5% of the density of CO2 and allows faster inflation-deflation cycles and greater precision in timing. In addition, helium is inert, although it is less soluble and potentially more toxic in case of gas leak in the circulation. The IABP was introduced in humans in 1967 (Fig. 7.1). Initial favorable experience with intra-aortic counterpulsation in critically ill patients in cardiogenic shock (CS)10,11 led to the first major multicenter trial. This trial demonstrated significant hemodynamic benefit but only a 17% survival to discharge rate.12 A number of incremental improvements over the next 4 decades resulted in introduction of a percutaneous approach,13 a second lumen for guidewire support of balloon advancement through the circulation,14 increasing automation of the control consoles, and prefolded and progressively lower-profile balloons.15
Physiology
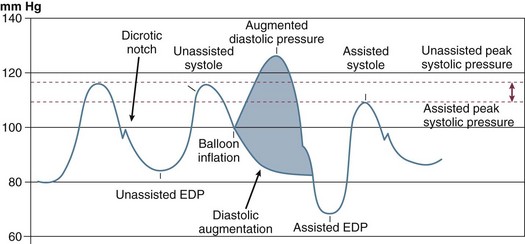
The classic concept of intra-aortic balloon counterpulsation involves inflation in synchrony with aortic valve closure, at the onset of isovolumic diastole and the appearance of the dicrotic notch, displacing blood comparable to the balloon’s volume into the peripheral circulation during diastole. To accomplish further unloading, and to prevent interference with left ventricular (LV) ejection, balloon deflation has traditionally begun before opening of the aortic valve and the beginning of LV ejection, although as discussed subsequently, this may not be the optimal algorithm. An example of the effects of balloon counterpulsation on systolic and diastolic pressure is seen in Figure 7.2. The hemodynamic response to institution of IABP is quite variable, and depends on a complex array consisting of the patient’s intrinsic blood pressure, heart rate, heart rhythm, aortic compliance, overall peripheral vascular resistance, intravascular volume status, cardiac function, adjunctive pharmacotherapy, disease state of the coronary vasculature, and degree of preservation of coronary flow autoregulation. In addition, the exact location of the IABP in the vasculature, the volume of the balloon, the frequency of inflation (frequencies from 1 : 1 to 1 : 8 are available depending on manufacturer), and timing of inflation and deflation all play important roles. Thus, the “classic” response consisting of lowering the systolic blood pressure and augmentation of diastolic pressure may not be seen; this classic response is based on the initial experience in CS, which led to a 20% drop in systolic pressure and a 30% rise in diastolic pressure.12 In fact, systolic pressure can increase secondary to improved cardiac output, can decrease, or can be unchanged, as can coronary blood flow. An important characteristic of the IABP in contrast to most mechanical assist devices is that it contributes to pulsatile flow, with theoretical benefits to end-organ perfusion beyond any actual change in mean flow or pressures.
Diastole and Coronary Blood Flow
Because the majority of coronary flow occurs in diastole, an increase in diastolic pressure has the potential to augment coronary flow as well as flow to other end organs. Diastolic pressure may in fact be augmented substantially, in part because balloon inflation is rapid, yielding an abrupt increase in volume and effecting a rapid rise on the pressure-volume curve. A variety of physical and biologic parameters, including compliance characteristics of the aorta and vascular bed, affect the degree of augmentation.16 The extent of peak pressure rise has been reported in a range from minimal to near doubling of diastolic pressure.17,18 However, increase in diastolic pressure and hence coronary perfusion pressure may not result in an increase in coronary flow because autoregulation modulates this potential and because in normal patients, end organs capable of autoregulation maintain flow without significant change.
Despite both several decades of experimentation in animal models and attempts to assess coronary flow in a variety of clinical settings in humans, the effect of IABP on coronary flow remains incompletely defined. Experimental and clinical data fail to show change in flow in native coronary arteries19,20 or in bypass grafts21 regardless of the severity of coronary stenosis.22 Although total coronary blood flow may not increase, coronary blood flow velocity has consistently been demonstrated to rise.17,23,24 In turn, data from a thrombolysis in myocardial infarction (MI) model suggest that the pulsatile waveform generated by IABP during diastole may contribute to improved time to coronary reperfusion without concomitant increase in coronary blood flow;25 in all likelihood this improvement reflects the enhancement in peak flow velocity that may also help prevent reocclusion.23 However, pulsatile flow may have additional benefit: in a randomized study comparing cardiopulmonary bypass with or without concomitant IABP, multiple parameters of whole-body perfusion were superior in the pulsatile flow setting.26 In general, augmentation of blood flow appears most likely to occur in patients in profound shock.17,24,27 Thus, most of the benefits of IABP are related to improvement in hemodynamics with secondary relief of ischemia. Amelioration of ischemia may in turn result in improved LV function and additional improvement in hemodynamics.
Systole and Myocardial Mechanics
Although the IABP is generally described as both improving myocardial oxygen supply and lowering myocardial oxygen demand, the predominant effect is the latter. This is based on decreased afterload and wall stress with increased stroke volume and cardiac output, in particular in patients with low-output states. In general, the magnitude of hemodynamic response is proportional to the extent of depression of cardiac function.16 Because stroke volume improves, heart rate stays the same or tends to decrease even as cardiac output rises. Reduction in LV end-diastolic pressure and improved cardiac output is associated with lower left ventricular heart filling and pulmonary arterial pressures, whereas systolic pressure typically decreases and mean blood pressure stays the same in hemodynamically stable patients. In patients in shock, mean blood pressure rises and systolic pressure may increase as well. When IABP placement results in improved end-organ perfusion, as evidenced by signs such as better urine output, the overall prognosis is generally improved.12 Reduction in left-sided heart filling and pulmonary artery pressures also leads to reduced right ventricular (RV) afterload; thus, IABP insertion may be helpful in some patients with right-sided heart failure.28 Although ejection phase indices tend to improve with unloading, the extent of augmentation of cardiac output is limited by the overall reserve in LV systolic function. Thus, at the extremes of ventricular dysfunction, even with excellent positioning of the balloon and timing as well as large IABP volumes, the IABP may not provide sufficient augmentation of cardiac output in some patients. A variety of other parameters have been used to assess the effect of IABP in shock, most prominently the cardiac power index (mean systemic pressure times cardiac index), which is the strongest hemodynamic correlate of outcomes.29
Effects on Organs Other Than the Heart
The effect of IABP on flow to end organs other than the heart is also incompletely characterized. In the cranial circulation, as in the heart, net flow does not appear to increase in patients with a stable hemodynamic state. Further, with balloon deflation, there is some evidence for transient reversal of blood flow as well,30 although this may be a consequence of early deflation (see discussion under “Timing”). Similarly, overall carotid flow is not changed in this setting.31 During hypothermia while patients are under cardiopulmonary bypass, pulsatile augmentation as obtained by the IABP does appear to improve cerebral oxygenation.32 Indirect evidence again suggests additional benefit in the setting of shock, with better cerebral blood flow, a setting in which there is modest clinical experience with using IABP in patients with refractory cerebral vasospasm.33 Despite improvement in cardiac output with IABP, gastric tonometry has not shown improvement in the splanchnic circulation in CS patients,34 and renal vein thermodilution has failed to show improvement in overall renal blood flow.35 In cardiac surgery patients who subsequently developed low-output syndrome, however, IABP did have a positive effect on gastric tonometery.36
The Intra-Aortic Balloon Pump Apparatus
With the introduction of a separate guidewire lumen in the early 1980s,14 the safety of IABP placement improved significantly. Previously, bulky 12 F or larger devices were placed via a surgical approach through an end-to-side graft to the femoral artery, with substantial associated morbidity. The device was then advanced through the femoral and iliac systems without a guidewire, frequently at the bedside with no fluoroscopic guidance. This led to a high rate of major vascular complications, in particular, iliac artery dissection but also aortic perforation and IABP malposition. The introduction of the percutaneous approach did not alleviate these problems until a guidewire lumen was added.14
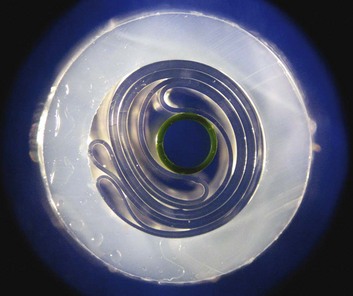
There were conflicting demands on balloon design: first, to minimize the central lumen size as part of reducing overall profile, and second, at the same time to maintain a large enough lumen both for guidewires that could provide safe passage through the circulation as well as sufficient diameter for high-fidelity hemodynamic recordings. One solution to the latter has been the introduction of a line of catheters with a fiber-optic pressure measurement sensor.37 In general, the guidewire lumens are designed to accommodate guidewires in the 0.018-inch to 0.030-inch range. The gas exchange lumen is concentrically placed around the guidewire lumen. Improvements in technology allowed for the introduction of tightly folded balloons prewrapped around the central lumen (Fig. 7.3); previously, a cumbersome process that sometimes led to device failure required the operator to wrap the balloon just before insertion.
Balloon Dimensions
The available IABP catheters range in gas volume from 25 to 50 mL and in shaft lengths from approximately 60 to 72 cm. The actual length of the balloon segment varies by manufacturer and balloon volume: Commercial balloon lengths are in the range of 16.5 to 26 cm. Although balloon volume does affect the degree of diastolic augmentation38 and cardiac output39and may be particularly important in patients with severe, refractory CS, little difference was found in IABP effectiveness between 32-mL and 40-mL balloons in one study,40 and risk to the patient is significantly higher if inflated balloon diameter approximates or exceeds aortic diameter (see “Complications”). Thus the decision for IABP size is typically based on patient size and severity of hemodynamic compromise, with the 40-mL balloon used in approximately three fourths of patients.41 A special consideration for balloon volumes is patient air transport; rapid changes in altitude will lead to increased (during ascent) or decreased (with descent) volume, requiring monitoring to ensure that appropriate balloon volume is maintained.42
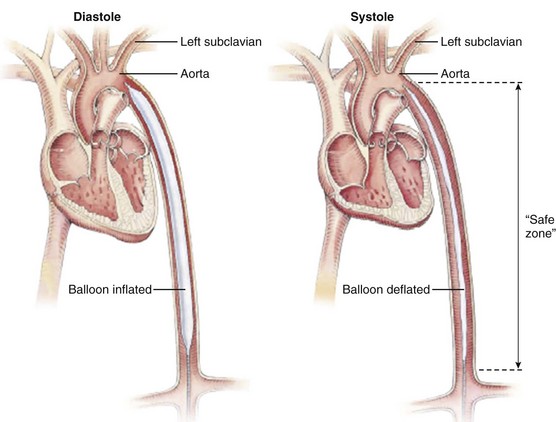
Because it is essential to locate the balloon below the origin of the left subclavian artery at the upper margin and above the renal arteries at the lower (a “safe zone”; Fig. 7.4), the tolerances are relatively low. Studies looking at the length of this segment in Japanese patients found a range of 21 to 25 cm, with good correlation between patient height and length of this segment (although in the relatively short Japanese population, the balloon lengths frequently exceeded the “safe zone” length for individual patients).43 Recommendations vary by manufacturer: A 50-mL size I is generally recommended for patients taller than 6 feet (183 cm), and the 25-mL is used for patients shorter than 5 feet (152 cm). Recent changes in technology have allowed for larger diameter balloons to be available for shorter patients (e.g., a 50-mL IABP on an 8 F shaft is now available for patients 5′4″ [162 cm] and taller). In general, larger balloon sizes lead to improved unloading and augmentation with greater blood volume displacement. Early balloon models were designed to be occlusive during inflation, and experimental data have demonstrated optimal augmentation with 100% occlusion of the aorta.38,39 Such full occlusion is generally avoided because of potential trauma to the aorta, ischemia to the spinal circulation, or abrasion of the balloon, which might contribute to a risk of rupture. The generally accepted value for ratio of balloon to aorta size is 80% to 90%; in the study by Igari43 in Japanese patients, the range of commercially available balloon diameters was noted to be 14 to 15 mm, whereas aortic diameter mean at the level of the renal arteries was 17.5 ± 3.2 mm, within the accepted 80% to 90% range, although at least one 50-mL balloon has an expanded diameter of 18 mm (Arrow International, Inc., Reading, PA). In an earlier study in Americans, 90% of midthoracic aortas were larger than 19 mm.44 Balloons are typically made of polyurethane or polyethylene, with materials chosen to allow rapid inflation-deflation cycles and tolerate an average of 100,000 to 150,000 cycles per day. Experimental materials with heparin and hydrophilic coatings have been developed to address thrombosis risk and trauma to the vasculature during device passage45,46 but are not generally available.
Balloon Console
IABPs are driven by complexly engineered consoles with extensive artificial intelligence designed to recognize electrocardiographic rhythms and hemodynamics, with the ability to trigger balloon inflation either from the ECG (including paced rhythms) or from pressure waveforms, although in some settings, such as during cardiopulmonary bypass, an automatic trigger at a preset rate can be used. Tachyarrhythmias and irregular rhythms have substantial influence on the effectiveness of the IABP, the former in part because of the disproportionately shorter diastolic filling periods, and the latter because of difficulties predicting the timing of occurrence of the dicrotic notch, although improved algorithms have been incorporated.47 The central lumen of the balloon is typically connected to a transducer and separate ECG leads are connected to the console. A series of alarms identify leaks in the IABP circuit, high or low pressure, loss of trigger signals, blood in the gas line, low battery, and other anomalies that foreshadow or indicate impending or existing system failures. (Figure 7.5 demonstrates the control panel of a modern IABP console.) Modern IABPs using fiber-optic technology permit automatic calibration in patients after insertion and automatic recalibration on a periodic basis or whenever the algorithms detect a change in patient condition.
Preparation
Balloon preparation requires establishing a vacuum in the gas lumen by drawing back on a large-volume syringe, and also flushing the central guidewire lumen (Fig. 7.6). If the procedure is to be done at the bedside without fluoroscopy, measuring the approximate distance along the course of the femoral and iliac arteries and then up the descending aorta is essential prior to IABP placement. The approximate depth to which it needs to be inserted should be noted on the shaft prior to beginning insertion. The ECG leads should be connected to the IABP console prior to introducing the catheter if ECG triggering is to be performed.
Balloon Insertion
Based on data from the Benchmark Registry, nearly two thirds of IABP insertions in the United States occur in the cardiac catheterization laboratory, one fourth in the operating room, and the remainder at the bedside in a variety of hospital locations. In contrast, outside the United States, the operating room and the catheterization laboratory each account for approximately 40% of placements, with the remaining 20% inserted at the bedside.4
The modern IABP is designed to be introduced percutaneously through the common femoral artery, which is the method used in 95% to 98% of cases, two thirds via the right common femoral artery.41,48 A variety of disease states affect the ability to freely pass the device to the central aorta, in particular atherosclerosis, but also spasm and congenitally small vessels. Preprocedure assessment of the vascular tree is important: Diabetics, women, and patients with small body surface area in particular have small femoral arteries,49 and there is a corresponding higher rate of vascular complications in these patients.50 Pulses at the common femoral artery and below must be carefully documented before catheter insertion, and if there is suspected or confirmed peripheral vascular disease, angiography of the abdominal aorta, iliac artery, and lower extremities should be considered. This step is frequently not practical in the emergency setting or in patients with renal failure; in elective situations noninvasive evaluation should be performed, including ankle-brachial indices with pulse volume recordings, computed tomography, angiography, or magnetic resonance angiography.
Femoral Access
Every effort should be made to ensure that femoral puncture is above the femoral bifurcation and below the inguinal ligament (Fig. 7.7). An excellent practice is to place a short 5 F or 6 F pilot sheath in the femoral artery and to perform angiography of the common femoral artery to confirm sheath entry below the inferior excursion of the inferior epigastric artery; punctures above this landmark correlate strongly with retroperitoneal hemorrhage.51 Puncture below the femoral head drastically increases the likelihood of puncture into the femoral bifurcation vessels (77% of femoral bifurcations are at or below the inferior margin of the femoral head49), which in turn is associated with acute leg ischemia due to vascular obstruction, and pseudoaneurysm formation upon balloon removal is more likely because of lack of the anvil of the femoral head against which to perform manual compression. If the pilot sheath is found to have entered outside the common femoral artery on femoral angiography, consideration should be given to switching to the contralateral side. Using fluoroscopy to aid in puncturing the common femoral artery at a point over the lower half of the femoral head is a recommended technique to ensure proper sheath placement.
Sheathless Insertion
The use of a sheathless insertion technique has been recommended to reduce complications.52 Sheaths typically add between 0.6 and 0.8 mm to the overall diameter required to place a device, so the sheath for an 8 F balloon approximates 10 F in outer diameter. “Going sheathless” therefore has the advantage of having the device occupy less space in the common femoral artery and has been described as reducing vascular complications with an odds ratio greater than 2 : 1.53,54 Although retrospective analysis of large patient subsets presents compelling data that the sheathless approach is superior for reducing complications,55 this finding has not been universally confirmed.50,56 The latest data suggest that about 80% of IABPs are inserted with a sheath.41 The sheathless approach is most compelling in diabetic patients, women, and patients with known peripheral vascular disease or small body surface area. Sheathless insertion of an IABP requires careful preparation of the tissue track to allow atraumatic entry of the balloon tip into the artery directly through the skin over a wire. Spreading of tissue and predilatation with a dilator are essential. Fibrosed tissue tracks or thickened/calcified arterial walls frequently resist sheathless entry. In some cases, a stiff guidewire provides an adequate rail along which to slide the naked balloon catheter into the vessel.
Balloon Advancement and Positioning
Once suitable access is gained, guidewire passage to the aortic arch is ideally performed under fluoroscopic guidance. Without fluoroscopy at the bedside, approximating the distance from the femoral puncture to a point near the top of the descending aorta is imprecise and adds to the risk of trauma/ischemia to head and neck vessels, the aorta, or the renal arteries. In the critical care setting, transesophageal echocardiography is a suitable alternative to fluoroscopy for enabling precise balloon placement.57 If fluoroscopy is not used, prompt postprocedure radiography to confirm location is imperative.
If guidewire passage meets resistance, alternative approaches include use of guidewires that are hydrophilic, steerable, or both.58 If significant iliac stenosis is noted, balloon dilatation or stenting of the iliac artery is an accepted practice with high success rate both for achieving passage of the IABP and as an adjunct to preventing distal ischemia.59,60 As more cardiologists become skilled in endovascular intervention, the ability to perform combined iliac stenting and IABP placement is expanding.61
Once the balloon has been advanced to a point 1 to 2 cm inferior to the left subclavian artery origin (see Fig. 7.4)—typically near the top of the descending aorta—the guidewire is withdrawn and the central lumen flushed. The vacuum port (minus its one-way valve) is connected to gas line tubing that in turn is connected to the console, and the dead space is purged and then filled with helium. The central catheter lumen is connected to a transducer on the console. After triggering is initiated, typically on every other beat, fluoroscopy should be used to confirm balloon location and filling, and pressure contours should be evaluated for appropriateness of timing (see later discussion under “Timing”). Once the timing is considered satisfactory, continuous pumping can be initiated. If necessary, the balloon should be repositioned with the console turned to standby to avoid trauma to the aorta.
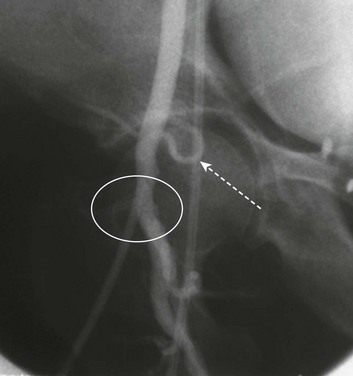
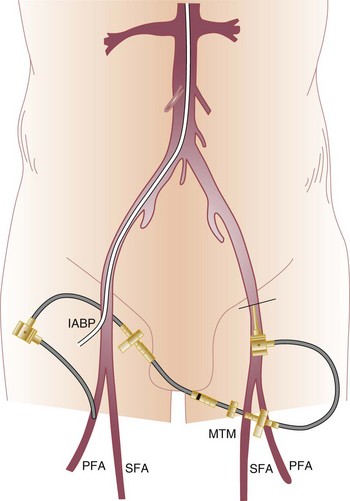
The distal circulation should be assessed carefully after IABP placement. Distal ischemia is relatively common. The most likely cause of acute ischemia is obstruction of the artery by the catheter shaft itself. If ischemia occurs hours or days after insertion, the possibility of thrombus, typically secondary to stagnant blood in the confined space of a small or diseased vessel, should be considered as well. A technique for addressing distal limb ischemia—placement of a small sheath retrograde in the contralateral femoral artery and antegrade in the ipsilateral common femoral or superficial femoral artery—if performed by expert hands, can occasionally salvage an ischemic limb without forcing removal of the IABP62 (Fig. 7.8). There is some evidence that IABP inflation properties and hemodynamic effects may be superior in the patient positioned horizontally than with the patient tilted at a 30-degree angle.63 Postprocedure and subsequent monitoring of left arm pressures can lead to early diagnosis of inadvertent balloon advancement obstructing left subclavian artery inflow, a phenomenon to which the restless patient who flexes the thigh is predisposed.
Alternate Access Routes
Approaches described to date include the brachial, subclavian, axillary, iliac, transthoracic, and translumbar arteries. Although the brachial approach has been successful in isolated cases,64,65 and sheathless entry reduces the overall diameter of lumen encroachment to less than 3 mm, the potential for complications is substantial. They include not only vascular injury and ischemia of the hand but also potential neurologic consequences from formation of thrombus on an indwelling catheter underneath the origin of the right common carotid artery (as the shaft traverses from the right subclavian artery to the innominate artery) as well as under the left common carotid artery and subclavian artery origin.66
Iliac insertion through a conduit has been used for patients in whom femoral access is not adequate or who are not candidates for ventricular assist devices: With retroperitoneal placement, patients can be at least partially ambulatory during prolonged counterpulsation.67 Other routes of access that have been described are via the subclavian68 and axillary arteries,69,70 with or without conduits, and generally with an eye toward allowing modest ambulation during prolonged IABP use. A transthoracic approach has been described, with placement via the ascending aorta into the standard descending aortic location.71,72 The morbidity rate associated with these surgical placements is significantly higher,73 in part because they typically involve longer IABP indwelling times as well as the comorbidity issues already mentioned.
Timing
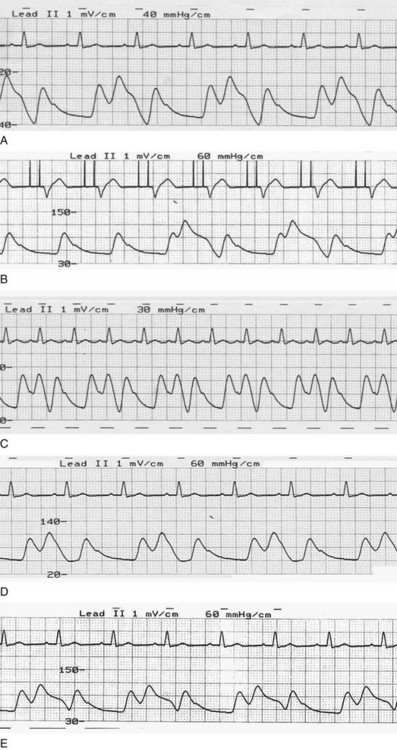
The importance of IABP timing was understood from the time of the original Moulopolous study in 1962.9 The timing of balloon inflation and deflation is designed to optimize afterload reduction and enhancement of diastolic pressure without interfering with ventricular ejection. Classically, it was considered optimal to inflate at the dicrotic notch, as soon as aortic valve closure occurred, and to deflate near the onset of ventricular depolarization, anticipating the beginning of mechanical systole (Fig. 7.9). In fact, significant enhancements to IABP efficiency can be obtained with refinements to these concepts.
Late Inflation and Early Deflation
Gross errors in inflation and deflation lead to failure to obtain benefit from IABP and occasionally to significant hemodynamic compromise. Early in the history of IABP deployment, it was appreciated that inflation throughout the period from closure of the aortic valve to its subsequent opening was necessary for optimal hemodynamic effect.16 Both late inflation and early deflation reduce augmented LV stroke volume and result in decreased peak diastolic coronary velocity;23 the latter is demonstrated with transthoracic Doppler ultrasound, a tool that can potentially be used to optimize timing. An additional concern exists with early deflation: The abrupt decrease in IABP volume during diastole can lead to reversal of both coronary and other end organ (e.g., cerebral) flow back into the aorta, shunting blood from vital end organs.30
Early Inflation
Early inflation results in increased afterload late in LV ejection with consequent impairment of LV systolic function. The ejection phase is shortened, LV end-systolic pressure rises, and stroke volume decreases; inflation, in the range of 130 to 190 ms before the dicrotic notch, results in a 20% decrease in stroke volume.74 This effect may not be seen if early balloon inflation is less pronounced; no hemodynamic effect was noted when IABP inflation occurred 50 ms before the dicrotic notch. Regardless, although early inflation theoretically lengthens diastole and allows for a longer period of diastolic augmentation, the net effect does not appear to be salutary. In addition, early inflation carries theoretical risks associated with the increased afterload and wall stress, including aneurysm formation and rupture in the peri-MI period.
Late Deflation
Similarly, late balloon deflation could be expected to interfere with ventricular ejection and decrease stroke volume. In fact, somewhat counterintuitively, a similar 110- to 180-ms delay in IABP deflation appears to have salutary effects and is associated with a stroke volume increase of 18%,74 which apparently results from both an increase in diastolic filling period and an augmented decrease in afterload later in the cycle. This finding confirms a prior observation by Kern and associates75 and suggests that, in general, most operators have timed deflation too early. The beneficial effect occurs with timing deflation to be simultaneous with LV ejection; delaying deflation beyond the range described, however, raises concerns similar to those described for early inflation.
Electrocardiogram Triggering
When the ECG is used as the trigger, the descending slope of the T wave correlates best with the onset of diastole38 and is the usual timing for balloon inflation. Deflation is typically timed to the R wave, which denotes a short time delay after the onset of electrical systole. Algorithms were developed early to effect prompt deflation and prevent inflation in the setting of ectopy, and IABP software recognizes pacemaker spikes in contrast to QRS complexes as well. As in the findings described previously,74,75 deflation timed to the J point, with adjustment for the delay between onset of isovolumic systole and aortic valve opening, and perhaps slightly later, was shown early in the IABP literature to improve stroke volume.38
Other Considerations
A common problem has been proper timing in patients with underlying arrhythmias. Atrial fibrillation has been particularly vexing, with unpredictable beat-to-beat intervals. An algorithm to predict the occurrence of the distance between the QRS and the dicrotic notch was developed in the mid-1990s.76 Newer dicrotic notch prediction algorithms using high-fidelity micromanometer pressure sensors have been described.47 However, atrial fibrillation poses difficulties beyond timing alone; rapid ventricular rates result in a disproportionate decrease in the diastolic interval and limit the effectiveness of IABP because of the inherently short period of counterpulsation,77 including subtraction of the fixed time interval required for shuttling helium into and out of the balloon. Operating at 1 : 2 rates may be required in this setting.
Lack of augmentation despite proper timing should result in troubleshooting the IABP console, checking with fluoroscopy to visualize inflation of the balloon and confirm the level of balloon placement, and excluding kinking of the gas line or other mechanical failures. Apparently normal IABP function with absence of hemodynamic improvement should raise a suspicion that the patient has a baseline hemodynamic state that will not benefit from LV unloading, in particular hypovolemia, sepsis, or profound hemodynamic collapse, as well as a number of conditions described subsequently (see “Contraindications”).
Adjunctive Pharmacotherapy
Adjunctive pharmacotherapy typically includes heparinization. There are two theoretical reasons for anticoagulation: First, in patients receiving less than 1 : 1 counterpulsation, there is concern regarding clot formation on the balloon apparatus, and second, stagnant blood around the catheter shaft, especially in the common femoral artery, has been thought to raise the risk of thrombosis. The general consensus has been that anticoagulation should be administered if not contraindicated. The balloons themselves have a thrombogenic surface,45 and occlusion of vessels requiring thromboembolectomy has been reported to be one of the most common complications, reaching nearly 3% in one series of 911 patients undergoing coronary artery bypass grafting (CABG).53 Nevertheless, the thinking on this is in flux, with a growing evidence base that routine anticoagulation does not prevent thrombosis or thromboembolism but does increase bleeding risk. Thus, a recent study78 compared routine heparin use versus selective anticoagulation only in those patients with an indication for heparin use other than IABP insertion. There was no difference in the rates of IABP-related complications including major limb ischemia, but anticoagulated patients had a higher incidence of non-access-site bleeding, predominantly gastrointestinal. A randomized trial had similar conclusions, although the course of counterpulsation was relatively brief.79 Review of the best evidence to date leads to the somewhat controversial conclusion that anticoagulation may be best targeted to selected patients who are at risk of thrombosis or thromboembolism for reasons other than IABP use alone.80
A second adjunctive pharmacotherapy issue is the use of prophylactic antibiotics. Although fever, bacteremia, and sepsis were reported to be common (occurring at rates of 47%, 15%, and 12%, respectively) in one small study,81 the infection rate in larger series has been less than 1%,53 and the consensus is that the evidence base is too thin and the public health implications too unfavorable to recommend routine antibiotic use unless otherwise clinically warranted.82 A third medication issue relates to sedation. Continuous bed rest in a critical care setting is frequently associated with disorientation or agitation. Balloon migration from leg bending and patient movement risks significant trauma to the aorta, renal arteries, and head and neck vessels. Careful sedation is essential. Finally, antiarrhythmic agents to slow and regularize the heart rate can have important benefits for lengthening diastole and optimizing balloon timing, both of which in turn enhance IABP augmentation.
Balloon Removal
Removal of the IABP poses several challenges. First, the ability to maintain hemodynamic stability without counterpulsation must be confirmed as part of the weaning process. Although cardiac output is disproportionately decreased with lowering of pumping ratios, such lowering appears superior to decreasing volumes as a weaning method, an approach confirmed by one small retrospective evaluation.83 The balloon should not be turned off completely until the activated clotting time value confirms that anticoagulation has been effectively discontinued and the patient is ready for balloon removal, because thrombosis on the balloon surface occurs rapidly.45 Most operators run the IABP at a low cycle rate, 1 : 3 to as low as 1 : 8 (depending on manufacturer), until anticoagulation has worn off sufficiently and the balloon can be removed. Nonfunctioning or stopped IABPs must be removed promptly, preferably within 20 minutes or less. A second challenge relates to the size of the deflated balloon. Because the balloon is delivered prefolded by the manufacturer (see Fig. 7.3), it passes readily through its delivery sheath during insertion. Once inflated, it will not refold when vacuum is applied, and the profile is too large for retrieval without bringing both the sheath and the balloon out of the body together.
Removing the balloon, especially after long indwelling times (mean indwelling time is 53 to 77 hours41,48), requires meticulous attention to several details. First, the balloon gas line should be aspirated to reduce the balloon profile. Second, it is essential to allow some bleeding after catheter removal and prior to compression to avoid stripping any clot off the balloon inside the common femoral artery. Because these are relatively large catheters, frequently deployed in patients with vascular disease who have a tenuous hemodynamic status even at the time of IABP removal, patients may not tolerate prolonged and aggressive compression of the groin. Recent discontinuation of heparin combined with a large arteriotomy size can result in significant difficulty in controlling hemorrhage and achieving hemostasis.
Surgical closure is sometimes preferable, particularly with severely obese patients, after very long indwelling times, with uncorrectable anticoagulation status, or with low or high punctures.84 Vascular closure devices have been used successfully in small series85,86 but require extreme caution; significant mortality risk is associated with infections related to vascular closure devices,87 and assuring sterility at the time of IABP removal is difficult. When use of a closure device is required, the authors typically place a 0.018-inch stiff guidewire inside the lumen after extensive efforts to achieve sterility of both the field and the device, withdraw the IABP, and place the closure device over the wire; we cannot recommend this off-label approach, however, until a better evidence base is available. Finally, it is important to continue to monitor patients for adverse events because nearly 25% of IABP-related complications have been reported to occur after IABP removal.88
Indications
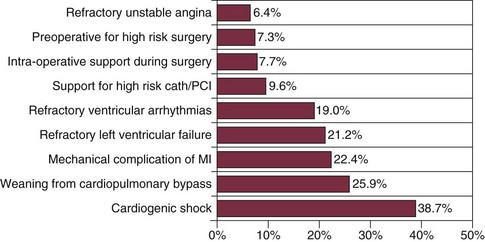
As with the original patients in 1968,10 CS remained the most common indication for several decades, although later data suggest that circulatory support for percutaneous intervention has replaced hemodynamic instability in the acute MI setting as the primary indication for IABP insertion.41 Other common indications are perioperative support for patients undergoing cardiac surgery, weaning from cardiopulmonary bypass, management of unstable angina, severe congestive heart failure, and with less evidence base, refractory ventricular arrhythmias or angina after MI as well as a host of miscellaneous settings largely defined by case reports (Box 7.1).
In general, indications can be divided into several categories: prophylactic versus therapeutic; hemodynamic support versus improved end-organ flow; and preoperative, intraoperative, or postoperative management. The rate of prophylactic use, defined as IABP insertion prior to percutaneous or surgical intervention, rose from 17.3% in 1992 to 31.3% in 2001 as a proportion of IABP use in the United States according to the Benchmark Registry.4 The management and particularly the complications associated with these subcategories are substantially different. Box 7.1 lists indications for IABP use. The class I indications include CS if not quickly reversible with pharmacologic therapy, acute mitral insufficiency or ventricular septal rupture, recurrent ischemia or infarction, hypotension that does not respond to other interventions, and a low output state.
In general, use of an IABP identifies a high-risk population, with in-hospital mortality rate exceeding 20% among more than 22,000 patients (Fig. 7.10).4,48,89 The Benchmark Registry examined the use of IABP in more than 5000 patients with acute MI; this represented 24% of all IABP placements at the 250 participating medical centers. Twenty-seven percent of the patients were in CS, an additional 12% needed support because of acute ventricular septal rupture or severe mitral insufficiency, and 5% underwent IABP placement for refractory LV failure. Thus, nearly half the patients with IABP placements and MI had hemodynamic settings in which balloon support was considered potentially life sustaining.48 Recent data confirm extensive IABP use in high-risk patients undergoing PCI: 44.4% of CS patients, 10.3% of patients presenting with ST-segment elevation myocardial infarction (STEMI), 28.1% of those undergoing left main PCI, and 13.9% of patients with LV function less than 30%.2
Despite general acceptance of its utility in salvaging myocardium and decreasing mortality risk (even without a strong evidence base), and despite a significant associated complication rate, there are no separate American College of Cardiology/American Heart Association guidelines for placing an IABP.90 However, indications for IABP use are included in the Guidelines for ST-Segment Elevation Myocardial Infaction91 and in the more recent Guidelines for Percutaneous Coronary Intervention92; hemodynamic support devices are currently considered a class I indication in the setting of CS, although as described subsequently, the evidence base is in flux. It is important to note that severe hemodynamic deterioration, including CS, is not limited to acute MI patients with ST-segment elevation.93
Cardiogenic Shock
The classic understanding of CS has been stunning or irreversible loss of a large amount of myocardium (≥40%) with resultant low output and compensatory elevated systemic vascular resistance to maintain central organ perfusion pressure. The definition of CS has varied, but the essential elements are persistent tissue hypoperfusion secondary to cardiac dysfunction in the presence of adequate filling pressure (and elevated left-sided heart filling pressure). The most common current definition is hypotension (blood pressure < 90 mm Hg systolic) for more than 30 minutes, with low cardiac index (less than 1.8 L/minute/m2 without support or less than 2.2 L/minute/m2 with support), and finally, elevated filling pressure (pulmonary artery wedge pressure > 15 mm Hg or LV end-diastolic pressure > 18 mm Hg or RV end-diastolic pressure > 10-15 mm Hg).94 Data from the SHOCK (SHould we emergently revascularize Occluded Coronaries in cardiogenic shocK) trial have called several classic assumptions into question,95 in part because some of the patients in this trial with CS had relatively preserved ejection fractions, whereas other patients with apparently much larger amounts of dysfunctional or nonfunctional heart muscle were hemodynamically compensated.
Although the incidence of CS has declined,96 it remains significant; the most recent estimates are that it is seen in approximately 6% of acute MIs. Mortality rate, once described as high as 80% (though variable in the literature, primarily because of the multiple definitions used), has declined in part because of better interventional tools,97 because patients with coronary artery disease are receiving better long-term medical therapy, which in turn helps limit infarct size, and because the pharmacotherapy for CS itself has improved. Mortality rate remains high, however, with a nearly 40% in-hospital death rate reported in the Benchmark Registry.48
IABP use in CS dates to the beginning of the IABP experience, and it results in predictable hemodynamic improvement in the majority of patients. IABPs have been shown to be beneficial in the setting of acute MI and CS when patients are not treated with PCI. The National Registry of Myocardial Infarction (NRMI) 2 compared results in patients with CS who received adjunct IABPs and those who did not.98 Although mortality rate was not affected in patients undergoing primary angioplasty, those receiving only thrombolytic therapy had a significantly higher mortality rate (67% without IABP vs. 49% with IABP). Similar findings were shown in the SHOCK trial99 as well as a meta-analysis of more than 10,000 STEMI patients with CS.100 A small and underpowered randomized trial also suggested that patients with Killip class III or IV heart failure after MI have lower mortality rates when treated with fibrinolysis combined with IABP than when treated with fibrinolysis alone.101 There is some basis for these findings from animal data, which suggest improved reperfusion when thrombolysis without other intervention is combined with IABP use.25
Intra-aortic Balloon Pump Use as an Adjunct to Revascularization in Cardiogenic Shock
Figure 7.11 provides an algorithm for CS management; it is notable that IABP is at the center of the algorithm, and part of early management in all patients except those with rapid response to initial pharmacologic interventions. Based largely on indirect evidence, it has been assumed that use of IABP as an adjunct to coronary intervention in CS improves outcomes.102 Findings have included a nearly 60% reduction in the rate of major adverse events (ventricular fibrillation, cardiopulmonary resuscitation, prolonged hypotension) in the cardiac catheterization laboratory (odds ratio 2 : 1) after IABP insertion.103 A trend toward lower 30-day and 1-year all-cause mortality rate has been demonstrated in patients in whom IABPs have been inserted within 1 day of presentation with CS,104 albeit with a higher associated complication rate. Stabilization of patients with IABP and thrombolytic therapy and subsequent transfer for coronary intervention also appeared to have favorable effects on survival,105 mimicking animal data showing that reperfusion combined with IABP is superior to reperfusion alone in salvaging heart muscle.106 IABP plus mechanical ventilation may also have incremental benefit in CS management.107
However, a growing body of evidence suggests that IABP use may have at best modest benefits in CS when combined with acute intervention for revascularization. Most notably, the IABP SHOCK-II trial randomized IABP use in CS patients undergoing PCI.108 No benefit of IABP was shown on 30-day mortality rate; a number of study design concerns exist (in particular between group crossovers and selection of a relatively low risk CS population) as well as the possibility that IABP placement before rather than after PCI (most patients had IABP placement after revascularization) would have shown additional benefit.109 A smaller study, IABP SHOCK,110 demonstrated no significant improvement in serial APACHE (Acute Physiology and Chronic Health Evaluation) II scores with IABP use. Several meta-analyses have come to similar conclusions,100,111 albeit with evidence that IABP does have more readily demonstrable hemodynamic benefit; a more detailed examination of a small subset of patients did not confirm these hemodynamic benefits when compared to medical therapy alone.112 The bottom line, that the routine use of IABP in CS patients undergoing PCI should remain a class I indication, is thus under considerable scrutiny.113
Finally, consideration has been given to the use of IABP in RV infarction; isolated RV failure occurs in approximately 3% of patients with CS.114 In this setting, patients commonly have severe hemodynamic decompensation and their overall prognosis is poor;115 within the cohort of patients with CS, however, RV dysfunction is associated with inferior MI and a relatively better prognosis than CS based on LV dysfunction alone.116 IABP use does not reliably result in hemodynamic improvement with RV infarction, and a variety of RV assist devices have been investigated, including pulmonary artery counterpulsation117 and a right atrium–pulmonary artery bypass pump.117,118 An occasional consideration in RV infarction is right-to-left shunting across a patent foramen ovale because of acute elevation in right-sided heart filling pressures, which result in a gradient that drives right-to-left atrial flow. Unloading of the left ventricle with an IABP can potentially exacerbate such shunting.119
Mechanical Complications of Acute Myocardial Infarction
Afterload reduction has significant hemodynamic benefits in patients with abnormal unloading of the left ventricle into the right ventricle (ventricular septal rupture) or left atrium (severe mitral regurgitation).120 The theoretical effect of counterpulsation in lowering afterload is in improving the ratio of forward flow through the aortic valve. The physiologic benefit in acute mitral insufficiency (mitral regurgitation) is widely accepted, resulting in a higher percentage of patients with severe mitral regurgitation and CS receiving IABP support than patients with CS alone.121 The mechanism of benefit appears to be lowered aortic impedance with consequent improvement in cardiac output, and modest decrease in regurgitant fraction.122 The IABP is in use in nearly all (98%) patients in this setting undergoing mitral valve repair, compared with less than half (43%) of those treated without surgery, a difference also influenced by selection issues. As with acute mitral regurgitation, 75% of ventricular septal rupture patients in the SHOCK registry underwent IABP placement.123 Although systolic pressure did rise (from a median 81 mm Hg to 102 mm Hg) with institution of IABP in patients with ventricular septal rupture, mortality rate was dismal in both groups. In-hospital survival rate was only 13% with ventricular septal rupture, compared with 45% with severe mitral regurgitation. Nevertheless, IABP use is an essential element of intervention in acute ventricular septal rupture, and one goal of therapy has been stabilization before closure of the defect is undertaken.124
Acute Myocardial Infarction without Shock
IABP use for uncomplicated acute MI is controversial. As with many modalities that lower myocardial oxygen demand, IABP theoretically helps decrease infarct size, even when reperfusion does not take place.106 However, a number of older trials and two randomized trials from the past decade did not demonstrate compelling risk-to-benefit ratios with routine IABP use in acute MI patients undergoing PCI,125,126 nor did a more recent study assessing myocardial infarct size using cardiac MRI.127 The findings conform to a meta-analysis that showed no benefit along with a small increase in stroke and bleeding.100 Economic analysis did not demonstrate significant increase in hospital costs in patients randomly assigned to undergo routine IABP insertion in this setting.128 The potential benefit of the IABP in maintaining higher infarct artery patency has been demonstrated in two randomized studies showing that an open artery is more likely at 5 days129 and at 3 weeks130 in patients randomly assigned to IABP placement.
IABP has been used to manage recurrent ischemia and malignant ventricular arrhythmias in the peri-infarction setting, although the evidence base for these indications is less compelling, and both are class II indications (see Box 7.1) in patients with STEMI.91
Unstable Angina
IABP use in unstable angina dates from an era in which the available alternatives were medical therapy and coronary bypass surgery, and IABP was utilized to stabilize patients before they were taken to the operating room. In certain settings, such as severe left main coronary artery disease discovered in the cardiac catheterization laboratory or in unstable angina with attendant hemodynamic instability, this approach is still appropriate.131 Reducing myocardial oxygen demand frequently stabilizes these patients, and the IABP may have additional benefits during subsequent revascularization in the cardiac catheterization laboratory or operating room, as discussed later.
Prophylactic Use for Coronary Intervention
Prophylactic use of an IABP prior to PCI has generally been considered to be a sound strategy for high-risk patients, typically defined as having acute coronary syndrome with hemodynamic instability, severe LV dysfunction and extensive coronary disease, or left main/last remaining vessel intervention. Prophylactic IABP insertion in this setting had substantially better outcomes than rescue IABP placement according to retrospective multivariate analysis,132 including evaluation of high-risk patients with severely depressed LV ejection fraction133 undergoing angioplasty and patients undergoing unprotected left main PCI,134 though the level of evidence base was generally weak. BCIS-1 (Balloon Pump-Assisted Coronary Intervention Study) randomly assigned patients to elective use of IABP prior to PCI in high-risk patients. Whereas it failed to demonstrate a significant benefit, a nonsignificant trend to decreased morality rate was seen (4.6% vs. 7.4%) and may reflect the underpowered nature of the trial. There was significant crossover in this study as well; crossover tends to dilute the power of studies that examine the use of already available technology, particularly when there is a perception on the part of clinicians that the device is efficacious, even if the efficacy is unproven.135 IABP use in this setting is currently class IIb,92 and is discussed further in this chapter under “Newer Technologies.”
Cardiac Surgery
IABP is utilized in approximately 10% to 15% of patients undergoing cardiac surgery, with substantial rise in the rate in the past decade.136 About half of this use is for coronary bypass patients; two-thirds of the CS patients in the Society of Thoracic Surgeons database had insertion of IABPs.137 A number of studies have demonstrated a favorable influence on outcomes, including mortality rate.138–140 Nevertheless, there is considerable variability in use among centers, reflecting a lack of consensus regarding indications for perioperative IABP use.136,141 The vast majority of IABP insertions occur preoperatively.136,137 The effectiveness of this approach has been controversial. One small randomized trial of high-risk patients demonstrated lower mortality rate, higher postoperative cardiac index, and shorter intubation time, intensive care unit (ICU) stay, and hospitalization in patients with preoperative IABP placement.131 Mortality rate benefit was also observed in a retrospective single center experience142 as well as a meta-analysis.143 In contrast, a propensity analysis suggested excess mortality rate in nearly 2,000 patients receiving preoperative IABP insertion compared with 28,000 who did not.144 Because of the limitations of propensity analysis, it is possible that selection bias determined the unfavorable outcomes associated with preoperative IABP use. Patients most likely to benefit from preoperative IABP insertion are those with depressed LV function, unstable angina or recent MI, or left main coronary artery disease or those who are undergoing repeat thoracotomy.
Several mechanisms can be postulated for superior outcomes with preoperative IABP insertion. Counterpulsation can provide hemodynamic support during anesthesia induction and during the stress of surgery before cardiopulmonary bypass is begun.140,145 As already described, IABP with pulsatile flow during cardiopulmonary bypass also appears to have favorable effects on end-organ perfusion26,146,147 with protection of both coronary and cerebral blood flow.148 IABP insertion can also be used to assess prognosis; requirement for catecholamine support and overall hemodynamics 1 hour after institution of perioperative balloon pump insertion is highly predictive of overall outcome.149
Although studies have reported the mortality rate in high-risk patients treated with IABP as high as 53%, preoperative placement of an IABP was associated with substantially lower morbidity and mortality rates (24%), while postoperative insertion, in this case creating bias toward late insertion in situations with bad outcomes, was associated with a 63% mortality rate.150 In the STS database, operative mortality rate in CS patients, the majority of whom received IABP, was high, ranging from 20% with isolated CABG to 33% for CABG and valve surgery and 58% for CABG and ventricular septal repair.137 Similarly, a nonrandomized study of patients with an ejection fraction of 25% or less compared patients who were treated with preoperative IABP with those who were not. Mortality rate was 2.7% in the former group compared with 11.9% in the latter group, despite the presence of New York Heart Association (NYHA) class III or IV heart failure in 92% of the former and only 55% of the latter.138 Several other post hoc analyses have found results consistent with advantages of preoperative IABP.151,152
Outcomes in addition to mortality rate that were superior in patients undergoing preoperative IABP insertion were duration of IABP support, length of hospital stay, and postoperative LV ejection fraction in a randomized study of high-risk patients with ejection fraction of 30% or less.153 Similarly, preoperative use of an IABP in high-risk off-pump CABG patients appears to be favorable.154,155 Thus, although the evidence base is incomplete, the overall preoperative use of IABP is growing.136 Cost analysis appears to be favorable; combining high-risk cohorts randomly assigned to receive preoperative IABP placement or not,131,145 costs were 36% less in patients with preoperative IABP insertion because of shorter IABP treatment time, shorter hospitalizations, and lower use of critical care facilities.156
Congestive Heart Failure
IABP has been used successfully in a variety of settings that results in congestive heart failure, including fulminant myocarditis157 and severe decompensated aortic stenosis.158 On the basis of case reports, the device has also been helpful as adjunctive therapy for myocardial depression secondary to drug toxicity,159,160 myocardial contusion,161 anaphylaxis,162 thyrotoxicosis,163 multiple sclerosis,164 and even lightning strike.165 Animal data suggest some benefit in the setting of RV failure;166 the mechanism appears to be lowered pulmonary vascular resistance with consequent improvement in RV ejection.28 IABP insertion has also improved outcomes in patients with acute RV failure after heart transplantation.167
Finally, IABP has been used as a bridge to transplantation, typically with placement in the axillary or external iliac arteries to allow ambulation during prolonged counterpulsation.168 With development of a range of LV assist devices designed for long-term implantation, the use of IABP for this indication has waned.169
Miscellaneous Indications
Limited data suggest that IABP placement during cardiopulmonary resuscitation has favorable effects.170,171 The device has been used in pregnancy in patients undergoing heart surgery with an eye toward preserving uterine and fetal flow during cardiopulmonary bypass.172 In patients at high risk for cardiac events during noncardiac surgery (e.g., recent MI, LV failure, unrevascularized ischemic myocardium),173,174 IABP has been shown to have significant benefits for outcome,175 although the evidence base consists largely of case reports;176,177 a randomized trial has not been performed. Prophylactic IABP insertion seems particularly appropriate in high-risk patients undergoing emergency noncardiac surgery.177
Use of the Intra-aortic Balloon Pump
Overall, the existing data suggest that IABP is underutilized. In more than 23,000 patients in CS reported by NRMI 2, only 31% were treated with IABP. As is the case for a number of other interventions, women were less likely to undergo IABP placement; there was an age and race difference as well, with lower rates in nonwhites and older patients.178 A number of studies from the 1990s demonstrated less than 25% use of IABP in CS;104,179 this figure contrasts with 86% utilization in the SHOCK trial.102 Although some exclusion criteria in the latter may have improved suitability for IABP in the cohort enrolled in the study, the threefold higher use of the device in the SHOCK trial is consistent with wide underuse in clinical practice, similar to findings for a variety of pharmacologic and invasive interventions in acute MI.90
Contraindications
The classic absolute contraindication to IABP use is aortic insufficiency (Box 7.2). Because the acute hemodynamic effects are so deleterious, animal and clinical investigations all date to the 1970s.180 Increased retrograde volume displacement into the left ventricle during diastole greatly exacerbates wall stress, with greater potential for hemodynamic decompensation as well as LV pseudoaneurysm formation and LV rupture in the post-MI setting. The amount of aortic insufficiency that constitutes an absolute contraindication has no objective basis, but most operators use a threshold of trivial to mild. Similarly, the presence of aortic dissection or aortic aneurysm is considered an absolute contraindication because of the risk of extending dissection or causing aneurysmal rupture. Patent ductus arteriosus, like aortic insufficiency, theoretically has deleterious results from shunting of blood flow from the aorta with IABP induction, in this case increasing left-to-right shunting into the pulmonary artery. Generally, patients who have severe comorbid conditions at end of life or who exhibit brain death are considered to have contraindications to IABP placement.
Relative contraindications include placement of an indwelling foreign body into a patient with active infection including sepsis, severe peripheral vascular disease likely to result in limb ischemia, bleeding diathesis (although in practice, many patients who have low fibrinogen levels or are receiving aggressive anticoagulant or antiplatelet treatment undergo IABP placement), and contraindications to afterload reduction, such as dynamic LV outflow obstruction,181 a condition that has on occasion been unmasked by institution of counterpulsation.182 Patients with shock due to severe hypovolemia will not benefit from insertion and may deteriorate with IABP-induced afterload reduction.
Complications
From the initial experiences with IABPs in the 1960s, the significant complication rate has been the single largest drawback to its use (Table 7.1). The predominant complications have related to the access site, with bleeding and limb ischemia being the most common, but they also include infection, thrombocytopenia, stroke, device failure, and a variety of vascular misadventures. Table 7.1 describes complications of IABP from multiple registries, trials, and case reports. The complication rate associated with IABP is affected by the insertion of indwelling, typically 7 to 10 F devices into the femoral artery of patients with a high prevalence of diabetes, peripheral vascular disease, and other major comorbid conditions. As would be expected, duration of IABP use correlates with risk of complications overall,183,184 including sepsis.185,186 Thus, frequent reevaluation of the patient to confirm ongoing need for IABP is prudent. Death due to IABP has been relatively uncommon, with the rate typically less than 0.3% to as low as 0.05%,41 and should be weighed against its considerable survival benefits.
Table 7.1
Complications with Use of Intra-aortic Balloon Pump (IABP) from the Benchmark Registry
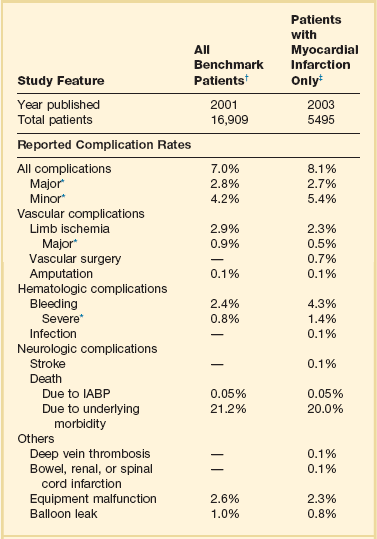
*Major complications are major limb ischemia, severe bleeding, balloon leak, and death attributable to IABP insertion or failure; major limb ischemia consists of loss of pulse, loss of sensation, or abnormal limb temperature or pallor requiring intervention, arterial repair, or amputation; severe bleeding is defined as bleeding that requires transfusion or surgical intervention or results in hemodynamic compromise. Independent risk factors for major complications were female gender, peripheral vascular disease, body surface area < 1.65 m2, and age ≥ 75 years.
†Based on data from Ferguson JJ III, Cohen M, Freedman RJ Jr, et al: The current practice of intra-aortic balloon counterpulsation: Results from the Benchmark Registry. J Am Coll Cardiol 2001;38:1456-1462.
‡Based on data from Stone GW, Ohman EM, Miller MF, et al: Contemporary utilization and outcomes of intra-aortic balloon counterpulsation in acute myocardial infarction: The Benchmark Registry. J Am Coll Cardiol 2003;41:1940-1945.
The influence of procedure volume on outcomes has been demonstrated in a wide variety of cardiac procedures. Data from NRMI 2 found a significant correlation after multivariate analysis between number of IABP implants per year and CS mortality rate.90 The study did not address complications related directly to IABP as a function of procedure volume for individual hospitals or operators. A higher rate of vascular complications was seen in the quartile of hospitals performing the most IABP insertions in high-risk PCI in the NCDR database,2 but after multivariate adjustment this difference was no longer present, likely reflecting substantially higher morbidity in that patient population.
Comparing overall complication rates among different series is hindered by lack of uniform definitions, variable demographics, comorbid conditions, duration of data collection, years when the data were collected (during which significant changes in technology may have occurred), and indications for IABP insertion. The rate of major complications has been reported to range from 2% to nearly 50%. However, the early data, which included surgical insertion, lack of a guidewire lumen for safer passage through the iliac arteries and aorta, and larger catheter and sheath sizes, were much worse than those in more recent series, with same center analyses describing as much as a fivefold decrease in major complications.187 Thus, Kantrowitz and coworkers188 reported a 47% complication rate over the first 15 years of IABP experience, and Alderman and colleagues189 described a 42% rate of limb ischemia alone in their mid-1980s study. With improvements in technology, periprocedure pharmacology, patient selection, and management, the overall complication rates have declined to 15% in a large single hospital review published in 200050 and 6.5% in the Benchmark Registry, which incorporates the largest experience to date.190 Although the series use variable definitions, the trend is unequivocal.
In general, diabetic patients and women have a higher complication rate,188 coincident with the finding that these two populations also have significantly smaller femoral arteries.49 A review of the existing literature on IABP complications shows peripheral vascular disease and female gender as nearly uniform markers of higher complication risk, with age, diabetes, size of catheter inserted, and smaller body surface area common but somewhat less consistent markers on multivariate analyses.41,50
Vascular Complications
Vascular complications including limb ischemia are the most common serious adverse events related to IABP insertion. Amputation is rare (0.1%),41 but major limb ischemia, defined in the Benchmark Registry as “loss of pulse or sensation, or abnormal limb temperature or pallor requiring surgical intervention,” occurred in 1.3% of cases.190 Minor limb ischemia, defined as not requiring surgery and improving with balloon removal, occurred in another 1.2% in the same series. These numbers are consistent with steady improvement over the past decade: A smaller but still substantial earlier series from India involving 911 patients (with a much higher proportion of diabetic patients and likely significantly smaller body surface area) reported a 5.9% incidence of major vascular complications, and 5.8% rate of minor vascular complications.53 This series used a 9.5 F shaft IABP, which has been shown to have higher vascular complication rates than the 8 F shaft balloons that have been available for the past decade.191 Vascular complications, likely in part because of comorbid conditions, are associated with a much higher overall mortality rate—as much as a twofold increase.88
Trauma to the aorta has been reported, with paraplegia caused by spinal necrosis due to subadventitial hematoma,192 cholesterol embolization to spinal and mesenteric arteries,193 and aortic dissection194 as well as no obvious cause in some patients. The presence of friable atheroma in the descending aorta has been associated with embolization.195 IABP use has been identified as an independent predictor for neurologic complications of percutaneous intervention, although whether this is due to embolic phenomena or confounding variables has not been established.196
A complication particular to IABP is thrombocytopenia, occurring presumably because of destruction of platelets that adhere to the IABP surface, although the mechanism remains unclear. Critical care patients who have IABPs in place and are heparinized have a 7 : 1 odds ratio of a 50% drop in platelet count compared with patients without IABPs who are placed on heparin therapy at similar doses,197 lowering the likelihood that heparin-induced thrombocytopenia is the etiologic factor.
Mechanical Failure
Several complications of mechanical failure of the balloon or console have serious consequences. Rupture of the balloon was more common early in the IABP era, with an incidence reported to range from 1.7%198 to 5.2%.199 Typically the diagnosis was made by the appearance of blood in the gas lumen, with triggering of alarms. The usual site of rupture has been at a point when the aorta is at its nadir in diameter along the course of the IABP. Small vessel size is associated with abrasion of the IABP (thus the observation that is it more common in women200 likely correlates with their smaller body surface area); similar concerns arise for larger balloon sizes. Rupture of the balloon with a major gas leak, a rare event, has been reported to cause stroke secondary to gas embolization.201 Hydrophilically coated balloons hold some promise for further reducing the risk of rupture through potentially decreasing abrasion of the balloon surface.46 Fracturing of the IABP can occur with entrapment, including separation and migration of part of the device.202 Clot may also form in the gas lumen after loss of balloon integrity and has been reported to interfere with balloon deflation, requiring use of a thrombolytic agent in the gas line to allow balloon deflation and removal of the balloon.203
Treating Complications Related to the Intra-aortic Balloon Pump
Several approaches to managing IABP-related complications have been proposed. Limb ischemia has traditionally been most successfully treated by removal of the IABP,53,204 even in patients wholly dependent on the balloon, in order to avoid loss of limb. This situation has occasionally forced physicians and families to choose between loss of limb and patient survival. Surgical femoral-femoral shunting performed at the bedside with exteriorization of the graft has been described as one potential approach.205 As previously mentioned, we have performed percutaneous nonsurgical external femoral-femoral shunting to effectively address limb ischemia by placing one sheath retrograde into the femoral artery contralateral to the IABP and another antegrade in the ipsilateral vessel, connecting the two sheaths with tubing and a flow regulator (see Fig. 7.5).62 Infusion of prostaglandin E1 through the balloon central lumen has been shown to relieve lower limb ischemia in a small series, presumably through increase in caliber of collateral vessels or relief of spasm in the common femoral or iliac system.206
Newer Technologies
Kantrowitz, who began this work more than a half century ago, attempted to develop a permanent implantable IABP. Initial results of a pilot trial demonstrated substantial improvement in hemodynamics. The ability to use the device intermittently rather than continuously, theoretically without thromboembolic risk once fully endothelialized, and its location downstream from the head and neck vessels differentiate it from other ventricular assist devices.207
A number of other percutaneous extracorporeal assist devices have been developed, although they lack the flexibility of bedside insertion and the low profile of the IABP (Fig. 7.12). The TandemHeart device (CardiacAssist, Inc., Pittsburgh, PA) is a left atrial to femoroiliac bypass, powered by an external centrifugal pump that provides up to 4 L/minute of forward flow. It requires transseptal puncture, institution of cardiac bypass with much larger arterial cannulas than the IABP (21 F in the left atrium, 15 F to 17 F in the iliac artery), and in general is more complex with greater risk of complications, as shown in a randomized study comparing the two approaches in patients presenting with CS being considered for PCI.208 It also cannot be shut off temporarily and requires more aggressive monitoring than balloon counterpulsation. Small randomized trials have been performed comparing the TandemHeart with IABP in patients with CS, demonstrating superior hemodynamics208,209 with the TandemHeart device.
The Impella device (Abiomed, Danvers, MA) uses a miniaturized axial flow rotary pump fitted onto a pigtail catheter. It is placed retrogradely across the aortic valve. It pumps blood into the aorta and directly unloads the left ventricle.210 The Impella 2.5 and 5.0 provide maximal flow of rates of 2.5 L/minute and 5.0 L/minute, respectively. Unlike the TandemHeart, it does not require transseptal puncture or extracorporeal circulation with the attendant complexity and risks but does require insertion of substantially larger hardware into the femoral artery than the IABP: A 13 F sheath is required for the Impella 2.5, whereas the shaft of the 5.0 device is 21 F. Both devices appear promising for circulatory support in a variety of settings but, unlike IABP, do not provide pulsatile flow.211 Registry data utilizing Impella for high-risk PCI and for acute MI and CS showed consistent improvement in hemodynamics.212,213 There are minimal data comparing IABP to Impella in patients with CS, with one small trial showing superior cardiac output during Impella use but an identical mortality rate.214 In the setting of high-risk PCI, the PROTECT II trial compared prophylactic IABP to Impella: Hemodynamics were superior with Impella, but overall outcomes including 30-day major adverse events were similar, with some later trends that appeared to favor Impella use, albeit with study design and enrollment issues that may have confounded results. A caveat to Impella use in this setting relates to a higher adverse event rate in the setting of rotational atherectomy.215 In a meta-analysis of three of the randomized clinical trials described previously,208,209,214 hemodynamics consistently superior to IABP use were shown with TandemHeart and Impella, but these findings did not translate into a survival benefit;216 it should be kept in mind that the TandemHeart and Impella devices are substantially more costly, complex to institute and manage, and in the case of TandemHeart, appears to be associated with a higher complication rate.
Potential contraindications for both TandemHeart and Impella include aortic insufficiency and peripheral vascular disease. Aortic stenosis and presence of LV thrombus are contraindications for Impella use. Although post-MI ventricular septal rupture is uncommon, TandemHeart and Impella should be used with caution in that setting, because use of the devices could theoretically trigger right-to-left shunting because the substantial volume extraction from the left-sided heart circulation preferentially lowers LV pressure; however, despite the theoretical concern, a small series has documented benefit in this setting as well.217 A number of other off-label uses of these devices have been described, including temporary support of the right ventricle218 in RV infarction and as a bridge to ventricular assist devices and transplantation.
A vast array of other technologies is under development. The use of IABP in conjunction with assist devices that do not provide pulsatile flow may provide symbiotic preservation of end-organ circulation.219,220
References
1. Kozak, LJ, DeFrances, CJ, Hall, MJ, National Hospital Discharge Survey: 2004 Annual summary with detailed diagnosis and procedure data. National Center for Health Statistics. Vital Health Stat. 2006; 13(162):176. http://www.cdc.gov/nchs/data/series/sr_13/sr13_162.pdf
2. Curtis, JP, Rathore, SS, Wang, Y, et al. Use and effectiveness of intra-aortic balloon pumps among patients undergoing high risk percutaneous coronary intervention: Insights from the National Cardiovascular Data Registry. Circ Cardiovasc Qual Outcomes. 2012; 5:21–30.
3. Torchiana, DF, Hirsch, G, Buckley, MJ, et al. Intraaortic balloon pumping for cardiac support: Trends in practice and outcome, 1968 to 1995. J Thorac Cardiovasc Surg. 1997; 113:758–764.
4. Cohen, M, Urban, P, Christenson, JT, et al. Intra-aortic balloon counterpulsation in US and non-US centres: Results of the Benchmark Registry. Eur Heart J. 2003; 24:1763–1770.
5. Kantrowitz, A. Experimental augmentation of coronary flow by retardation of the arterial pressure pulse. Surgery. 1953; 34:678–687.
6. Kantrowitz, A. Moments in history. Introduction of left ventricular assistance. ASAIO Trans. 1987; 33:39–48.
7. Kantrowitz, A, McKinnon, WM. The experimental use of the diaphragm as an auxiliary myocardium. Surg Forum. 1958; 9:266–268.
8. Clauss, RH, Birtwell, WC, Albertal, G, et al. Assisted circulation. I. The arterial counterpulsator. J Thorac Cardiovasc Surg. 1961; 41:447–458.
9. Moulopoulos, SD, Topaz, S, Kolff, WJ. Diastolic balloon pumping (with carbon dioxide) in the aorta—A mechanical assistance to the failing circulation. Am Heart J. 1962; 63:669–675.
10. Kantrowitz, A, Tjonneland, S, Freed, PS, et al. Initial clinical experience with intraaortic balloon pumping in cardiogenic shock. JAMA. 1968; 203:113–118.
11. Kantrowitz, A, Tjonneland, S, Krakauer, JS, et al. Mechanical intraaortic cardiac assistance in cardiogenic shock. Hemodynamic effects. Arch Surg. 1968; 97:1000–1004.
12. Scheidt, S, Wilner, G, Mueller, H, et al. Intra-aortic balloon counterpulsation in cardiogenic shock. Report of a co-operative clinical trial. N Engl J Med. 1973; 288:979–984.
13. Bregman, D, Casarella, WJ. Percutaneous intraaortic balloon pumping: Initial clinical experience. Ann Thorac Surg. 1980; 29:153–155.
14. Merav, AD, Solomon, N, Montefusco, CM, et al. A new guidable double lumen percutaneous intra-aortic balloon. Trans Am Soc Artif Intern Organs. 1981; 27:593–597.
15. Wolvek, S. The development of a safe and highly durable balloon catheter for the IAB. Artif Organs. 1993; 17:891–892.
16. Weber, KT, Janicki, JS. Intraaortic balloon counterpulsation. A review of physiological principles, clinical results, and device safety. Ann Thorac Surg. 1974; 17:602–636.
17. Kern, MJ, Aguirre, FV, Tatineni, S, et al. Enhanced coronary blood flow velocity during intraaortic balloon counterpulsation in critically ill patients. J Am Coll Cardiol. 1993; 21:359–368.
18. Stefanadis, C, Dernellis, J, Tsiamis, E, et al. Aortic function in patients during intra-aortic balloon pumping determined by the pressure-diameter relation. J Thorac Cardiovasc Surg. 1998; 116:1052–1059.
19. Anderson, RD, Gurbel, PA. The effect of intra-aortic balloon counterpulsation on coronary blood flow velocity distal to coronary artery stenoses. Cardiology. 1996; 87:306–312.
20. Toyota, E, Songfang, L, Kimura, A, et al. Evaluation of intramyocardial coronary blood flow waveform during intraaortic balloon pumping in the absence or presence of coronary stenosis. Artif Organs. 1996; 20:166–168.
21. Gitter, R, Cate, CM, Smart, K, et al. Influence of ascending versus descending balloon counterpulsation on bypass graft blood flow. Ann Thorac Surg. 1998; 65:365–370.
22. Kimura, A, Toyota, E, Lu, S, et al. Effects of intraaortic balloon pumping on septal arterial blood flow velocity waveform during severe left main coronary artery stenosis. J Am Coll Cardiol. 1996; 27:810–816.
23. Takeuchi, M, Nohtomi, Y, Yoshitani, H, et al. Enhanced coronary flow velocity during intra-aortic balloon pumping assessed by transthoracic Doppler echocardiography. J Am Coll Cardiol. 2004; 43:368–376.
24. Zehetgruber, M, Mundigler, G, Christ, G, et al. Relation of hemodynamic variables to augmentation of left anterior descending coronary flow by intraaortic balloon pulsation in coronary artery disease. Am J Cardiol. 1997; 80:951–955.
25. Gurbel, PA, Anderson, RD, MacCord, CS, et al. Arterial diastolic pressure augmentation by intra-aortic balloon counterpulsation enhances the onset of coronary artery reperfusion by thrombolytic therapy. Circulation. 1994; 89:361–365.
26. Onorati, F, Santarpino, G, Presta, P, et al. Pulsatile perfusion with intra-aortic balloon pumping ameliorates whole body response to cardiopulmonary bypass in the elderly. Crit Care Med. 2009; 37:902–911.
27. Kveim, M, Cappelen, C, Jr., Frooysaker, T, et al. Intra-aortic balloon pumping in the treatment of cardiogenic shock following open-heart surgery. Scand J Thorac Cardiovasc Surg. 1976; 10:231–235.
28. Nordhaug, D, Steensrud, T, Muller, S, et al. Intraaortic balloon pumping improves hemodynamics and right ventricular efficiency in acute ischemic right ventricular failure. Ann Thorac Surg. 2004; 78:1426–1432.
29. Fincke, R, Hochman, JS, Lowe, AM, et al. Cardiac power is the strongest hemodynamic correlate of mortality in cardiogenic shock: A report from the SHOCK trial registry. J Am Coll Cardiol. 2004; 44:340–348.
30. Schachtrupp, A, Wrigge, H, Busch, T, et al. Influence of intra-aortic balloon pumping on cerebral blood flow pattern in patients after cardiac surgery. Eur J Anaesthesiol. 2005; 22:165–170.
31. Applebaum, RM, Wun, HH, Katz, ES, et al. Effects of intraaortic balloon counterpulsation on carotid artery blood flow. Am Heart J. 1998; 135:850–854.
32. Hashimoto, K, Onoguchi, K, Takakura, H, et al. Beneficial effect of balloon-induced pulsatility on brain oxygenation in hypothermic cardiopulmonary bypass. J Cardiovasc Surg (Torino). 2001; 42:587–593.
33. Rosen, CL, Sekhar, LN, Duong, DH. Use of intra-aortic balloon pump counterpulsation for refractory symptomatic vasospasm. Acta Neurochir (Wien). 2000; 142:25–32.
34. Janssens, U, Graf, J, Koch, KC, et al. Gastric tonometry in patients with cardiogenic shock and intra-aortic balloon counterpulsation. Crit Care Med. 2000; 28:3449–3455.
35. Haywood, GA, Keeling, PJ, Parker, DJ, et al. Short-term effects of intra-aortic balloon pumping on renal blood flow and renal oxygen consumption in cardiogenic shock. J Card Fail. 1995; 1:217–222.
36. Heinze, H, Heringlake, M, Schmucker, P, et al. Effects of intra-aortic balloon counterpulsation on parameters of tissue oxygenation. Eur J Anaesthesiol. 2006; 23:555–562.
37. Reesink, KD, van der, NT, Bovelander, J, et al. Feasibility study of a fiber-optic system for invasive blood pressure measurements. Catheter Cardiovasc Intervent. 2002; 57:272–276.
38. Weber, KT, Janicki, JS, Walker, AA. Intra-aortic balloon pumping: An analysis of several variables affecting balloon performance. Trans Am Soc Artif Intern Organs. 1972; 18:486–492.
39. Stamatelopoulos, SF, Nanas, JN, Saridakis, NS, et al. Treating severe cardiogenic shock by large counterpulsation volumes. Ann Thorac Surg. 1996; 62:1110–1117.
40. Cohen, M, Fasseas, P, Singh, VP, et al. Impact of intra-aortic balloon counterpulsation with different balloon volumes on cardiac performance in humans. Catheter Cardiovasc Intervent. 2002; 57:199–204.
41. Ferguson, JJ, III., Cohen, M, Freedman, RJ, Jr., et al. The current practice of intra-aortic balloon counterpulsation: Results from the Benchmark Registry. J Am Coll Cardiol. 2001; 38:1456–1462.
42. Hatlestad, DC, Van Horn, J. Air transport of the IABP patient. Intra-aortic balloon pump. Air Med J. 2002; 21:42–48.
43. Igari, T. The length of the aorta from the subclavian artery to the renal artery based on computed tomographic measurements in Japanese adults. J Artif Organs. 2006; 9:267–270.
44. Weikel, AM, Jones, RT, Dinsmore, R, et al. Size limits and pumping effectiveness of intraaortic balloons. Ann Thorac Surg. 1971; 12:45–53.
45. Mueller, XM, Tevaearai, HT, Hayoz, D, et al. Thrombogenicity of deflated intraaortic balloon: Impact of heparin coating. Artif Organs. 1999; 23:195–198.
46. Winters, KJ, Smith, SC, Cohen, M, et al. Reduction in ischemic vascular complications with a hydrophilic-coated intra-aortic balloon catheter. Catheter Cardiovasc Intervent. 1999; 46:357–362.
47. Schreuder, JJ, Castiglioni, A, Donelli, A, et al. Automatic intraaortic balloon pump timing using an intrabeat dicrotic notch prediction algorithm. Ann Thorac Surg. 2005; 79:1017–1022.
48. Stone, GW, Ohman, EM, Miller, MF, et al. Contemporary utilization and outcomes of intra-aortic balloon counterpulsation in acute myocardial infarction: The benchmark registry. J Am Coll Cardiol. 2003; 41:1940–1945.
49. Schnyder, G, Sawhney, N, Whisenant, B, et al. Common femoral artery anatomy is influenced by demographics and comorbidity: Implications for cardiac and peripheral invasive studies. Catheter Cardiovasc Intervent. 2001; 53:289–295.
50. Cohen, M, Dawson, MS, Kopistansky, C, et al. Sex and other predictors of intra-aortic balloon counterpulsation-related complications: Prospective study of 1119 consecutive patients. Am Heart J. 2000; 139:282–287.
51. Ellis, SG, Bhatt, D, Kapadia, S, et al. Correlates and outcomes of retroperitoneal hemorrhage complicating percutaneous coronary intervention. Catheter Cardiovasc Intervent. 2006; 67:541–545.
52. Meisel, S, Shochat, M, Sheikha, SA, et al. Utilization of low-profile intra-aortic balloon catheters inserted by the sheathless technique in acute cardiac patients: Clinical efficacy with a very low complication rate. Clin Cardiol. 2004; 27:600–604.
53. Meharwal, ZS, Trehan, N. Vascular complications of intra-aortic balloon insertion in patients undergoing coronary reavscularization: Analysis of 911 cases. Eur J Cardiothorac Surg. 2002; 21:741–747.
54. Menon, P, Totaro, P, Youhana, A, et al. Reduced vascular complication after IABP insertion using smaller sized catheter and sheathless technique. Eur J Cardiothorac Surg. 2002; 22:491–492.
55. Erdogan, HB, Goksedef, D, Erentug, V, et al. In which patients should sheathless IABP be used? An analysis of vascular complications in 1211 cases. J Card Surg. 2006; 21:342–346.
56. Patel, JJ, Kopisyansky, C, Boston, B, et al. Prospective evaluation of complications associated with percutaneous intraaortic balloon counterpulsation. Am J Cardiol. 1995; 76:1205–1207.
57. Klopman, MA, Chen, EP, Sniecinski, RM. Positioning an intraaortic balloon pump using intraoperative transesophageal echocardiogram guidance. Anesth Analg. 2011; 113:40–43.
58. Corcos, T, Guerin, Y, Favereau, X. Stiff hydrophilic glidewire: A useful tool for percutaneous insertion of intraaortic balloon counterpulsation catheters. Catheter Cardiovasc Intervent. 1999; 46:497.
59. Colyer, WR, Jr., Burket, MW, Ansel, GM, et al. Intra-aortic balloon pump placement following aorto-iliac angioplasty and stent placement. Catheter Cardiovasc Intervent. 2002; 55:163–168.
60. Lewis, BE, Sumida, C, Hwang, MH, et al. New approach to management of intraaortic balloon pumps in patients with peripheral vascular disease: Case reports of four patients requiring urgent IABP insertion. Cathet Cardiovasc Diagn. 1992; 26:295–299.
61. Turi, ZG. Vascular turf wars: Getting a leg up on iliac artery disease during intraaortic balloon pumping [editorial; comment]. Cathet Cardiovasc Diagn. 1997; 42:7.
62. Merhi, WM, Turi, ZG, Dixon, S, et al. Percutaneous ex-vivo femoral arterial bypass: A novel approach for treatment of acute limb ischemia as a complication of femoral arterial catheterization. Catheter Cardiovasc Intervent. 2006; 68:435–440.
63. Khir, AW, Price, S, Hale, C, et al. Intra-aortic balloon pumping: Does posture matter? Artif Organs. 2005; 29:36–40.
64. Noel, BM, Gleeton, O, Barbeau, GR. Transbrachial insertion of an intra-aortic balloon pump for complex coronary angioplasty. Catheter Cardiovasc Intervent. 2003; 60:36–39.
65. Onorati, F, Bilotta, M, Pezzo, F, et al. Transbrachial insertion of a 7. 5-Fr intra-aortic balloon pump in a severely atherosclerotic patient. Crit Care Med. 2006; 34:2231–2233.
66. Werns, SW. Should the transbrachial route be used for intra-aortic balloon pumps? Almost never!. Crit Care Med. 2006; 34:2259–2261.
67. Patel, MR, Buchanan, SA, Bergin, JD, et al. As originally published in 1994: Ambulatory intraaortic balloon counterpulsation. Updated in 2001. Ann Thorac Surg. 2001; 72:975.
68. Rubenstein, RB, Karhade, NV. Supraclavicular subclavian technique of intra-aortic balloon insertion. J Vasc Surg. 1984; 1:577–578.
69. Cochran, RP, Starkey, TD, Panos, AL, et al. Ambulatory intraaortic balloon pump use as bridge to heart transplant. Ann Thorac Surg. 2002; 74:746–751.
70. H’Doubler, PB, Jr., H’Doubler, WZ, Bien, RC, et al. A novel technique for intraaortic balloon pump placement via the left axillary artery in patients awaiting cardiac transplantation. Cardiovasc Surg. 2000; 8:463–465.
71. Arafa, OE, Geiran, OR, Svennevig, JL. Transthoracic intra-aortic balloon pump in open heart operations: Techniques and outcome. Scand Cardiovasc J. 2001; 35:40–44.
72. Santini, F, Mazzucco, A. Transthoracic intraaortic counterpulsation: A simple method for balloon catheter positioning. Ann Thorac Surg. 1997; 64:859–860.
73. Mueller, DK, Stout, M, Blakeman, BM. Morbidity and mortality of intra-aortic balloon pumps placed through the aortic arch. Chest. 1998; 114:85–88.
74. Schreuder, JJ, Maisano, F, Donelli, A, et al. Beat-to-beat effects of intraaortic balloon pump timing on left ventricular performance in patients with low ejection fraction. Ann Thorac Surg. 2005; 79:872–880.
75. Kern, MJ, Aguirre, FV, Caracciolo, EA, et al. Hemodynamic effects of new intra-aortic balloon counterpulsation timing methods in patients: A multicenter evaluation. Am Heart J. 1999; 137:1129–1136.
76. Sakamoto, T, Arai, H, Maruyama, T, et al. New algorithm of intra aortic balloon pumping in patients with atrial fibrillation. ASAIO J. 1995; 41:79–83.
77. Papaioannou, TG, Terrovitis, J, Kanakakis, J, et al. Heart rate effect on hemodynamics during mechanical assistance by the intra-aortic balloon pump. Int J Artif Organs. 2002; 25:1160–1165.
78. Cooper, HA, Thompson, E, Panza, JA. The role of heparin anticoagulation during intra-aortic balloon counterpulsation in the coronary care unit. Acute Card Care. 2008; 10:214–220.
79. Jiang, CY, Zhao, LL, Wang, JA, et al. Anticoagulation therapy in intra-aortic balloon counterpulsation: Does IABP really need anti-coagulation? J Zhejiang Univ Sci. 2003; 4:607–611.
80. Pucher, PH, Cummings, IG, Shipolini, AR, et al. Is heparin needed for patients with an intra-aortic balloon pump? Interact Cardiovasc Thorac Surg. 2012; 15:136–139.
81. Crystal, E, Borer, A, Gilad, J, et al. Incidence and clinical significance of bacteremia and sepsis among cardiac patients treated with intra-aortic balloon counterpulsation pump. Am J Cardiol. 2000; 86:1281–1284.
82. Niederhauser, U, Vogt, M, Vogt, P, et al. Cardiac surgery in a high-risk group of patients: Is prolonged postoperative antibiotic prophylaxis effective? J Thorac Cardiovasc Surg. 1997; 114:162–168.
83. Lewis, PA, Mullany, DV, Courtney, M, et al. Australasian trends in intra-aortic balloon counterpulsation weaning: Results of a postal survey. Crit Care Resusc. 2006; 8:361–367.
84. Manord, JD, Garrard, CL, Mehra, MR, et al. Implications for the vascular surgeon with prolonged (3 to 89 days) intraaortic balloon pump counterpulsation. J Vasc Surg. 1997; 26:511–515.
85. Chadow, HL, Hauptman, RE, Strizik, B, et al. Vasoseal after intra-aortic balloon pump removal: A pilot study. Catheter Cardiovasc Intervent. 2000; 50(4):495–497.
86. Kato, K, Sato, N, Yamamoto, T, et al. Initial experiences of removal of intra-aortic balloon pumps with the Angio-Seal. J Invasive Cardiol. 2006; 18:130–132.
87. Sohail, MR, Khan, AH, Holmes, DR, Jr., et al. Infectious complications of percutaneous vascular closure devices. Mayo Clin Proc. 2005; 80:1011–1015.
88. Busch, T, Sirbu, H, Zenker, D, et al. Vascular complications related to intraaortic balloon counterpulsation: An analysis of ten years experience. Thorac Cardiovasc Surg. 1997; 45:55–59.
89. Urban, PM, Freedman, RJ, Ohman, EM, et al. In-hospital mortality associated with the use of intra-aortic balloon counterpulsation. Am J Cardiol. 2004; 94:181–185.
90. Chen, EW, Canto, JG, Parsons, LS, et al. Relation between hospital intra-aortic balloon counterpulsation volume and mortality in acute myocardial infarction complicated by cardiogenic shock. Circulation. 2003; 108:951–957.
91. Antman, EM, Anbe, DT, Armstrong, PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of patients with acute myocardial infarction). J Am Coll Cardiol. 2004; 44:E1–E211.
92. Levine, GN, Bates, ER, Blankenship, JC, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol. 2011; 58:e44–122.
93. Holmes, DR, Jr., Berger, PB, Hochman, JS, et al. Cardiogenic shock in patients with acute ischemic syndromes with and without ST-segment elevation. Circulation. 1999; 100:2067–2073.
94. Reynolds, HR, Hochman, JS. Cardiogenic shock: Current concepts and improving outcomes. Circulation. 2008; 117:686–697.
95. Hochman, JS. Cardiogenic shock complicating acute myocardial infarction: Expanding the paradigm. Circulation. 2003; 107:2998–3002.
96. Goldberg, RJ, Gore, JM, Thompson, CA, et al. Recent magnitude of and temporal trends (1994-1997) in the incidence and hospital death rates of cardiogenic shock complicating acute myocardial infarction: The second national registry of myocardial infarction. Am Heart J. 2001; 141:65–72.
97. Dauerman, HL, Goldberg, RJ, White, K, et al. Revascularization, stenting, and outcomes of patients with acute myocardial infarction complicated by cardiogenic shock. Am J Cardiol. 2002; 90:838–842.
98. Barron, HV, Every, NR, Parsons, LS, et al. The use of intra-aortic balloon counterpulsation in patients with cardiogenic shock complicating acute myocardial infarction: Data from the National Registry of Myocardial Infarction 2. Am Heart J. 2001; 141:933–939.
99. Sanborn, TA, Sleeper, LA, Bates, ER, et al. Impact of thrombolysis, intra-aortic balloon pump counterpulsation, and their combination in cardiogenic shock complicating acute myocardial infarction: A report from the SHOCK Trial Registry. SHould we emergently revascularize Occluded Coronaries for cardiogenic shocK? J Am Coll Cardiol. 2000; 36:1123–1129.
100. Sjauw, KD, Engstrom, AE, Vis, MM, et al. A systematic review and meta-analysis of intra-aortic balloon pump therapy in ST-elevation myocardial infarction: Should we change the guidelines? Eur Heart J. 2009; 30:459–468.
101. Ohman, EM, Nanas, J, Stomel, RJ, et al. Thrombolysis and counterpulsation to improve survival in myocardial infarction complicated by hypotension and suspected cardiogenic shock or heart failure: Results of the TACTICS Trial. J Thromb Thrombolysis. 2005; 19:33–39.
102. Hochman, JS, Sleeper, LA, Webb, JG, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. SHould we emergently revascularize Occluded Coronaries for cardiogenic Shock. N Engl J Med. 1999; 341:625–634.
103. Brodie, BR, Stuckey, TD, Hansen, C, et al. Intra-aortic balloon counterpulsation before primary percutaneous transluminal coronary angioplasty reduces catheterization laboratory events in high-risk patients with acute myocardial infarction. Am J Cardiol. 1999; 84:18–23.
104. Anderson, RD, Ohman, EM, Holmes, DR, Jr., et al. Use of intraaortic balloon counterpulsation in patients presenting with cardiogenic shock: Observations from the GUSTO-I Study. Global Utilization of Streptokinase and TPA for Occluded Coronary Arteries. J Am Coll Cardiol. 1997; 30:708–715.
105. Kovack, PJ, Rasak, MA, Bates, ER, et al. Thrombolysis plus aortic counterpulsation: Improved survival in patients who present to community hospitals with cardiogenic shock. J Am Coll Cardiol. 1997; 29:1454–1458.
106. Nanas, JN, Nanas, SN, Kontoyannis, DA, et al. Myocardial salvage by the use of reperfusion and intraaortic balloon pump: Experimental study. Ann Thorac Surg. 1996; 61:629–634.
107. Kontoyannis, DA, Nanas, JN, Kontoyannis, SA, et al. Mechanical ventilation in conjunction with the intra-aortic balloon pump improves the outcome of patients in profound cardiogenic shock. Intensive Care Med. 1999; 25:835–838.
108. Thiele, H, Zeymer, U, Neumann, FJ, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012; 367(14):1287–1296.
109. Abdel-Wahab, M, Saad, M, Kynast, J, et al. Comparison of hospital mortality with intra-aortic balloon counterpulsation insertion before versus after primary percutaneous coronary intervention for cardiogenic shock complicating acute myocardial infarction. Am J Cardiol. 2010; 105:967–971.
110. Prondzinsky, R, Lemm, H, Swyter, M, et al. Intra-aortic balloon counterpulsation in patients with acute myocardial infarction complicated by cardiogenic shock: The prospective, randomized IABP SHOCK Trial for attenuation of multiorgan dysfunction syndrome. Crit Care Med. 2010; 38:152–160.
111. Unverzagt, S, Machemer, MT, Solms, A, et al. Intra-aortic balloon pump counterpulsation (IABP) for myocardial infarction complicated by cardiogenic shock. Cochrane Database Syst Rev. 2011.
112. Prondzinsky, R, Unverzagt, S, Russ, M, et al. Hemodynamic effects of intra-aortic balloon counterpulsation in patients with acute myocardial infarction complicated by cardiogenic shock: The prospective, randomized IABP shock trial. Shock. 2012; 37:378–384.
113. O’Connor, CM, Rogers, JG. Evidence for overturning the guidelines in cardiogenic shock. N Engl J Med. 2012; 367(14):1349–1350.
114. Hochman, JS, Buller, CE, Sleeper, LA, et al. Cardiogenic shock complicating acute myocardial infarction—Etiologies, management and outcome: A report from the SHOCK Trial Registry. SHould we emergently revascularize Occluded Coronaries for cardiogenic shocK? J Am Coll Cardiol. 2000; 36:1063–1070.
115. Jacobs, AK, Leopold, JA, Bates, E, et al. Cardiogenic shock caused by right ventricular infarction: A report from the SHOCK registry. J Am Coll Cardiol. 2003; 41:1273–1279.
116. Mendes, LA, Picard, MH, Sleeper, LA, et al. Cardiogenic shock: Predictors of outcome based on right and left ventricular size and function at presentation. Coron Artery Dis. 2005; 16:209–215.
117. Skillington, PD, Couper, GS, Peigh, PS, et al. Pulmonary artery balloon counterpulsation for intraoperative right ventricular failure. Ann Thorac Surg. 1991; 51:658–660.
118. Giesler, GM, Gomez, JS, Letsou, G, et al. Initial report of percutaneous right ventricular assist for right ventricular shock secondary to right ventricular infarction. Catheter Cardiovasc Intervent. 2006; 68:263–266.
119. Hasan, RI, Deiranyia, AK, Yonan, NA. Effect of intra-aortic balloon counterpulsation on right-left shunt following right ventricular infarction. Int J Cardiol. 1991; 33:439–442.
120. Liuzzo, JP, Shin, YT, Choi, C, et al. Simultaneous papillary muscle avulsion and free wall rupture during acute myocardial infarction. Intra-aortic balloon pump: A bridge to survival. J Invasive Cardiol. 2006; 18:135–140.
121. Thompson, CR, Buller, CE, Sleeper, LA, et al. Cardiogenic shock due to acute severe mitral regurgitation complicating acute myocardial infarction: A report from the SHOCK Trial Registry. SHould we use emergently revascularize Occluded Coronaries in cardiogenic shocK? J Am Coll Cardiol. 2000; 36:1104–1109.
122. Dekker, AL, Reesink, KD, van der Veen, FH, et al. Intra-aortic balloon pumping in acute mitral regurgitation reduces aortic impedance and regurgitant fraction. Shock. 2003; 19:334–338.
123. Menon, V, Webb, JG, Hillis, LD, et al. Outcome and profile of ventricular septal rupture with cardiogenic shock after myocardial infarction: A report from the SHOCK Trial Registry. SHould we emergently revascularize Occluded Coronaries in cardiogenic shocK? J Am Coll Cardiol. 2000; 36:1110–1116.
124. Thiele, H, Lauer, B, Hambrecht, R, et al. Short- and long-term hemodynamic effects of intra-aortic balloon support in ventricular septal defect complicating acute myocardial infarction. Am J Cardiol. 2003; 92:450–454.
125. Stone, GW, Marsalese, D, Brodie, BR, et al. A prospective, randomized evaluation of prophylactic intraaortic balloon counterpulsation in high risk patients with acute myocardial infarction treated with primary angioplasty. Second Primary Angioplasty in Myocardial Infarction (PAMI-II) Trial Investigators. J Am Coll Cardiol. 1997; 29:1459–1467.
126. ‘t Hof, AW, Liem, AL, de Boer, MJ, et al. A randomized comparison of intra-aortic balloon pumping after primary coronary angioplasty in high risk patients with acute myocardial infarction. Eur Heart J. 1999; 20:659–665.
127. Patel, MR, Smalling, RW, Thiele, H, et al. Intra-aortic balloon counterpulsation and infarct size in patients with acute anterior myocardial infarction without shock: The CRISP AMI randomized trial. JAMA. 2011; 306:1329–1337.
128. Talley, JD, Ohman, EM, Mark, DB, et al. Economic implications of the prophylactic use of intraaortic balloon counterpulsation in the setting of acute myocardial infarction. The Randomized IABP Study Group. Intraaortic balloon pump. Am J Cardiol. 1997; 79:590–594.
129. Ohman, EM, George, BS, White, CJ, et al. Use of aortic counterpulsation to improve sustained coronary artery patency during acute myocardial infarction. Results of a randomized trial. The Randomized IABP Study Group. Circulation. 1994; 90:792–799.
130. Kono, T, Morita, H, Nishina, T, et al. Aortic counterpulsation may improve late patency of the occluded coronary artery in patients with early failure of thrombolytic therapy. J Am Coll Cardiol. 1996; 28:876–881.
131. Christenson, JT, Simonet, F, Badel, P, et al. Optimal timing of preoperative intraaortic balloon pump support in high-risk coronary patients. Ann Thorac Surg. 1999; 68:934–939.
132. Mishra, S, Chu, WW, Torguson, R, et al. Role of prophylactic intra-aortic balloon pump in high-risk patients undergoing percutaneous coronary intervention. Am J Cardiol. 2006; 98:608–612.
133. Briguori, C, Sarais, C, Pagnotta, P, et al. Elective versus provisional intra-aortic balloon pumping in high-risk percutaneous transluminal coronary angioplasty. Am Heart J. 2003; 145:700–707.
134. Briguori, C, Airoldi, F, Chieffo, A, et al. Elective versus provisional intraaortic balloon pumping in unprotected left main stenting. Am Heart J. 2006; 152:565–572.
135. Perera, D, Stables, R, Thomas, M, et al. Elective intra-aortic balloon counterpulsation during high-risk percutaneous coronary intervention: A randomized controlled trial. JAMA. 2010; 304:867–874.
136. Baskett, RJ, O’Connor, GT, Hirsch, GM, et al. A multicenter comparison of intraaortic balloon pump utilization in isolated coronary artery bypass graft surgery. Ann Thorac Surg. 2003; 76:1988–1992.
137. Mehta, RH, Grab, JD, O’Brien, SM, et al. Clinical characteristics and in-hospital outcomes of patients with cardiogenic shock undergoing coronary artery bypass surgery: Insights from the Society of Thoracic Surgeons National Cardiac Database. Circulation. 2008; 117:876–885.
138. Dietl, CA, Berkheimer, MD, Woods, EL, et al. Efficacy and cost-effectiveness of preoperative IABP in patients with ejection fraction of 0. 25 or less. Ann Thorac Surg. 1996; 62:401–408.
139. Craver, JM, Murrah, CP. Elective intraaortic balloon counterpulsation for high-risk off-pump coronary artery bypass operations. Ann Thorac Surg. 2001; 71:1220–1223.
140. Gutfinger, DE, Ott, RA, Miller, M, et al. Aggressive preoperative use of intraaortic balloon pump in elderly patients undergoing coronary artery bypass grafting. Ann Thorac Surg. 1999; 67:610–613.
141. Ghali, WA, Ash, AS, Hall, RE, et al. Variation in hospital rates of intraaortic balloon pump use in coronary artery bypass operations. Ann Thorac Surg. 1999; 67:441–445.
142. Lavana, JD, Fraser, JF, Smith, SE, et al. Influence of timing of intraaortic balloon placement in cardiac surgical patients. J Thorac Cardiovasc Surg. 2010; 140:80–85.
143. Dyub, AM, Whitlock, RP, Abouzahr, LL, et al. Preoperative intra-aortic balloon pump in patients undergoing coronary bypass surgery: A systematic review and meta-analysis. J Card Surg. 2008; 23:79–86.
144. Baskett, RJ, O’Connor, GT, Hirsch, GM, et al. The preoperative intraaortic balloon pump in coronary bypass surgery: A lack of evidence of effectiveness. Am Heart J. 2005; 150:1122–1127.
145. Christenson, JT, Badel, P, Simonet, F, et al. Preoperative intraaortic balloon pump enhances cardiac performance and improves the outcome of redo CABG. Ann Thorac Surg. 1997; 64:1237–1244.
146. Onorati, F, Cristodoro, L, Bilotta, M, et al. Intraaortic balloon pumping during cardioplegic arrest preserves lung function in patients with chronic obstructive pulmonary disease. Ann Thorac Surg. 2006; 82:35–43.
147. Onorati, F, Cristodoro, L, Mastroroberto, P, et al. Should we discontinue intraaortic balloon during cardioplegic arrest? Splanchnic function results of a prospective randomized trial. Ann Thorac Surg. 2005; 80:2221–2228.
148. Geppert, A, Frey, B, Gabriel, H, et al. Effects of intraaortic balloon pumping on coronary and carotid flow during percutaneous cardiopulmonary support. Ann Thorac Surg. 1996; 61:1539–1541.
149. Hausmann, H, Potapov, EV, Koster, A, et al. Predictors of survival 1 hour after implantation of an intra-aortic balloon pump in cardiac surgery. J Card Surg. 2001; 16:72–77.
150. Arafa, OE, Pedersen, TH, Svennevig, JL, et al. Intraaortic balloon pump in open heart operations: 10-year follow-up with risk analysis. Ann Thorac Surg. 1998; 65:741–747.
151. Fasseas, P, Cohen, M, Kopistansky, C, et al. Pre-operative intra-aortic balloon counterpulsation in stable patients with left main coronary disease. J Invasive Cardiol. 2001; 13:679–683.
152. Pfeiffer, S, Frisch, P, Weyand, M, et al. The use of preoperative intra-aortic balloon pump in open heart surgery. J Cardiovasc Surg (Torino). 2005; 46:55–60.
153. Marra, C, De Santo, LS, Amarelli, C, et al. Coronary artery bypass grafting in patients with severe left ventricular dysfunction: A prospective randomized study on the timing of perioperative intraaortic balloon pump support. Int J Artif Organs. 2002; 25:141–146.
154. Vohra, HA, Dimitri, WR. Elective intraaortic balloon counterpulsation in high-risk off-pump coronary artery bypass grafting. J Card Surg. 2006; 21:1–5.
155. Christenson, JT, Licker, M, Kalangos, A. The role of intra-aortic counterpulsation in high-risk OPCAB surgery: A prospective randomized study. J Card Surg. 2003; 18:286–294.
156. Christenson, JT, Simonet, F, Schmuziger, M. Economic impact of preoperative intraaortic balloon pump therapy in high-risk coronary patients. Ann Thorac Surg. 2000; 70:510–515.
157. Ahmar, W, Leet, A, Morton, J. Diagnostic dilemmas and management of fulminant myocarditis. Anaesth Intensive Care. 2007; 35:117–120.
158. Gu, YL, Jessurun, GA, van den Merkhof, LF, et al. Intra-aortic balloon counterpulsation for complex aortic stenosis in hybrid strategy. Int J Cardiol. 2007; 117:e46–e48.
159. Timperley, J, Mitchell, AR, Brown, PD, et al. Flecainide overdose—Support using an intra-aortic balloon pump. BMC Emerg Med. 2005; 5:10.
160. David, JS, Gueugniaud, PY, Hepp, A, et al. Severe heart failure secondary to 5-fluorouracil and low-doses of folinic acid: Usefulness of an intra-aortic balloon pump. Crit Care Med. 2000; 28:3558–3560.
161. Penney, DJ, Bannon, PG, Parr, MJ. Intra-aortic balloon counterpulsation for cardiogenic shock due to cardiac contusion in an elderly trauma patient. Resuscitation. 2002; 55:337–340.
162. Yeguiayan, JM, Ravisy, J, Lenfant, F, et al. Anaphylactic shock: The advantages of intraaortic balloon counter pulsation for the treatment of heart failure. Resuscitation. 2007; 72:493–495.
163. Ngo, AS, Lung, T. Thyrotoxic heart disease. Resuscitation. 2006; 70:287–290.
164. Uriel, N, Kaluski, E, Hendler, A, et al. Cardiogenic shock in a young female with multiple sclerosis. Resuscitation. 2006; 70:153–157.
165. Rivera, J, Romero, KA, Gonzalez-Chon, O, et al. Severe stunned myocardium after lightning strike. Crit Care Med. 2007; 35:280–285.
166. Darrah, WC, Sharpe, MD, Guiraudon, GM, et al. Intraaortic balloon counterpulsation improves right ventricular failure resulting from pressure overload. Ann Thorac Surg. 1997; 64:1718–1723.
167. Arafa, OE, Geiran, OR, Andersen, K, et al. Intraaortic balloon pumping for predominantly right ventricular failure after heart transplantation. Ann Thorac Surg. 2000; 70:1587–1593.
168. Boehmer, JP, Popjes, E. Cardiac failure: Mechanical support strategies. Crit Care Med. 2006; 34:S268–S277.
169. Mather, PJ, Konstam, MA. Percutaneous mechanical devices in the management of decompensated heart failure. Curr Heart Fail Rep. 2007; 4:43–47.
170. Emerman, CL, Pinchak, AC, Hagen, JF, et al. Hemodynamic effects of the intra-aortic balloon pump during experimental cardiac arrest. Am J Emerg Med. 1989; 7:378–383.
171. Lurie, KG. Recent advances in mechanical methods of cardiopulmonary resuscitation. Acta Anaesthesiol Scand Suppl. 1997; 111:49–52.
172. Willcox, TW, Stone, P, Milsom, FP, et al. Cardiopulmonary bypass in pregnancy: Possible new role for the intra-aortic balloon pump. J Extra Corpor Technol. 2005; 37:189–191.
173. Georgeson, S, Coombs, AT, Eckman, MH. Prophylactic use of the intra-aortic balloon pump in high-risk cardiac patients undergoing noncardiac surgery: A decision analytic view. Am J Med. 1992; 92:665–678.
174. Browner, WS, Li, J, Mangano, DT. In-hospital and long-term mortality in male veterans following noncardiac surgery. The Study of Perioperative Ischemia Research Group. JAMA. 1992; 268:228–232.
175. Jafary, FH. Preoperative use of intra-aortic balloon counterpulsation in very high-risk patients prior to urgent noncardiac surgery. Acta Cardiol. 2005; 60:557–560.
176. Masaki, E, Takinami, M, Kurata, Y, et al. Anesthetic management of high-risk cardiac patients undergoing noncardiac surgery under the support of intraaortic balloon pump. J Clin Anesth. 1999; 11:342–345.
177. Shayani, V, Watson, WC, Mansour, MA, et al. Intra-aortic balloon counterpulsation in patients with severe cardiac dysfunction undergoing abdominal operations. Arch Surg. 1998; 133:632–635.
178. Goldberg, RJ, Gore, JM, Alpert, JS, et al. Cardiogenic shock after acute myocardial infarction. Incidence and mortality from a community-wide perspective, 1975 to 1988. N Engl J Med. 1991; 325:1117–1122.
179. Hasdai, D, Holmes, DR, Jr., Topol, EJ, et al. Frequency and clinical outcome of cardiogenic shock during acute myocardial infarction among patients receiving reteplase or alteplase. Results from GUSTO-III. Global Use of Strategies to Open Occluded Coronary Arteries. Eur Heart J. 1999; 20:128–135.
180. Yellin, E, Levy, L, Bregman, D, et al. Hemodynamic effects of intra-aortic balloon pumping in dogs with aortic incompetence. Trans Am Soc Artif Intern Organs. 1973; 19:389–394.
181. Tse, RW, Masindet, S, Stavola, T, et al. Acute myocardial infarction with dynamic outflow obstruction precipitated by intra-aortic balloon counterpulsation. Cathet Cardiovasc Diagn. 1996; 39:62–66.
182. Cohen, R, Rivagorda, J, Elhadad, S. Asymmetric septal hypertrophy complicated by dynamic left ventricular obstruction after intra-aortic balloon counterpulsation placement in the setting of anterior myocardial infarction. J Invasive Cardiol. 2006; 18:E207–E208.
183. Scholz, KH, Ragab, S, von zur, MF, et al. Complications of intra-aortic balloon counterpulsation. The role of catheter size and duration of support in a multivariate analysis of risk. Eur Heart J. 1998; 19:458–465.
184. Cook, L, Pillar, B, McCord, G, et al. Intra-aortic balloon pump complications: A five-year retrospective study of 283 patients. Heart Lung. 1999; 28:195–202.
185. Arafa, OE, Pedersen, TH, Svennevig, JL, et al. Vascular complications of the intraaortic balloon pump in patients undergoing open heart operations: 15-year experience. Ann Thorac Surg. 1999; 67:645–651.
186. Pawar, M, Mehta, Y, Ansari, A, et al. Nosocomial infections and balloon counterpulsation: Risk factors and outcome. Asian Cardiovasc Thorac Ann. 2005; 13:316–320.
187. Elahi, MM, Chetty, GK, Kirke, R, et al. Complications related to intra-aortic balloon pump in cardiac surgery: A decade later. Eur J Vasc Endovasc Surg. 2005; 29:591–594.
188. Kantrowitz, A, Wasfie, T, Freed, PS, et al. Intraaortic balloon pumping 1967 through 1982: Analysis of complications in 733 patients. Am J Cardiol. 1986; 57:976–983.
189. Alderman, JD, Gabliani, GI, McCabe, CH, et al. Incidence and management of limb ischemia with percutaneous wire-guided intraaortic balloon catheters. J Am Coll Cardiol. 1987; 9:524–530.
190. Christenson, JT, Cohen, M, Ferguson, JJ, III., et al. Trends in intraaortic balloon counterpulsation complications and outcomes in cardiac surgery. Ann Thorac Surg. 2002; 74:1086–1090.
191. Cohen, M, Ferguson, JJ, III., Freedman, RJ, Jr., et al. Comparison of outcomes after 8 vs. 9. 5 French size intra-aortic balloon counterpulsation catheters based on 9,332 patients in the prospective Benchmark registry. Catheter Cardiovasc Intervent. 2002; 56:200–206.
192. Tyras, DH, Willman, VL. Paraplegia following intraaortic balloon assistance. Ann Thorac Surg. 1978; 25:164–166.
193. Harris, RE, Reimer, KA, Crain, BJ, et al. Spinal cord infarction following intraaortic balloon support. Ann Thorac Surg. 1986; 42:206–207.
194. Beholz, S, Braun, J, Ansorge, K, et al. Paraplegia caused by aortic dissection after intraaortic balloon pump assist. Ann Thorac Surg. 1998; 65:603–604.
195. Karalis, DG, Quinn, V, Victor, MF, et al. Risk of catheter-related emboli in patients with atherosclerotic debris in the thoracic aorta. Am Heart J. 1996; 131:1149–1155.
196. Wong, SC, Minutello, R, Hong, MK. Neurological complications following percutaneous coronary interventions (a report from the 2000-2001 New York State Angioplasty Registry). Am J Cardiol. 2005; 96:1248–1250.
197. Vonderheide, RH, Thadhani, R, Kuter, DJ. Association of thrombocytopenia with the use of intra-aortic balloon pumps. Am J Med. 1998; 105:27–32.
198. Nishida, H, Koyanagi, H, Abe, T, et al. Comparative study of five types of IABP balloons in terms of incidence of balloon rupture and other complications: A multi-institutional study. Artif Organs. 1994; 18:746–751.
199. Patel, JJ, Kopistansky, C, Boston, B, et al. Prospective evaluation of factors associated with intraaortic balloon rupture. ASAIO J. 1996; 42:37–40.
200. Sutter, FP, Joyce, DH, Bailey, BM, et al. Events associated with rupture of intra-aortic balloon counterpulsation devices. ASAIO Trans. 1991; 37:38–40.
201. Cruz-Flores, S, Diamond, AL, Leira, EC. Cerebral air embolism secondary to intra-aortic balloon pump rupture. Neurocrit Care. 2005; 2:49–50.
202. Totaro, P, Degno, N, Smith, J, et al. The missing intra-aortic balloon pump catheter. Ital Heart J. 2005; 6:361–362.
203. Fukushima, Y, Yoshioka, M, Hirayama, N, et al. Management of intraaortic balloon entrapment. Ann Thorac Surg. 1995; 60:1109–1111.
204. Barnett, MG, Swartz, MT, Peterson, GJ, et al. Vascular complications from intraaortic balloons: Risk analysis. J Vasc Surg. 1994; 19:81–87.
205. Dosluoglu, HH, Dryjski, ML. External femorofemoral bypass to relieve acute leg ischemia during circulatory assist. Vascular. 2004; 12:198–201.
206. Nakano, T, Tominaga, R, Shiraishi, K, et al. Prostaglandin E1 from the tip of an intraaortic balloon catheter for lower limb ischemia. Ann Thorac Surg. 1998; 65:1158–1160.
207. Jeevanandam, V, Jayakar, D, Anderson, AS, et al. Circulatory assistance with a permanent implantable IABP: Initial human experience. Circulation. 2002; 106:I183–I188.
208. Thiele, H, Sick, P, Boudriot, E, et al. Randomized comparison of intra-aortic balloon support with a percutaneous left ventricular assist device in patients with revascularized acute myocardial infarction complicated by cardiogenic shock. Eur Heart J. 2005; 26:1276–1283.
209. Burkhoff, D, Cohen, H, Brunckhorst, C, et al. A randomized multicenter clinical study to evaluate the safety and efficacy of the TandemHeart percutaneous ventricular assist device versus conventional therapy with intraaortic balloon pumping for treatment of cardiogenic shock. Am Heart J. 2006; 152:469. e1–469. e8.
210. Henriques, JP, Remmelink, M, Baan, J, Jr., et al. Safety and feasibility of elective high-risk percutaneous coronary intervention procedures with left ventricular support of the Impella Recover LP 2. 5. Am J Cardiol. 2006; 97:990–992.
211. Lee, MS, Makkar, RR. Percutaneous left ventricular support devices. Cardiol Clin. 2006; 24:265–275.
212. Remmelink, M, Sjauw, KD, Henriques, JP, et al. Effects of mechanical left ventricular unloading by Impella on left ventricular dynamics in high-risk and primary percutaneous coronary intervention patients. Catheter Cardiovasc Intervent. 2010; 75:187–194.
213. Sjauw, KD, Konorza, T, Erbel, R, et al. Supported high-risk percutaneous coronary intervention with the Impella 2. 5 device the Europella registry. J Am Coll Cardiol. 2009; 54:2430–2434.
214. Seyfarth, M, Sibbing, D, Bauer, I, et al. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J Am Coll Cardiol. 2008; 52:1584–1588.
215. O’Neill, WW, Kleiman, NS, Moses, J, et al. A prospective randomized clinical trial of hemodynamic support with impella 2. 5TM versus intra-aortic balloon pump in patients undergoing high-risk percutaneous coronary intervention: The PROTECT II Study. Circulation. 2012; 126(14):1717–1727.
216. Cheng, JM, den Uil, CA, Hoeks, SE, et al. Percutaneous left ventricular assist devices vs. intra-aortic balloon pump counterpulsation for treatment of cardiogenic shock: A meta-analysis of controlled trials. Eur Heart J. 2009; 30:2102–2108.
217. La Torre, MW, Centofanti, P, Attisani, M, et al. Posterior ventricular septal defect in presence of cardiogenic shock: Early implantation of the Impella Recover LP 5. 0 as a bridge to surgery. Tex Heart Inst J. 2011; 38:42–49.
218. Kiernan, MS, Krishnamurthy, B, Kapur, NK. Percutaneous right ventricular assist via the internal jugular vein in cardiogenic shock complicating an acute inferior myocardial infarction. J Invasive Cardiol. 2010; 22:E23–E26.
219. Collart, F, Kerbaul, F, Mekkaoui, C, et al. Balloon-pump-induced pulsatility improves coronary and carotid flows in an experimental model of BioMedicus left ventricular assistance. Artif Organs. 2004; 28:743–746.
220. Drakos, SG, Charitos, CE, Ntalianis, A, et al. Comparison of pulsatile with nonpulsatile mechanical support in a porcine model of profound cardiogenic shock. ASAIO J. 2005; 51:26–29.