CHAPTER 114 Intestinal Ischemia
Since the development and widespread use of colonoscopy, angiography, computed tomography (CT), and other imaging modalities, various types of ischemic injury to the gastrointestinal tract have been recognized and increasingly appreciated (Table 114-1; see also Tables 114-2 and 114-4). Our concepts of their pathogenesis, diagnosis, and management have been so altered since the 1980s that much of what has been written in the past is no longer applicable. In this chapter we describe the spectrum of ischemic damage to the gastrointestinal tract and discuss the management of these conditions in light of recent advances.
Table 114-1 Types and Approximate Frequencies of Intestinal Ischemia
| TYPE | FREQUENCY (%) |
|---|---|
| Colon ischemia* | 75 |
| Acute mesenteric ischemia* | 25 |
| Focal segmental ischemia* | <5 |
| Chronic mesenteric ischemia | <5 |
* Includes mesenteric venous thrombosis. Mesenteric venous thrombosis can manifest as colon ischemia, acute mesenteric ischemia, or focal segmental ischemia.
ANATOMY OF THE SPLANCHNIC CIRCULATION
The celiac axis (CA), superior mesenteric artery (SMA), and inferior mesenteric artery (IMA) supply almost all of the blood flow to the digestive tract.1 There is marked variability of vascular anatomy among individuals, but typical patterns have emerged from anatomic dissections and abdominal angiographic studies.
CELIAC AXIS
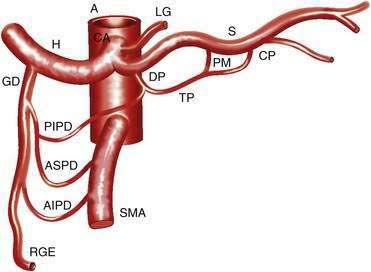
The CA (Fig. 114-1) arises from the anterior aorta and typically gives rise to three major branches: the left gastric artery, the common hepatic artery, and the splenic artery. The common hepatic artery gives rise to the gastroduodenal, right gastroepiploic, and anterior superior pancreaticoduodenal arterial branches. The splenic artery gives off pancreatic and left gastroepiploic arterial branches. The CA and its branches supply the stomach, duodenum, pancreas, and liver.
SUPERIOR MESENTERIC ARTERY
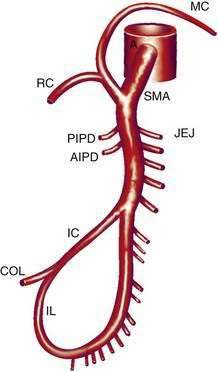
The SMA (Fig. 114-2) has its origin from the anterior aorta near the neck of the pancreas. It gives rise to five major vessels: the anterior and posterior inferior pancreaticoduodenal vessels, middle colic, right colic, and ileocolic arteries, as well as to a series of jejunal and ileal branches, all of which supply their named portions of intestine. These intestinal branches typically form a series of arcades, and from the terminal arcade, numerous straight vessels arise that enter the intestinal wall.
INFERIOR MESENTERIC ARTERY
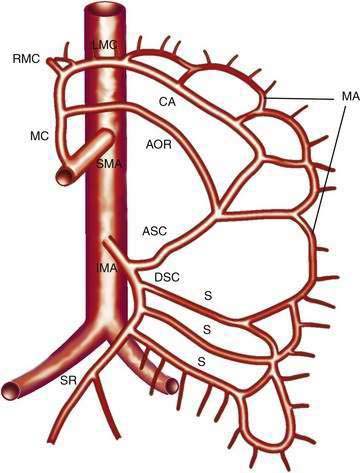
The IMA (Fig. 114-3) arises 3 to 4 cm above the aortic bifurcation close to the inferior border of the duodenum. It branches into the left colic artery, gives off multiple sigmoid branches, and terminates as the superior rectal artery. The IMA and its branches supply the large intestine from the distal transverse colon to the proximal rectum. The distal rectum is supplied by branches of the internal iliac (hypogastric) artery.
COLLATERAL AND ANASTOMOTIC CIRCULATION
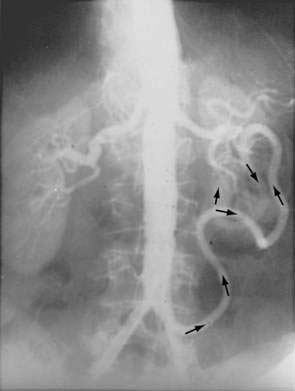
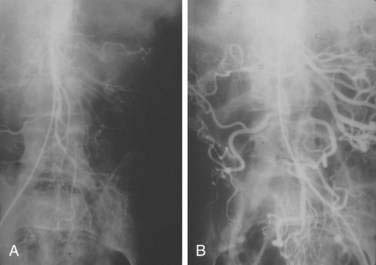
There are three potential paths of communication between the SMA and IMA: the marginal artery of Drummond, which is closest to and parallel with the wall of the intestine; the central anastomotic artery, a larger and more centrally placed vessel; and the arc of Riolan, an artery in the base of the mesentery. In the presence of SMA or IMA occlusion, a large collateral termed the meandering artery may be identified angiographically and represents a dilated central anastomotic artery or arc of Riolan (Fig. 114-4). It is critical to determine the direction of flow within a meandering artery before sacrificing the IMA, such as during aortic aneurysm surgery, lest the IMA be the main vessel supplying blood to the small bowel because of an occluded SMA.
PATHOPHYSIOLOGY AND PATHOLOGY
Ischemic injury of the intestine results from deprivation of oxygen and nutrients necessary for cellular integrity. Remarkably, the bowel can tolerate a 75% reduction of mesenteric blood flow and oxygen consumption for 12 hours with no changes on light microscopy, because only one fifth of the mesenteric capillaries is open at any time, and when oxygen delivery is decreased, the bowel adapts by increasing oxygen extraction.2 Below a critical level of blood flow, however, these compensatory mechanisms are overwhelmed and no longer protective.
Ischemic damage results both from hypoxia during the period of ischemia and reperfusion injury when blood flow is re-established. More reinjury from brief ischemia appears during reperfusion, but as the ischemic period lengthens, hypoxia becomes more detrimental than reperfusion3; the injury after three hours of ischemia and one hour of reperfusion is more severe than that after four hours of ischemia. Reperfusion injury has been attributed to many factors, including reactive oxygen radicals. When molecular oxygen is reduced in univalent steps, superoxide, hydrogen peroxide, and hydroxyl radicals are formed. These oxygen radicals damage an array of molecules found in tissues, including nucleic acids, membrane lipids, enzymes, and receptors; such widespread damage can result in cell lysis, impaired cell function, and necrosis on reperfusion of ischemic tissues.
Neutrophils are another source of reactive oxygen metabolites. During reperfusion, XO-derived oxidants initiate the production and release of leukotriene B4 and platelet-activating factor, which lead to neutrophil adherence and migration. The adherent leukocytes mediate microvascular injury by release of proteases and physical disruption of the endothelial barrier. Oxygen radical scavengers (superoxide dismutase, dimethyl sulfoxide), XO inhibitors, and agents that inhibit leukocyte adherence and migration have been shown experimentally to protect various organs against reperfusion injury, but are not yet used clinically because, in large measure, they must be given before or coincident with the ischemic injury to have protective effects.3
ACUTE MESENTERIC ISCHEMIA
Intestinal ischemia can be classified as acute or chronic and of venous or arterial origin. In the acute forms, intestinal viability is threatened, whereas in the chronic forms, blood flow is inadequate to support the functional demands of the intestine. Acute mesenteric ischemia (AMI) is much more common than the chronic type, and arterial disease is more common than venous disease. Arterial forms of AMI include SMA embolus (SMAE), nonocclusive mesenteric ischemia (NOMI), SMA thrombosis (SMAT), and focal segmental ischemia (FSI) (Table 114-2). Acute mesenteric venous thrombosis (MVT) and FSI are the venous forms of AMI.
Table 114-2 Causes and Approximate Frequencies of Acute Mesenteric Ischemia
| CAUSE | FREQUENCY (%) |
|---|---|
| SMA embolus | 50 |
| Nonocclusive mesenteric ischemia | 25 |
| SMA thrombosis | 10 |
| Mesenteric venous thrombosis | 10 |
| Focal segmental ischemia | 5 |
SMA, superior mesenteric artery.
INCIDENCE
Most series of AMI reported in the late 1970s and early 1980s showed that SMAE was responsible for 40% to 50%, NOMI for 20% to 30%, and SMAT for 10% to 20% of cases. The incidence of NOMI has now declined, however, likely because intensive care unit monitoring enables prompt correction of hypotension and blood volume deficits, and the widespread use of calcium channel blockers and other systemic vasodilators might protect the vascular bed from spasm. Today, SMAE is the most common cause of AMI. In one study of autopsies on patients who died from acute mesenteric thromboembolic occlusion the embolus-to-thrombus ratio was 1.4 : 1.4
CLINICAL FEATURES
Unexplained abdominal distention or gastrointestinal bleeding may be the only indications of AMI when pain is absent, especially when AMI is due to NOMI. Distention, although absent early in the course of AMI, is often the first sign of intestinal infarction. The stool contains occult blood in 75% of patients. Right-sided abdominal pain associated with the passage of maroon or bright red blood in the stool, although characteristic of colon ischemia, also may be seen with AMI, because the blood supply to the right colon and small intestine originates from the SMA. Elderly patients with AMI have been reported to develop mental confusion acutely in as many as 30% of cases.5 In patients who survive cardiopulmonary resuscitation and who then develop recurrent bacteremia or sepsis, the suspected cause of sepsis should be NOMI that resulted in a segment of bowel with subacute ischemic injury, acting as a portal for bacterial translocation.6 Although episodes of sepsis may be treated successfully with antibiotics, the length of damaged bowel must be removed to prevent recurrent sepsis.
LABORATORY FEATURES AND DIAGNOSIS
On admission to the hospital, approximately 75% of patients with AMI have leukocytosis greater than 15,000 cells/mm3 and about 50% have metabolic acidemia. A normal white blood cell (WBC) count cannot be used to exclude early AMI, just as a high WBC count does not make the diagnosis. Elevated levels of serum phosphate, d-lactate, amylase, and other enzymes have been noted, as have high peritoneal fluid amylase and intestinal alkaline phosphatase activity, but the sensitivity and specificity of these markers of intestinal ischemia have not been established.7 More-specific intestinal enzymes including diamine oxidase, hexosaminidase, glutathione S-transferase,8 and intestinal fatty acid-binding protein9 also lack sufficient sensitivity and specificity to diagnose AMI. Moreover, serum markers, when elevated, usually indicate late-stage disease.
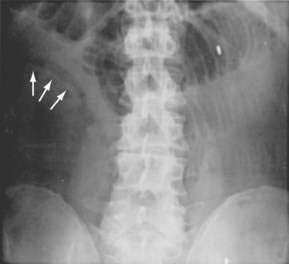
Although they are poorly sensitive (30%) and nonspecific, plain films of the abdomen still are obtained in evaluating patients with suspected AMI. Plain films of the abdomen usually are normal in AMI before infarction. Later on, formless loops of small intestine, ileus, thumbprinting of the small bowel or right colon (Fig. 114-5), or still later, pneumatosis and portal or mesenteric vascular gas may be seen. In one study, the mortality rate of patients with normal plain film studies was 29%, whereas it was 78% in those with abnormal findings.10 The primary purpose of plain films (or CT scans) is to exclude causes of abdominal pain other than ischemia that might mandate a different therapeutic approach.
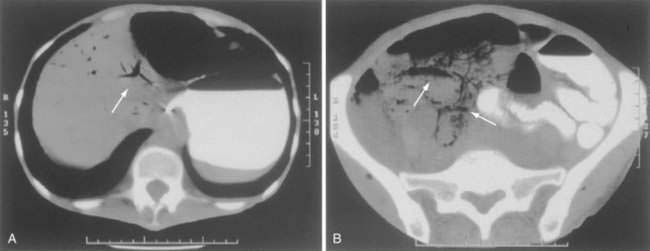
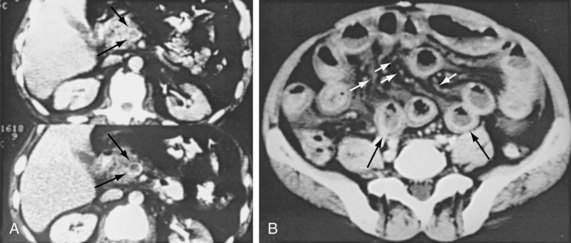
CT has largely replaced plain film study of the abdomen for diagnosis and is used to identify arterial and venous thromboses as well as ischemic bowel.11–14 Findings on CT include colon dilatation, bowel wall thickening, abnormal bowel wall enhancement, lack of enhancement of arterial vasculature with timed intravenous contrast injections, arterial occlusion, venous thrombosis, engorgement of mesenteric veins, intramural gas and mesenteric or portal venous gas (Fig. 114-6), infarction of other organs, ascites, and signs related to the cause of the infarcted bowel such as hernia.11 There are three relatively specific findings of AMI that are better depicted on CT scans compared with plain films: gas in the bowel wall or portal system, acute embolic infarction of other intra-abdominal organs, and thrombi in the mesenteric vessels.12 Unfortunately, the early signs on CT are nonspecific and the late signs reflect necrotic bowel.
In a study of 26 patients with AMI who had a preoperative multislice CT scan followed by exploratory laparotomy, CT scanning identified mesenteric arterial thrombosis in 16 of 17 patients and mesenteric vein thrombosis in 7 of 7 patients, all confirmed at operation. In this study, the sensitivity and specificity of CT scanning for occlusive AMI was 92% and 100%, respectively.15 The predictive value of CT scanning in the community might not be as high as in this report, because this study used only highly trained radiologists; improved CT scanner technology, however, likely will yield higher detection rates than in the past.
CT angiography has been shown to be promising in the diagnosis of AMI and, in one study, the added CT angiographic findings were believed to alter clinical management in 19% of 62 patients by making the diagnosis of AMI when CT alone did not.16 Magnetic resonance (MR) angiography and venography are newer imaging techniques used to diagnose AMI; they not only can image the vasculature but might be useful in determining metabolic consequences of inadequate blood flow.17,18
Selective mesenteric angiography, often with papaverine infusion, currently is the mainstay of diagnosis and initial treatment of both occlusive and nonocclusive forms of AMI, and it should be performed promptly if AMI is suspected or diagnosed on other imaging tests. Sensitivity and specificity of mesenteric angiography for diagnosing AMI in most studies are 90% to 100% and 100%, respectively.19 Opponents of routine angiography for patients with suspected AMI cite several problems with this approach:
TREATMENT
Our approach to the management of AMI is based on several observations. First, if the diagnosis is not made before intestinal infarction, the mortality rate is 70% to 90%. Second, diagnosis of both the occlusive and nonocclusive forms of AMI can be made in most patients by angiography. Third, vasoconstriction, which can persist even after the cause of the ischemia is corrected, is the basis of NOMI and a contributing factor in the other forms of AMI. Finally, vasoconstriction can be relieved by vasodilators infused into the SMA. The cornerstones of our approach, therefore, are the earlier and more liberal use of angiography and the incorporation of intra-arterial papaverine in the treatment of both occlusive AMI and NOMI. Duration of symptoms parallels mortality, and therefore early diagnosis and treatment is paramount to increase the chance for survival.20
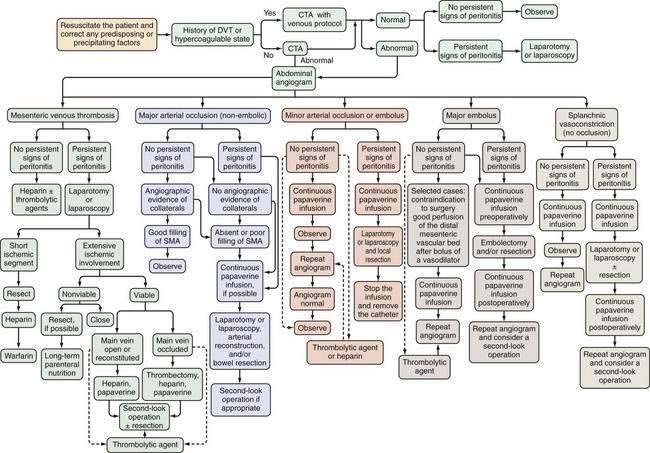
AMI should be suspected in patients older than 50 years who have the risk factors previously described and in younger patients—especially those with atrial fibrillation, vasculitis, a coagulation disorder, and those on vasoactive medications—who seek medical attention for sudden, severe abdominal pain that lasts longer than several hours. These patients should be managed according to the algorithm shown in Figure 114-7. Less-absolute indications for inclusion into this protocol consist of unexplained acute abdominal distention, colonoscopic evidence of isolated right-sided colonic ischemia, and acidosis without an identifiable cause.
Initial management of patients with suspected AMI includes resuscitation and diagnostic imaging studies. Resuscitation includes relief of acute congestive heart failure and correction of hypotension, hypovolemia, and cardiac arrhythmias. Broad-spectrum antibiotics (e.g., levofloxacin, metronidazole, piperacillin-tazobactam) are given immediately because of the high incidence of positive blood cultures in AMI and because they reduce the extent and severity of ischemic injury in experimental animals.21 There are no randomized, controlled trials showing the benefit of antibiotics in AMI, and it is unlikely that such trials will ever be done. After resuscitation, plain films or CT scan of the abdomen are obtained, not to establish the diagnosis of AMI but rather to exclude other causes of abdominal pain. A normal plain film or CT scan does not exclude AMI; ideally, patients are studied before radiologic signs appear because these signs connote irreversibly damaged bowel. If no alternative diagnosis is made on these studies, selective SMA angiography is performed. Based on the angiographic findings and the presence or absence of peritoneal signs, the patient is treated according to the algorithm in Figure 114-7.
Laparotomy is performed in AMI to restore arterial flow obstructed by embolus or thrombosis or to resect irreparably damaged bowel, or both. Embolectomy, thrombectomy, or arterial bypass precedes evaluation of intestinal viability because bowel that initially appears infarcted can show surprising recovery after adequate blood flow is restored. In the operating room, intestinal viability can be assessed clinically, by qualitative or quantitative surface fluorescence or by Doppler ultrasonography.22 Animal models show that administration of intravenous glucagon, intravenous heparin-binding epidermal growth factor (HB-EGF)-like growth factor or intraluminal nitroglycerin after revascularization of an acute arterial occlusion can improve mucosal viability and minimize reperfusion damage.23,24
Specific Types of Acute Mesenteric Ischemia
Superior Mesenteric Artery Embolus
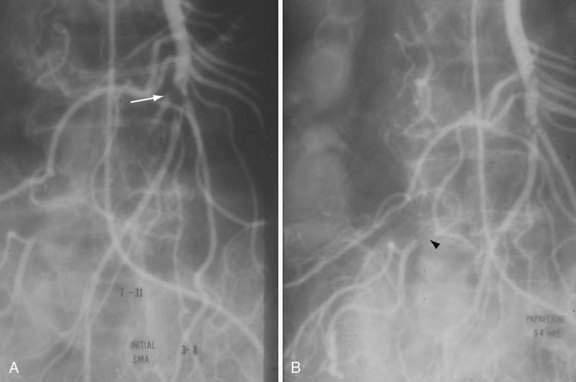
SMAE is responsible for 40% to 50% of AMI episodes. Emboli usually originate from a left atrial or ventricular mural thrombus. Many patients with SMAE have had previous peripheral artery emboli, and approximately 20% have synchronous emboli. SMAEs lodge at points of normal anatomic narrowing, usually immediately distal to the origin of a major branch. Angiography typically reveals a rounded filling defect with nearly complete obstruction to flow. Mesenteric atherosclerosis is usually not as severe as in SMAT. Emboli proximal to the origin of the ileocolic artery are considered major emboli. Minor emboli are those that lodge in the SMA distal to the takeoff of the ileocolic artery or in the distal branches of the SMA (Fig. 114-8).
Various therapeutic approaches have been proposed for SMAE, depending on the presence or absence of peritoneal signs, whether the embolus is partially or completely occluding, and whether the embolus is above the origin of the ileocolic artery or more distal. Therapy for SMAE has included surgical revascularization, intra-arterial perfusion with vasodilators or thrombolytic agents, and anticoagulation.19 In the absence of peritoneal signs, minor SMA emboli have been treated successfully with all of these agents without the need for surgery. Exploration is usually performed in patients with major emboli after papaverine infusion is begun. Nonoperative therapy using only papaverine infusion is attempted if there are significant contraindications to surgery, no peritoneal signs, and adequate perfusion of the vascular bed distal to the embolus after a bolus of vasodilator into the SMA.
Exploratory laparotomy is mandatory when peritonitis is present; embolectomy and bowel resection are performed as necessary. If possible, intra-arterial papaverine is begun before surgery and is continued during surgery. If no “second-look” operation is planned, infusion is continued for 12 to 24 hours postoperatively; persistent vasospasm is excluded by angiography before the catheter is removed (see Fig. 114-8). If a second operation is planned, the infusion is continued through the second procedure until angiography shows the vasoconstriction is ceased. Recognition of persistent vasoconstriction has prompted some authorities to recommend routine use of intra-arterial papaverine in all patients with SMAE; the best survival rates are seen in patients treated by this approach.19
Use of transcatheter thrombolytic therapy (e.g., alteplase or urokinase) can be considered before exploratory laparotomy if the patient does not have signs of peritonitis. Prospective studies and meta-analyses have shown that thrombolysis may be effective in resolving thrombi, improving symptoms, and avoiding surgery in patients with lesions amenable to such therapy.25,26 Thrombolytic therapy is most likely to be successful when the embolus is partially occluding or is minor and when the study is performed within 12 hours of the onset of symptoms.27 A canine study showed that intra-arterial streptokinase was more effective than intra-arterial papaverine in lysing clots implanted into the SMA, although greater ischemic damage occurred with streptokinase than with papaverine because of papaverine’s action to cause vasodilation and open collateral pathways for blood flow around the obstructing clot. When streptokinase and papaverine were administered simultaneously, neither medication functioned as well as it did alone and intestinal damage was intensified.28 Given the shortage of supporting evidence for thrombolytics in AMI and the high complication rate attending their use, this treatment remains controversial.27
Nonocclusive Mesenteric Ischemia
NOMI is responsible for 20% to 30% of AMI and usually is due to splanchnic vasoconstriction consequent to a preceding cardiovascular event. AMI can appear hours to days after the event, and vasoconstriction, which initially is reversible, can persist even after the precipitating event has been corrected. Precipitating causes for NOMI include acute myocardial infarction, congestive heart failure, arrhythmias, shock, cirrhosis, medications (e.g., digitalis), cardiopulmonary bypass surgery, and chronic kidney disease, especially when patients are on either hemodialysis or peritoneal dialysis. When presenting with abdominal pain, patients on peritoneal dialysis may be thought to have peritonitis, thereby delaying the diagnosis of NOMI and resulting in a poor outcome.29
NOMI is diagnosed by angiography using four criteria: narrowing of the origins of SMA branches, irregularities in the intestinal branches, spasm of the arcades, and impaired filling of intramural vessels. Patients with these signs who are neither in shock nor on vasopressors and who do not have pancreatitis can be considered to have NOMI (Fig. 114-9).
Acute Thrombosis of the Superior Mesenteric Artery
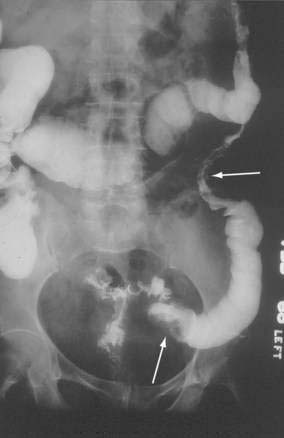
SMAT is demonstrated on flush aortography, which usually shows occlusion of the SMA 1 to 2 cm from its origin. Some distal filling of the SMA via collaterals is common. Branches proximal and distal to the obstruction can show localized or diffuse vasoconstriction. In patients with abdominal pain, no abdominal tenderness, and complete occlusion of the SMA on aortography, it is important, though difficult, to distinguish between acute thrombosis and long-standing, coincidental chronic occlusion. Prominent collaterals between the SMA and other major splanchnic vessels indicate chronic SMA occlusion. If there is good filling of the SMA, the occlusion is considered chronic and the abdominal pain is considered unrelated to mesenteric vascular disease (see Fig. 114-4). The absence of collateral vessels or the presence of collaterals with inadequate filling of the SMA indicates an acute occlusion and demands prompt intervention. If possible, an angiographic catheter is placed in the proximal SMA, and papaverine infusion is begun before surgery is undertaken.
Results
Although mortality rates of 70% to 90% were reported through the 1980s for patients whose AMI was diagnosed and treated conventionally, the approach described here can reduce these catastrophic figures. The best survival is reported in series in which angiography has been used routinely in the management of AMI.30–35
MESENTERIC VENOUS THROMBOSIS
INCIDENCE
In early studies, MVT was believed to be the major cause of AMI, but most of these cases probably represented NOMI. Today, only 5% to 10% of patients with AMI have MVT. The mean age at presentation with MVT is in the mid-60s.36 A Swedish study showed that the highest incidence of MVT was 11.3 per 100,000 person years among those 70 to 79 years old.37
PREDISPOSING CONDITIONS
Previously, a cause of MVT was identified in fewer than half of patients. The discoveries of the primary and secondary hypercoagulable states and the use of estrogens for contraception and hormone replacement have led to identification of the cause in almost 90% of patients.38 Arterial hypertension is the most commonly associated medical comorbidity with this disorder, and neoplasms (e.g., acute lymphocytic leukemia, adenocarcinoma of the pancreas, stomach) and coagulation disorders (e.g., lupus anticoagulant, factor V Leiden, and protein S deficiency) also are commonly seen.36,39 A list of predisposing conditions for MVT is given in Table 114-3.
Table 114-3 Conditions Associated with Mesenteric Venous Thrombosis
CLINICAL FEATURES
MVT can have an acute, subacute (weeks to months), or chronic onset. Except for late complications, chronic MVT is asymptomatic. As many as 60% of patients have a history of peripheral vein thromboses.40
Acute MVT manifests with abdominal pain in more than 90% of patients and, as with acute arterial ischemia, the pain initially is out of proportion to the physical findings. The mean duration of pain before admission is five to 14 days, but it is more than one month in as many as 25% of patients.41 Other symptoms, including nausea and vomiting, occur in more than 50%. Lower gastrointestinal bleeding, bloody diarrhea, or hematemesis occur in 15% and indicate bowel infarction. Fecal occult blood is found in more than half of instances during the course of MVT. Initial physical findings vary at different stages and with different degrees of ischemic injury, but guarding and rebound tenderness develop as bowel infarction evolves. Most patients have a temperature higher than 38°C (100.4°F), and 25% exhibit signs of septic shock.
Chronic MVT is seen in patients who are asymptomatic at the time of thrombosis but who may develop gastrointestinal bleeding from varices.42 Most patients who bleed do so from gastroesophageal varices secondary to portal or splenic vein thrombosis, and they have physical findings of portal hypertension. Laboratory studies may show secondary hypersplenism with pancytopenia or thrombocytopenia.
DIAGNOSIS
Acute Mesenteric Venous Thrombosis
Selective mesenteric arteriography can establish a definitive diagnosis before bowel infarction, can differentiate venous thrombosis from arterial forms of ischemia, and can provide access for vasodilator therapy. Angiographic findings of acute MVT include thrombus in the SMV with partial or complete occlusion, failure to visualize the SMV or portal vein, slow or absent filling of the mesenteric veins, arterial spasm, failure of the arterial arcades to empty, reflux of contrast medium into the artery, and prolonged blush in the involved segment.32
Ultrasonography, CT, and MR imaging all have been used to demonstrate thrombi in the SMV and the portal vein.43,44 CT can diagnose MVT in more than 90% of patients and is the diagnostic study of choice. Specific findings include thickening and enhancement of the bowel wall, enlargement of the SMV, a central lucency in the lumen of the vein (representing a thrombus), a sharply defined vein wall with a rim of increased density, and dilated collateral vessels in a thickened mesentery (Fig. 114-10). CT also is very accurate in differentiating transmural infarction from non-transmural ischemia.45 When MVT is diagnosed on CT scanning, angiography might not be necessary, but in selected symptomatic patients it better delineates thrombosed veins and provides access for intra-arterial vasodilators. Magnetic resonance venography also appears to be highly sensitive for diagnosing MVT and is a diagnostic alternative to CT.46 Esophagogastroduodenoscopy and colonoscopy rarely are helpful, because the duodenum and colon are infrequently involved. As in other forms of AMI, laparoscopy may be useful either when other studies are contraindicated or in concert with imaging tests.47
TREATMENT
Acute Mesenteric Venous Thrombosis
Most patients with acute MVT initially are believed to have some form of AMI and are treated as discussed in earlier sections and as outlined in the algorithm of Figure 114-7. In asymptomatic persons in whom the diagnosis is made on a CT scan done for other than abdominal pain, either three to six months of anticoagulation or no therapy, in some cases, is reasonable. In symptomatic patients, treatment is determined by the presence or absence of peritoneal signs; signs of peritonitis mandate laparotomy and resection of infarcted bowel. If long segments of questionably viable bowel are found, papaverine is infused, and if arterial spasm is relieved and the SMV or portal vein is visualized, thrombectomy or a second look may be attempted to determine whether resection should be performed. Following surgery, heparin should be administered. Immediate heparinization for seven to 10 days has been shown to diminish recurrence and progression of thrombosis and to improve survival.48,49 In the absence of peritoneal signs, immediate heparinization followed by a three- to six-month course of warfarin may be all that is needed. A comparison of patients who were treated surgically with those who were managed medically suggested that nonoperative management is a reasonable option provided the diagnosis on CT scan is certain and there is no transmural necrosis or perforation.50 A few case reports have documented the use of thrombolytic agents in the treatment of acute MVT.
Chronic Mesenteric Venous Thrombosis
Treatment of chronic MVT is aimed at controlling bleeding, usually from esophageal varices. Sclerotherapy, variceal banding, portosystemic shunts, transjugular intrahepatic portosystemic procedures, devascularization procedures, and bowel resection all have a place in treating selected patients. Use of beta blockers and anticoagulation was found to be associated with improved survival in these patients.51 No treatment is indicated for patients with asymptomatic chronic MVT.
PROGNOSIS
Mortality associated with acute MVT is lower than that for other forms of AMI and is approximately 20%. Intestinal infarction, not having a CT scan performed, and treatment on a nonsurgical ward all are associated with increased mortality.37 Recurrence rates of 20% to 25% fall to about 15% if heparin therapy is begun promptly. The natural history of chronic MVT is not known, but from postmortem studies it appears that almost 50% of patients with MVT have no bowel infarction and many have no symptoms.
COLON ISCHEMIA
Colon ischemia (CI) is a common disorder of the large bowel in older persons and is the most common form of intestinal ischemic injury. It comprises a spectrum (Table 114-4) that includes reversible colopathy (submucosal or intramural hemorrhage), transient colitis, chronic colitis, stricture, gangrene, and fulminant universal colitis. The initial presentation usually is the same among these types and does not necessarily predict the course of disease, with the exception of ischemia involving the ascending colon. This latter pattern can simultaneously involve the small intestine, usually is caused by SMAE or NOMI, can have associated shock, and carries a mortality rate of more than 50%.52–54
Table 114-4 Types and Approximate Frequencies of Colon Ischemia in Patients Seen at a Tertiary Referral Hospital
| TYPE | FREQUENCY (%)* |
|---|---|
| Reversible colopathy and transient colitis | >50 |
| Transient colitis | 10 |
| Chronic ulcerating colitis | 20 |
| Stricture | 10 |
| Gangrene | 15 |
| Fulminant universal colitis | <5 |
* Because of the approximate nature of the frequencies, the total of the frequencies of all types of colon ischemia is not 100%.
INCIDENCE
In our tertiary care hospital, CI accounts for approximately 1 in 2000 hospital admissions and is seen in approximately 1 in 100 flexible sigmoidoscopies and colonoscopies. A study using medical claims data from a large health care organization calculated a crude incidence rate of 7.2 cases per 100,000 person-years of observation in the general population, in contrast to 42.8 cases per 100,000 person-years for patients with irritable bowel syndrome (IBS). After adjustment for age, sex, and calendar year, the incidence of CI in people with IBS was 3.4 times higher than it was in persons without IBS.55
PATHOPHYSIOLOGY AND CAUSES
CI can result from alterations in the systemic circulation or from anatomic or functional changes in the mesenteric vasculature, and it is thought to result from local hypoperfusion and reperfusion injury. In most cases, no specific cause for the ischemia is identified, and such episodes are viewed as localized nonocclusive ischemia, likely a result of small-vessel disease. An increasing variety of causes of CI is being defined, including hematologic disorders, thrombophilic states, and medications (see later) (Table 114-5).
Table 114-5 Causes of Colon Ischemia
Medications as a Cause of Colon Ischemia
Medications should always be considered as a possible etiology for CI.56
Antibiotics
Antibiotic-associated hemorrhagic colitis (AAHC) is believed to be mediated by CI. The penicillins and their derivatives, including amoxicillin and ampicillin, most commonly have been associated, although macrolides, cephalosporins, chloramphenicol, fluoroquinolones, and tetracyclines also are known precipitants. AAHC typically manifests two to seven days after antibiotics are initiated, beginning with lower abdominal pain and loose stools and followed by hematochezia several hours later.57 Some believe the mechanism of AAHC is a hypersensitivity or allergic reaction, and others believe it results from a cross reaction between the infecting agent and the antibiotic. Regardless of mechanism, if AAHC is suspected, it is recommended that the antibiotic be stopped and an alternative regimen started.
Chemotherapeutic Agents
When associated with chemotherapy, CI usually is associated with the alkaloid and taxane classes of chemotherapeutic agents, such as vinorelibine tartrate (alkaloid) and paclitaxel and docetaxel (taxanes).58 Although it is not proved, the mechanism of injury of CI with these compounds is believed to be either a direct toxic injury of the colonic epithelium or anti-angiogenic toxicity.59 If patients develop CI while taking these medications, the clinician must reconsider the risks and benefits of their use.
Constipation-Inducing Agents
Patients taking medications that have constipation as a known adverse effect are potentially at increased risk for CI.60 More than 250 different medications from a variety of medication classes fit this category.56 One potential mechanism of CI caused by constipation-inducing agents is based upon the observation that patients with idiopathic colonic slow transit have reduced baseline rectal and mucosal blood flow, possibly as a result of impaired efferent vagal cholinergic activity. Impaired cholinergic innervation is a side effect of many constipation-inducing medications, and the resultant unopposed sympathetic input leaves the colon susceptible to ischemic injury.
Decongestants
Pseudoephedrine is used for relief of nasal congestion because direct application to α1-adrenergic receptors constricts vessels and relieves symptoms. The drug can be absorbed, however, and cause mesenteric vasoconstriction with resultant CI. Women are affected more often than men, and the splenic flexure watershed region seems to be most susceptible to injury by this agent. Patients with a history of CI or vasculitis or with a known thrombophilic state should be alerted to this potential complication.56
Diuretics
Diuretics, such as furosemide, have been implicated in NOMI and CI. The presumed mechanism for CI with these agents is a decrease in extracellular fluid volume and reduction in peripheral resistance, prompting a steal of blood from the bowel to the limbs.61 Improved fluid balance should treat this condition.
Hormonal Therapies
Oral contraceptive pills (OCPs) usually are combinations of low-dose ethinyl estradiol and progestin, whereas hormone replacement therapy (HRT) often consists of ethinyl estradiol, with progestin added if the patient has an intact uterus. Young women taking OCPs are at a six-fold greater risk of CI compared with an age-matched cohort not taking these agents.62 OCPs and HRT are known to result in a hypercoagulable state with ensuing microthromboembolic events and resultant CI. In any patient with other risk factors for CI, the use of OCPs or HRT, if clinically appropriate, should be monitored closely. Patients experiencing CI while taking these medications should stop taking them and consider other options.
Controlled or Illicit Pharmacologic Agents
Amphetamines are sympathomimetic vasoconstricting medications used for medicinal and recreational purposes. Significant increases in morbidity from a variety of ischemic insults attributed to their use have been reported, including myocardial ischemia and intestinal gangrene.63 Amphetamine-induced CI is rare and usually manifests with hematochezia and mild to moderate abdominal pain. These medications tend to affect the ascending colon, possibly as a result of selective vasoconstriction of the SMA.56
Cocaine is known to induce CI.64 The main mechanisms of injury with this agent include mesenteric vasoconstriction, a hypercoagulable state, and direct toxicity to the vasculature.65 Patients present 12 to 24 hours after intranasal or intravenous cocaine use with rectal bleeding with or without abdominal pain. Involvement of the descending colon, sigmoid, and occasionally the rectum are typical of cocaine-induced CI.
Laxatives
Sodium polystyrene sulfonate or sorbitol (SPS, Kayexelate) commonly is used to treat hyperkalemia. Case series have shown that patients who have uremia and are given SPS are at increased risk for intestinal and colonic necrosis.66 The exact mechanism of disease is not clear, although some believe that sorbitol-induced osmotic mucosal injury combined with elevated renin levels predisposes patients to NOMI via angiotensin-mediated vasoconstriction.67 When using SPS, creatinine and urine output should be closely monitored.56
Magnesium citrate and sodium phosphate have been shown to cause CI,68 which can manifest with abdominal pain and diarrhea as quickly as within one hour of taking the medication. It is believed that rapid fluid shifts from the mesenteric circulation to the colonic lumen results in transient hypoperfusion and ischemia. All reported cases have been self-limited.
Bisacodyl, a stimulant laxative, has been associated with CI in young healthy patients.69 It is believed that the mechanism of this disease is enhanced colonic motility resulting in decreased mucosal perfusion. Patients usually develop hematochezia and abdominal pain several hours after ingesting bisacodyl. In patients with a history of CI, this medication is relatively contraindicated.
Glycerin enemas have been reported to cause hematochezia six hours after their use, with a flexible sigmoidoscopic appearance consistent with CI.70 One possible mechanism of this insult is inferior mesenteric artery spasm (seen on mesenteric angiography) resulting from increased colonic intraluminal pressure.
Nonsteroidal Anti-inflammatory Drugs
NSAID-induced colitis manifests with abdominal pain, diarrhea, and hematochezia, with occasional fevers and weight loss.71 It is associated with an endoscopic and histologic appearance similar to CI. Injury induced by NSAIDS is believed to result from long-term use of the medication, with a consequent decrease of vasodilating prostaglandins and increase of vasoconstricting leukotrienes.
Serotonin Agonists and Antagonists
Serotonin (5-hydroxytryptamine, 5-HT) plays a critical role in modulating enteric neurotransmission and central nervous system signaling. The mechanisms for CI associated with serotonin agonists and antagonists are unproved, although several possibilities have been put forth, including cross-talk among 5-HT receptors and 5-HT3-modulated neurotransmitter release, which possibly is aggravated in atherosclerosis.72
Sumatriptan, a 5-HT1 serotonin receptor agonist used to treat migraine headaches, is an uncommon cause of CI. Its primary mechanism is intracranial vasoconstriction, but in patients with CI, it is believed there is vasoconstriction of the colonic vascular bed.73 Women are more commonly affected than men and typically present with abrupt onset of abdominal pain and hematochezia approximately two days after starting the medication. If CI is suspected, the medication should be discontinued.
Alosetron, a 5-HT3 antagonist used to treat diarrhea-predominant irritable bowel syndrome (IBS-D) in women, was removed from the United States market in 2000 as a result of its observed association with CI. Alosetron subsequently was reintroduced in 2002 for use in patients with IBS-D who had not responded to conventional therapies. The estimated incidence rate of CI during alosetron treatment (since its reissuance in 2002) is 1.53 cases per 1000 patient-years.74 Approximately one quarter of patients present within one week and one half present within one month of beginning the drug; abdominal pain and bloody diarrhea are typical.74 Alosetron-induced CI is usually reversible and rarely has caused stricture or gangrene.
PATHOLOGY
Morphologic changes after CI vary with the duration and severity of the injury. The mildest injury is mucosal and submucosal hemorrhage and edema, with or without partial necrosis and ulceration of the mucosa. With more severe injury, chronic ulcerations, crypt abscesses, and pseudopolyps develop, changes that can mimic inflammatory bowel disease (Fig. 114-11).75 Pseudomembranes also may be seen. Iron-laden macrophages and submucosal fibrosis are characteristic of ischemic injury. With severe ischemia, the muscularis propria is replaced by fibrous tissue, forming a stricture. The most-severe form of ischemic damage causes transmural infarction.
CLINICAL FEATURES AND DIAGNOSIS
CI usually manifests with sudden cramping, mild, left lower abdominal pain; an urgent desire to defecate; and passage within 24 hours of bright red or maroon blood or bloody diarrhea. Bleeding is not sufficient to require transfusion. Mild to moderate abdominal tenderness usually is present over the involved segment of bowel. Patients with ischemia isolated to the right side of the colon more often present with lower abdominal pain than they do with rectal bleeding or bloody diarrhea.54
A large retrospective study of patients with biopsy-proven CI showed that no region of the colon is spared from involvement. A segmental pattern of involvement is seen most commonly and the sigmoid is affected most often (22.9%), followed by the descending-to-sigmoid colon segment (11.0%), the cecum-to-hepatic flexure segment (8.0%), the descending colon alone (8.0%), and a pancolonic pattern (6.6%).76 Although no specific etiology was associated with any specific anatomic distribution, pancolitis and isolated right-sided colonic disease were seen frequently in patients with sepsis.76 In older reports, certain causes were believed to affect particular segments: local nonocclusive ischemic injuries, the watershed areas (the splenic flexure and rectosigmoid); ligation of the IMA, the sigmoid. The length of affected bowel can depend on the cause of CI: atheromatous emboli involve short segments, and nonocclusive injuries involve longer portions of colon.
If CI is suspected, a CT scan might support the clinical suspicion and also diagnose potential complications.77 If the CT scan shows only nonspecific findings such as a thickened segment of colon, or if the abdominal plain film appears normal, colonoscopy should be performed on the unprepared colon within 48 hours of the onset of symptoms. During colonoscopy and barium enema examination, care should be taken not to overdistend the colon because high intraluminal pressure diminishes intestinal blood flow and can aggravate ischemic damage, particularly in patients with vasculitis.78
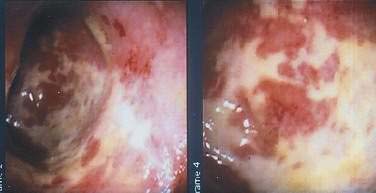
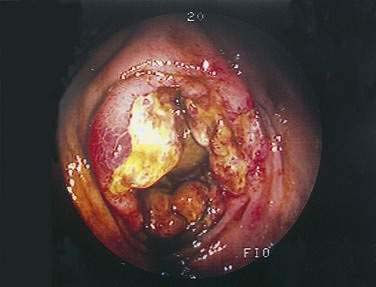
Colonoscopy is preferable to barium enema because it is more sensitive in diagnosing mucosal abnormalities, and biopsy specimens may be obtained. Hemorrhagic nodules seen at colonoscopy represent bleeding into the submucosa and are equivalent to thumbprints on barium enema studies (Fig. 114-12). Segmental distribution of these findings, with or without ulceration, is highly suggestive of CI, but the diagnosis of CI cannot be made conclusively on the basis of a single examination unless mucosal gangrene is seen (Fig. 114-13). A colonoscopic finding called the colon single-stripe sign has been described in patients with CI, referring to a single line of erythema with erosion or ulceration oriented along the longitudinal axis of the colon; it had a 75% histopathologic yield in making the diagnosis of ischemic injury and signified a milder course than did a circumferential ulcer.79 Segmental disease, rectal sparing, and rapid spontaneous evolution usually resulting in resolution of disease are characteristics of CI.
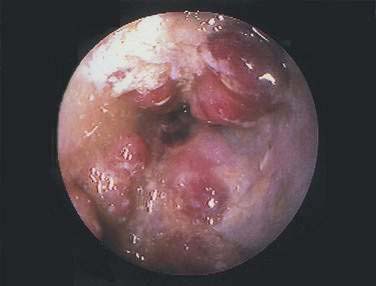
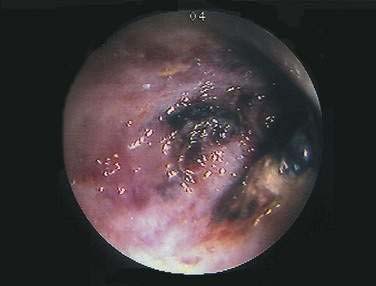
Figure 114-12. Colonoscopic equivalent of a radiologic thumbprint, caused by subepithelial hemorrhage and edema, in a patient with colon ischemia.
The initial diagnostic study should be performed within 48 hours, because thumbprinting disappears within days as the submucosal hemorrhages are resorbed or the overlying mucosa sloughs. Studies performed one week after the initial study should reflect evolution of the injury—either normalization of the colon or replacement of the thumbprints with a segmental ulcerative colitis-type pattern (Fig. 114-14). Universal colonic involvement, however, favors true ulcerative colitis, whereas fistula formation suggests Crohn’s disease. Occasionally, an abundant inflammatory response can produce heaping up of mucosa and submucosa that resembles a stricture or neoplasm (Fig. 114-15).
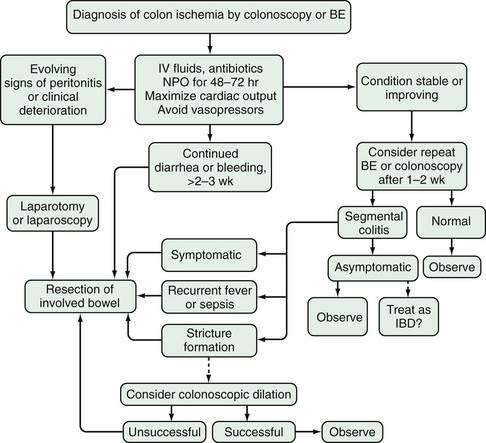
CLINICAL COURSE AND TREATMENT (Fig 114-16)
When CI is diagnosed and physical examination does not suggest gangrene or perforation, the patient is treated expectantly. Parenteral fluids are administered and the bowel is placed at rest. Broad-spectrum antibiotics are given to cover the fecal flora because in experimental models, antibiotics reduce the extent and severity of bowel damage. No randomized, controlled, blinded trials have been done to prove the validity of this recommendation. An electrocardiogram, Holter monitoring, and transthoracic echocardiogram should be obtained to exclude or confirm a cardiac source of embolism. Patients with segmental, nongangrenous CI undergoing such evaluation are 2.5 times more likely to have their cardiac risk factor identified compared with other patients with CI.80 Cardiac failure and arrhythmias are treated, and medications that can cause mesenteric vasoconstriction are withdrawn. If the colon appears distended, it is decompressed with a rectal tube. Serial imaging tests of the colon and continued monitoring of the hemoglobin level, WBC count, and electrolyte levels are indicated until the patient’s condition stabilizes.
In more than half of patients with CI, the disease is reversible. Generally, the symptoms of CI resolve within 48 to 72 hours and the colon heals in one to two weeks. With severe injury, it can take one to six months for the colon to heal; however, during this time the patient is usually asymptomatic. A retrospective study of 350 patients with biopsy-proven CI showed that those with isolated right-sided colon ischemia had a worse outcome than those with CI isolated to other segments, including a five-fold increase in the need for surgery and a two-fold increase in mortality.54 Another retrospective study showed that patients’ ages, leukocyte counts, lactate dehydrogenase levels, blood lactate levels, and absence of vascular flow to the colonic wall on abdominal Doppler ultrasonography were independent predictors of complicated CI; only absence of arterial flow was a significant predictor of complicated disease when confounding for other factors.81 Symptoms that persist for more than two weeks also are associated with a higher incidence of acute complications and irreversible disease: gangrene and perforation, segmental ulcerating colitis, or stricture.
SPECIAL CLINICAL PROBLEMS
Isolated Ischemia of the Right Colon
Ischemia that only involves the right side of the colon (IRCI) recently has been shown to occur in 23% of cases, an incidence more than twice the conventionally accepted incidence of 8% to 10%.54,76 Such a pattern of involvement is important to document, because these patients require surgery twice as often as and have a mortality rate five times greater than patients with involvement of other areas of the colon, including those in whom the right side is involved synchronously with other segments (surgery: 54.9% vs. 10.9%; 30-day mortality 22.5% vs. 11.9%).76 Because the SMA supplies blood to the right side of the colon—as well as to the small intestine—and because a substantial number of patients with IRCI have it as the heralding presentation of otherwise silent SMA obstructive disease, we currently recommend evaluating the splanchnic vasculature in these patients. A CTA will identify patients at risk for AMI and, if an SMA occlusion is found, revascularization should be strongly considered. IRCI is an exception to our general practice of not evaluating the splanchnic vascular system in a patient with CI.
Colon Ischemia in Patients with Carcinoma of the Colon and Other Potentially Obstructive Lesions
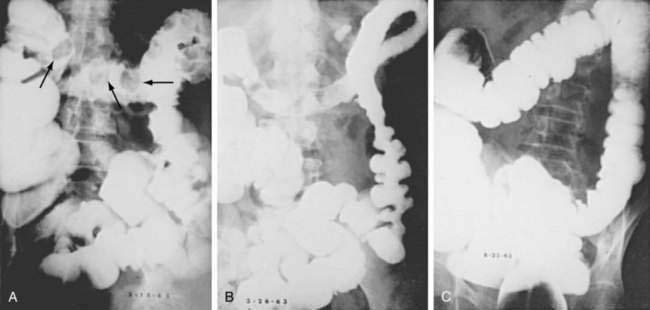
Less than 5% of patients with CI have a distal and potentially obstructing lesion or disorder, including carcinoma of the colon, diverticulitis, volvulus, fecal impaction, postoperative stricture, prior ischemic stenosis, or radiation stricture. Typically, the associated lesion is distal, and there is a segment of normal colon between the distal lesion and the proximal colitis (Fig. 114-17). The mechanism of this association may involve increased intracolonic pressure proximal to the lesion with resultant decreased colon blood flow.
Colon Ischemia in Irritable Bowel Syndrome
CI occurs 3.4 to 3.9 times more frequently in the presence of IBS than without it.55,82 Although some authors believe that patients with IBS visit their doctor more frequently and therefore are more likely to have CI diagnosed than the general population, others hypothesize there is a common pathophysiology in patients with IBS, such as hypersensitivity of the colonic vasculature, autonomic hyper-responsiveness, or differences in the sensitivity of serotonin receptors. Clinical studies and experimental models have shown that alosetron increases risk for CI (see earlier73,83,84). The incidence of CI, however, has been shown to be higher in IBS patients regardless of therapy used.85 More studies, better data, and a greater understanding of the mechanisms of actions of serotoninergic agents are needed to further elucidate any such association.
Colon Ischemia Complicating Aortic Surgery
CI complicates elective aortic surgery in up to 7% of cases and surgery for ruptured abdominal aortic aneurysms in up to 60% of cases.86 CI is responsible for approximately 10% of the deaths after aortic replacement. Factors that contribute to postoperative CI include rupture of aneurysm, hypotension, operative trauma to the colon, hypoxemia, arrhythmias, prolonged cross-clamp time, and improper management of the IMA during aneurysmectomy. Tonometric determination of intramural pH of the sigmoid before and after cross-clamping the aorta has been used successfully to predict which patients will develop CI after aneurysmectomy.87
CHRONIC MESENTERIC ISCHEMIA (INTESTINAL ANGINA)
CMI is uncommon, accounting for less than 5% of intestinal ischemic diseases; it almost always is caused by mesenteric atherosclerosis, although rare causes such as collagen vascular disease and inflammatory vasculopathy are known. There is no specific association between CMI and smoking, although 75% of patients with CMI have a history of smoking.88 Abdominal pain is likely caused by ischemia in the small intestine as blood is stolen from this organ to meet the increased demand for gastric blood flow as food enters the stomach.89 This rationale for why the pain occurs so soon after eating, when food still remains in the stomach, is preferable to the traditional explanation that a fixed and limited blood supply is incapable of meeting the increased metabolic demands of the small intestine during digestion.
DIAGNOSIS
Ultrasonography, MR angiography, and even traditional mesenteric angiography all merely reveal morphologic abnormalities and anatomic limitations of splanchnic blood flow; they do not establish the presence or absence of intestinal ischemia. Duplex ultrasonography can be used to identify splanchnic artery stenoses but not to establish the diagnosis of CMI.90 Elevated peak systolic velocity in the SMA and CA of 275 and 200 cm/sec, respectively, is a reliable sign of at least 70% stenosis of these vessels.91 Duplex ultrasonography and phase-contrast cine MR imaging of the SMA and CA have been used to measure the effect of eating on mesenteric blood flow, all based on the principle that eating normally increases blood flow to the small intestine, whereas in CMI, this fails to occur; however, postprandial studies are no better than fasting examinations, especially at lesser degrees of vascular stenosis.91
Gastric tonometry exercise testing (GET) uses a nasogastric tube and peripheral arterial catheter to obtain gastric juice and arterial blood from a fasting patient who has been given acid-suppression medication. Determination is made of the gastric-arterial Pco2 gradient before, during, and after exercise. An increase in the gradient after exercise is an indicator of gastrointestinal ischemia. In a study of the diagnostic accuracy for chronic gastrointestinal ischemia, GET and duplex sonography, alone and in combination, were compared with the gold standard, angiography. Combined GET and duplex sonography would not have missed any patients with symptomatic gastrointestinal ischemia; therefore, they are currently believed to constitute the best diagnostic workup strategy.92 More experience with these provocative tests is needed, however, before firm conclusions can be made about their diagnostic usefulness.
In the absence of any specific, reliable diagnostic test, the diagnosis of CMI is based on clinical symptoms, in combination with radiologic demonstration of an occlusive process of the splanchnic vessels, and, to a great measure, the exclusion of other gastrointestinal disorders. Angiography should show occlusion of two or more splanchnic arteries to allow the diagnosis of CMI; however, such occlusions, even of all three vessels, do not by themselves make the diagnosis of CMI, because they may be present with no corresponding clinical symptoms. In most patients with CMI, at least two of the three splanchnic vessels either are completely obstructed or severely stenotic. In a review of series of patients with CMI,88 91% had occlusion of at least two vessels and 55% had involvement of all three; 7% and 2% had isolated occlusion of the SMA and CA, respectively.
TREATMENT
CMI is not considered to require urgent therapy, although acute complete occlusion of the gastrointestinal blood supply can occur if thrombosis is superimposed on already narrowed arteries. A patient with the typical pain of CMI and unexplained weight loss whose diagnostic evaluation has excluded other gastrointestinal disease and whose angiogram shows occlusive involvement of at least two of the three major arteries should undergo revascularization (Fig. 114-18).
The true efficacy of surgical revascularization and PTMA is difficult to determine because of the varied criteria used by different investigators to define a successful outcome. Thus, some authors use graft or vessel patency rates, whereas others define success by relief of symptoms, recurrence rates, or long-term survival. A tabulation of 17 series of surgical revascularization for CMI totaling 614 patients yielded perioperative mortality rates that ranged from 0%93–98 to 16%,99 success rates of 59%100 to 100%,94–98 and recurrence rates of 0%85,93,94,99,101,102 to 26.5%.103 Most recent series have reported mortality rates less than 10%, success rates of more than 90%, and recurrence rates generally less than 10%.19 Several long-term studies have shown that patients surviving surgical revascularization have cumulative 5-year survival rates of approximately 80% to 90%.19
The rates of success for PTMA are similar to those for surgical revascularization. The experience with PTMA is more limited but has been achieved in patients often considered too high risk for a surgical procedure. In 10 representative series of PTMA for CMI, totaling 128 patients, clinical success rates (i.e., relief of symptoms) have varied from 63%104 to 100%,105 with little mortality.19 Recurrence of symptoms, however, has been much higher than after surgical revascularization, varying from 10% to 67% in the larger series.19 Intraluminal stenting is an adjunctive treatment to PTMA and attempts to decrease the incidence of recurrent stenoses. Percutaneous transluminal mesenteric angioplasty with stenting (PTMAS) results in the same short-term patency rates for both stenotic and occluded vessels.106 Long-term studies of PTMAS patency rates indicate that approximately 70% are patent at seven years and 56% of patients are free from recurrent symptoms. When compared with open surgical bypass, PTMAS shows a decreased primary patency, decreased primary assisted patency, and earlier required reinterventions.107
VASCULITIS AND ANGIOPATHY OF THE SPLANCHNIC CIRCULATION
Inflammation and necrosis can affect splanchnic blood vessels of all sizes: arteries, veins, the vasa recta, arterioles, and venules.108 Symptoms depend on the size of the involved vessel and may be indistinguishable from AMI caused by emboli or thromboses; associated systemic features such as renal failure, cutaneous nodules, and pulmonary infiltrates suggest a systemic disorder.
ALLERGIC GRANULOMATOUS ANGIITIS (CHURG-STRAUSS SYNDROME)
Allergic granulomatous angiitis is a disorder that is typified by asthma, glomerulonephritis, eosinophilia, and granulomatous inflammation associated with antineutrophil cytoplasmic autoantibodies.109 Necrotizing vasculitis affects small and medium-sized vessels and involves the gastrointestinal tract in almost half the patients. As in other vasculitides, abdominal pain and bleeding secondary to ischemia are the usual manifestations. Glucocorticoid therapy usually is effective.
BEHÇET’S DISEASE
Behçet’s disease (see Chapter 35) is characterized by oral and genital ulcers, recurrent iritis or chorioretinitis, and skin lesions. It is most often seen in Eastern Mediterranean men and is strongly associated with the B51 allele. Small-vessel vasculitis accounts for much of the damage, but large-vessel involvement of arteries and veins is not uncommon. Gastrointestinal disease, present in 50% of patients, typically involves the ileocecal area, although involvement of the esophagus and small intestine has been reported.110 Attacks are recurrent and usually self-limited except for the uveitis which may be chronic. The most common gastrointestinal symptoms are abdominal pain, diarrhea, and bleeding; deep ulcers are responsible for the most common intestinal complications: severe bleeding and perforation. Mortality is low in Behçet’s disease; however, intestinal perforation is one of the common causes of death. Therapy with glucocorticoids, immunosuppressive agents, and colchicine has been tried, with varying success.
BUERGER’S DISEASE
Also called thromboangiitis obliterans, Buerger’s disease involves medium-sized and small peripheral arteries and veins, especially the infrapopliteal vessels; foot claudication and rubor are the most common symptoms. It is largely a disease of men, especially those who have smoked cigarettes from an early age, and typically has its onset before the age of 50 years; there is a distinct absence of other atherosclerotic risk factors. Intestinal involvement is unusual, but most common is involvement of the vessels supplying the small intestine.111 In the acute lesion, inflammation spreads outward from the thrombus-endothelium interface through the vessel wall. Later, microabscesses, necrotizing granulomas, and multinucleated giant cells occur in the thrombus, after which the thrombus organizes and becomes occlusive. Intestinal involvement usually requires resection.
COGAN’S SYNDROME
Cogan’s syndrome is a rare disorder of young people characterized by vasculitis of the conjunctiva, cornea, and cochlea.112 Although this vasculitis usually is localized, it is considered to be a hypersensitivity reaction to an unknown viral agent, and the disease can become disseminated. Three percent to 10% of patients develop gastrointestinal symptoms, with diarrhea and bloody stools. High-dose glucocorticoids, and occasionally cytotoxic agents, are required. Vascular surgery may be needed after inflammation is controlled.
FIBROMUSCULAR DYSPLASIA
FMD is a rare angiopathy that is neither related to atherosclerosis nor to inflammation.113 Its cause is unknown, although genetic factors might play a role and there is an association with cigarette smoking and hypertension. There are several types of FMD depending on which arterial layer is involved: intima, media, or adventitia. The renal arteries are most commonly involved, followed by the carotid and vertebral arteries and then other vasculature including the mesenteric arteries. Splanchnic involvement can manifest with symptoms of AMI or CMI.114 Diagnosis is based on the same techniques used to image other vascular disorders; the classic string-of-beads appearance is typical of only the medial type of FMD, whereas aneurysms and dissection are known complications of all types. Therapy consists of percutaneous transluminal angioplasty, surgical revascularization, and resection of necrotic bowel as indicated.
HENOCH-SCHÖNLEIN PURPURA
Henoch-Schönlein purpura (see Chapter 35) typically affects children aged 4 to 7 years. It is characterized by IgA immune complexes deposited within the small vessels of the skin, gastrointestinal tract, joints, and kidneys and is often preceded by an upper respiratory infection. The classic clinical triad consists of palpable purpura (usually below the waist), arthritis (knees and ankles), and abdominal pain; the gastrointestinal tract is involved in up to 75% of patients.115 Abdominal pain and gastrointestinal bleeding are the most common gastrointestinal symptoms and are caused by mucosal and submucosal hemorrhage; a submucosal hematoma may be the lead point of an intussusception. Gastrointestinal involvement may be documented by endoscopy116 or by CT study.117 The disease is usually self-limited, but the outlook may be less favorable in adults, in large measure because of the development of renal failure.
KAWASAKI’S DISEASE
Kawasaki’s disease, also called infantile febrile mucocutaneous lymph node syndrome, is a necrotizing vasculitis of medium-sized arteries.118 It manifests as fever, rash on the palms and soles, desquamation, conjunctival congestion, strawberry tongue, and cervical lymphadenopathy in infants and children. Many patients have nausea, vomiting, abdominal pain, and diarrhea, and they can suffer ileus, small bowel obstruction, bleeding, and perforation. Death may be due to coronary artery aneurysms and myocardial infarction. Treatment is aspirin for the acute phase and large intravenous doses of gamma globulin for the prevention of coronary artery aneurysms.
KÖHLMEIER-DEGOS DISEASE (MALIGNANT ATROPHIC PAPULOSIS)
Köhlmeier-Degos disease is a rare form of progressive occlusive vascular disease of young men that affects the small and medium-sized arteries, mainly those of the skin and intestine.119 Typically, skin lesions of porcelain-white punctate scars with erythematous borders are found on the trunk and upper extremities. The rash is followed, within months to years, by the development of abdominal pain and spontaneous intestinal perforation. Thrombosis of small and medium-sized vessels is found, without inflammatory cell infiltration. There is no known therapy for this disease, and it is generally fatal.
POLYARTERITIS NODOSA
Polyarteritis nodosa (see Chapter 35) is a necrotizing vasculitis of medium-sized and small arteries characterized by aneurysms at branch points. Abdominal symptoms are reported in up to half of patients with the disorder, the most common of which is abdominal pain, usually from ischemia.120 Involvement of the small intestine is most common, followed by lesions of colon, liver, and pancreas. Diagnosis is suggested by typical angiographic findings of aneurysms in the mesenteric, renal, and hepatic vasculature. Treatment with glucocorticoids and cyclophosphamide or azathioprine has improved survival greatly.
Vasculitis resembling polyarteritis also is associated with hepatitis B and C virus infections.121,122 Fifty percent of patients with classic polyarteritis are hepatitis B surface antigen positive, but unlike in classic polyarteritis, only small arteries are involved. Patients develop a polyarteritis picture following the viral infection, presumably from deposition of antigen-associated immune complexes on the vessel wall.
RHEUMATOID VASCULITIS
Rheumatoid vasculitis affects the gastrointestinal tract in approximately 10% of patients, usually those who have subcutaneous nodules and who are seropositive for rheumatoid factor.123 As with all vasculitides, ischemia manifests with abdominal pain, bleeding, perforation, and gangrene. Other diseases have been noted in association with rheumatoid arthritis, including atrophic gastritis, gastric antral vascular ectasia, inflammatory bowel disease, collagenous colitis, and amyloidosis.
SYSTEMIC LUPUS ERYTHEMATOSUS
Systemic lupus erythematosus (see Chapter 35) affects the gastrointestinal system in about half of cases and can involve any of the hollow and solid gastrointestinal organs.108 The most common symptoms are nausea, vomiting, and abdominal pain, but diarrhea, malabsorption, pseudo-obstruction, peritonitis, pancreatitis, protein-losing enteropathy, and ascites are also well-known occurrences. The systemic nature of the disorder makes differential diagnosis complicated, but vasculitis-induced ischemia underlies many of the presentations. The vasculitis typically involves small vessels and causes FSI and gastrointestinal bleeding, both of which are associated with high mortality rates if not diagnosed promptly.
TAKAYASU’S DISEASE
Takayasu’s disease (pulseless disease) is an idiopathic chronic inflammatory disorder that most often affects the aorta and its branches in young women of Asian heritage; it is unusual that the splanchnic vessels are involved.124 Fibrotic occlusion of the involved vessels is the end result of the inflammatory process. Takayasu’s disease rarely has been associated with Crohn’s disease and ulcerative colitis, and in the serum of some of these patients, antibodies to colonic mucosa and aorta have been detected. Treatment is large doses of glucocorticoids before reconstructive surgery. The five-year survival rate is higher than 90%.
Acosta S, Ogren M, Sternby NH, et al. Clinical implications for the management of acute thromboembolic occlusion of the superior mesenteric artery: autopsy findings in 213 patients. Ann Surg. 2005;241:516-22. (Ref 4.)
Acosta-Merida MA, Marchena-Gomez J, Hemmersbach-Miller M, et al. Mesenteric venous thrombosis. Associated systemic disorders and hypercoagulability status of 21 surgical patients. Hepatogastroenterology. 2007;54:1080-4. (Ref 36.)
Atkins MD, Kwolek CJ, LaMuraglia GM, et al. Surgical revascularization versus endovascular therapy for chronic mesenteric ischemia: a comparative experience. J Vasc Surg. 2007;45:1162-71. (Ref 107.)
Boley SJ, Feinstein FR, Sammartano R, et al. New concepts in the management of emboli of the superior mesenteric artery. Surg Gynecol Obstet. 1981;153:561-9. (Ref 31.)
Boley SJ, Sprayregan S, Siegelman SS, Veith FJ. Initial results from an agressive roentgenological and surgical approach to acute mesenteric ischemia. Surgery. 1977;82:848-55. (Ref 30.)
Brandt LJ, Boley SJ. AGA technical review on intestinal ischemia. American Gastrointestinal Association. Gastroenterology. 2000;118:954-68. (Ref 19.)
Cole JA, Cook SF, Sands BE, et al. Occurrence of colon ischemia in relation to irritable bowel syndrome. Am J Gastroenterol. 2004;99:486-91. (Ref 55.)
Hass DJ, Kozuch P, Brandt LJ. Pharmacologically mediated colon ischemia. Am J Gastroenterol. 2007;102:1765-80. (Ref 56.)
Higgins PD, Davis KJ, Laine L. Systematic review: The epidemiology of ischaemic colitis. Aliment Pharmacol Ther. 2004;19:729-38. (Ref 82.)
Kougias P, Lau D, El Sayed HF, et al. Determinants of mortality and treatment outcome following surgical interventions for acute mesenteric ischemia. J Vasc Surg. 2007;46:467-74. (Ref 20.)
Lee SS, Ha HK, Park SH, et al. Usefulness of computed tomography in differentiating transmural infarction from nontransmural ischemia of the small intestine in patients with acute mesenteric venous thrombosis. J Comput Assist Tomogr. 2008;32:730-7. (Ref 45.)
Otte JA, Geelkerken RH, Huisman AB, Kolkman JJ. What is the best diagnostic approach for chronic gastrointestinal ischemia? Am J Gastroenterol. 2007;102:2005-10. (Ref 92.)
Poole JW, Sammartano RJ, Boley SJ. Hemodynamic basis of the pain of chronic mesenteric ischemia. Am J Surg. 1987;153:171-6. (Ref 89.)
Sotiriadis J, Brandt LJ, Behin DS, Southern WN. Ischemic colitis has a worse prognosis when isolated to the right side of the colon. Am J Gastroenterol. 2007;102:2247-52. (Ref 54.)
Zandrino F, Musante F, Gallesio I, Benzi L. Assessment of patients with acute mesenteric ischemia: multislice computed tomography signs and clinical performance in a group of patients with surgical correlation. Minerva Gastroenterol Dietol. 2006;52:317-25. (Ref 15.)
1. Kornblith PL, Boley SJ, Whitehouse BS. Anatomy of the splanchnic circulation. Surg Clin North Am. 1992;72:1-30.
2. Boley SJ, Treiber W, Winslow PR, et al. Circulatory responses to acute reduction of superior mesenteric arterial blood flow. Physiologist. 1969;12:180.
3. Zimmerman BJ, Granger DN. Reperfusion injury. Surg Clin North Am. 1992;72:65-83.
4. Acosta S, Ogren M, Sternby NH, et al. Clinical implications for the management of acute thromboembolic occlusion of the superior mesenteric artery: autopsy findings in 213 patients. Ann Surg. 2005;241:516-22.
5. Finucane PM, Arunachalam T, O’Dowd J, Pathy MS. Acute mesenteric infarction in elderly patients. J Am Geriatr Soc. 1989;37:355-8.
6. Gaussorgues P, Gueugniaud PY, Vedrinne JM, et al. Bacteremia following cardiac arrest and cardiopulmonary resuscitation. Intensive Care Med. 1988;14:575-7.
7. Kurland B, Brandt LJ, Delany HM. Diagnostic tests for intestinal ischemia. Surg Clin North Am. 1992;72:85-105.
8. Khurana S, Corbally MT, Manning F, et al. Glutathione S-transferase: a potential new marker of intestinal ischemia. J Pediatr Surg. 2002;37:1543-8.
9. Kanda T, Fujii H, Tani T, et al. Intestinal fatty acid–binding protein is a useful diagnostic marker for mesenteric infarction in humans. Gastroenterology. 1996;110:339-43.
10. Ritz JP, Runkel N, Berger G, Buhr HJ. [Prognostic factors in mesenteric infarct]. Zentralbl Chir. 1997;122:332-8.
11. Yamada K, Saeki M, Yamaguchi T, et al. Acute mesenteric ischemia. CT and plain radiographic analysis of 26 cases. Clin Imaging. 1998;22:34-41.
12. Lee R, Tung HK, Tung PH, et al. CT in acute mesenteric ischaemia. Clin Radiol. 2003;58:279-87.
13. Taourel PG, Deneuville M, Pradel JA, et al. Acute mesenteric ischemia: diagnosis with contrast-enhanced CT. Radiology. 1996;199:632-6.
14. Smerud MJ, Johnson CD, Stephens DH. Diagnosis of bowel infarction: a comparison of plain films and CT scans in 23 cases. AJR Am J Roentgenol. 1990;154:99-103.
15. Zandrino F, Musante F, Gallesio I, Benzi L. Assessment of patients with acute mesenteric ischemia: multislice computed tomography signs and clinical performance in a group of patients with surgical correlation. Minerva Gastroenterol Dietol. 2006;52:317-25.
16. Kirkpatrick ID, Kroeker MA, Greenberg HM. Biphasic CT with mesenteric CT angiography in the evaluation of acute mesenteric ischemia: initial experience. Radiology. 2003;229:91-8.
17. Li KC, Pelc LR, Dalman RL, et al. In vivo magnetic resonance evaluation of blood oxygen saturation in the superior mesenteric vein as a measure of the degree of acute flow reduction in the superior mesenteric artery: findings in a canine model. Acad Radiol. 1997;4:21-5.
18. Hagspiel KD, Leung DA, Angle JF, et al. MR angiography of the mesenteric vasculature. Radiol Clin North Am. 2002;40:867-86.
19. Brandt LJ, Boley SJ. AGA technical review on intestinal ischemia. American Gastrointestinal Association. Gastroenterology. 2000;118:954-68.
20. Kougias P, Lau D, El Sayed HF, et al. Determinants of mortality and treatment outcome following surgical interventions for acute mesenteric ischemia. J Vasc Surg. 2007;46:467-74.
21. Jamieson WG, Pliagus G, Marchuk S, et al. Effect of antibiotic and fluid resuscitation upon survival time in experimental intestinal ischemia. Surg Gynecol Obstet. 1988;167:103-8.
22. Horgan PG, Gorey TF. Operative assessment of intestinal viability. Surg Clin North Am. 1992;72:143-55.
23. Gangadharan SP, Wagner RJ, Cronenwett JL. Effect of intravenous glucagon on intestinal viability after segmental mesenteric ischemia. J Vasc Surg. 1995;21:900-7.
24. Khanna A, Rossman J, Caty MG, Fung HL. Beneficial effects of intraluminal nitroglycerin in intestinal ischemia-reperfusion injury in rats. J Surg Res. 2003;114:15-24.
25. Hollingshead M, Burke CT, Mauro MA, et al. Transcatheter thrombolytic therapy for acute mesenteric and portal vein thrombosis. J Vasc Interv Radiol. 2005;16:651-61.
26. Schoots IG, Levi MM, Reekers JA, et al. Thrombolytic therapy for acute superior mesenteric artery occlusion. J Vasc Interv Radiol. 2005;16:317-29.
27. Schoenbaum SW, Pena C, Koenigsberg P, Katzen BT. Superior mesenteric artery embolism: treatment with intraarterial urokinase. J Vasc Interv Radiol. 1992;3:485-90.
28. Boley SJ, Sammartino RJ, Brandt LJ, et al. Vasodilators and thrombolytic agents in experimental superior mesenteric artery embolus. Gastroenterology. 1982;82:1021.
29. Archodovassilis F, Lagoudiannakis EE, Tsekouras DK, et al. Nonocclusive mesenteric ischemia: a lethal complication in peritoneal dialysis patients. Perit Dial Int. 2007;27:136-41.
30. Boley SJ, Sprayregan S, Siegelman SS, Veith FJ. Initial results from an agressive roentgenological and surgical approach to acute mesenteric ischemia. Surgery. 1977;82:848-55.
31. Boley SJ, Feinstein FR, Sammartano R, et al. New concepts in the management of emboli of the superior mesenteric artery. Surg Gynecol Obstet. 1981;153:561-9.
32. Clark RA, Gallant TE. Acute mesenteric ischemia: angiographic spectrum. AJR Am J Roentgenol. 1984;142:555-62.
33. Boos S. [Angiography of the mesenteric artery 1976 to 1991. A change in the indications during mesenteric circulatory disorders?]. Radiologe. 1992;32:154-7.
34. Bottger T, Schafer W, Weber W, Junginger T. [Value of preoperative diagnosis in mesenteric vascular occlusion. A prospective study]. Langenbecks Arch Chir. 1990;375:278-82.
35. Czerny M, Trubel W, Claeys L, et al. [Acute mesenteric ischemia]. Zentralbl Chir. 1997;122:538-44.
36. Acosta-Merida MA, Marchena-Gomez J, Hemmersbach-Miller M, et al. Mesenteric venous thrombosis. Associated systemic disorders and hypercoagulability status of 21 surgical patients. Hepatogastroenterology. 2007;54:1080-4.
37. Acosta S, Alhadad A, Svensson P, Ekberg O. Epidemiology, risk and prognostic factors in mesenteric venous thrombosis. Br J Surg. 2008;95:1245-51.
38. Harward TR, Green D, Bergan JJ, et al. Mesenteric venous thrombosis. J Vasc Surg. 1989;9:328-33.
39. Morasch MD, Ebaugh JL, Chiou AC, et al. Mesenteric venous thrombosis: a changing clinical entity. J Vasc Surg. 2001;34:680-4.
40. Clavien PA, Durig M, Harder F. Venous mesenteric infarction: a particular entity. Br J Surg. 1988;75:252-5.
41. Font VE, Hermann RE, Longworth DL. Chronic mesenteric venous thrombosis: difficult diagnosis and therapy. Cleve Clin J Med. 1989;56:823-8.
42. Warshaw AL, Jin GL, Ottinger LW. Recognition and clinical implications of mesenteric and portal vein obstruction in chronic pancreatitis. Arch Surg. 1987;122:410-15.
43. Clavien PA, Huber O, Mirescu D, Rohner A. Contrast enhanced CT scan as a diagnostic procedure in mesenteric ischaemia due to mesenteric venous thrombosis. Br J Surg. 1989;76:93-4.
44. Al Karawi MA, Quaiz M, Clark D, et al. Mesenteric vein thrombosis, non-invasive diagnosis and followup (US + MRI) and non-invasive therapy by streptokinase and anti-coagulants. Hepatogastroenterology. 1990;37:507.
45. Lee SS, Ha HK, Park SH, et al. Usefulness of computed tomography in differentiating transmural infarction from nontransmural ischemia of the small intestine in patients with acute mesenteric venous thrombosis. J Comput Assist Tomogr. 2008;32:730-7.
46. Bradbury MS, Kavanagh PV, Chen MY, et al. Noninvasive assessment of portomesenteric venous thrombosis: current concepts and imaging strategies. J Comput Assist Tomogr. 2002;26:392-404.
47. Cho YP, Jung SM, Han MS, et al. Role of diagnostic laparoscopy in managing acute mesenteric venous thrombosis. Surg Laparosc Endosc Percutan Tech. 2003;13:215-17.
48. Rhee RY, Gloviczki P, Mendonca CT, et al. Mesenteric venous thrombosis: still a lethal disease in the 1990s. J Vasc Surg. 1994;20:688-97.
49. Grieshop RJ, Dalsing MC, Cikrit DF, et al. Acute mesenteric venous thrombosis. Revisited in a time of diagnostic clarity. Am Surg. 1991;57:573-7.
50. Brunaud L, Antunes L, Collinet-Adler S, et al. Acute mesenteric venous thrombosis: case for nonoperative management. J Vasc Surg. 2001;34:673-9.
51. Orr DW, Harrison PM, Devlin J, et al. Chronic mesenteric venous thrombosis: evaluation and determinants of survival during long-term follow-up. Clin Gastroenterol Hepatol. 2007;5:80-6.
52. Sakai L, Keltner R, Kaminski D. Spontaneous and shock-associated ischemic colitis. Am J Surg. 1980;140:755-60.
53. Guttormson NL, Burbrick MP. Mortality from ischemic colitis. Dis Colon Rectum. 1989;32:469-72.
54. Sotiriadis J, Brandt LJ, Behin DS, Southern WN. Ischemic colitis has a worse prognosis when isolated to the right side of the colon. Am J Gastroenterol. 2007;102:2247-52.
55. Cole JA, Cook SF, Sands BE, et al. Occurrence of colon ischemia in relation to irritable bowel syndrome. Am J Gastroenterol. 2004;99:486-91.
56. Hass DJ, Kozuch P, Brandt LJ. Pharmacologically mediated colon ischemia. Am J Gastroenterol. 2007;102:1765-80.
57. Moulis H, Vender RJ. Antibiotic-associated hemorrhagic colitis. J Clin Gastroenterol. 1994;18:227-31.
58. Tashiro M, Yoshikawa I, Kume K, Otsuki M. Ischemic colitis associated with paclitaxel and carboplatin chemotherapy. Am J Gastroenterol. 2003;98:231-2.
59. Sollott SJ, Cheng L, Pauly RR, et al. Taxol inhibits neointimal smooth muscle cell accumulation after angioplasty in the rat. J Clin Invest. 1995;95:1869-76.
60. Walker AM, Bohn RL, Cali C, et al. Risk factors for colon ischemia. Am J Gastroenterol. 2004;99:1333-7.
61. Sharefkin JB, Silen W. Diuretic agents: inciting factor in nonocclusive mesenteric infarction? JAMA. 1974;229:1451-3.
62. Deana DG, Dean PJ. Reversible ischemic colitis in young women. Association with oral contraceptive use. Am J Surg Pathol. 1995;19:454-62.
63. Beyer KL, Bickel JT, Butt JH. Ischemic colitis associated with dextroamphetamine use. J Clin Gastroenterol. 1991;13:198-201.
64. Fishel R, Hamamoto G, Barbul A, et al. Cocaine colitis. Is this a new syndrome? Dis Colon Rectum. 1985;28:264-6.
65. Boutros HH, Pautler S, Chakrabarti S. Cocaine-induced ischemic colitis with small-vessel thrombosis of colon and gallbladder. J Clin Gastroenterol. 1997;24:49-53.
66. Rogers FB, Li SC. Acute colonic necrosis associated with sodium polystyrene sulfonate (Kayexalate) enemas in a critically ill patient: case report and review of the literature. J Trauma. 2001;51:395-7.
67. Rashid A, Hamilton SR. Necrosis of the gastrointestinal tract in uremic patients as a result of sodium polystyrene sulfonate (Kayexalate) in sorbitol: an underrecognized condition. Am J Surg Pathol. 1997;21:60-9.
68. Oh JK, Meiselman M, Lataif LEJr. Ischemic colitis caused by oral hyperosmotic saline laxatives. Gastrointest Endosc. 1997;45:319-22.
69. Lopez Morra HA, Fine SN, Dickstein G. Colonic ischemia with laxative use in young adults. Am J Gastroenterol. 2005;100:2134-6.
70. Chang RY, Tsai CH, Chou YS, Wu TC. Nonocclusive ischemic colitis following glycerin enema in a patient with coronary artery disease. A case report. Angiology. 1995;46:747-52.
71. Gleeson M, Ramsay D, Hutchinson S, et al. Colitis associated with non-steroidal anti-inflammatory drugs. Lancet. 1994;344:1028.
72. Beck IT. Possible mechanisms for ischemic colitis during alosetron therapy. Gastroenterology. 2001;121:231-2.
73. Knudsen JF, Friedman B, Chen M, Goldwasser JE. Ischemic colitis and sumatriptan use. Arch Intern Med. 1998;158:1946-8.
74. Chang L, Chey WD, Harris L, et al. Incidence of ischemic colitis and serious complications of constipation among patients using alosetron: systematic review of clinical trials and post-marketing surveillance data. Am J Gastroenterol. 2006;101:1069-79.
75. Brandt L, Boley S, Goldberg L, et al. Colitis in the elderly. A reappraisal. Am J Gastroenterol. 1981;76:239-45.
76. Blaszka M, Brandt L. Patterns of involvement in 350 cases of biopsy-proven ischemic colitis. Am J Gastroenterol. 2008;103:s193.
77. Balthazar EJ, Yen BC, Gordon RB. Ischemic colitis: CT evaluation of 54 cases. Radiology. 1999;211:381-8.
78. Church JM. Ischemic colitis complicating flexible endoscopy in a patient with connective tissue disease. Gastrointest Endosc. 1995;41:181-2.
79. Zuckerman GR, Prakash C, Merriman RB, et al. The colon single-stripe sign and its relationship to ischemic colitis. Am J Gastroenterol. 2003;98:2018-22.
80. Hourmand-Ollivier I, Bouin M, Saloux E, et al. Cardiac sources of embolism should be routinely screened in ischemic colitis. Am J Gastroenterol. 2003;98:1573-7.
81. Danse EM, Van Beers BE, Jamart J, et al. Prognosis of ischemic colitis: comparison of color Doppler sonography with early clinical and laboratory findings. AJR Am J Roentgenol. 2000;175:1151-4.
82. Higgins PD, Davis KJ, Laine L. Systematic review: the epidemiology of ischaemic colitis. Aliment Pharmacol Ther. 2004 Apr 1;19:729-38.
83. Holzer P, Painsipp E, Weber E. 5HT3 receptor antagonists, alosetron and cilansetron, impair mesenteric blood flow in rats. Gastroenterology. 2003;124:A148-9.
84. FDA Gastrointestinal Drugs Advisory Committee and Drug Safety and Risk Management Subcommitte Meeting. In: Administration FaD, editor.; April 23, 2002.
85. Singh G, Mithal A, Triadafilopoulos G. Patients with irritable bowel syndrome have a high risk of developing ischemic colitis. Gastroenterology. 2004;126:A41. [Abstract 349]
86. Zelenock GB, Strodel WE, Knol JA, et al. A prospective study of clinically and endoscopically documented colonic ischemia in 100 patients undergoing aortic reconstructive surgery with aggressive colonic and direct pelvic revascularization, compared with historic controls. Surgery. 1989;106:771-9.
87. Schiedler MG, Cutler BS, Fiddian-Green RG. Sigmoid intramural pH for prediction of ischemic colitis during aortic surgery. A comparison with risk factors and inferior mesenteric artery stump pressures. Arch Surg. 1987;122:881-6.
88. Moawad J, Gewertz BL. Chronic mesenteric ischemia. Clinical presentation and diagnosis. Surg Clin North Am. 1997;77:357-69.
89. Poole JW, Sammartano RJ, Boley SJ. Hemodynamic basis of the pain of chronic mesenteric ischemia. Am J Surg. 1987;153:171-6.
90. Moneta GL. Screening for mesenteric vascular insufficiency and follow-up of mesenteric artery bypass procedures. Semin Vasc Surg. 2001;14:186-92.
91. Gentile AT, Moneta GL, Lee RW, et al. Usefulness of fasting and postprandial duplex ultrasound examinations for predicting high-grade superior mesenteric artery stenosis. Am J Surg. 1995;169:476-9.
92. Otte JA, Geelkerken RH, Huisman AB, Kolkman JJ. What is the best diagnostic approach for chronic gastrointestinal ischemia? Am J Gastroenterol. 2007;102:2005-10.
93. Beebe HG, MacFarlane S, Raker EJ. Supraceliac aortomesenteric bypass for intestinal ischemia. J Vasc Surg. 1987;5:749-54.
94. Calderon M, Reul GJ, Gregoric ID, et al. Long-term results of the surgical management of symptomatic chronic intestinal ischemia. J Cardiovasc Surg (Torino). 1992;33:723-8.
95. Christensen MG, Lorentzen JE, Schroeder TV. Revascularization of atherosclerotic mesenteric arteries: Experience in 90 consecutive patients. Eur J Vasc Surg. 1994;8:297-302.
96. Gentile AT, Moneta GL, Taylor LMJr, et al. Isolated bypass to the superior mesenteric artery for intestinal ischemia. Arch Surg. 1994;129:926-31.
97. Johnston KW, Lindsay TF, Walker PM, Kalman PG. Mesenteric arterial bypass grafts: early and late results and suggested surgical approach for chronic and acute mesenteric ischemia. Surgery. 1995;118:1-7.
98. Wolf YG, Verstandig A, Sasson T, et al. Mesenteric bypass for chronic mesenteric ischaemia. Cardiovasc Surg. 1998;6:34-41.
99. Van Damme H, Creemers E, Limet E. Surgical treatment of chronic mesenteric ischemia. Acta Gastroenterologia Belgium. 1989;52:406-20.
100. Sandmann W, Bohner H, Kniemeyer HW, Schramm J. [Chronic mesenteric ischemia]. Dtsch Med Wochenschr. 1994;119:979-84.
101. Geelkerken RH, van Bockel JH, de Roos WK, et al. Chronic mesenteric vascular syndrome. Results of reconstructive surgery. Arch Surg. 1991;126:1101-6.
102. Moawad J, McKinsey JF, Wyble CW, et al. Current results of surgical therapy for chronic mesenteric ischemia. Arch Surg. 1997;132:613-18.
103. Hollier LH, Bernatz PE, Pairolero PC, et al. Surgical management of chronic intestinal ischemia: a reappraisal. Surgery. 1981;90:940-6.
104. Matsumoto AH, Tegtmeyer CJ, Fitzcharles EK, et al. Percutaneous transluminal angioplasty of visceral arterial stenoses: results and long-term clinical follow-up. J Vasc Interv Radiol. 1995;6:165-74.
105. Roberts LJr, Wertman DAJr, Mills SR, et al. Transluminal angioplasty of the superior mesenteric artery: an alternative to surgical revascularization. AJR Am J Roentgenol. 1983;141:1039-42.
106. Sarac TP, Altinel O, Kashyap V, et al. Endovascular treatment of stenotic and occluded visceral arteries for chronic mesenteric ischemia. J Vasc Surg. 2008;47:485-91.
107. Atkins MD, Kwolek CJ, LaMuraglia GM, et al. Surgical revascularization versus endovascular therapy for chronic mesenteric ischemia: a comparative experience. J Vasc Surg. 2007;45:1162-71.
108. Bailey M, Chapin W, Licht H, Reynolds JC. The effects of vasculitis on the gastrointestinal tract and liver. Gastroenterol Clin North Am. 1998;27:747-82. v-vi
109. Lhote F, Guillevin L. Polyarteritis nodosa, microscopic polyangiitis, and Churg-Strauss syndrome. Clinical aspects and treatment. Rheum Dis Clin North Am. 1995;21:911-47.
110. Lee RG. The colitis of Behçet’s syndrome. Am J Surg Pathol. 1986;10:888-93.
111. Lie JT. Visceral intestinal Buerger’s disease. Int J Cardiol. 1998;66:S249-56.
112. Haynes BF, Kaiser-Kupfer MI, Mason P, Fauci AS. Cogan syndrome: studies in thirteen patients, long-term follow-up, and a review of the literature. Medicine (Baltimore). 1980;59:426-41.
113. Slovut DP, Olin JW. Fibromuscular dysplasia. N Engl J Med. 2004;350:1862-71.
114. Guill CK, Benavides DC, Rees C, et al. Fatal mesenteric fibromuscular dysplasia: a case report and review of the literature. Arch Intern Med. 2004;164:1148-53.
115. Robson WL, Leung AK. Henoch-Schönlein purpura. Adv Pediatr. 1994;41:163-94.
116. Nakasone H, Hokama A, Fukuchi J, et al. Colonoscopic findings in an adult patient with Henoch-Schönlein purpura. Gastrointest Endosc. 2000;52:392.
117. Jeong YK, Ha HK, Yoon CH, et al. Gastrointestinal involvement in Henoch-Schönlein syndrome: CT findings. AJR Am J Roentgenol. 1997;168:965-8.
118. Kato H, Hara T, Inoue O, et al. Current issues in Kawasaki disease. Acta Paediatr Jpn. 1993;35:464-71.
119. Fruhwirth J, Mischinger HJ, Werkgartner G, et al. Kohlmeier-Degos’s disease with primary intestinal manifestation. Scand J Gastroenterol. 1997;32:1066-70.
120. Bassel K, Harford W. Gastrointestinal manifestations of collagen-vascular diseases. Semin Gastrointest Dis. 1995;6:228-40.
121. Deny P, Guillevin L, Bonacorsi S, Quint L. Association between hepatitis C virus and polyarteritis nodosa. Clin Exp Rheumatol. 1992;10:319.
122. Guillevin L, Lhote F, Cohen P, Sauvaget F, et al. Polyarteritis nodosa related to hepatitis B virus. A prospective study with long-term observation of 41 patients. Medicine (Baltimore). 1995;74:238-53.
123. Scott DG, Bacon PA, Tribe CR. Systemic rheumatoid vasculitis: a clinical and laboratory study of 50 cases. Medicine (Baltimore). 1981;60:288-97.
124. Sharma BK, Jain S, Sagar S. Systemic manifestations of Takayasu arteritis: the expanding spectrum. Int J Cardiol. 1996;54:S149-54.