Intestinal Protozoa
1. Describe the basic life cycle, distinguishing morphologic characteristics, clinical disease (if pathogenic), laboratory diagnosis, and prevention for the organisms listed in the following table.
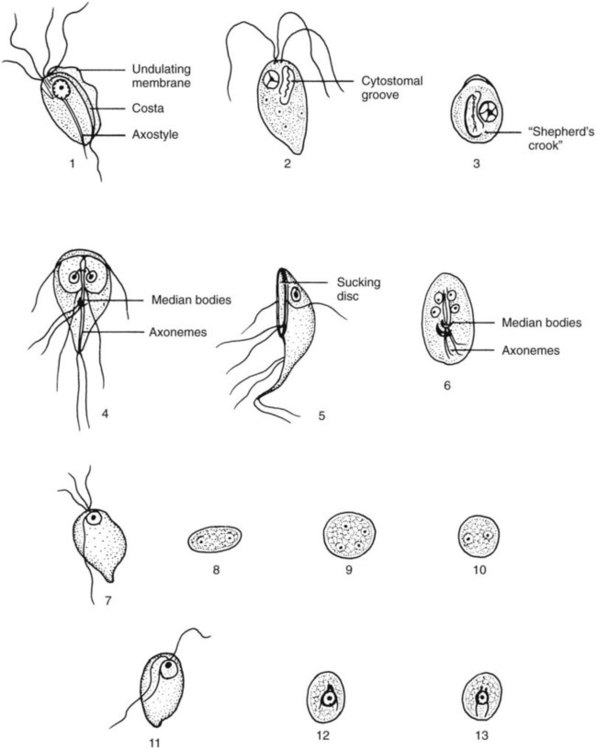
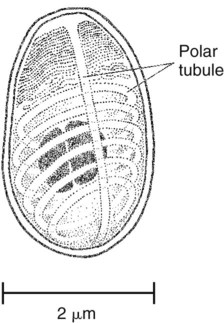
2. Define and identify the following parasitic structures: trophozoite, cyst, oocyst, spore, pseudopodia, flagella, cilia, chromatoidal bars, karysome, central vacuole cyst form, axoneme, cytosotome, spiral groove, undulating membrane, ventral disc, shepherd’s crook, axostyle, macronucleus, micronucleus, apical complex, sporocyst, sporozoite, spore, sarcocyst, and polar tubule.
3. Define life cycle processes, including merogony, gametogony, sporogony, schizogony, and the associated organism or organisms and associated stages.
4. Correlate the parasitic life cycles with the specific diagnostic stages for the organisms listed.
The important characteristics of the intestinal protozoa are presented in Tables 48-1 to 48-7. The clinically relevant intestinal protozoa are generally considered to be Entamoeba histolytica, Blastocystis hominis, Giardia lamblia, Dientamoeba fragilis, Balantidium coli, Isospora (Cystoisospora) belli, Cryptosporidium spp., Cyclospora cayetanensis, and the microsporidia. Nonpathogenic intestinal protozoa are listed in various figures and tables but are not discussed in detail.
TABLE 48-1
Intestinal Protozoa: Trophozoites of Common Amebae
| Characteristic | Entamoeba histolytica | Entamoeba dispar | Entamoeba hartmanni | Entamoeba coli | Endolimax nana | Iodamoeba bütschlii |
| Size* (diameter or length) | 12-60 µm (usual range, 15-20 µm); invasive forms may be > 20 µm | Same size range as E. histolytica | 5-12 µm (usual range, 8-10 µm) | 15-50 µm (usual range, 20-25 µm) | 6-12 µm (usual range, 8-10 µm) | 8-20 µm (usual range, 12-15 µm) |
| Motility | Progressive, with hyaline, finger-like pseudopodia; motility may be rapid | Same motility as E. histolytica | Usually nonprogressive | Sluggish, nondirectional; blunt, granular pseudopodia | Sluggish, usually nonprogressive | Sluggish, usually nonprogressive |
| Nucleus (single) and visibility | Difficult to see in unstained preparations | Difficult to see in unstained preparations | Usually not seen in unstained preparations | Often visible in unstained preparation | Occasionally visible in unstained preparations | Usually not visible in unstained preparations |
| Peripheral chromatin (stained) | Fine granules, uniform in size and usually evenly distributed; may have beaded appearance | Fine granules, uniform in size and usually evenly distributed; may have beaded appearance | Nucleus may stain more darkly than in E. histolytica, although morphology is similar; chromatin may appear as solid ring rather than beaded (trichrome) | May be clumped and unevenly arranged on the membrane; may also appear as solid, dark ring with no beads or clumps | Usually no peripheral chromatin; nuclear chromatin may be quite variable | Usually no peripheral chromatin |
| Karyosome (stained) | Small, usually compact; centrally located but may also be eccentric | Small, usually compact; centrally located but may also be eccentric | Usually small and compact; may be centrally located or eccentric | Large, not compact; may or may not be eccentric; may be diffuse and darkly stained | Large, irregularly shaped; may appear blotlike; many nuclear variations are common; may mimic E. hartmanni or Dientamoeba fragilis | Large, may be surrounded by refractile granules that are difficult to see (“basket nucleus”) |
| Cytoplasm appearance (stained) | Finely granular, “ground glass” appearance; clear differentiation of ectoplasm and endoplasm; if present, vacuoles are usually small | Finely granular, “ground glass” appearance; clear differentiation of ectoplasm and endoplasm; if present, vacuoles are usually small | Finely granular | Granular, with little differentiation into ectoplasm and endoplasm; usually vacuolated | Granular, vacuolated | Granular, may be heavily vacuolated |
| Inclusions (stained) | Noninvasive organism may contain bacteria; presence of red blood cells (RBCs) is diagnostic; presence of RBCs is the only characteristic that allows differentiation between pathogenic E. histolytica and nonpathogenic E. dispar | Organisms usually contain bacteria; RBCs not present in cytoplasm | May contain bacteria; no RBCs | Bacteria, yeast, other debris | Bacteria | Bacteria |
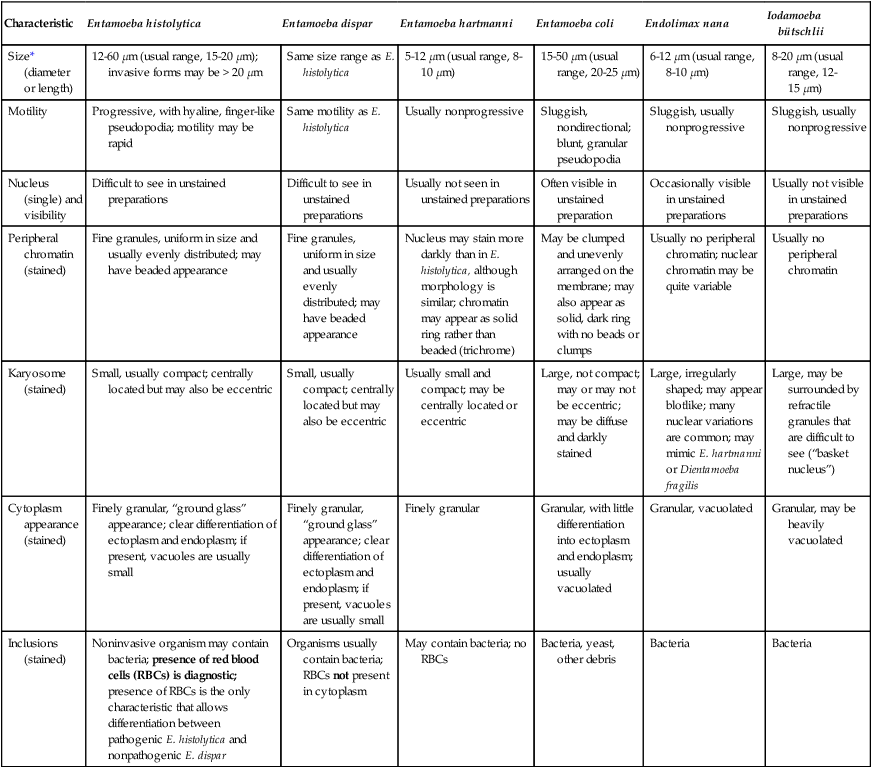
*These sizes refer to wet preparation measurements. Organisms on a permanent stained smear may be 1 to 1.5 µm smaller as a result of artificial shrinkage.
TABLE 48-2
Intestinal Protozoa—Cysts of Common Amebae
| Characteristic | Entamoeba Histolytica/Entamoeba dispar | Entamoeba hartmanni | Entamoeba coli | Endolimax nana | Iodamoeba bütschlii |
| Size* (diameter or length) | 10-20 µm (usual range, 12-15 µm) | 5-10 µm (usual range, 6-8 µm) | 10-35 µm (usual range, 15-25 µm) | 5-10 µm (usual range, 6-8 µm) | 5-20 µm (usual range, 10-12 µm) |
| Shape | Usually spherical | Usually spherical | Usually spherical; may be oval, triangular, or other shapes; may be distorted on permanent stained slide because of inadequate fixative penetration | Usually oval, may be round | May vary from oval to round; cyst may collapse because of large glycogen vacuole space |
| Nucleus (number and visibility) | Mature cyst: 4 nuclei Immature cyst: 1-2 nuclei; nuclear characteristics difficult to see on wet preparation |
Mature cyst: 4 nuclei; Immature cyst: 1-2 nuclei (2-nucleated cysts very common) | Mature cyst: 8 (occasionally 16 or more nuclei may be seen) Immature cysts with 2 or more nuclei are occasionally seen | Mature cyst: 4 Immature cysts: 2 Very rarely seen and may resemble cysts of Enteromonas hominis | Mature cyst: 1 |
| Peripheral chromatin (stained) | Peripheral chromatin present; fine, uniform granules, evenly distributed; nuclear characteristics may not be as clearly visible as in trophozoite | Fine granules evenly distributed on the membrane; nuclear characteristics may be difficult to see | Coarsely granular; may be clumped and unevenly arranged on membrane; nuclear characteristics not as clearly defined as in trophozoite; may resemble E. histolytica | No peripheral chromatin | No peripheral chromatin |
| Karyosome (stained) | Small, compact, usually centrally located but occasionally may be eccentric | Small, compact, usually centrally located | Large, may or may not be compact and/or eccentric; occasionally may be centrally located | Smaller than karyosome seen in trophozoites but generally larger than those of genus Entamoeba | Larger, usually eccentric refractile granules may be on one side of karyosome (“basket nucleus”) |
| Cytoplasm, chromatoidal bodies (stained) | May be present; bodies usually elongate, with blunt, rounded, smooth edges; may be round or oval | Usually present; bodies usually elongate with blunt, rounded, smooth edges; may be round or oval | May be present (less frequently than in E. histolytica); splinter shaped with rough, pointed ends | Rare chromatoidal bodies present; occasionally small granules or inclusions seen; fine linear chromatoidals may be faintly visible on well-stained smears | No chromatoidal bodies present; occasionally small granules may be present |
| Glycogen (stained with iodine) | May be diffuse or absent in mature cyst; clumped chromatin mass may be present in early cysts (stains reddish brown with iodine) | May or may not be present, as in E. histolytica | May be diffuse or absent in mature cyst; clumped mass occasionally seen in mature cysts (stains reddish brown with iodine) | Usually diffuse if present (stains reddish brown with iodine) | Large, compact, well-defined mass (stains reddish brown with iodine) |
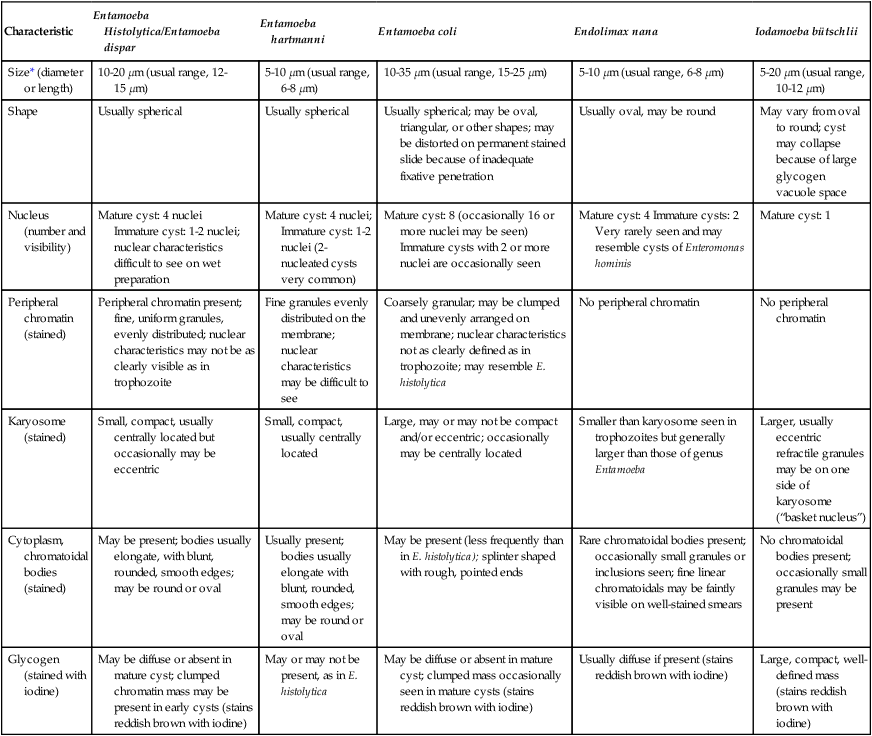
*Wet preparation measurements; in permanent stains, organisms usually are 1 to 2 µm smaller.
TABLE 48-3
Intestinal Protozoa—Trophozoites of Flagellates
| Protozoa | Shape and Size | Motility | Number of Nuclei and Visibility | Number of Flagella (Usually Difficult to See) | Other Features |
| Dientamoeba fragilis | Shaped like amebae; 5-15 µm (usual range, 9-12 µm) | Usually nonprogressive; pseudopodia are angular, serrated, or broad lobed and almost transparent | Percentage may vary, but 40% of organisms have 1 nucleus and 60% have 2 nuclei; not visible in unstained preparations; no peripheral chromatin; karyosome is composed of a cluster of 4-8 granules | Internal flagella; not visible | Cytoplasm finely granular and may be vacuolated with ingested bacteria, yeasts, and other debris; may be great variation in size and shape on a single smear |
| Giardia lamblia | Pear-shaped; length 10-20 µm; width, 5-15 µm | “Falling leaf” motility may be difficult to see if organism is in mucus; slight flutter of flagella may be visible using low light (duodenal aspirate or mucus from Entero-Test capsule) | 2; not visible in unstained mounts | 4 lateral; 2 ventral, 2 caudal | Sucking disk occupies one half to three fourths of ventral surface; pear-shaped front view, spoon-shaped side view |
| Chilomastix mesnili | Pear-shaped; length 6-24 µm (usual range, 10-15 µm); width, 4-8 µm | Stiff, rotary | 1; not visible in unstained mounts | 3 anterior, 1 in cytostome | Prominent cytostome extending one third to one half the length of the body; spiral groove across ventral surface |
| Pentatrichomonas hominis | Pear-shaped; length 5-15 µm (usual range, 7-9 µm); width 7-10 µm | Jerky, rapid | 1; not visible in unstained mounts | 3-5 anterior, 1 posterior | Undulating membrane extends the length of the body; posterior flagellum extends free beyond end of body |
| Trichomonas tenax | Pear shaped; length 5-12 µm; average of 6.5-7.5 µm; width, 7-9 µm | Jerky, rapid | 1; not visible in unstained mounts | 4 anterior, 1 posterior | Seen only in preparations from mouth; axostyle (slender rod) protrudes beyond the posterior end and may be visible; posterior flagellum extends only halfway down the body; no free end |
| Enteromonas hominis | Oval; 4-10 µm (usual range, 8-9 µm); width, 5-6 µm | Jerky | 1; not visible in unstained mounts | 3 anterior, 1 posterior | One side of the body is flattened; posterior flagellum extends free posteriorly or laterally |
| Retortamonas intestinalis | Pear-shaped or oval; 4-9 µm (usual range, 6-7 µm); width, 3-4 µm | Jerky | 1; not visible in unstained mount | 1 anterior, 1 posterior | Prominent cytostome extends approximately one half the length of the body |
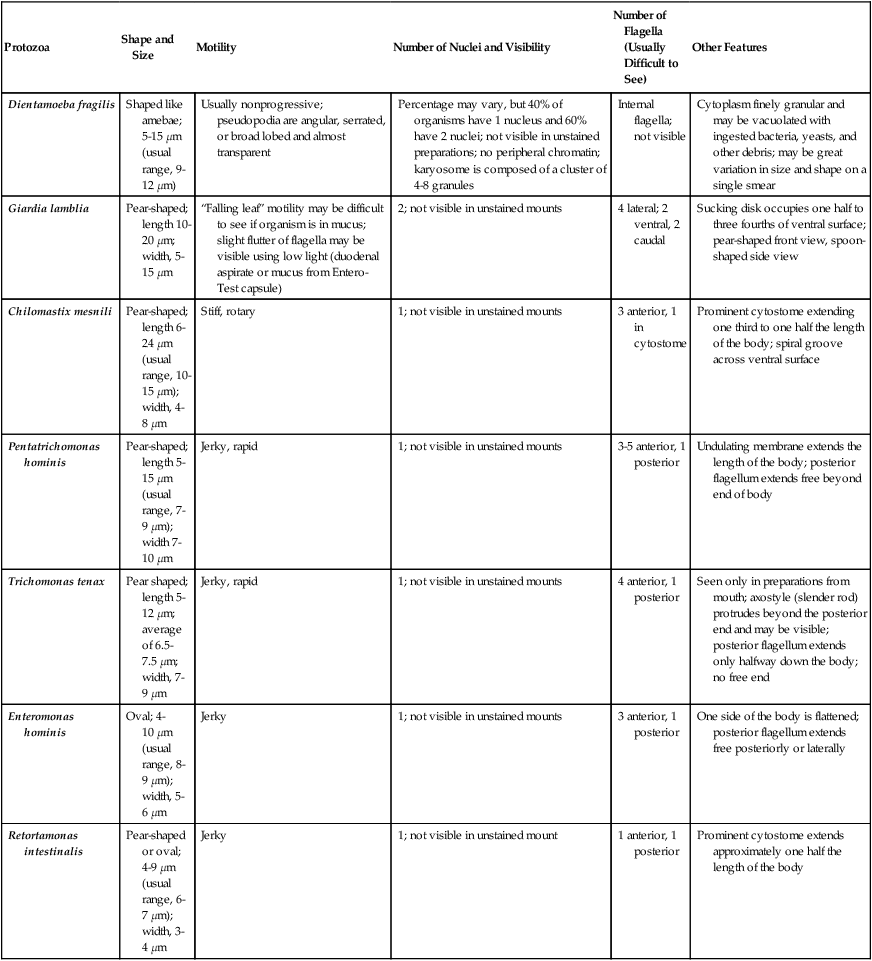
TABLE 48-4
Intestinal Protozoa—Cysts of Flagellates
| Protozoa | Size | Shape | Number of Nuclei | Other Features |
| Dientamoeba fragilis, Pentatrichomonas hominis, Trichomonas tenax | No cyst stage | |||
| Giardia lamblia | 8-19 µm (usual range, 11-14 µm); width, 7-10 µm | Oval, ellipsoidal, or may appear round | 4; not distinct in unstained preparations; usually located at one end | Longitudinal fibers in cysts may be visible in unstained preparations; deep staining median bodies usually lie across the longitudinal fibers. Shrinkage is common, with the cytoplasm pulling away from the cyst wall; “halo” effect may be seen around the outside of the cyst wall because of shrinkage caused by dehydrating reagents |
| Chilomastix mesnili | 6-10 µm (usual range, 7-9 µm); width, 4-6 µm | Lemon or pear shaped with anterior hyaline knob | 1; not distinct in unstained preparations | Cytostome with supporting fibrils, usually visible in stained preparation; curved fibril along side of cytostome, usually referred to as a “shepherd’s crook” |
| Enteromonas hominis | 4-10 µm (usual range, 6-8 µm); width, 4-6 µm | Elongate or oval | 1-4; usually 2 lying at opposite ends of cyst; not visible in unstained mounts | Resembles Endolimax nana cyst; fibrils or flagella usually not seen |
| Retortamonas intestinalis | 4-9 µm (usual range, 4-7 µm); width, 5 µm | Pear shaped or slightly lemon shaped | 1; not visible in unstained mounts | Resembles Chilomastix cyst; shadow outline of cytostome with supporting fibrils extends above nucleus; “bird beak” fibril arrangement |
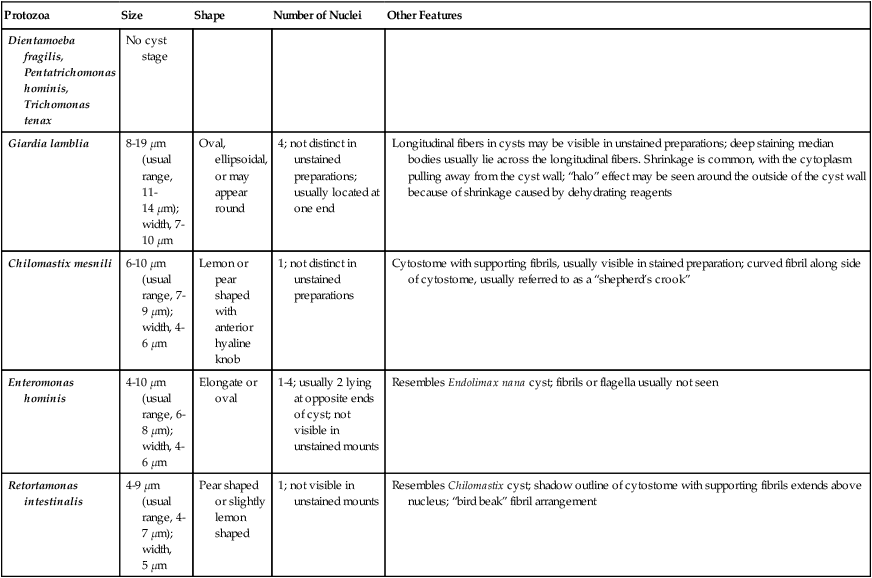
TABLE 48-5
| Protozoa | Shape and Size | Motility | Number of Nuclei | Other Features |
| Balantidium coli trophozoite |
Ovoid with tapering anterior end; 50-100 µm long, 40-70 µm wide (usual range, 40-50 µm) | Ciliates: rotary, boring; may be rapid | 1 large kidney-shaped macronucleus; 1 small round micronucleus, which is difficult to see even in stained smear; macronucleus may be visible in unstained preparation | Body covered with cilia, which tend to be longer near cytostome; cytoplasm may be vacuolated |
| Cyst | Spherical or oval; 50-70 µm (usual range, 50-55 µm) | 1 large macronucleus visible in unstained preparation; micronucleus difficult to see | Macronucleus and contractile vacuole are visible in young cysts; in older cysts, internal structure appears granular; cilia difficult to see in cyst wall |
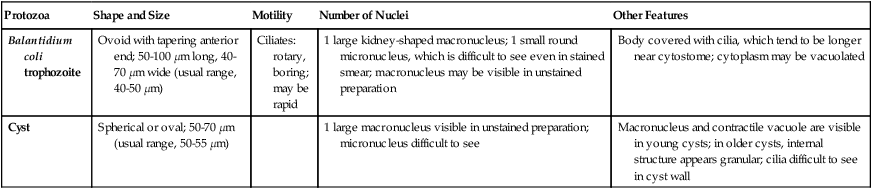
TABLE 48-6
Morphologic Criteria Used to Identify Intestinal Protozoa (Coccidia, Blastocystis hominis)
| Protozoa | Shape and Size | Other Features |
| Cryptosporidium spp. C. parvum (humans and animals) C. hominis (humans) |
Oocyst generally round, 4-6 µm; each mature oocyst contains four sporozoites | Oocyst, diagnostic stage in stool, sporozoites occasionally visible within oocyst wall; acid-fast positive using modified acid-fast stains; various other stages in life cycle can be seen in biopsy specimens taken from gastrointestinal tract (brush border of epithelial cells) and other tissues; disseminated infection well documented in compromised host; oocysts immediately infective (in both formed and/or watery specimens); nosocomial infections documented; use enteric precautions for inpatients. |
| Cyclospora cayetanensis | Oocyst generally round, 8-10 µm; oocysts are not mature, no visible internal structure; oocysts may appear wrinkled | Oocyst, diagnostic stage in stool; acid-fast variable using modified acid-fast stains; color range from clear to deep purple (tremendous variation); best results obtained with decolorizing solution consisting of 1% acid, 3% maximum; oocysts may appear wrinkled (like crumpled cellophane); mimic Cryptosporidium oocysts but are twice as large. |
| Isospora (Cystoisospora) belli | Ellipsoidal oocyst; range 20-30 µm long, 10-19 µm wide; sporocysts rarely seen broken out of oocysts but measure 9-11µm | Mature oocyst contains two sporocysts with four sporozoites each; usual diagnostic stage in feces is immature oocyst containing spherical mass of protoplasm (intestinal tract). Oocysts are modified acid-fast positive. Whole oocyst may stain pink, but just the internal sporocysts stain if the oocyst is mature. |
| Sarcocystis hominis S. suihominis S. bovihominis |
Oocyst thin-walled and contains two mature sporocysts, each containing four sporozoites; frequently thin oocyst wall ruptures; ovoid sporocysts each measure 10-16 µm long and 7.5-12 µm wide | Thin-walled oocyst or ovoid sporocysts occur in stool (intestinal tract) |
| S. “lindemanni” | Shapes and sizes of skeletal and cardiac muscle sarcocysts vary considerably | Sarcocysts contain several hundred to several thousand trophozoites, each measuring 12-16µm long and 4-9µm wide. Sarcocysts may also be divided into compartments by septa, which are not seen in Toxoplasma cysts (tissue/muscle). |
| Blastocystis hominis | Organisms are generally round, measure approximately 6-40 µm, and are usually characterized by a large, central body (looks like a large vacuole); this stage has been called the central body form | The more amebic form can be seen in diarrheal fluid but is difficult to identify. The central body forms vary tremendously in size, even on a single fecal smear; this is the most common form seen. Routine fecal examinations may indicate a positive rate much higher than other protozoa; some laboratories report figures of 20% and higher. |
TABLE 48-7
Microsporidia That Cause Human Infection
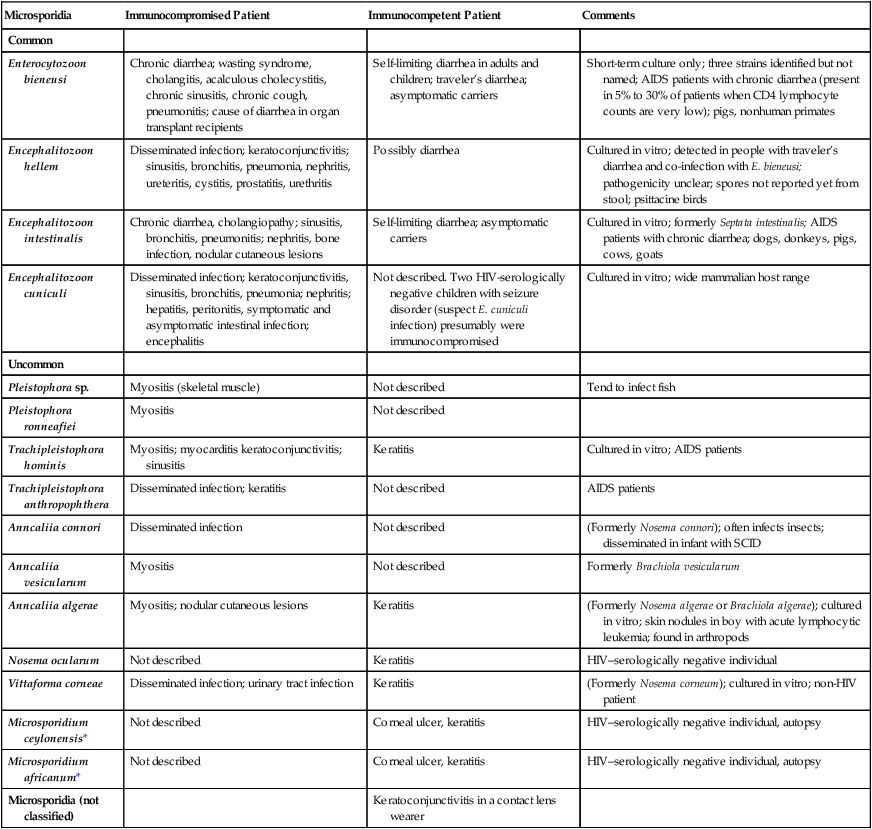
| Microsporidia | Immunocompromised Patient | Immunocompetent Patient | Comments |
| Common | |||
| Enterocytozoon bieneusi | Chronic diarrhea; wasting syndrome, cholangitis, acalculous cholecystitis, chronic sinusitis, chronic cough, pneumonitis; cause of diarrhea in organ transplant recipients | Self-limiting diarrhea in adults and children; traveler’s diarrhea; asymptomatic carriers | Short-term culture only; three strains identified but not named; AIDS patients with chronic diarrhea (present in 5% to 30% of patients when CD4 lymphocyte counts are very low); pigs, nonhuman primates |
| Encephalitozoon hellem | Disseminated infection; keratoconjunctivitis; sinusitis, bronchitis, pneumonia, nephritis, ureteritis, cystitis, prostatitis, urethritis | Possibly diarrhea | Cultured in vitro; detected in people with traveler’s diarrhea and co-infection with E. bieneusi; pathogenicity unclear; spores not reported yet from stool; psittacine birds |
| Encephalitozoon intestinalis | Chronic diarrhea, cholangiopathy; sinusitis, bronchitis, pneumonitis; nephritis, bone infection, nodular cutaneous lesions | Self-limiting diarrhea; asymptomatic carriers | Cultured in vitro; formerly Septata intestinalis; AIDS patients with chronic diarrhea; dogs, donkeys, pigs, cows, goats |
| Encephalitozoon cuniculi | Disseminated infection; keratoconjunctivitis, sinusitis, bronchitis, pneumonia; nephritis; hepatitis, peritonitis, symptomatic and asymptomatic intestinal infection; encephalitis | Not described. Two HIV-serologically negative children with seizure disorder (suspect E. cuniculi infection) presumably were immunocompromised | Cultured in vitro; wide mammalian host range |
| Uncommon | |||
| Pleistophora sp. | Myositis (skeletal muscle) | Not described | Tend to infect fish |
| Pleistophora ronneafiei | Myositis | Not described | |
| Trachipleistophora hominis | Myositis; myocarditis keratoconjunctivitis; sinusitis | Keratitis | Cultured in vitro; AIDS patients |
| Trachipleistophora anthropophthera | Disseminated infection; keratitis | Not described | AIDS patients |
| Anncaliia connori | Disseminated infection | Not described | (Formerly Nosema connori); often infects insects; disseminated in infant with SCID |
| Anncaliia vesicularum | Myositis | Not described | Formerly Brachiola vesicularum |
| Anncaliia algerae | Myositis; nodular cutaneous lesions | Keratitis | (Formerly Nosema algerae or Brachiola algerae); cultured in vitro; skin nodules in boy with acute lymphocytic leukemia; found in arthropods |
| Nosema ocularum | Not described | Keratitis | HIV–serologically negative individual |
| Vittaforma corneae | Disseminated infection; urinary tract infection | Keratitis | (Formerly Nosema corneum); cultured in vitro; non-HIV patient |
| Microsporidium ceylonensis* | Not described | Corneal ulcer, keratitis | HIV–serologically negative individual, autopsy |
| Microsporidium africanum* | Not described | Corneal ulcer, keratitis | HIV–serologically negative individual, autopsy |
| Microsporidia (not classified) | Keratoconjunctivitis in a contact lens wearer |
AIDS, Acquired immunodeficiency syndrome; HIV, human immunodeficiency virus; SCID, severe combined immunodeficiency
*Microsporidium is a collective generic name for microsporidia that cannot be classified
Amebae
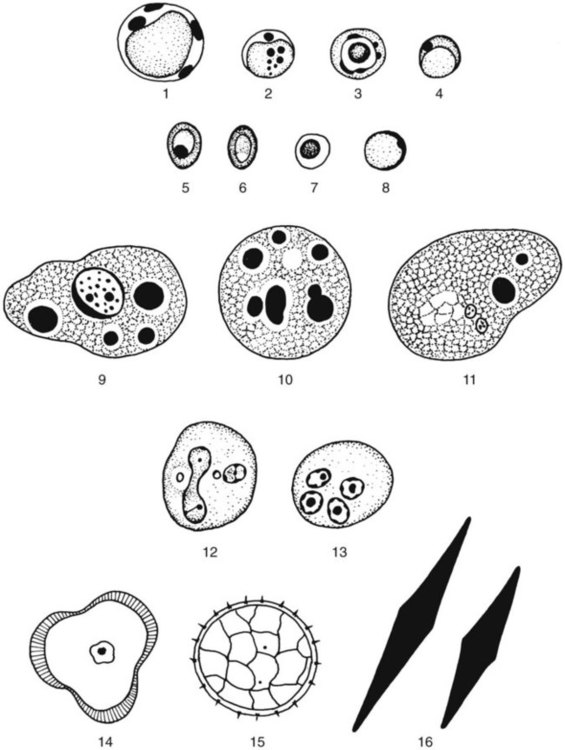
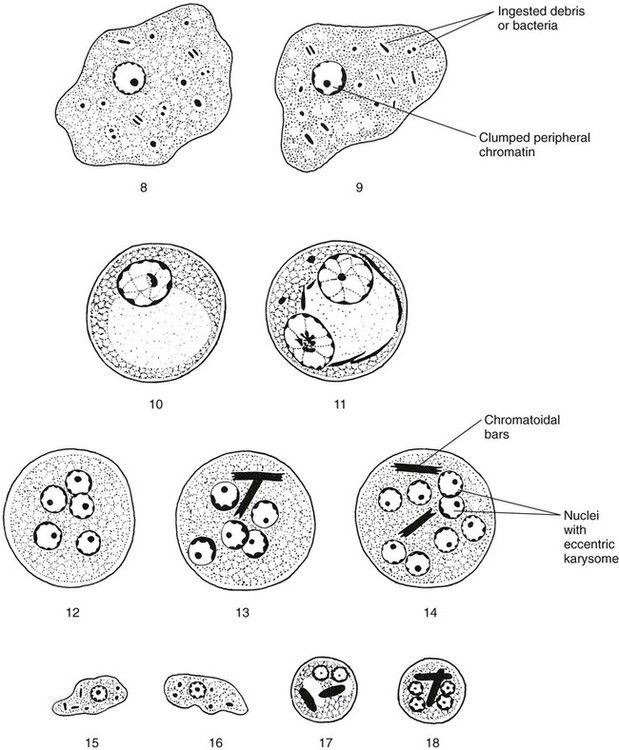
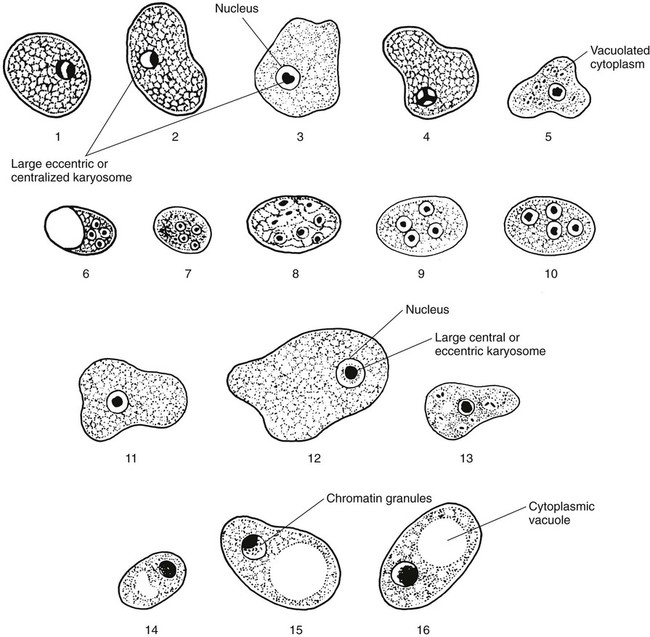
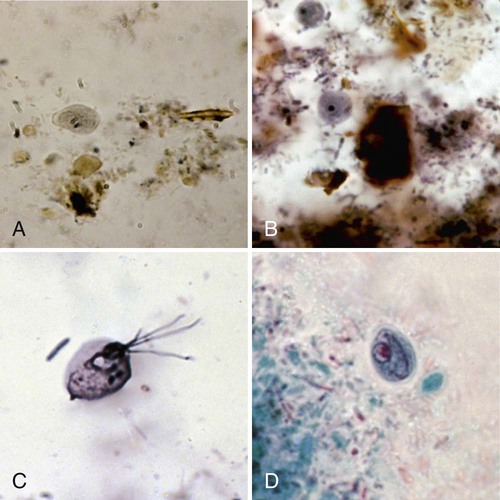
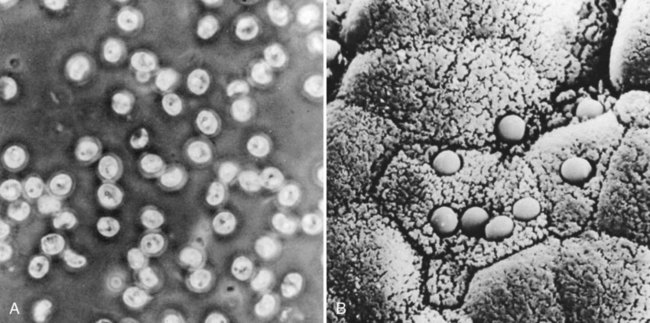
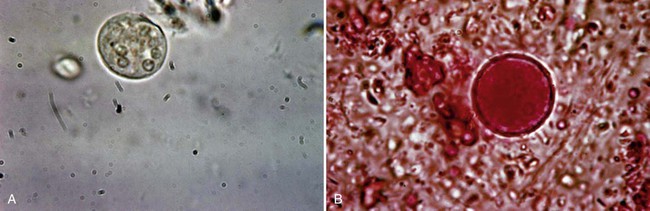
The class Sarcodina, or Amebae, includes the organisms capable of movement by means of cytoplasmic protrusions called pseudopodia. This group includes free-living organisms, in addition to nonpathogenic and pathogenic organisms found in the intestinal tract and other areas of the body (see Tables 48-1 and 48-2). Occasionally, when fresh stool material is examined as a direct wet mount, motile trophozoites may be seen, as well as other, nonparasitic structures (Figure 48-1).
Entamoeba histolytica
General Characteristics
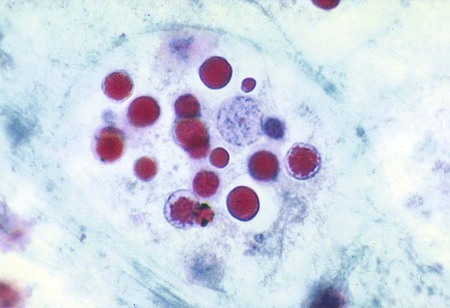
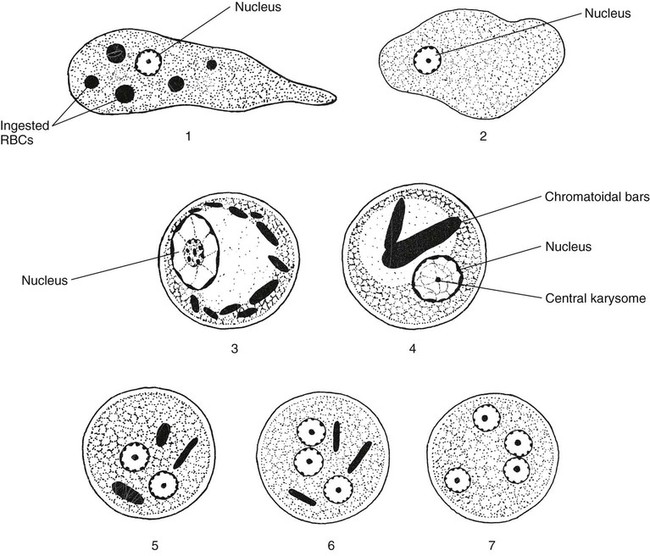
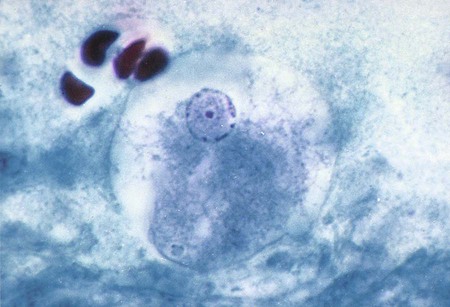
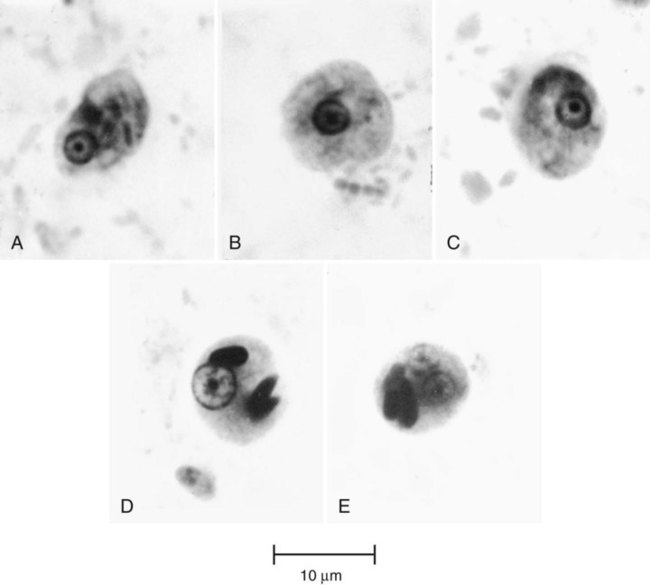
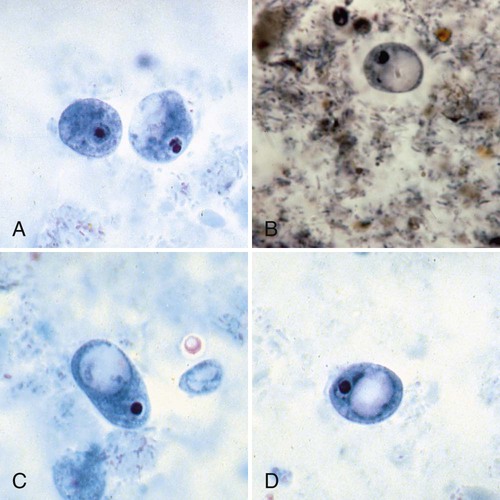
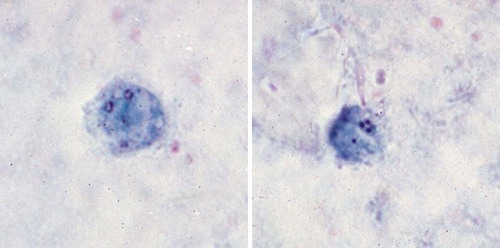
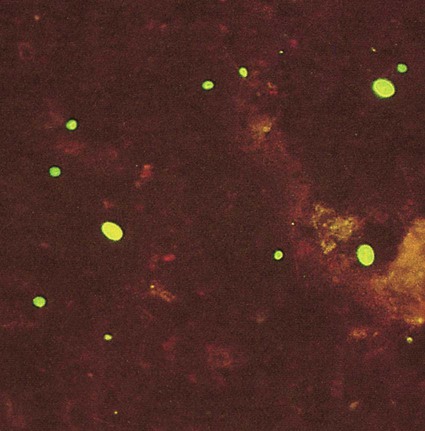
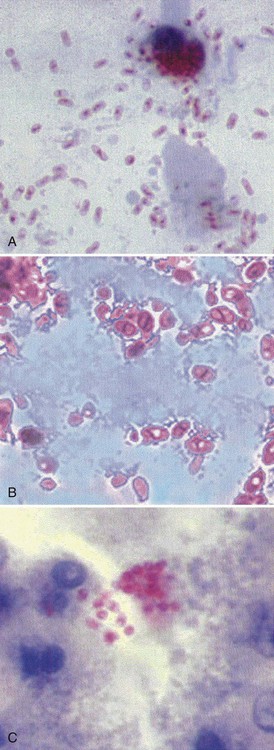
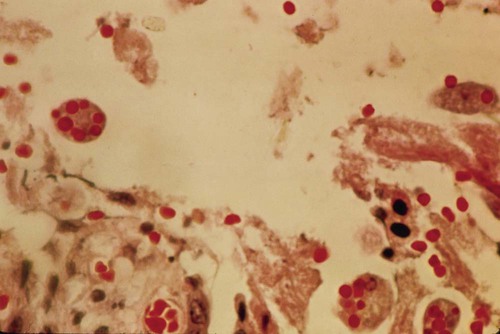
E. histolytica has directional and progressive motility, whereas the other amebae tend to move more slowly and at random. However, motility is rarely seen even in a fresh wet mount from a patient with diarrhea or dysentery. The cytoplasm is generally more finely granular, and the presence of red blood cells (RBCs) in the cytoplasm is considered diagnostic for E. histolytica (Figure 48-2).
Permanent stained smears demonstrate accurate morphology compared with other techniques. When the organism is examined on a permanent stained smear (trichrome or iron-hematoxylin stain), the morphologic characteristics of E. histolytica/E. dispar are readily seen. The nucleus is characterized by evenly arranged chromatin on the nuclear membrane and a small, compact, centrally located karyosome (condensed chromatin). As mentioned, the cytoplasm usually is described as finely granular, with few ingested bacteria and scant debris in vacuoles. As stated previously, in organisms isolated from a patient with dysentery, RBCs may be visible in the cytoplasm, a feature diagnostic for E. histolytica (Figure 48-3). Most often, infection with E. histolytica is diagnosed on the basis of the organism’s morphology, without the presence of RBCs.
As part of the life cycle, the trophozoites may condense into a round mass (precyst), and a thin wall is secreted around the immature cyst. Two types of inclusions may be found in this immature cyst: a glycogen mass and highly refractile chromatoidal bars (refractile chromatin structure) with smooth, rounded edges. As the cyst matures (metacyst) (see Figure 48-3; Figure 48-4), nuclear division occurs, with the production of four nuclei. Often chromatoidals may be absent in the mature cyst. Cyst morphology does not differentiate E. histolytica from E. dispar. Cyst formation occurs only in the intestinal tract; once the stool has left the body, cyst formation does not occur. The one-, two-, and four-nucleated cysts are infective and represent the mode of transmission from one host to another.
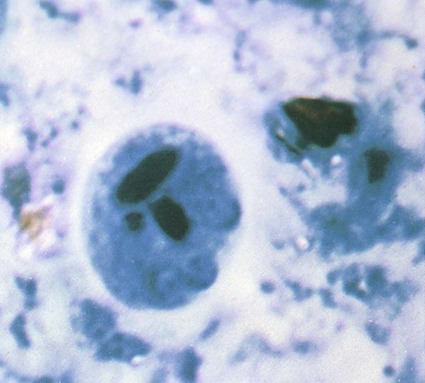
Epidemiology
Amebiasis is caused by infection with the true pathogen, Entamoeba histolytica. Recent evidence from molecular studies confirms the differentiation of pathogenic E. histolytica and nonpathogenic E. dispar (Figure 48-5) as two distinct species. E. histolytica is considered the etiologic agent of amebic colitis and extraintestinal abscesses (amebic liver abscess), whereas nonpathogenic E. dispar produces no intestinal symptoms and is not invasive in humans.
Entamoeba coli
General Characteristics
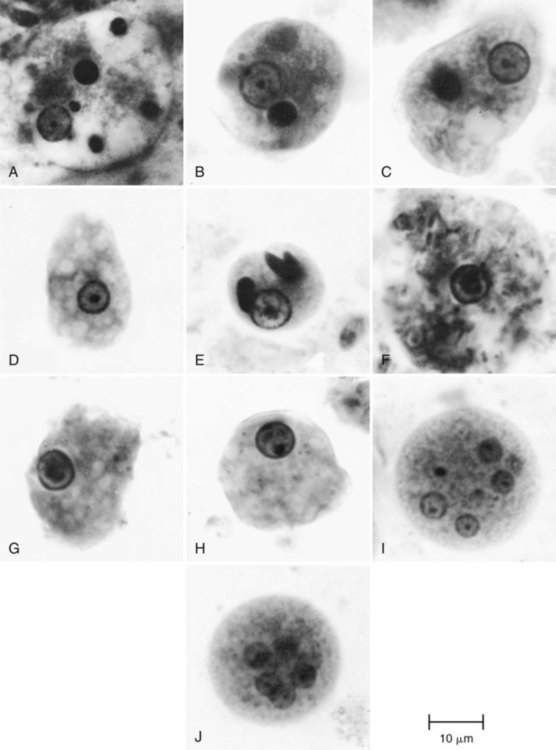
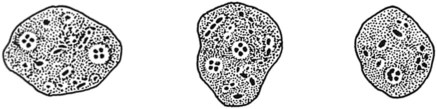
E. coli trophozoites are somewhat larger than those of E. histolytica and E. dispar and range from 15 to 50 µm in diameter (see Table 48-1; Figures 48-6 and 48-7; see Figure 48-3). Motility is sluggish with broad, short pseudopods. In wet preparations, differentiating nonpathogenic E. coli from pathogenic E. histolytica is almost impossible. On the permanent stained smear viewed at a higher magnification, the cytoplasm is granular with vacuoles containing bacteria, yeasts, and other food materials. The nucleus has a large blotlike karyosome that may be eccentric rather than centrally located. The chromatin on the nuclear membrane tends to be clumped and irregular. Although rare, if RBCs are present in the intestinal tract, E. coli may ingest them rather than bacteria.
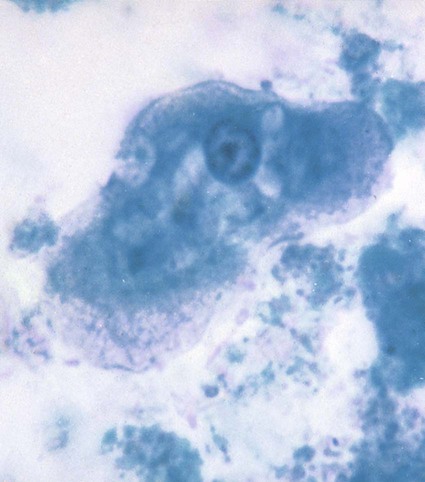
Early cysts often contain chromatoidal bars, which tend to be splinter shaped and irregular. Eventually, the nuclei divide until the mature cyst, containing eight nuclei, is formed (see Table 48-2; see Figures 48-3 and 48-6). In rare cases, the number of nuclei reaches 16. The cysts measure 10 to 35 µm in diameter, and as they mature, the chromatoidal bars disappear. When the cyst of E. coli matures, it becomes more refractive to fixation; therefore, the cyst may be seen on the wet preparation but not on the permanent stained smear. Occasionally, on trichrome smears, the cysts appear distorted and somewhat pink (Figure 48-8).
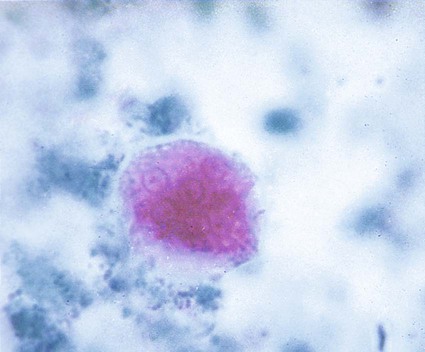
Entamoeba hartmanni
General Characteristics
The life cycle of E. hartmanni is similar to that of E. dispar, with differences in size (Figures 48-9 and 48-10). In wet preparations, E. hartmanni trophozoites range in size from 4 to 12 µm in diameter, and cysts range in size from 5 to 10 µm in diameter. On the permanent stained smear, the cysts, primarily, tend to shrink as a result of dehydration; therefore, the sizes of all the organisms, including pathogenic E. histolytica, may be somewhat smaller (1 to 1.5 µm) than the wet preparation measurements.
Trophozoites do not ingest RBCs, and the motility is usually less rapid (see Table 48-1; see Figures 48-3, 48-9, and 48-10). The morphologic characteristics of E. hartmanni are very similar to those of E. histolytica, with two exceptions. Frequently, E. hartmanni cysts may contain only one or two nuclei, even though the mature cyst contains four nuclei. Mature cysts of E. hartmanni also retain their chromatoidal bars, a characteristic not usually seen in E. histolytica/E. dispar. E. hartmanni’s chromatoidal bars are similar to those of E. histolytica and E. dispar but smaller and more numerous (see Table 48-2; see Figures 48-9 and 48-10). At the species level, differentiation between E. hartmanni and E. histolytica/E. dispar depends on size; therefore, laboratories are required to use calibrated microscopes that are checked periodically for accuracy.
Endolimax nana
General Characteristics
E. nana has the same life cycle stages as E. dispar and the other nonpathogenic amebae. The trophozoite usually measures 6 to 12 µm in diameter (normal range, 8 to 10 µm) (see Table 48-1; Figures 48-11 to 48-13). Although rarely seen, motility is sluggish and nonprogressive with blunt, hyaline pseudopods. In the permanent stained smear, normally no peripheral chromatin is seen on the nuclear membrane, and the karyosome is large, with either a central or an eccentric location in the nucleus (see Figures 48-12 and 48-13). E. nana shows more nuclear variation than any of the other amebae, and occasionally E. nana can mimic D. fragilis or E. hartmanni. The cytoplasm may have small vacuoles containing ingested debris or bacteria, but it also may appear relatively clean.
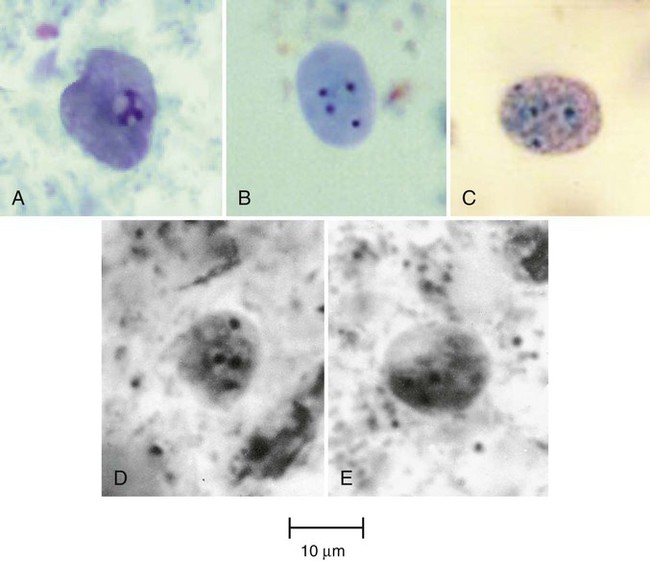
Cysts usually measure 5 to 10 µm in diameter (normal range, 6 to 8 µm) (see Table 48-2). Cysts as large as 14 µm have been seen. The cyst is usually oval to round, with the mature cyst containing four nuclei. The nuclei typically have no peripheral chromatin and are somewhat evenly distributed in the cyst. Occasionally, very small, slightly curved chromatoidal bars are present. The two-nucleated stage is not commonly seen, and frequently both trophozoites and cysts are present in clinical specimens.
Iodamoeba bütschlii
General Characteristics
The life cycle stages of I. bütschlii are exactly the same as those of E. nana. The trophozoite varies from 8 to 20 µm in diameter and has fairly active motility in a fresh stool preparation (see Table 48-1). The cytoplasm is granular, containing numerous vacuoles with ingested debris and bacteria. The cytoplasm is more vacuolated than in E. nana trophozoites. The nucleus has a large karyosome, which can be either centrally located or eccentric (Figures 48-14 and 48-15). On the permanent stained smear, the nucleus may appear to have a halo, and chromatin granules fan out around the karyosome. If the granules are on one side, the nucleus may appear to have a “basket nucleus” arrangement of chromatin, more commonly seen in the cyst stage. The trophozoites of I. bütschlii and E. nana may appear similar and are difficult to differentiate at the species level, even on the permanent stained smear. Both organisms are considered nonpathogenic. E. nana is recovered in clinical specimens much more frequently than is I. bütschlii.
I. bütschlii cysts are round to oval (see Table 48-2). The glycogen vacuole is so large that occasionally the cyst collapses on itself. Because nuclear multiplication does not occur in the cyst form, the mature cyst contains a single nucleus. The cysts measure approximately 5 to 20 µm in diameter and are rarely confused with those of other amebae (see Figures 48-14 and 48-15).
Blastocystis hominis
General Characteristics
Blastocystis hominis (see Figure 48-1; see Table 48-6) comprises a number of different strains that are indistinguishable morphologically; some of which are pathogenic, and some are nonpathogenic. Although usually listed with the amebae, the organism’s classification is still under review; different strains eventually may be classified as different species. Although the true role of this organism in terms of disease has been controversial, it is now generally considered a causative agent of intestinal disease. The current recommendation is to report the presence of B. hominis and quantitate from the permanent stained smear (i.e., rare, few, moderate, many, packed); this information may be valuable in helping to assess the pathogenicity of the organism in the individual patient.
Flagellates
Four common species of flagellates are found in the intestinal tract: Giardia lamblia, Dientamoeba fragilis, Chilomastix mesnili, and Pentatrichomonas hominis (Figures 48-16 to 48-23) (see Tables 48-3 and 48-4). Several other smaller, nonpathogenic flagellates, such as Enteromonas hominis and Retortamonas intestinalis (see Figure 48-16), are rarely seen, and none are identified in the intestinal tract. The sucking disk and axonemes of G. lamblia, the cytostome and spiral groove of C. mesnili, and the undulating membrane of Trichomonas spp. are all distinctive criteria for identification (see Figures 48-16 to 48-23).
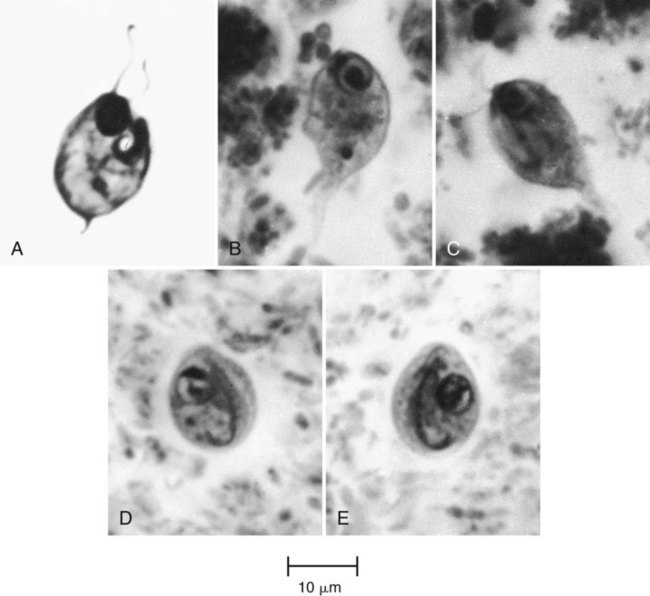
Giardia lamblia
General Characteristics
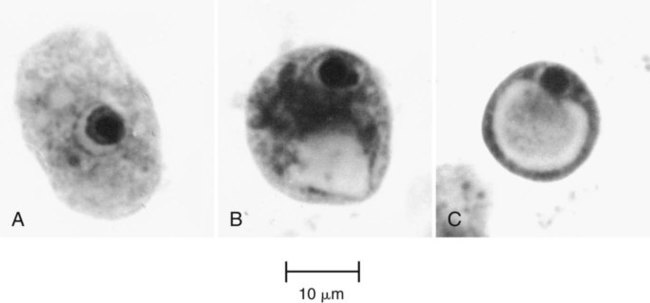
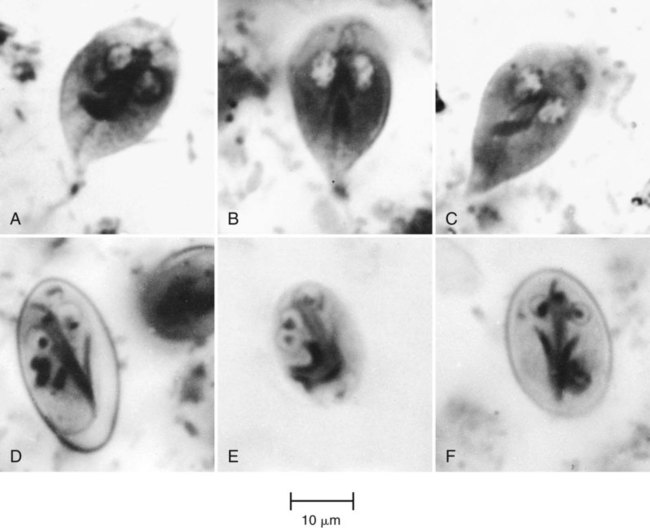
Both the trophozoite and cyst stages are included in the life cycle of G. lamblia. Trophozoites divide by means of longitudinal binary fission, producing two daughter trophozoites. The organism is found most commonly in the crypts in the duodenum. Trophozoites are the intestinal dwelling stage and attach to the epithelium of the host villi by means of the ventral disk. The attachment is substantial and results in disk “impression prints” when the organism detaches from the surface of the epithelium. Trophozoites may remain attached to or may detach from the mucosal surface. Because the epithelial surface sloughs off the tip of the villus every 72 hours, the trophozoites apparently detach at that time. G. lamblia trophozoites are teardrop shaped and have been described as “someone looking at you” (see Figures 48-16 to 48-18).
Cyst formation takes place as the organisms move down through the jejunum after exposure to biliary secretions. The trophozoites retract the flagella into the axonemes, the cytoplasm becomes condensed, and the cyst wall is secreted (see Figures 48-16 to 48-18). As the cyst matures, the internal structures are doubled, so that when excystation occurs, the cytoplasm divides, producing two trophozoites. Excystation occurs in the duodenum or appropriate culture medium.
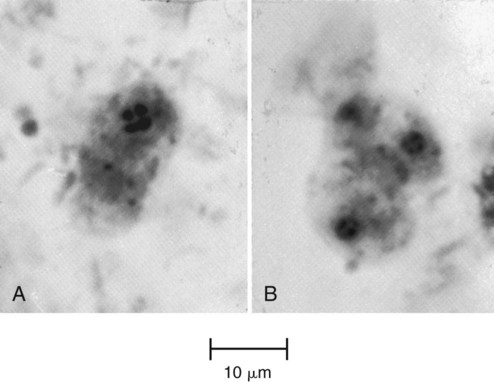
Chilomastix mesnili
General Characteristics
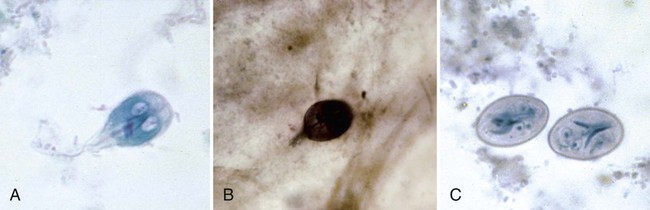
C. mesnili has both trophozoite and cyst stages and is somewhat more easily identified than are some of the smaller flagellates, such as E. hominis and R. intestinalis (see Tables 48-3 and 48-4; see Figure 48-19). The C. mesnili trophozoite is pear shaped, measuring 6 to 24 µm long and 4 to 8 µm wide. It has a single nucleus and a distinct oral groove, or cytostome (mouth), close to the nucleus. Flagella are difficult to see without obvious motility in a wet preparation. The morphology can be seen on the permanent stained smear; the cytostome may be visible in some trophozoites. The cysts are pear or lemon shaped and range from 6 to 10 µm long and 4 to 6 µm wide (see Figure 48-20). They have a single nucleus and a typical curved cytostomal fibril, called the shepherd’s crook. The cyst’s definitive morphology can be seen on a permanent stain.
Pentatrichomonas hominis
P. hominis is probably the most commonly identified flagellate, other than G. lamblia and D. fragilis. P. hominis has been recovered from all parts of the world, in both warm and temperate climates, and is considered nonpathogenic and noninvasive. It is not known to have a cyst stage (see Figure 48-16). P. hominis trophozoites live in the cecum and feed on bacteria. The trophozoite measures 5 to 15 µm long and 7 to 10 µm wide. It has a pyriform shape and has both an axostyle and an undulating membrane, which aid identification of the organism. The undulating membrane extends the entire length of the body, in contrast to that seen in the pathogen T. vaginalis (on which the membrane extends halfway down the body).
Epidemiology
Because P. hominis is not known to have a cyst stage, transmission probably occurs in the trophic form. If ingested in a substance such as milk, these organisms apparently can survive passage through the stomach and small intestine in patients with achlorhydria. P. hominis cannot be transplanted into the vagina, the natural habitat of T. vaginalis. The incidence of this organism is relatively low, but it tends to be recovered more often than E. hominis or R. intestinalis, two small nonpathogenic flagellates that are rarely seen and extremely difficult to identify (see Figure 48-16).
Ciliates
Balantidium coli
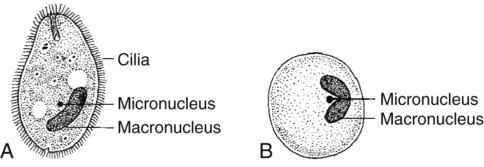
General Characteristics
The life cycle of B. coli includes both the trophozoite and cyst stages (Figures 48-24 and 48-25). The cyst form is the infective stage. After ingestion of the cysts and excystation, trophozoites secrete hyaluronidase, which aids the invasion of the colonic tissue.
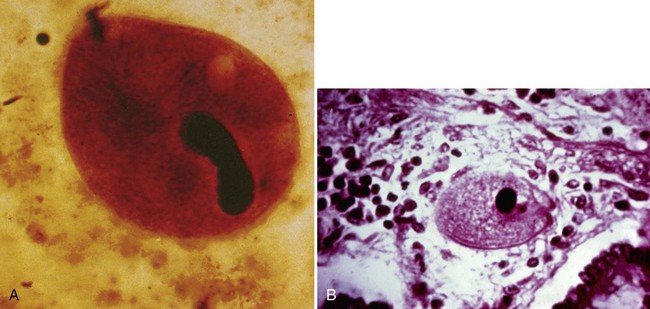
The trophozoite is quite large, oval, and covered with short cilia. It measures approximately 50 to 150 µm long and 40 to 70 µm wide. The organism can be seen in a wet preparation on lower power. The anterior end is somewhat pointed and has a cytostome (primitive mouth opening); in contrast, the posterior end is broadly rounded. The cytoplasm contains many vacuoles with ingested bacteria and debris. The trophozoite has two nuclei: one very large, bean-shaped macronucleus and a smaller, round micronucleus. The organisms live in the large intestine. The trophozoites have a rapid, rotatory, boring motion because of the movement of the cilia. The cyst is formed as the trophozoite moves down the intestine. Nuclear division does not occur in the cyst; therefore, only two nuclei are present, the macronucleus and the micronucleus. The cysts measure 50 to 70 µm in diameter (see Table 48-5).
Sporozoa (Apicomplexa)
All the Apicomplexa are unicellular and have an apical complex. These structures can be seen in electron microscopy studies and are used to help classify the various organisms. Genera that develop in the gastrointestinal tract of vertebrates throughout their entire life cycle include Isospora, Cyclospora, and Cryptosporidium. Genera capable of or requiring extraintestinal development are referred to as cyst-forming coccidia; they include Sarcocystis and Toxoplasma spp. The genera that cause disease in humans include Cryptosporidium, Cyclospora, Isospora, Sarcocystis, and Toxoplasma (see Chapter 50 for a discussion of Toxoplasma).
Cryptosporidium spp.
General Characteristics
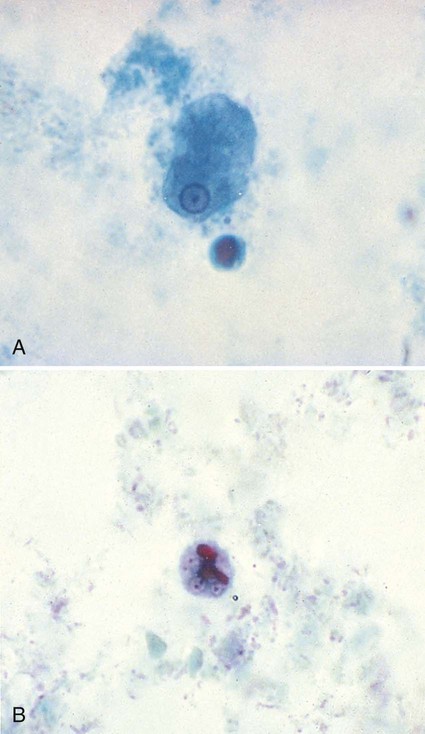
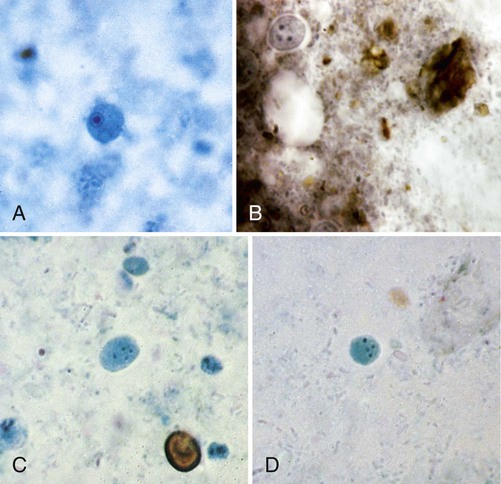
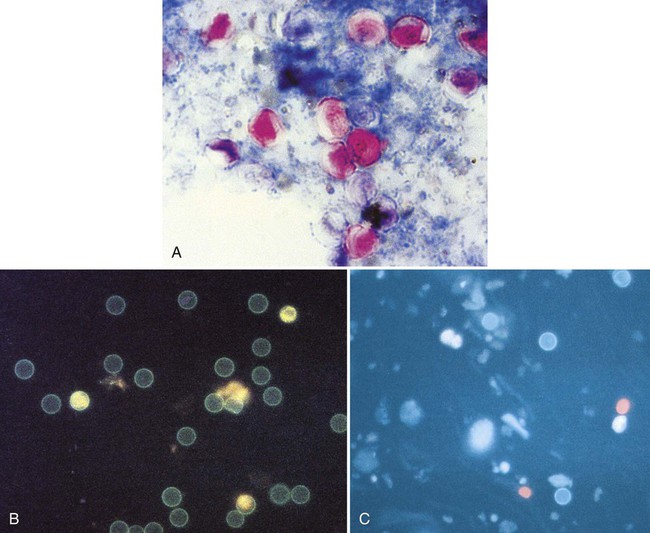
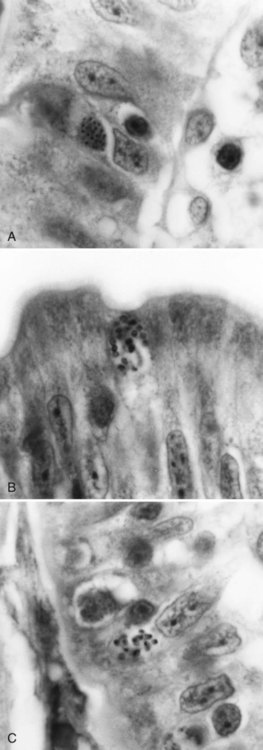
Cryptosporidium spp. are intracellular parasites that primarily infect epithelial cells of the stomach, intestine, and biliary ducts. The organism previously called Cryptosporidium parvum, thought to be the primary Cryptosporidium species infecting humans, now is classified as two species, C. parvum (mammals, including humans) and C. hominis (primarily humans) (see Table 48-6, Figures 48-26 to 48-28). Differentiation of these two species based on oocyst morphology is not possible. Currently, more than 20 established Cryptosporidium species have been identified in vertebrates, and more than 10 Cryptosporidium spp. have been reported in humans.
Cyclospora cayetanensis
General Characteristics
The life cycle of C. cayetanensis involves only humans as hosts. Oocysts are passed in the feces unsporulated (Figure 48-29). At room temperature (23° to 25°C), small numbers of oocysts may sporulate within 10 to 12 days.
Isospora (cystoisospora) belli
General Characteristics
Although isosporiasis is found worldwide, certain tropical areas in the Western Hemisphere have specific locations where endemic infections occur. These organisms infect both adults and children, and intestinal involvement and symptoms are generally transient unless the patient is immunocompromised. I. belli has also been implicated in traveler’s diarrhea. However, unlike with Cryptosporidium spp. and C. cayetanensis, large outbreaks of isosporiasis have not been reported (see Table 48-6, Figure 48-30).
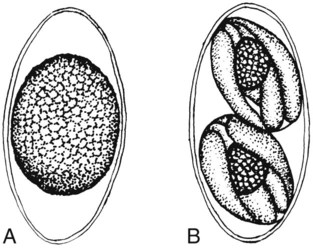
Sarcocystis spp.
General Characteristics
The sporocysts found in the stool are broadly oval and slightly tapered at the ends. They measure 9 to 16 µm long and contain four mature sporozoites and the residual body (see Table 48-6). Normally, the oocyst contains two sporocysts (similar to I. belli); however, in Sarcocystis infections, the sporocysts are released from the oocyst and normally are seen singly. These sporocysts tend to be larger than Cryptosporidium oocysts that contain four sporozoites, so they look totally different. The oocysts are fully sporulated when passed in the stool.
Microsporidia
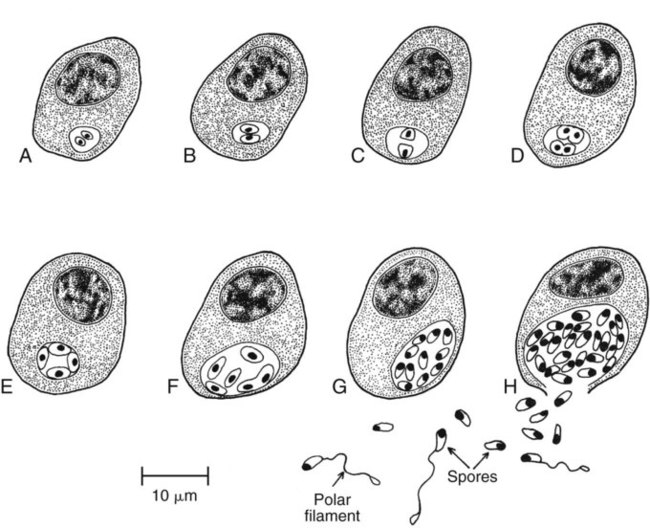
General Characteristics
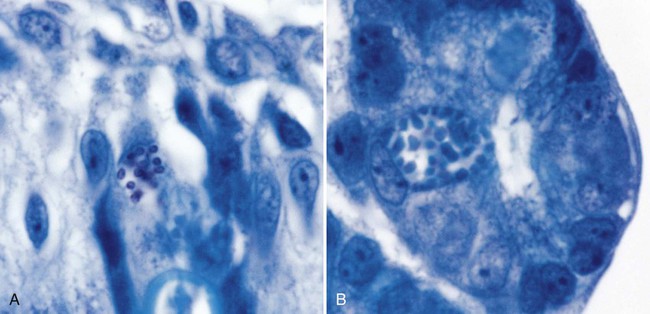
Human microsporidial infections have been documented worldwide. The spore, the only life cycle stage able to survive outside the host cell, is the infective stage (see Table 48-7 and Figures 48-31 to 48-35). Infection occurs with ingestion or inhalation of the infective spores, from which the infective sporoplasm (spore protoplasm) enters the host cell through the polar tubule. The microsporidia multiply extensively in the host cell cytoplasm; the life cycle includes repeated divisions by binary fission (merogony) or multiple fission (schizogony) and spore production (sporogony). Both merogony and sporogony can occur in the same cell at the same time. During sporogony, a thick spore wall is formed, providing environmental protection for the spore.
1. Which of the following is the best technique for identifying Dientamoeba fragilis in stool?
2. Which of the following protozoan organisms has been widely implicated in waterborne and food-borne outbreaks in the United States?
3. An Entamoeba histolytica trophozoite has which characteristics?
a. Central karyosome in the nucleus, ingested RBCs, and clear pseudopodia
b. Ingested RBCs, clear pseudopodia, and uneven chromatin on the nuclear membrane
c. Ingested RBCs, clear pseudopodia, and large glycogen vacuoles in cytoplasm
d. Large, blotlike karyosome, ingested white blood cells (WBCs), and granular pseudopods
e. Uneven karyosome, no peripheral chromatin, granular pseudopods
4. Entamoeba dispar is most easily confused morphologically with:
_____ Microsporidia can cause intestinal symptoms and also disease in other tissues, particularly in immunocompromised patients.
_____ Oocysts of Cryptosporidium spp. can be detected in stool specimens using the modified acid-fast stain.
_____ Fecal immunoassays for antigen detection have become more commonly used to diagnose infections with Dientamoeba fragilis and Blastocystis hominis.
_____ In the United States, sporadic minioutbreaks of diarrheal disease have been associated with the ingestion of strawberries, raspberries, fresh basil, mesclun (baby lettuce leaves), and snow peas. The most likely causative agent is Cryptosporidium spp.
_____ Although the pathogenicity of Blastocystis hominis has been controversial, newer information suggests that numerous strains and species are included in the name, some of which are pathogenic and some nonpathogenic.