CHAPTER 110 Intestinal Infections by Parasitic Worms
Videos for this chapter can be found on www.expertconsult.com.
Parasitic worms are found worldwide. Modern travel, emigration,1 and consumption of “exotic” cuisines allow intestinal helminths to appear in any locale. People now acquire tropical helminths without leaving their industrialized temperate cities. Travel history is a critical, but often overlooked, aspect of the patient interview. Many helminths survive for decades within a host, so even a remote history of visits to or emigration from countries where they are endemic is important. Fresh food is flown around the world and often consumed raw.
In developed countries, we usually diagnose an intestinal helminth because we stumble across it rather than because we actively pursue it. Helminths are complex organisms well adapted to their hosts; like quiet house guests, most cause no symptoms. Worms rarely cause diarrhea, but many medical laboratories do not assay formed stool routinely for parasite eggs. Physicians need to communicate their concerns of possible helminthic infection to laboratory personnel. A telephone call to the local laboratory before a sample is sent can improve diagnostic results dramatically. Occasionally, alarmed patients bring proglottids or whole worms that they passed with their stools. These specimens should be fixed in 5% aqueous formalin and sent for identification.2 All specimens should be handled carefully with full precautions to avoid accidental exposure.
Some helminths can cause severe disease, but this is unusual. Most persons colonized with helminths have no symptoms or illness attributable to the parasites. Only with heavy infections does disease result. Well-adapted worms usually act more as commensals than as pathogens. It is even possible that exposure to helminths affords some protection against disease due to excessive immune reactions.3,4 Helminths induce immune regulatory pathways.5 Recent studies in mice and rats show that exposure to helminths can be used to prevent or treat colitis,3,6–8 insulin-dependent diabetes,9 and autoimmune encephalitis.10,11 Studies in humans show that helminth exposure improves ulcerative colitis12 and probably Crohn’s disease13,14 and that helminth eradication increases atopy.15 Although it remains important to treat helminthic infections when they are discovered, further research on these organisms can enable discovery of new approaches to treat immune-mediated disease.
NEMATODES
ASCARIS LUMBRICOIDES
Ascaris lumbricoides is the largest of the nematode parasites that colonize humans. Females can grow to 49 cm (19 inches).16 The name “lumbricoides” alludes to its resemblance to earth worms (Lumbricus sp.). The parasite is acquired by ingesting its eggs. Ascaris can cause intestinal obstruction and pancreaticobiliary symptoms. Treatment is albendazole.
Epidemiology
A. lumbricoides has a worldwide distribution, although these parasites are most numerous in less-developed countries and in areas with poor sanitation. About 25% of the world’s population (1.2 billion people) harbor A. lumbricoides.17,18 Children acquire the parasite by playing in dirt contaminated with eggs, whereas adults most often are infected by farming or eating raw vegetables from plants fertilized with untreated sewage. Pigs harbor Ascaris suum, which is closely related to A. lumbricoides, but cross-infection is rare.19
Clinical Features and Pathophysiology
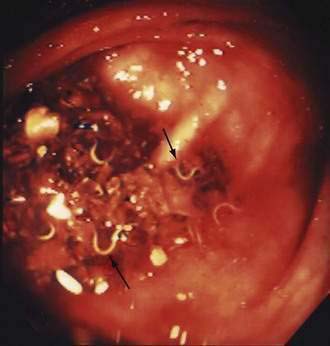
A. lumbricoides produces no symptoms in most infected persons. Often, worms are found unexpectedly on endoscopy20,21 (Video 110-1) or are seen on radiologic imaging,22 or eggs are identified in stool specimens of patients with symptoms not directly attributable to the worms. Disease usually develops only in those with heavy worm burdens: pulmonary, intestinal, and hepatobiliary ascariasis are well described.
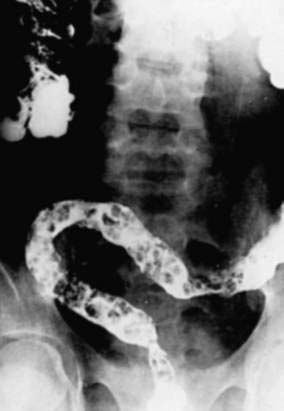
Large numbers of mature worms can cause severe intestinal symptoms including abdominal pain, distention, nausea, and vomiting. The most common complication of intestinal ascariasis is partial or complete small bowel obstruction; such patients often have a history of passing mature worms in their stool or vomitus. Patients with intestinal obstruction generally have more than 60 worms,23 and the rare patients with fatal cases often have more than 600 worms. Fatality results from intestinal necrosis caused by obstruction, intussusception, or volvulus (Fig. 110-1).24 Most cases of obstruction, absent signs of peritonitis or perforation, can be managed conservatively.
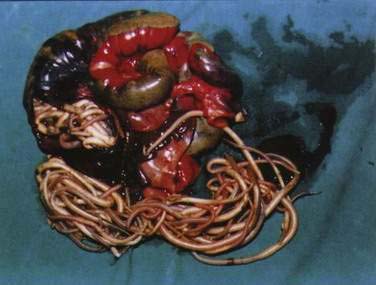
Figure 110-1. Small intestinal obstruction caused by Ascaris lumbricoides.
(From Wasadikar PP, Kulkarni AB. Intestinal obstruction due to ascariasis. Br J Surg 1997; 84:410.)
A. lumbricoides are highly motile. Mature worms can enter the ampulla of Vater (Fig. 110-2) and migrate into the bile or pancreatic ducts, causing biliary colic, obstructive jaundice, ascending cholangitis, acalculous cholecystitis, or acute pancreatitis.16 Pregnancy can promote biliary trespass.25 The worms can move in and out of the papilla, producing intermittent symptoms and fluctuating laboratory tests. Recurrent ascending cholangitis or acute pancreatitis from ascariasis is rare in highly developed Western countries but can be fatal if the diagnosis is not entertained.26
Diagnosis
Ascaris eggs are visible in direct smears of stool (Fig. 110-3). The eggs begin to appear in the stool about two months after initial exposure. Fertilized eggs are 35 by 55 µm and have a thick shell and outer layer; females also lay unfertilized eggs that are larger (90 by 44 µm) and have a thin shell and outer layer. Ascaris eggs that lose their outer layer resemble the eggs of hookworms.

Figure 110-3. Stool specimen containing helminth eggs. A, Ascaris lumbricoides. B, Hookworm. C, Trichuris trichiura. D, Fasciolopsis buski.
(A to D, Courtesy of Mae Melvin, MD, Atlanta, Ga.)
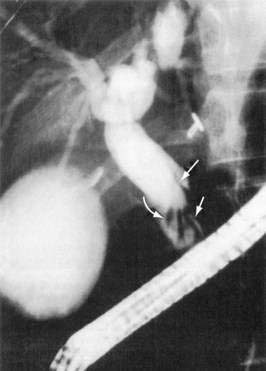
Adults worms may be seen at endoscopy,21 or identified on upper gastrointestinal series as long, linear, filling defects within the small intestine.22 The worms retain barium after it has cleared from the patient’s gastrointestinal tract, producing linear opacities. Similar findings are seen on endoscopic retrograde cholangiopancreatography (ERCP) if a worm is in the bile or pancreatic duct (Fig. 110-4). Ascaris also has a characteristic appearance on ultrasound examination of the biliary tree or pancreas: They appear as long, linear echogenic strips that do not cast acoustic shadows.22
Treatment
Asymptomatic colonization with A. lumbricoides is treated easily with a single 400-mg oral dose of albendazole. Albendazole inhibits glucose uptake and microtubule formation, effectively paralyzing the worms. Albendazole is poorly absorbed but is teratogenic, and therefore it should not be used in pregnant women. When possible, treatment with this agent should be delayed until after delivery. Single-dose mebendazole also is efficacious for Ascaris.27 A study of 1042 pregnant women in Peru found no adverse effect of a single 500-mg oral dose of mebendazole on birth outcomes.28
Hepatobiliary ascariasis also can be treated conservatively with fluid resuscitation, bowel rest, and antibiotics.29 Worms in the bile duct are not effectively treated with albendazole because it is poorly absorbed and not concentrated in the bile. This feature of albendazole is advantageous because were paralyzed worms unable to pass through the sphincter of Oddi, they could become trapped in the bile duct. Patients with hepatobiliary ascariasis should be treated with albendazole each day for several days because the worms only become susceptible when they migrate out of the bile duct.
Worms also can invade the pancreatic duct and can be treated conservatively, as for hepatobiliary ascariasis.30 Ascending cholangitis, acute obstructive jaundice, or acute pancreatitis requires emergent ERCP with worm extraction from the ducts by balloon, basket, or forceps—preferably without sphincterotomy. Ampullary sphincterotomy permits worms easier access to the ducts and can increase the risk of recurrent pancreaticobiliary ascariasis.31
STRONGYLOIDES STERCORALIS
Clinical Features and Pathophysiology
Most patients with S. stercoralis have no abdominal symptoms. Patients might have a serpiginous urticarial rash (larva currens) caused by the rapid (5 to 10 cm/hour) dermal migration of filariform larvae. This rash often occurs on the buttocks from larvae entering the perianal skin after they exit the anus during autoinfection. A study of prisoners of war found this creeping eruption to be a far more common symptom of chronic strongyloidiasis than were gastrointestinal complaints.32 Occasionally, patients have nausea, abdominal pain, or unexplained occult gastrointestinal blood loss from S. stercoralis. The parasite also can cause colonic inflammation that resembles ulcerative colitis but is more right-sided and strongly eosinophilic.33–35
While the parasite burden remains balanced, symptoms are minimal or absent. Immunosuppression or glucocorticoid administration upsets this balance. Previously asymptomatic, but chronically infested, patients develop fulminant, potentially fatal strongyloidiasis due to massive autoinfection.36,37 The mechanisms that permit massive autoinfection are unknown, but events that inhibit Th2-directed immune responses can release eosinophil-mediated control of the parasites. In addition, glucocorticoids can act directly on the parasites to increase the development of infective filariform larvae.38 Fulminant disseminated strongyloidiasis rarely complicates HIV and AIDS.39
Massive autoinfection produces disseminated fulminant strongyloidiasis. Migrating filariform larvae injure the intestinal mucosa and carry luminal bacteria into the bloodstream, resulting in polymicrobial sepsis with enteric organisms. Streptococcus bovis endocarditis or meningitis40 also can result. Numerous larvae migrating through the lungs cause pneumonitis, and worms can arrive in unusual locations such as the brain. Fulminant strongyloidiasis often is fatal.
Diagnosis
A recent survey of United States physicians-in-training demonstrated very poor ability to identify or even consider strongyloidiasis.41 Patients with chronic strongyloidiasis often are asymptomatic. Peripheral blood eosinophils may be elevated, but a normal eosinophil count does not argue against infestation with the parasite. Currently, the best method for detecting exposure is enzyme-linked immunosorbent assay (ELISA) for immunoglobulin (Ig) G antibodies against S. stercoralis. This assay is performed by the Centers for Disease Control and Prevention (CDC) in the United States and is 95% sensitive,42 sensitivity being highest for immigrants with prolonged exposure and lowest for returning visitors with lower-level recently acquired infestation.43
False-positive reactions can occur in patients exposed to other helminthic parasites,44 and serologic positivity can indicate prior exposure to S. stercoralis, not necessarily active infestation. Because chronic strongyloidiasis can remain subclinical and difficult to detect for decades, however, treatment of seropositive patients is warranted. Indeed, some argue that patients with only suspected strongyloidiasis, such as immigrants from endemic countries who have elevated eosinophil counts, should be treated empirically before glucocorticoid therapy.45
Active infestation can be diagnosed by finding rhabditiform larvae in direct smears of the stool, though this is an insensitive method. A 10-fold more sensitive technique is to spread stool on an agar plate and look for serpentine tracks left by migrating larvae.46 Intestinal biopsy is also an insensitive means of diagnosis.
Treatment
Chronic strongyloidiasis is best treated with one dose of ivermectin (200 µg/kg) given orally; this dose is used in both adult and pediatric patients. Ivermectin is better tolerated than thiabendazole. Ivermectin paralyzes the intestinal adult worms but not the larvae migrating through tissue, and therefore patients can develop recurrent infestation from migrating larvae; a repeat dose after two weeks helps to prevent this outcome. Successful treatment causes a fall in antibody titer by six months in most (about 90%) patients.42 Immunocompromised patients require repeat doses given 2, 15, and 16 days after the first dose.37
CAPILLARIA (PARACAPILLARIA) PHILIPPINENSIS
Capillariasis is acquired by eating raw fish that are infested with the parasite.47 The nematode causing capillariasis has been renamed from Capillaria philippinensis to Paracapillaria philippinensis,48 but by any name, it is deadly. The parasite replicates in the host, producing an ever-increasing number of intestinal worms. Patients develop protein-losing, sprue-like diarrhea with progressive emaciation and anasarca, which ultimately leads to death. Treatment is albendazole.
Epidemiology
The first known human case of capillariasis was reported in 1964. It remains a rare but deadly parasitic infestation. From 1965 through 1968, an epidemic in the rural Philippines involved 229 cases, with an overall mortality rate of 30%.49 As the name implies, Paracapillaria phillippinensis is endemic to the Philippines, but it also is endemic in Thailand and cases occur in Japan, Taiwan, Egypt, and Iran. Modern travel transports cases worldwide.50
Life Cycle
People become infested with the worm by eating raw or undercooked freshwater or brackish-water fish that contain the parasitic larvae. Some female adult P. philippinensis are larviparous, producing infective larvae instead of eggs. These larvae then mature in the small intestine and increase the parasite burden. This pathway of autoinfection permits a massive increase in parasite numbers. A rhesus monkey originally fed 27 larvae had more than 30,000 worms by 162 days of infection.51
Clinical Features and Pathophysiology
The progressive disease is believed to result from an ever-increasing number of poorly adapted intestinal parasites. In autopsy studies, the jejunal intestinal mucosa showed flattened, denuded villi with numerous plasma cells, lymphocytes, macrophages, and neutrophils infiltrating the lamina propria.47
Diagnosis
Diagnosis is made by finding eggs and larvae in stool specimens. No serologic tests for capillariasis are available. Symptomatic patients have detectable eggs in their stool. The eggs are easily confused with those of Trichuris trichiura, but T. trichiura eggs have prominent bipolar plugs that appear cut off in P. phillipinensis.47
HOOKWORMS (NECATOR AMERICANUS, ANCYLOSTOMA DUODENALE, AND ANCYLOSTOMA CANINUM)
Worldwide, an estimated 740 million people are infested with hookworm,17 usually by Necator americanus, Ancylostoma duodenale, or a mixture of the two. Hookworm is acquired by skin contact with contaminated soil. Moderate infestation contributes to iron deficiency. Hookworm should be suspected in patients with eosinophilia and iron-deficiency anemia. The dog and cat parasite Ancylostoma caninum is a cause of eosinophilic enteritis. Treatment is albendazole.
Necator americanus and Ancylostoma duodenale
Life Cycle
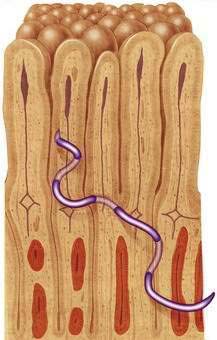
Infective third-stage hookworm larvae penetrate intact skin, such as between the toes of bare feet while walking on contaminated ground. Larvae migrate through the dermis to reach blood vessels. This migration can cause a pruritic, serpiginous rash, cutaneous larva migrans (Fig. 110-5). Ancylostoma braziliense normally infests dogs and cats, but it produces a similar rash during infective dermal wandering in humans and is the usual cause of cutaneous larva migrans. Larvae of N. americanus and A. duodenale enter blood vessels in the skin and migrate with venous flow through the right side of the heart to the lungs. A. duodenale larvae can arrest their migration and become dormant for many months before proceeding to the lungs.52 In the lungs, larvae penetrate the alveoli and enter the air spaces, after which they migrate up the pulmonary tree, are swallowed with saliva, and pass into the small intestine, where they mature. Patients also can acquire A. duodenale by directly ingesting larvae crawling on contaminated fresh vegetables. Adult worms develop large buccal cavities and graze on the intestinal mucosa, ingesting epithelial cells and blood (Figs. 110-6 and 110-7). Adults are about one centimeter long and can live for up to 14 years. Mature worms mate and lay eggs. Each female N. americanus lays about 10,000 eggs a day, and each female A. duodenale lays about 20,000 eggs a day. Eggs are deposited with feces in moist, shady soil, where they hatch to release larvae. The larvae molt twice after which they move to the soil surface and seek a suitable host.
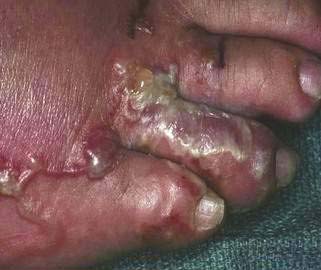
Figure 110-5. Serpiginous rash caused by hookworm larvae migrating through the dermis.
(Courtesy of the University of Iowa Department of Dermatology, Iowa City, Ia.)
Clinical Features and Pathophysiology
Light infestations with N. americanus and A. duodenale cause no symptoms.53 The major consequence of moderate and heavy hookworm infestation is iron deficiency. Adult worms feed on intestinal epithelial cells and blood. The closely related A. caninum (see later) secretes anticoagulant peptides that inhibit clotting factors54 and platelet aggregation,55 thereby preventing hemostasis and permitting the hematophagous parasites to feed on host blood. Intestinal blood loss is estimated to be 0.01 to 0.04 mL/day per adult N. americanus and 0.05 to 0.3 mL/day per adult A. duodenale.56 With a moderate number of worms, this blood loss becomes appreciable (Table 110-1). Iron deficiency results when iron loss outstrips iron absorption. The average North American diet is high in iron so anemia might not develop, and men with a diet high in iron (more than 20 mg/day) can tolerate up to 800 adult hookworms without developing anemia.
Table 110-1 A Comparison of Daily Physiologic Iron Losses and Iron Losses Due to Hookworm Infection in Women*
| CONDITION | IRON LOSS (MG/DAY) |
|---|---|
| Physiologic Losses | |
| Menstruation | 0.44 |
| Pregnancy | 2.14 |
| Lactation | 0.23 |
| Losses Due to Hookworm Infection | |
| Necator americanus (60-200 worms) | 1.10 |
| Ancylostoma duodenale (20-100 worms) | 2.30 |
* Losses shown are in addition to the basal iron loss of 0.72 mg/day.
Adapted from Stoltzfuss RJ, Dreyfuss ML, Chwaya HM, Albonico M. Hookworm control as a strategy to prevent iron deficiency. Nutr Rev 1997; 55:223-32.
Infestation with hookworm can modulate immune responses.57 Clinical trials are under way to determine if subclinical infestation with hookworm inhibits immune-mediated disease such as Crohn’s disease and asthma.58,59 Dose-ranging studies on healthy volunteers suggested that low-level hookworm infestation (10 larvae) is well tolerated.58
Diagnosis
Hookworms can be visible endoscopically (Fig. 110-8),60 but diagnosis is made by identifying eggs on direct smears of formalin-fixed stool (see Fig. 110-3). Evaluation of three stool specimens obtained on separate days should permit diagnosis of hookworm,61 but light infestations can require concentration techniques. Eggs mature rapidly at room temperature and can hatch to release larvae. It is difficult to distinguish N. americanus eggs from those of A. duodenale simply by morphology.
Ancylostoma caninum
Clinical Features and Pathophysiology
A. caninum is a well-recognized cause of cutaneous larva migrans, a distinctive serpiginous rash caused by an abortive migration of the parasite in an unsupportive host. A. caninum also can cause eosinophilic enteritis, although not all eosinophilic enteritis is caused by this parasite (see Chapter 27). Patients with eosinophilic enteritis from A. caninum often are dog owners and present with colicky mid-abdominal pain and peripheral eosinophilia,62 but they do not recall having cutaneous larva migrans. Intestinal biopsies show high numbers (>45/high-power field) of mucosal eosinophils,63 and eosinophilic inflammation is most prevalent in distal small bowel. Unlike eosinophilic gastroenteritis, tissue eosinophilia is not present in the stomach. On endoscopy of the terminal ileum, patients might have scattered small superficial aphthous ulcers and mucosal hemorrhage.64 Serologic evidence suggests that A. caninum also may be a cause of abdominal pain without eosinophilia or eosinophilic enteritis.62
WHIPWORM (TRICHURIS TRICHIURA)
Epidemiology
An estimated 800 million people harbor T. trichiura. It occurs in temperate and tropical countries and remains prevalent in areas with suboptimal sanitation. In one equatorial Cameroon province, 97% of the school-age children had T. trichiura.65 Whipworm eggs are sensitive to desiccation, so prevalence is low in desert climates.
Life Cycle
T. trichiura has a simple life cycle. Colonization occurs by ingesting the parasite egg, each of which contains one developed larva. The eggs hatch in the intestine, and larvae migrate to the cecum, where they mature, mate, and lay eggs. This process takes about eight to 12 weeks. Adult worms are approximately three centimeters long and have a thin tapered anterior region so that the worm resembles a whip (Fig. 110-9, Video 110-2).66 A mature female worm lays about 20,000 eggs a day and can live for three years. Eggs are deposited with feces into the soil. Over the next two to six weeks, one larva develops within each egg, but the egg is not infective until it has fully embryonated. Therefore, T. trichiura does not multiply in the host and is not directly transmitted to other persons.
Clinical Features and Pathophysiology
Most persons with T. trichiura infestation have no symptoms attributable to the parasite. Most people in an endemic area are colonized by small numbers (less than 15) of worms and for them, the parasite is a commensal organism rather than a pathogen. Some people harbor hundreds or even thousands of worms,67 and they are the ones who develop symptoms68; this bimodal distribution of infestation persists after patients are treated and then become reinfected naturally, suggesting that unique host factors (genetic or behavioral) contribute to determining an individual patient’s worm burden.
Rectal prolapse can occur in children with extremely high numbers of T. trichiura worms.69 Some persons with numerous worms have mucoid diarrhea and occasional bleeding, a combination of symptoms called the Trichuris dysentery syndrome (TDS). Children with this condition have growth retardation,70 but studies attributing these symptoms to T. trichiura are complicated, because persons with TDS often are socioeconomically deprived and may be coinfected with other pathogens. Colonic biopsy specimens from children with TDS show few or no abnormalities compared with healthy local children,71 other than an increase in mast cells72 and in the number of cells that express TNF-α and calprotectin.73
A different but closely related species, Trichuris muris, infests mice. Mouse strains that react to the parasite with a strong Th2 response, characterized by production of interleukin (IL)-4, IL-5, and IL-13, are able to expel the worms, whereas strains that respond with a Th1 response (interferon [IFN]-γ) have difficulty expelling the worms.74 Blocking IL-4 makes resistant strains susceptible, and blocking IFN-γ makes susceptible strains resistant to chronic infestation with T. muris.75 The type of immune response developed by inbred mice to T. muris is an important factor in determining length and intensity of infestation. A similar response in humans might explain why some people repeatedly acquire heavy infestations whereas others carry only a few worms.
Diagnosis
Diagnosis is made by identifying T. trichiura eggs in stool specimens. Trichuris eggs are 23 µm by 50 µm and have characteristic plugs at each end (see Fig. 110-3).
Treatment
T. trichiura is treated with mebendazole 100 mg twice a day for three days; alternatively, patients can take albendazole 400 mg each day for three days. Heavily infested patients might require seven days of treatment.76 Single-dose treatment with albendazole is ineffective27 but one treatment with a combination of albendazole (400 mg) and ivermectin (200 µg/kg) appears quite effective, with cure rates of up to 80% and egg reduction rates of 94%.77,78
PINWORM (ENTEROBIUS VERMICULARIS)
Epidemiology
E. vermicularis is a quintessential intestinal parasite with no geographic constraints. It is transmissible by close contact with colonized persons. People have had pinworm for thousands of years, and before modern sanitation, colonization by pinworm probably was universal. E. vermicularis eggs were identified in a 10,000-year-old human coprolite found in Utah.79 The pinworm Enterobius gregorii, originally thought to be a separate species of pinworm,80,81 actually may be just a young adult form of E. vermicularis.82
Pinworm remains common in many areas, but it appears to be decreasing in prevalence. A survey of positive cellophane tape tests (see later) in New York City documented a sharp decline in positivity from 57 of 248 tests in 1971 to 17 of 165 in 1978 to 0 of 38 in 1986.83 Similar trends are reported from California.
Life Cycle
Eggs hatch in the duodenum, releasing larvae that molt twice as they mature and migrate to the cecum and ascending colon (Fig. 110-10, Video 110-3).84 The parasites are small: adult males measure 0.2 mm by 2 to 5 mm, and adult females measure 0.5 mm by 8 to 13 mm. After mating, gravid females migrate to the rectum. During the night, egg-laden females migrate out of the anal canal and onto the perianal skin. Each female deposits up to 17,000 eggs, which mature rapidly, becoming infective within six hours. Pinworm infestation typically causes perianal itching, and scratching gathers eggs onto the hands, promoting reinfection and transmission to others.
Clinical Features and Pathophysiology
E. vermicularis is an extremely well adapted parasite that produces no specific symptoms in the vast majority of colonized persons. Most symptoms are minor, such as pruritus ani and restless sleeping. Rarely, pinworm causes eosinophilia or eosinophilic enteritis.85
Vulvovaginitis is more common in girls with pinworm than in girls without this infection. Vulvovaginitis may be caused by migration of the worms into the introitus and genital tract. Dead worms and eggs encased in granulomas have been found in the cervix, endometrium, fallopian tubes, and peritoneum, attesting to the migratory effort of female worms.86 Ectopic enterobiasis is rare and causes no or very little overt pathology.
Infestation with E. vermicularis can influence mucosal immune responses. One case report described a 12-year-old girl with pinworm and apparently latent ulcerative colitis, who developed severe ulcerative colitis after treatment with pyrantel to remove the worms.87 While she was colonized with E. vermicularis, intestinal biopsies showed increased expression of mRNA for IL-4, transforming growth factor (TGF)-β, IL-10, and FOXP3 compared with biopsy specimens taken after anthelminthic treatment; these transcripts are associated with immune regulatory pathways that suppress inflammation.
TRICHINELLA SPECIES
Epidemiology
Trichinosis is acquired by eating raw or undercooked meat that contains parasite larvae of Trichinella species. Worldwide, domestic pigs are the most common carriers. Trichinella species are divided into two groups,88 one that forms encapsulated muscle cysts and only infests mammals (Trichinella spiralis, Trichinella britovi, Trichinella nelsoni, Trichinella native, Trichinella murrelli), and one that does not form encapsulated cysts and infests mammals and birds (Trichinella pseudospiralis) or mammals and reptiles (Trichinella papuae, Trichinella zimbabwensis). To date only T. zimbabwensis has not been implicated in human disease.
Trichinosis was much more common in the United States than it is now. In the late 1940s, about 400 cases per year of symptomatic trichinosis were reported to various health agencies, and this number dropped to an average of 14.4 cases per year in the time period 1997 to 200189; reports from Germany show a similar pattern.90 This decrease is explained by two major factors: First is the strong admonition to thoroughly cook all pork products; second is a change in farming practice to now feed pigs only grain. Industrialized pig farms in North America have been free of trichinosis for more than 50 years, but trichinosis is a reemerging illness in eastern Europe, related to relaxed enforcement of regulations.91
Currently, most reported cases involve a discrete exposure. For example, a 1991 outbreak in Wisconsin involved 40 people who ate pork sausage from one shop. A 1995 outbreak in Idaho involved 10 people who ate cougar jerky.92 A 2005 outbreak in Canada involved at least 14 people who ate frozen then stewed black bear meat.93 In France, several outbreaks have resulted from eating raw horse meat.94 This emphasizes that all mammals including herbivores can transmit Trichinella.
Life Cycle
The same host harbors both the adult and larval form of Trichinella.95 People acquire the parasite by eating raw or undercooked meat that contains encapsulated parasite larvae. Each cyst dissolves in the digestive tract, releasing one larva that invades the small intestinal mucosa and lives within the cytoplasm of about 45 villus cells (Fig. 110-11). Larvae mature rapidly and mate within 30 hours. Adults are minute: Male worms measure 60 µm by 1.2 mm and female worms measure 90 µm by 2.2 mm. Females are viviparous and begin releasing larvae about one week after their initial ingestion. Adults are short-lived, producing larvae for only four weeks, by which time they are expelled by the host.
Clinical Features and Pathophysiology
Although most infestations with Trichinella are asymptomatic, significant exposure produces illness and even death.96 Clinical trichinosis has two phases caused by the enteral (adult) and parenteral (larval) stages of the parasite. Intestinal symptoms result from enteritis due to adult worms that have embedded themselves in the intestinal epithelium. Enteritis produces abdominal pain, nausea, vomiting, diarrhea, and low-grade fever. Intestinal symptoms begin about two days to one week and peak at two weeks after ingestion of contaminated meat. The timing and severity of symptoms vary with intensity of exposure. The intestinal phase of trichinosis often is misdiagnosed as viral gastroenteritis or food poisoning.
T. spiralis also infests mice and rats, permitting detailed study of the intestinal phase of infection.97 Mice begin to expel adult worms about two weeks after initial infestation. Type 2 (Th2) cytokines (IL-4 and IL-5) promote worm expulsion. Expulsion of adult worms results from focal immune attack, increased secretions, and enhanced intestinal motility; T lymphocytes, eosinophils, and mast cells assist this primary response. Rats previously exposed to T. spiralis rapidly expel the parasite upon rechallenge, a protection likely resulting from an immediate-type hypersensitivity response to the parasite triggered by IgE-armed mast cells.
Treatment
Although adults are short-lived, treatment with albendazole 400 mg twice a day or mebendazole 5 mg/kg/day for 10 to 15 days98 is warranted and abbreviates the production of larvae by adult worms. Addition of glucocorticoids reduces inflammation and systemic symptoms; however, glucocorticoids given in the absence of a benzimidazole can prolong the intestinal phase, increasing the number of larvae released.
ANISAKIS SIMPLEX
Epidemiology and Life Cycle
A. simplex and P. decipiens infest fish and marine mammals.99 People become accidental hosts by eating raw or pickled fish. Anisakidosis has become more common with the increased popularity of eating raw fish (e.g., sushi). Many species of saltwater fish harbor A. simplex larvae including herring, mackerel, salmon, plaice, and squid. The parasite larvae initially infest crustaceans that are consumed by fish. The larvae migrate to the fish musculature and, if a parasitized fish is eaten by another fish, the larvae again migrate to the musculature of their new host. Eventually, a parasitized fish is eaten by a marine mammal that serves as the definitive host. In the marine mammal, the parasite larvae mature into adult intestinal worms and lay eggs that are passed with feces, the eggs hatch to release larvae that infest crustaceans, and the life cycle is thus renewed.
Clinical Features and Pathophysiology
A. simplex and P. decipiens cause transient infestations in humans. They do not reach full maturity in humans and therefore produce no eggs. The most common gastrointestinal symptom is acute severe stomach pain with nausea and hematemesis shortly after eating larva-infested raw fish. Endoscopy may demonstrate a small larva partially penetrating the gastric or intestinal wall.100,101 Rarely, A. simplex can enter the intestinal wall and cause a strong inflammatory reaction that can mimic acute appendicitis102 or Crohn’s disease. Human infestations with either A. simplex or P. decipiens is termed anisakidosis after the family name (Anisakidae) for these parasites.
A. simplex is a potent allergen, and many cases of seafood (fish) allergy actually may be reactions to A. simplex,103 including anaphylaxis from well-cooked marine fish.99,104 In Spain, 12% to 22% of persons are seropositive for IgE against A. simplex.105,106
Diagnosis and Treatment
A history of recent (within three days) ingestion of raw fish suggests anisakidosis in the appropriately symptomatic patient. Diagnosis is made by finding the larvae on endoscopy or in surgically excised specimens. Gastric anisakidosis is diagnosed by endoscopy, and endoscopic removal of the anisakid alleviates symptoms. Intestinal anisakidosis can prompt surgery for patients presenting with symptoms of acute small bowel obstruction or peritonitis,107 but surgery may be avoidable if a recent history of eating raw fish is elicited and conservative treatment is tolerated.108
CESTODES
DIPHYLLOBOTHRIUM SPECIES
Epidemiology
D. latum is most common but other Diphyllobothrium species (e.g., Diphyllobothrium dendriticum, Diphyllobothrium nihonkaiense) can colonize humans.109,110 D. latum is endemic in northern Europe, Russia, and Alaska, but fish tapeworm has been reported in Africa, Japan, Taiwan, Australia, South America, North America, and Canada.111
Life Cycle
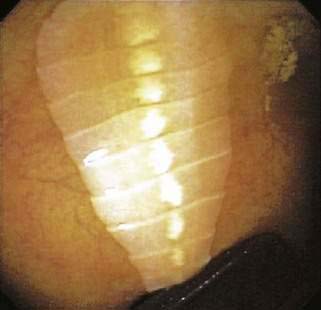
Trout, salmon, pike, perch, and whitefish all can harbor D. latum. People acquire the parasite by eating raw or undercooked fish. D. latum also can colonize many other mammals such as dogs, cats, bears, and seals. In mammals, the ingested plerocercoid larva attaches to the wall of the small intestine and matures into an adult worm. A long chain of proglottids, called the strobila, develops off of the scolex (Fig. 110-12). D. latum is the largest parasite of humans, reaching 12 meters (40 feet) in length. The proglottids release eggs into the lumen that pass with the feces.
Clinical Features and Pathophysiology
Fish tapeworm is not invasive and causes no direct symptoms. The worm obtains nutrients by absorbing luminal contents through its surface. D. latum produces a substance that splits B12 from intrinsic factor in the intestine,112 interfering with host absorption of the vitamin. The tapeworm also avidly absorbs B12, effectively competing with its host’s use of the vitamin. D. latum is long-lived and, over time, can cause significant B12 deficiency in patients with limited dietary cobalamin. Rarely, severe B12 deficiency results in megaloblastic anemia and neurologic symptoms.
Diagnosis and Treatment
Fish tapeworm is diagnosed by identifying D. latum eggs in stool specimens. Occasionally, diagnosis is made because the patient passes proglottids and brings them in for identification or the worm is seen on endoscopy.113 Praziquantel is effective in a single oral dose of 10 mg/kg. Patients should be warned that they might pass a rather long worm two to five hours after taking the medication. Albendazole 400 mg each day for three days also kills the tapeworms.
TAENIA SAGINATA AND TAENIA SOLIUM
An estimated 80 million people are colonized with beef (Taenia saginata) or pork (Taenia solium) tapeworm. Colonization occurs by eating raw or undercooked meat infested with cysticerci. Tapeworms usually cause no symptoms and can surprise an endoscopist who finds the unsuspected jejunal or colonic inhabitant (Videos 110-4 and 110-5).114,115 Ingestion of T. solium eggs causes cysticercosis, a potentially fatal disease. Treatment is praziquantel or albendazole.
Epidemiology
Beef and pork tapeworm occur where livestock are exposed to untreated human waste and people eat raw or undercooked meat. Both parasites have a worldwide distribution, although infestations originating in the United States and Europe are rare. Beef tapeworm is endemic in Africa, the Middle East, Eastern Europe, Asia, and Latin America. Pork tapeworm is endemic in Africa, India, China, Asia, and Latin America. T. solium is rare in Muslim countries, where pork consumption is prohibited. T. solium is considered an eradicable parasite,116 though progress in such eradication is hindered by socioeconomic barriers.117
Clinical Features and Pathophysiology
The most feared complication of T. solium infestation is cysticercosis,118 which occurs when people inadvertently consume T. solium eggs. Just as in pigs, the eggs release oncospheres that penetrate the intestinal wall, disseminate through the body, and form cysticerci. Cysticerci produce localized inflammation in the brain, spinal cord, eye, and heart, with dire consequences. Neurocysticercosis is a common cause of epilepsy in countries where T. solium is endemic. Worldwide, an estimated 50,000 people die of neurocysticercosis each year. In the United States, 221 people died from cysticercosis between 1990 and 2002.119 Because the disease occurs after ingestion of parasite eggs, neurocysticercosis in a patient who has not visited or emigrated from an endemic country should prompt an effort to identify local carriers.
Diagnosis
Beef and pork tapeworm are diagnosed by identifying eggs or proglottids in stool specimens. The eggs of the two species are indistinguishable microscopically. The proglottids of T. saginata are two centimeters long and have more than 12 uterine branches; those of T. solium measure 1.2 cm and have fewer than 10 uterine branches.112 Egg and proglottid production can be sporadic, necessitating repeated stool tests. Cysticercosis usually is diagnosed by computed tomography (CT) or magnetic resonance imaging (MRI) and confirmed by serology using a larval cyst antigen-specific immunoblot.120
HYMENOLEPIS NANA AND HYMENOLEPIS DIMINUTA
Epidemiology
H. nana is the most common tapeworm of humans. Unlike other tapeworms, it can be transmitted from person to person without an intermediate host. Dwarf tapeworm has a worldwide distribution, with highest prevalence in warm and arid regions. A survey of Egyptian children found that 16% carried H. nana.121 In the United States, a 1987 survey of state diagnostic laboratories found that 900 of 216,000 submitted stool specimens demonstrated H. nana, with 34 states reporting positive specimens.122 H. nana also colonizes mice and rats; however, the strains that colonize people appear to differ from those of rodents.
Human colonization with H. diminuta is rare, but it too has worldwide distribution. Rats and mice are the parasite’s usual hosts. People acquire rodent tapeworm by ingesting fleas, grain beetles, mealworms, or cockroaches infested with larval forms of the parasite. Most cases involve young children. The incorporation of beetles in traditional oriental medications also permits transmission.123
Clinical Features and Pathophysiology
Mice can harbor Hymenolepis, permitting investigation of the mechanisms that limit worm density. It appears that a Th1-mediated IFN-γ response provides protective immunity against cysticercoid larvae,124,125 and a Th2 response involving IgE and mast cells assists in the expulsion of adult worms.126,127 The mucosal immune response to the tapeworm can alter intestinal inflammation elicited by other agents; for example, mice colonized with H. dimunita are protected from dinitrobenzene sulfonic acid (DNBS)-induced colitis128 but are more susceptible to oxazalone-induced colitis.129
DIPYLIDIUM CANINUM
Echinococcus species also are tapeworms of dogs. Ingestion of Echinococcus granulosus, Echinococcus multilocularis, or Echinococcus vogeli eggs causes severe disease due to formation of hydatid cysts (see Chapter 82).
TREMATODES
INTESTINAL FLUKES
Most intestinal trematodes have a broad host range, and more than 50 different species are capable of colonizing humans.130 Many of these are geographically restricted and are acquired because of specific indigenous dietary behavior. The more common intestinal trematodes are Fasciolopsis buski, Heterophyes species, and Echinostoma species. These parasites are acquired by ingesting larval metacercariae encysted on freshwater plants (F. buski) or in freshwater fish (Heterophyes, Echinostoma). The parasites usually cause no specific symptoms, but heavy infestations can cause diarrhea and abdominal pain. Treatment is with praziquantel.
Fasciolopsis buski
F. buski is the largest intestinal trematode that colonizes humans. Adults measure 7.5 cm long and 2 cm wide. F. buski is endemic in southeast Asia and Indonesia131 and is acquired by ingesting metacercariae encysted on freshwater plants. The metacercariae excyst in the duodenum and attach to the small intestinal mucosa. Within three months, they mature to adult flatworms and begin to lay eggs. The eggs pass with feces, and if they are deposited into fresh water, they embryonate. Each egg releases a ciliated miracidium that seeks a suitable snail to infect. The miracidium enters the snail and develops into a sporocyst that asexually multiplies, releasing numerous cercariae. The cercariae swim to freshwater plants, and each encysts to form a metacercaria on the plant’s surface, awaiting ingestion by a mammal.
Adult F. buski live for about one year and cause no symptoms in most people.132 Histology of jejunal biopsy specimens along with carbohydrate, fat, and protein absorption were normal in one study of patients harboring F. buski133; however, in 1952, a 15-year-old Thai girl, hospitalized for diarrhea and abdominal pain, died of anasarca with more than 470 adult worms in her small intestine.134 Diagnosis is by finding parasite eggs in the stool (see Fig 110-3). Rarely the large flatworm is found on endoscopy (Video 110-6).135 Treatment is one dose of praziquantel 15 mg/kg given orally.
Heterophyes Species
People acquire these parasites by eating raw or undercooked fish that contain metacercariae. In the United States, a case of H. heterophyes involved a Pennsylvanian woman who ate sushi flown in from Asia.136 The metacercariae ingested in raw fish excyst in the intestine, attach to the small intestinal mucosa, and develop into adults. The adults lay eggs that are deposited with feces. If passed into fresh or brackish water, the eggs release miracidia that swim in search of a suitable snail. A miracidium enters a snail and develops into a sporocyst that asexually multiplies, releasing numerous cercariae. The cercariae swim away from the snail in search of a fish to infect. Either freshwater fish or saltwater fish feeding in brackish outlets can become infected.
Echinostoma Species
There are at least 16 species of Echinostoma that can colonize humans.137 Adults are 2 to 6 mm long and 1 to 1.5 mm wide, depending on the species. Echinostoma species are endemic in Taiwan, Korea, Thailand, Japan, Indonesia, and the Philippines. One outbreak of probable echinostomiasis involved 18 of 20 American travelers returning from Kenya.138
Echinostoma species produce no symptoms in most people, but these parasites can cause epigastric pain, abdominal cramps, and diarrhea.138 Diagnosis is by finding eggs in the stool or adults on endoscopy.139 Echinostoma eggs resemble those of F. buski but are smaller. Treatment is one 25-mg/kg dose of praziquantel given orally.
LIVER FLUKES
Clonorchis sinensis, Opisthorchis viverrini, and Opisthorchis felineus
C. sinensis and Opisthorchis species are closely related parasites that have similar life cycles and cause similar disease. C. sinensis is endemic to China, Hong Kong, Taiwan, the Republic of Korea, and North Vietnam. O. viverrini is endemic to Thailand and Laos.140 O. felineus is endemic to Russia and the Ukraine. Infection with C. sinensis and other food-borne trematodes is increasing in prevalence, possibly due to fish farming.141 People acquire these parasites by eating metacercariae present in raw or undercooked fish such as grass carp (Ctenopharyngodon idellus) or pond smelt (Hypomesus olidus). Studies in Korea show that at least 80 species of freshwater fish can harbor metacercariae.142
Most infections with C. sinensis or Opisthorchis are asymptomatic. With heavy exposures, patients develop fever, malaise, hepatic tenderness, and eosinophilia,143 symptoms and signs that abate as the worms mature and begin laying eggs in the bile ducts (Video 110-7).144 In a minority of patients, these parasites can cause relapsing cholangitis (see Chapter 82). The worms elicit a fibrotic and adenomatous reaction in the smaller branches of the biliary ducts, which can produce a localized obstruction and hepatic abscess. The flukes also can migrate into the pancreatic duct and cause pancreatitis.
The most important complication of chronic infection with C. sinensis or O. viverrini is cholangiocarcinoma (see Chapter 69).145 Infection with these parasites dramatically increases the risk of developing this otherwise rare cancer (Table 110-2)146,147: Parasites damage the bile duct, causing cellular desquamation followed by hyperplasia, adenomatous hyperplasia, periductal fibrosis, dysplasia, and finally cholangiocarcinoma.151 Cancer can result from increased sensitivity to carcinogens. Hamsters infected with O. viverrini develop cholangiocarcinoma when treated with subcarcinogenic doses of dimethylnitrosamine. C. sinensis and O. viverrini can sensitize patients to dietary or endogenously produced N-nitroso compounds and thereby increase the risk for cholangiocarcinoma.152 This is an important consideration in Western countries as well. A 1977 study found that 26% of Chinese immigrants relocating to New York had C. sinensis.153 Because of the increased cancer risk associated with these parasites, it is advisable to look for them in any patient from an endemic area.154
Table 110-2 Relative Risks of Cholangiocarcinoma in Patients with Clonorchis or Opisthorchis Infestation
| REFERENCE | RELATIVE RISK | 95% CI |
|---|---|---|
| Clonorchis sinensis | ||
| 146 | 3.1 | 0.13-8.4 |
| 147 | 6.5 | 3.7-12 |
| 148 | 6.0 | 2.8-13 |
| Opisthorchis viverrini | ||
| 149 | 5.0 | 2.3-11.0 |
| 152* | ||
| Light | 1.7 | 0.2-16.3 |
| Medium | 3.2 | 0.4-30 |
| Heavy | 14.0 | 1.7-119 |
CI, confidence interval.
* Light, <1500 eggs/g stool; medium, 1501-6000 eggs/g stool; heavy, >6000 eggs/g stool.
Diagnosis is by finding parasite eggs in the stool or duodenal aspirate. Symptomatic patients might have curvilinear lucencies in the biliary and pancreatic ducts on ERCP.155 Ultrasound findings include increased periductal echogenicity and floating echogenic foci in the gallbladder.156 The recommended treatment is praziquantel 25 mg/kg every eight hours for three doses. Heavy infections may require two days of therapy.157 An alternative treatment is albendazole 10 mg/kg twice a day for seven days. Albendazole is teratogenic and should not be given to pregnant women.
Fasciola hepatica and Fasciola gigantica
Humans acquire these parasites by ingesting metacercariae encysted on freshwater plants such as watercress. Ingested metacercariae excyst in the small intestine, penetrate through the bowel wall, and enter the peritoneal cavity, where they migrate to the liver, penetrate the capsule, and travel through the hepatic parenchyma in search of a bile duct. They reside within the bile ducts, reaching maturity within three or four months, after which they lay eggs. Adult F. hepatica are 1.3 cm by 4.0 cm, and F. gigantica grow up to 7.0 cm in length. Adults of both species are only one millimeter thick and resemble leaves. Fasciola are long-lived; one documented infection persisted for 16 years.158 Adults lay eggs that pass with the bile into the intestinal lumen, from which they are excreted. Upon reaching fresh water, Fasciola eggs embryonate, hatch, and release miracidia that swim in search of a suitable snail. A miracidium enters a snail and develops into a sporocyst that asexually multiplies, eventually releasing numerous cercariae. The cercariae swim to a freshwater plant and encyst on the wall, awaiting ingestion by a mammal.
Fasciola infestations usually are asymptomatic. In the acute phase, patients can have abdominal pain and hepatomegaly as the parasites penetrate the intestinal wall and hepatic capsule. Abdominal CT scan may show low-density areas in the periphery of the liver. Patients also develop symptoms from migration of the parasites to other sites such as subcutaneous fat.159 Acute symptoms wane as the parasites enter the bile ducts. During the chronic phase of fascioliasis, patients can have symptoms of intermittent biliary obstruction and cholangitis. Rarely, patients develop pancreatitis. ERCP may show curvilinear lucencies in the bile duct (Fig. 110-13).160
Diagnosis is by finding eggs in the stool. Fasciola release low numbers of eggs, however, making this test insensitive. Duodenal or bile aspirates also can demonstrate eggs. The most sensitive method to detect Fasciola infection is ELISA for antibodies against the worms161; antibody titer drops after successful drug treatment.
Unlike other trematodes, Fasciola are resistant to praziquantel. Triclabendazole is the drug of choice for fascioliasis. In one study, a single oral dose of triclabendazole (10 mg/kg) cured 79% of patients as measured by fecal egg counts and ELISA.162
BLOOD FLUKES
Epidemiology
Construction of water reservoirs and irrigation canals has expanded the snail habitat in many countries, a practice that has increased the risk of acquiring schistosomiasis. Mice and other mammals can harbor schistosomes and might allow spread of the parasite even were sanitation to be improved,163 thereby making it difficult to eradicate. Nonetheless, Japan successfully eradicated S. japonicum, and S. mansoni is vanishing from areas of Puerto Rico.164
Clinical Features and Pathophysiology
Dermal invasion and migration by infecting cercariae usually produce no symptoms. Patients with repeated contact can develop a mild papular rash, in contrast to the intensely pruritic papular rash that develops after exposure to avian schistosomes such as Trichobilharzia ocellata. These avian trematodes infect water fowl but are unable to live in mammals, and so the cercariae and schisosomules die in a person’s skin, eliciting an immunologic response that produces swimmer’s itch. Swimmer’s itch is common in the Great Lakes region and has been found as far north as Iceland.165 Swimmer’s itch is not dangerous, but repetitive scratching can cause secondary cellulitis.
Schistosomules migrate through the body without producing symptoms. Juvenile and adult worms evade immune attack elegantly: Their tegument is coated with histocompatibility and blood group antigens derived from the host.166 The tegument contains immunoglobulin receptors and proteases that might help cleave any bound antibody. Moreover, schistosomes produce several proteins that prevent complement, neutrophils, macrophages, or lymphocytes from injuring them.167 Such immune evasion allows adult worms to survive in the blood vessels without causing much direct damage. The average life span of worms is thought to be about five years, but there are documented cases of adult worms surviving for more than 35 years after persons had left an endemic area.168
Each schistosome egg secretes antigens that provoke a focal granulomatous inflammatory reaction that helps move the egg from the inside of a capillary, through the intestinal wall, and out into the lumen.169 Thus, inflammation actually benefits the parasite. Passage of eggs through the bowel wall causes intestinal schistosomiasis with guaiac-positive stools or even bloody diarrhea. Patients also can have tenesmus and tenderness over the sigmoid colon. Patients with S. mansoni can develop colitis with inflammatory pseudopolyps (Fig. 110-14) composed of numerous eosinophils and occasional eggs170—a picture that can resemble Crohn’s disease or ulcerative colitis. S. japonicum prefers to dwell in veins drained by the superior mesenteric vein and lays thousands of eggs at a time. S. japonicum can produce upper abdominal pain unrelated to meals, gastric bleeding, and pyloric obstruction due to inflammation and fibrosis.
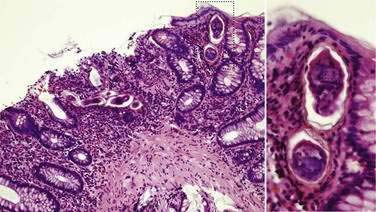
About half of the eggs pass out of the body; the other half lodge in the host’s tissues and cause the pathology of chronic schistosomiasis. Eggs are carried by the portal flow and some lodge in the liver. Other eggs lodge in the mesenteric and portal veins or remain in the intestinal wall. In these locations, the eggs elicit granulomatous inflammation with eosinophils, macrophages, lymphocytes, fibroblasts, and mast cells (Fig. 110-15). Eosinophils account for 50% of the schistosome egg granuloma cell population. When eosinophils degranulate, they deposit major basic protein that produces an eosinophilic halo around the eggs, termed the Splendore-Hoeppli phenomenon. This phenomenon is nonspecific and can be seen with bacterial, fungal, and parasitic infections. Eosinophils likely assist in killing the miracidia protected by the tough egg shell. After one or two weeks the miracidium dies, antigen release wanes, and the granuloma involutes to leave a fibrotic scar.
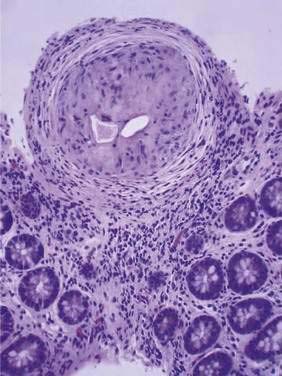
Figure 110-15. Histopathology of a colonic biopsy specimen from a patient with schistosomiasis. A schistosome egg granuloma is seen.
Over the years, the daily production of eggs, granulomas, and scars accumulates enough damage to produce disease. Eggs that lodge in the hepatic and portal vessels produce a unique pattern of scarring called Symmers’ pipe stem fibrosis, in which the vessels become fibrotic and resemble clay pipe stems on cross section; this process causes the presinusoidal venous obstruction and portal hypertension characteristic of hepatosplenic schistosomiasis (see Chapter 82). Patients typically have an enlarged left hepatic lobe, splenomegaly, and thrombocytopenia due to platelet sequestration. Hepatocellular function remains normal because the blood supply to the liver is maintained by increased hepatic artery flow. Patients have normal serum aminotransferase levels and mildly elevated serum levels of alkaline phosphatase and gamma glutamyl transpeptidase. Patients with hepatosplenic schistosomiasis do not develop cirrhosis unless they are coinfected with hepatitis B or C, and so they lack stigmata of chronic liver disease. The classic presentation of decompensated hepatosplenic schistosomiasis is variceal hemorrhage.
Hepatosplenic schistosomiasis results from accumulated injury and requires prolonged, moderately intense infection. Patients with hepatosplenic schistosomiasis typically range in age from adolescence to late 20s and have had schistosomiasis for five to 15 years. Compensated disease improves, however, after schistosomes are killed by drug therapy, permitting the portal tributaries to heal and remodel.171,172
Patients with schistosomiasis can present with recurrent bacteremia. Adult schistosome worms can ingest enteric bacteria transiently present in the portal circulation, harbor these bacteria, and serve as reservoirs for infection. Recurrent salmonella infection is particularly common in patients with schistosomiasis.173
Schistosomiasis can cause membranoproliferative glomerulonephritis or focal glomerulosclerosis with proteinuria, nephrotic syndrome, and end-stage renal disease. Schistosomal nephropathy results from deposition of immune complexes of parasite antigens and antibodies, and the renal disease can be progressive even if the parasites are killed with drug therapy.174
Diagnosis
Schistosome eggs are present in stool, but not in high numbers. The classic method for detecting eggs is the Kato-Katz thick smear.175 This technique is not performed as part of the standard ova and parasite test, and standard evaluation is not sensitive enough to find the relatively rare schistosome eggs. Even Kato-Katz thick smears are not highly sensitive and are unlikely to detect eggs at very low levels of infection.
The vast majority of patients with intestinal schistosomiasis are asymptomatic; patients come to medical attention during evaluation of mild anemia, positive fecal occult blood tests, or unexpected variceal hemorrhage. On endoscopy, a patient might have inflammatory polyps that contain eggs,170 but usually, the intestinal mucosa appears normal. Subtle changes in the vascular pattern can result from egg emboli producing a terminal curling of small blood vessels.176 Occasionally, histopathology of random biopsies of the colonic mucosa show schistosome eggs (Fig. 110-16), but this is an insensitive means of diagnosis. Biopsy of the rectum can demonstrate eggs, especially when the biopsy is crushed between two glass slides and the whole biopsy specimen is surveyed microscopically. Evaluation of six crush biopsies is more sensitive than two Kato-Katz smears for S. mansoni.177
Present or past exposure to schistosomes is detectable by serology. Antischistosome antibodies are detected by ELISA using adult microsomal antigens. Sensitivity varies depending on whether the infecting schistosome is the same species as that used to prepare the antigens. The ELISA uses S. mansoni microsomal antigens, and immunoblot tests using antigens from S. japonicum and S. hematobium (the schistosome responsible for urinary schistosomiasis) also can be performed.178 The antibody assay also is useful to diagnose acute schistosomiasis (Katayama fever) because there are few or no eggs in the stool during the peak of the reaction. The ELISA does not distinguish active from prior infections, and therefore it is most useful for diagnosis in recent travelers rather than in expatriates. Because schistosomes can be long-lived, one-time treatment of antibody-positive patients is reasonable.
Active infection can be demonstrated by detecting circulating schistosome gut-associated protein antigens CCA (circulating cathodic antigen) and CAA (circulating anodic antigen) in the patient’s serum.179 Serologic detection of CCA and CAA has an equivalent or higher sensitivity than the Kato-Katz thick smear, but each test misses some low-level infections.180 Measurement of circulating antigens also can prove useful to document response to treatment,181 but these tests are not yet commercially available in the United States.
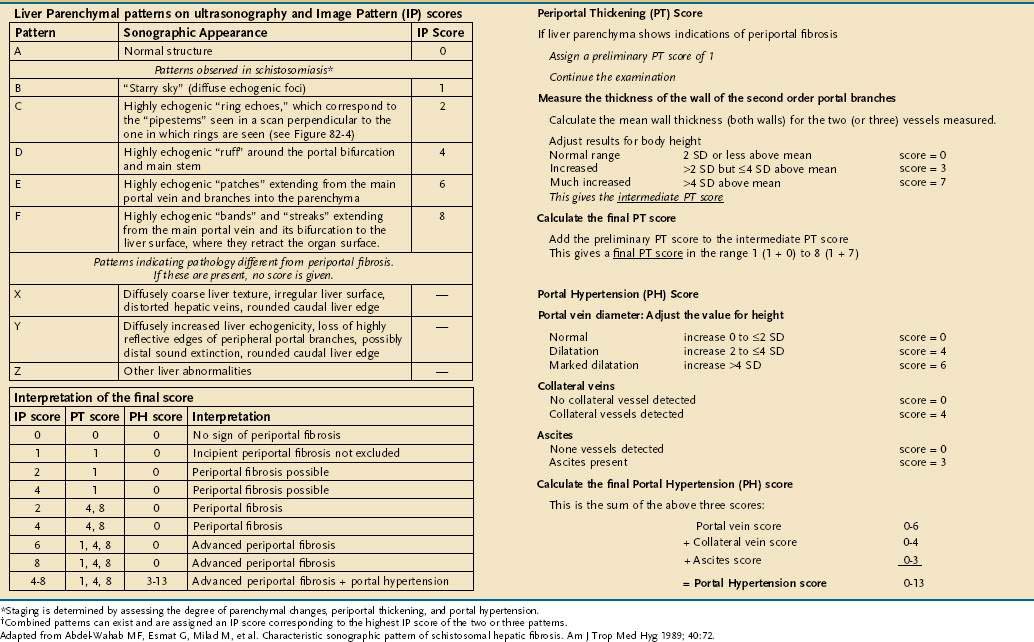
Abdominal ultrasound is an important additional test in hepatosplenic schistosomiasis. Ultrasound evaluation documents periportal fibrosis, splenomegaly, portal blood flow, and collateral vessels. Periportal fibrosis has a characteristic appearance: multiple echogenic areas, each with central echolucency.182,183 A scoring system exists that uses a liver parenchyma and image pattern (IP), a portal thickening (PT), and a portal hypertension (PH) score to stage the disease (Table 110-3).184
Treatment
Praziquantel is the drug of choice to treat schistosomiasis. It is the safest schistosomicide in current use. Praziquantel given orally in three doses of 20 mg/kg, each four hours apart (total dose, 60 mg/kg), gives the best cure rates of 60% to 98%, depending on the series. Eggs continue to be shed in the stool for up to two weeks after drug treatment, because eggs that were deposited before treatment can take this long to work through the intestinal wall. Patients who are not cured with a single course of praziquantel have a dramatic decrease in egg counts and respond to a second course of treatment. Periportal fibrosis improves after the worms are killed, halting the daily deluge of eggs and permitting the portal tributaries to heal and remodel.171,172
Audicana MT, Kennedy MW. Anisakis simplex: From obscure infectious worm to inducer of immune hypersensitivity. Clin Microbiol Rev. 2008;21:360-79. (Ref 99.)
Boulware DR, Stauffer WM, Hendel-Paterson BR, et al. Maltreatment of Strongyloides infection: Case series and worldwide physicians-in-training survey. Am J Med. 2007;120:545-8. (Ref 41.)
Crompton DW. How much human helminthiasis is there in the world? J Parasitol. 1999;85:397-403. (Ref 18.)
Das CJ, Kumar J, Debnath J, Chaudhry A. Imaging of ascariasis. Australas Radiol. 2007;51:500-6. (Ref 22.)
Dick TA, Nelson PA, Choudhury A. Diphyllobothriasis: Update on human cases, foci, patterns and sources of human infections and future considerations. Southeast Asian J Trop Med Public Health. 2001;32(Suppl 2):59-76. (Ref 109.)
Elliott DE, Summers RW, Weinstock JV. Helminths and the modulation of mucosal inflammation. Curr Opin Gastroenterol. 2005;21:51-8. (Ref 5.)
Garcia HH, Del Brutto OH. Neurocysticercosis: Updated concepts about an old disease. Lancet Neurol. 2005;4:653-61. (Ref 120.)
Kaewpitoon N, Kaewpitoon SJ, Pengsaa P, Sripa B. Opisthorchis viverrini: The carcinogenic human liver fluke. World J Gastroenterol. 2008;14:666-74. (Ref 140.)
Keiser J, Utzinger J. Emerging foodborne trematodiasis. Emerg Infect Dis. 2005;11:1507-14. (Ref 141.)
Keiser PB, Nutman TB. Strongyloides stercoralis in the immunocompromised population. Clin Microbiol Rev. 2004;17:208-17. (Ref 37.)
Loukas A, Constant SL, Bethony JM. Immunobiology of hookworm infection. FEMS Immunol Med Microbiol. 2005;43:115-24. (Ref 57.)
Pozio E, Zarlenga DS. Recent advances on the taxonomy, systematics and epidemiology of Trichinella. Int J Parasitol. 2005;35:1191-204. (Ref 88.)
Richter J, Hatz C, Campagne G, et al. Ultrasound in Schistosomiasis: A Practical Guide to the Standardized Use of Ultrasonography for the Assessment of Schistosomiasis-related Morbidity. Niamey, Niger: World Health Organization: Second International Workshop; 2000. (Ref 184.)
Sorvillo FJ, DeGiorgio C, Waterman SH. Deaths from cysticercosis, United States. Emerg Infect Dis. 2007;13:230-5. (Ref 119.)
Varkey P, Jerath AU, Bagniewski S, Lesnick T. Intestinal parasitic infection among new refugees to Minnesota, 1996-2001. Travel Med Infect Dis. 2007;5:223-9. (Ref 1.)
1. Varkey P, Jerath AU, Bagniewski S, Lesnick T. Intestinal parasitic infection among new refugees to Minnesota, 1996-2001. Travel Med Infect Dis. 2007;5:223-9.
2. Little MD. Laboratory diagnosis of worms and miscellaneous specimens. Clin Lab Med. 1991;11:1041-50.
3. Elliott DE, Urban JFJ, Argo CK, Weinstock JV. Does the failure to acquire helminthic parasites predispose to Crohn’s disease? FASEB J. 2000;14:1848-55.
4. Yazdanbakhsh M, Kremsner PG, van Ree R. Allergy, parasites, and the hygiene hypothesis. Science. 2002;296:490-4.
5. Elliott DE, Summers RW, Weinstock JV. Helminths and the modulation of mucosal inflammation. Curr Opin Gastroenterol. 2005;21:51-8.
6. Elliott D, Li J, Blum A, et al. Exposure to schistosome eggs protects mice from TNBS-induced colitis. Am J Physiol. 2003;284:G385-91.
7. Khan WI, Blennerhasset PA, Varghese AK, et al. Intestinal nematode infection ameliorates experimental colitis in mice. Infect Immun. 2002;70:5931-7.
8. Moreels TG, Nieuwendijk RJ, De Man JG, et al. Concurrent infection with Schistosoma mansoni attenuates inflammation induced changes in colonic morphology, cytokine levels, and smooth muscle contractility of trinitrobenzene sulphonic acid induced colitis in rats. Gut. 2004;53:99-107.
9. Cooke A, Tonks P, Jones FM, et al. Infection with Schistosoma mansoni prevents insulin dependent diabetes mellitus in non-obese diabetic mice. Parasite Immunol. 1999;21:169-76.
10. La Flamme AC, Ruddenklau K, Backstrom BT. Schistosomiasis decreases central nervous system inflammation and alters the progression of experimental autoimmune encephalomyelitis. Infect Immun. 2003;71:4996-5004.
11. Sewell D, Qing Z, Reinke E, et al. Immunomodulation of experimental autoimmune encephalomyelitis by helminth ova immunization. Int Immunol. 2003;15:59-69.
12. Summers RW, Elliott DE, Urban JFJr, et al. Trichuris suis therapy for active ulcerative colitis: A randomized controlled trial. Gastroenterology. 2005;128:825-32.
13. Summers RW, Elliott DE, Qadir K, et al. Trichuris suis seems to be safe and possibly effective in the treatment of inflammatory bowel disease. Am J Gastroenterol. 2003;98:2034-41.
14. Summers RW, Elliott DE, Urban JFJr, et al. Trichuris suis therapy in Crohn’s disease. Gut. 2005;54:87-90.
15. van den Biggelaar AH, Rodrigues LC, van Ree R, et al. Long-term treatment of intestinal helminths increases mite skin-test reactivity in Gabonese schoolchildren. J Infect Dis. 2004;189:892-900.
16. Khuroo MS. Ascariasis. Gastroenterol Clin North Am. 1996;25:553-77.
17. Bethony J, Brooker S, Albonico M, et al. Soil-transmitted helminth infections: Ascariasis, trichuriasis, and hookworm. Lancet. 2006;367:1521-32.
18. Crompton DW. How much human helminthiasis is there in the world? J Parasitol. 1999;85:397-403.
19. Anderson TJ, Jaenike J. Host specificity, evolutionary relationships and macrogeographic differentiation among Ascaris populations from humans and pigs. Parasitology. 1997;115:325-42.
20. Eckardt AJ, Barnard GF. Endoscopic diagnosis and removal of Ascaris lumbricoides during colonoscopy for polyp surveillance (with video). Gastrointest Endosc. 2006;63:708-9.
21. Jang MK, Lee KS. Images in clinical medicine. Ascariasis. N Engl J Med. 2008;358:e16.
22. Das CJ, Kumar J, Debnath J, Chaudhry A. Imaging of ascariasis. Australas Radiol. 2007;51:500-6.
23. de Silva NR, Guyatt HL, Bundy DA. Worm burden in intestinal obstruction caused by Ascaris lumbricoides. Trop Med Int Health. 1997;2:189-90.
24. Wasadikar PP, Kulkarni AB. Intestinal obstruction due to ascariasis. Br J Surg. 1997;84:410-2.
25. Shah OJ, Robanni I, Khan F, et al. Management of biliary ascariasis in pregnancy. World J Surg. 2005;29:1294-8.
26. Maddern GJ, Dennison AR, Blumgart LH. Fatal ascaris pancreatitis: An uncommon problem in the west. Gut. 1992;33:402-3.
27. Keiser J, Utzinger J. Efficacy of current drugs against soil-transmitted helminth infections: Systematic review and meta-analysis. JAMA. 2008;299:1937-48.
28. Gyorkos TW, Larocque R, Casapia M, Gotuzzo E. Lack of risk of adverse birth outcomes after deworming in pregnant women. Pediatr Infect Dis J. 2006;25:791-4.
29. Gonzalez AH, Regalado VC, Van den Ende J. Non-invasive management of Ascaris lumbricoides biliary tact migration: A prospective study in 69 patients from Ecuador. Trop Med Int Health. 2001;6:146-50.
30. Malik AH, Saima BD, Wani MY. Management of hepatobiliary and pancreatic ascariasis in children of an endemic area. Pediatr Surg Int. 2006;22:164-8.
31. Shah OJ, Zargar SA, Robbani I. Biliary ascariasis: A review. World J Surg. 2006;30:1500-6.
32. Gill GV, Bell DR. Strongyloides stercoralis infection in former Far East prisoners of war. Br Med J. 1979;2:572-4.
33. Al Samman M, Haque S, Long JD. Strongyloidiasis colitis: A case report and review of the literature. J Clin Gastroenterol. 1999;28:77-80.
34. Sridhara S, Simon N, Raghuraman U, et al. Strongyloides stercoralis pancolitis in an immunocompetent patient. Gastrointest Endosc. 2008;68:196-9.
35. Weight SC, Barrie WW. Colonic Strongyloides stercoralis infection masquerading as ulcerative colitis. J R Coll Surg Edinb. 1997;42:202-3.
36. Segarra-Newnham M. Manifestations, diagnosis, and treatment of Strongyloides stercoralis infection. Ann Pharmacother. 2007;41:1992-2001.
37. Keiser PB, Nutman TB. Strongyloides stercoralis in the immunocompromised population. Clin Microbiol Rev. 2004;17:208-17.
38. Genta RM. Dysregulation of strongyloidiasis: A new hypothesis. Clin Microbiol Rev. 1992;5:345-55.
39. Viney ME, Brown M, Omoding NE, et al. Why does HIV infection not lead to disseminated strongyloidiasis? J Infect Dis. 2004;190:2175-80.
40. Link K, Orenstein R. Bacterial complications of strongyloidiasis: Streptococcus bovis meningitis. South Med J. 1999;92:728-31.
41. Boulware DR, Stauffer WM, Hendel-Paterson BR, et al. Maltreatment of Strongyloides infection: Case series and worldwide physicians-in-training survey. Am J Med. 2007;120:545-8.
42. Loutfy MR, Wilson M, Keystone JS, Kain KC. Serology and eosinophil count in the diagnosis and management of strongyloidiasis in a non-endemic area. Am J Trop Med Hyg. 2002;66:749-52.
43. Sudarshi S, Stumpfle R, Armstrong M, et al. Clinical presentation and diagnostic sensitivity of laboratory tests for Strongyloides stercoralis in travellers compared with immigrants in a non-endemic country. Trop Med Int Health. 2003;8:728-32.
44. Siddiqui AA, Berk SL. Diagnosis of Strongyloides stercoralis infection. Clin Infect Dis. 2001;33:1040-7.
45. Klein RA, Cleri DJ, Doshi V, Brasitus TA. Disseminated Strongyloides stercoralis: A fatal case eluding diagnosis. South Med J. 1983;76:1438-40.
46. Jongwutiwes S, Charoenkorn M, Sitthichareonchai P, et al. Increased sensitivity of routine laboratory detection of Strongyloides stercoralis and hookworm by agar-plate culture. Trans R Soc Trop Med Hyg. 1999;93:398-400.
47. Cross JH. Intestinal capillariasis. Clin Microbiol Rev. 1992;5:120-9.
48. Moravec F. Redescription and systematic status of Capillaria philippinensis, an intestinal parasite of human beings. J Parasitol. 2001;87:161-4.
49. Detels R, Gutman L, Jaramillo J, et al. An epidemic of intestinal capillariasis in man. A study in a barrio in Northern Luzon. Am J Trop Med Hyg. 1969;18:676-82.
50. Fan E, Soong C, Kain KC, Detsky AS. Clinical problem-solving. A gut feeling. N Engl J Med. 2008;359:75-80.
51. Cross JH, Banzon T, Clarke MD, et al. Studies on the experimental transmission of Capillaria philippinensis in monkeys. Trans R Soc Trop Med Hyg. 1972;66:819-27.
52. Nawalinski TA, Schad GA. Arrested development in Ancylostoma duodenale: Course of a self-induced infection in man. Am J Trop Med Hyg. 1974;23:895-8.
53. Pritchard DI, Brown A. Is Necator americanus approaching a mutualistic symbiotic relationship with humans? Trends Parasitol. 2001;17:169-72.
54. Stassens P, Bergum PW, Gansemans Y, et al. Anticoagulant repertoire of the hookworm Ancylostoma caninum. Proc Natl Acad Sci U S A. 1996;93:2149-54.
55. Chadderdon RC, Cappello M. The hookworm platelet inhibitor: Functional blockade of integrins GPIIb/IIIa (αIIbβ3) and GPIa/IIa (α2β1) inhibits platelet aggregation and adhesion in vitro. J Infect Dis. 1999;179:1235-41.
56. Roche M, Layrisse M. The nature and causes of “hookworm anemia.”. Am J Trop Med Hyg. 1966;15:1029-102.
57. Loukas A, Constant SL, Bethony JM. Immunobiology of hookworm infection. FEMS Immunol Med Microbiol. 2005;43:115-24.
58. Mortimer K, Brown A, Feary J, et al. Dose-ranging study for trials of therapeutic infection with Necator americanus in humans. Am J Trop Med Hyg. 2006;75:914-20.
59. Croese J, O’Neil J, Masson J, et al. A proof of concept study establishing Necator americanus in Crohn’s patients and reservoir donors. Gut. 2006;55:136-7.
60. Reddy SC, Vega KJ. Endoscopic diagnosis of chronic severe upper GI bleeding due to helminthic infection. Gastrointest Endosc. 2008;67:990-2.
61. Knopp S, Mgeni AF, Khamis IS, et al. Diagnosis of soil-transmitted helminths in the era of preventive chemotherapy: Effect of multiple stool sampling and use of different diagnostic techniques. PLoS Negl Trop Dis. 2008;2:e331.
62. Croese J, Loukas A, Opdebeeck J, Prociv P. Occult enteric infection by Ancylostoma caninum: A previously unrecognized zoonosis. Gastroenterology. 1994;106:3-12.
63. Walker NI, Croese J, Clouston AD, et al. Eosinophilic enteritis in northeastern Australia. Pathology, association with Ancylostoma caninum, and implications. Am J Surg Pathol. 1995;19:328-37.
64. Croese J, Fairley S, Loukas A, et al. A distinctive aphthous ileitis linked to Ancylostoma caninum. J Gastroenterol Hepatol. 1996;11:524-31.
65. Ratard RC, Kouemeni LE, Ekani BM, et al. Ascariasis and trichuriasis in Cameroon. Trans R Soc Trop Med Hyg. 1991;85:84-8.
66. Taguchi H, Yamamoto H, Miyata T, et al. In vivo diagnosis of whipworm (Trichuris trichiura) with high-definition magnifying colonoscope (with video). Gastrointest Endosc. 2008;68:376-7.
67. Bundy DA, Cooper ES, Thompson DE, et al. Predisposition to Trichuris trichiura infection in humans. Epidemiol Infect. 1987;98:65-71.
68. Stephenson LS, Holland CV, Cooper ES. The public health significance of Trichuris trichiura. Parasitology. 2000;121(Suppl):S73-5.
69. Jung RC, Beaver PC. Clinical observations on Trichocephalus trichiurus (whipworm) infestation in children. Pediatrics. 1951;18:548-57.
70. Cooper ES, Bundy DA, MacDonald TT, Golden MH. Growth suppression in the Trichuris dysentery syndrome. Eur J Clin Nutr. 1990;44:285-91.
71. Pearce EJ, Caspar P, Grzych JM, et al. Downregulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth, Schistosoma mansoni. J Exp Med. 1991;173:159-66.
72. Strober W, Ehrhardt RO. Chronic intestinal inflammation: An unexpected outcome in cytokine or T cell receptor mutant mice. Cell. 1993;75:203-5.
73. MacDonald TT, Spencer J, Murch SH, et al. Immunoepidemiology of intestinal helminthic infections. 3. Mucosal macrophages and cytokine production in the colon of children with Trichuris trichiura dysentery. Trans R Soc Trop Med Hyg. 1994;88:265-8.
74. Else KJ, Hultner L, Grencis RK. Cellular immune responses to the murine nematode parasite Trichuris muris. II. Differential induction of TH-cell subsets in resistant versus susceptible mice. Immunology. 1992;75:232-7.
75. Else KJ, Finkelman FD, Maliszewski CR, Grencis RK. Cytokine-mediated regulation of chronic intestinal helminth infection. J Exp Med. 1994;179:347-51.
76. Sirivichayakul C, Pojjaroen-Anant C, Wisetsing P, et al. The effectiveness of 3, 5 or 7 days of albendazole for the treatment of Trichuris trichiura infection. Ann Trop Med Parasitol. 2003;97:847-53.
77. Ismail MM, Jayakody RL. Efficacy of albendazole and its combinations with ivermectin or diethylcarbamazine (DEC) in the treatment of Trichuris trichiura infections in Sri Lanka. Ann Trop Med Parasitol. 1999;93:501-4.
78. Belizario VY, Amarillo ME, de Leon WU, et al. A comparison of the efficacy of single doses of albendazole, ivermectin, and diethylcarbamazine alone or in combinations against Ascaris and Trichuris spp. Bull World Health Organ. 2003;81:35-42.
79. Fry GF, Moore JG. Enterobius vermicularis: 10,000 year old human infection. Science. 1969;166:1620.
80. Hugot JP. Enterobius gregorii (Oxyuridae, Nematoda), a new human parasite. Ann Parasitol Hum Comp. 1983;58:403-4.
81. Chittenden AM, Ashford RW. Enterobius gregorii Hugot 1983; first report in the U.K. Ann Trop Med Parasitol. 1987;81:195-8.
82. Hasegawa H, Takao Y, Nakao M, et al. Is Enterobius gregorii Hugot, 1983 (Nematoda: Oxyuridae) a distinct species? J Parasitol. 1998;84:131-4.
83. Vermund SH, MacLeod S. Is pinworm a vanishing infection? Laboratory surveillance in a New York City medical center from 1971 to 1986. Am J Dis Child. 1988;142:566-8.
84. Brown MD. Images in clinical medicine. Enterobius vermicularis. N Engl J Med. 2006;354:e12.
85. Tsibouris P, Galeas T, Moussia M, et al. Two cases of eosinophilic gastroenteritis and malabsorption due to Enterobius vermicularis. Dig Dis Sci. 2005;50:2389-92.
86. Sinniah B, Leopairut J, Neafie RC, et al. Enterobiasis: A histopathological study of 259 patients. Ann Trop Med Parasitol. 1991;85:625-35.
87. Büning J, Homann N, von Smolinski D, et al. Helminths as governors of inflammatory bowel disease. Gut. 2008;57:1182-3.
88. Pozio E, Zarlenga DS. Recent advances on the taxonomy, systematics and epidemiology of Trichinella. Int J Parasitol. 2005;35:1191-204.
89. Roy SL, Lopez AS, Schantz PM. Trichinellosis surveillance—United States, 1997-2001. MMWR Surveillance Summaries. 2003;52(6):1-8.
90. Hinz E. Trichinellosis and trichinellosis control in Germany. Southeast Asian J Trop Med Public Health. 1991;22(Suppl):329-33.
91. Pozio E. New patterns of Trichinella infection. Vet Parasitol. 2001;98:133-48.
92. Moorhead A, Grunenwald PE, Dietz VJ, Schantz PM. Trichinellosis in the United States, 1991-1996: Declining but not gone. Am J Trop Med Hyg. 1999;60:66-9.
93. McIntyre L, Pollock SL, Fyfe M, et al. Trichinellosis from consumption of wild game meat. CMAJ. 2007;176:449-51.
94. Ancelle T, Dupouy-Camet J, Desenclos JC, et al. A multifocal outbreak of trichinellosis linked to horse meat imported from North America to France in 1993. Am J Trop Med Hyg. 1998;59:615-9.
95. Despommier DD. Trichinella spiralis and the concept of niche. J Parasitol. 1993;79:472-82.
96. Capo V, Despommier DD. Clinical aspects of infection with Trichinella spp. Clin Microbiol Rev. 1996;9:47-54.
97. Finkelman FD, Shea-Donohue T, Goldhill J, et al. Cytokine regulation of host defense against parasitic gastrointestinal nematodes: Lessons from studies with rodent models. Ann Rev Immunol. 1997;15:505-33.
98. Dupouy-Camet J, Kociecka W, Bruschi F, et al. Opinion on the diagnosis and treatment of human trichinellosis. Exp Opin Pharmacother. 2002;3:1117-301.
99. Audicana MT, Kennedy MW. Anisakis simplex: From obscure infectious worm to inducer of immune hypersensitivity. Clin Microbiol Rev. 2008;21:360-79.
100. Ikeda K, Kumashiro R, Kifune T. Nine cases of acute gastric anisakiasis. Gastrointest Endosc. 1989;35:304-8.
101. Deardorff TL, Kayes SG, Fukumura T. Human anisakiasis transmitted by marine food products. Hawaii Med J. 1991;50:9-16.
102. Kark AE, McAlpine JC. Anisakiasis (“herring worm disease”) as a cause of acute abdominal crisis. Br J Clin Pract. 1994;48:216-7.
103. del Pozo MD, Moneo I, de Corres LF, et al. Laboratory determinations in Anisakis simplex allergy. J Allergy Clin Immunol. 1996;97:977-84.
104. Audicana MT, Ansotegui IJ, de Corres LF, Kennedy MW. Anisakis simplex: Dangerous—dead and alive? Trends Parasitol. 2002;18:20-5.
105. Del Rey MA, Valero A, Mayorga C, et al. Sensitization to Anisakis simplex s.l. in a healthy population. Acta Trop. 2006;97:265-9.
106. Puente P, Anadon AM, Rodero M, et al. Anisakis simplex: The high prevalence in Madrid (Spain) and its relation with fish consumption. Exp Parasitol. 2008;118:271-4.
107. Caramello P, Vitali A, Canta F, et al. Intestinal localization of anisakiasis manifested as acute abdomen. Clin Microbiol Infect. 2003;9:734-7.
108. Pellegrini M, Occhini R, Tordini G, et al. Acute abdomen due to small bowel anisakiasis. Dig Liver Dis. 2005;37:65-7.
109. Dick TA, Nelson PA, Choudhury A. Diphyllobothriasis: Update on human cases, foci, patterns and sources of human infections and future considerations. Southeast Asian J Trop Med Public Health. 2001;32(Suppl 2):59-76.
110. Ohnishi K, Kato Y. Single low-dose treatment with praziquantel for Diphyllobothrium nihonkaiense infections. Intern Med. 2003;42:41-3.
111. Schantz PM. Tapeworms (cestodiasis). Gastroenterol Clin North Am. 1996;25:637-53.
112. Nyberg W. The influence of Diphyllobothrium latum on the vitamin B12–intrinsic factor complex: II. In vitro studies. Acta Med Scand. 1960;167:189-92.
113. Hirata M, Yamaguchi Y, Ikei Y, et al. A case of Diphyllobothrium latum/nihonkaiense infection identified by capsule endoscopy in small intestine. Gastrointest Endosc. 2006;64:129.
114. Liao WS, Bair MJ. Images in clinical medicine. Taenia in the gastrointestinal tract. N Engl J Med. 2007;357:1028.
115. Martines H, Fanciulli E, Menardo G. Incidental video-capsule diagnosis of small-bowel Taenia saginata in a patient with recurrent hemorrhage due to angiodysplasias. Endoscopy. 2006;38(Suppl 2):e35.
116. Centers for Disease Control and Prevention (CDC). Recommendations of the International Task Force for Disease Eradication. MMWR. 1993;42:1-46.
117. Morales J, Martinez JJ, Garcia-Castella J, et al. Taenia solium: The complex interactions, of biological, social, geographical and commercial factors, involved in the transmission dynamics of pig cysticercosis in highly endemic areas. Ann Trop Med Parasitol. 2006;100:123-35.
118. Garcia HH, Gonzalez AE, Evans CA, Gilman RH, Cysticercosis Working Group in Peru. Taenia solium cysticercosis. Lancet. 2003;362:547-56.
119. Sorvillo FJ, DeGiorgio C, Waterman SH. Deaths from cysticercosis, United States. Emerg Infect Dis. 2007;13:230-5.
120. Garcia HH, Del Brutto OH. Neurocysticercosis: Updated concepts about an old disease. Lancet Neurol. 2005;4:653-61.
121. Khalil HM, el Shimi S, Sarwat MA, et al. Recent study of Hymenolepis nana infection in Egyptian children. J Egypt Soc Parasitol. 1991;21:293-300.
122. Kappus KD, Lundgren RGJ, Juranek DD, et al. Intestinal parasitism in the United States: Update on a continuing problem. Am J Trop Med Hyg. 1994;50:705-13.
123. Chu GS, Palmieri JR, Sullivan JT. Beetle-eating: A Malaysia folk medical practice and its public health implications. Trop Geogr Med. 1977;29:422-7.
124. Asano K, Okamoto K. Murine T cell clones specific for Hymenolepis nana: Generation and functional analysis in vivo and in vitro. Int J Parasitol. 1991;21:891-6.
125. Asano K, Muramatsu K. Importance of interferon-γ in protective immunity against Hymenolepis nana cysticercoids derived from challenge infection with eggs in BALB/c mice. Int J Parasitol. 1997;27:1437-43.
126. Watanabe N, Nawa Y, Okamoto K, Kobayashi A. Expulsion of Hymenolepis nana from mice with congenital deficiencies of IgE production or of mast cell development. Parasite Immunol. 1994;16:137-44.
127. Conchedda M, Bortoletti G, Gabriele F, et al. Immune response to the cestode Hymenolepis nana: Cytokine production during infection with eggs or cysts. Int J Parasitol. 1997;27:321-7.
128. Hunter MM, Wang A, Hirota CL, McKay DM. Neutralizing anti–IL-10 antibody blocks the protective effect of tapeworm infection in a murine model of chemically induced colitis. J Immunol. 2005;174(11):7368-75.
129. Hunter MM, Wang A, McKay DM. Helminth infection enhances disease in a murine TH2 model of colitis. Gastroenterology. 2007;132:1320-30.
130. Liu LX, Harinasuta KT. Liver and intestinal flukes. Gastroenterol Clin North Am. 1996;25:627-36.
131. Mas-Coma S, Bargues MD, Valero MA. Fascioliasis and other plant-borne trematode zoonoses. Int J Parasitol. 2005;35:1255-78.
132. Plaut AG, Kampanart-Sanyakorn C, Manning GS. A clinical study of Fasciolopsis buski infection in Thailand. Trans R Soc Trop Med Hyg. 1969;63:470-8.
133. Jaroonvesama N, Charoenlarp K, Areekul S. Intestinal absorption studies in Fasciolopsis buski infection. Southeast Asian J Trop Med Public Health. 1986;17:582-6.
134. Sadun EH, Maiphoom C. Studies on the epidemiology of the human intestinal fluke, Fasciolopsis buski (Lankester) in central Thailand. Am J Trop Med Hyg. 1953;2:1070-84.
135. Murugesh M, Veerendra S, Madhu S, et al. Endoscopic extraction of Fasciolopsis buski. Endoscopy. 2007;39(Suppl 1):E129.
136. Adams KO, Jungkind DL, Bergquist EJ, Wirts CW. Intestinal fluke infection as a result of eating sushi. Am J Clin Pathol. 1986;86:688-9.
137. Huffman JE, Fried B. Echinostoma and echinostomiasis. Adv Parasitol. 1990;29:215-69.
138. Poland GA, Navin TR, Sarosi GA. Outbreak of parasitic gastroenteritis among travelers returning from Africa. Arch Intern Med. 1985;145:2220-1.
139. Cho CM, Tak WY, Kweon YO, et al. A human case of Echinostoma hortense (Trematoda: Echinostomatidae) infection diagnosed by gastroduodenal endoscopy in Korea. Korean J Parasitol. 2003;41:117-20.
140. Kaewpitoon N, Kaewpitoon SJ, Pengsaa P, Sripa B. Opisthorchis viverrini: The carcinogenic human liver fluke. World J Gastroenterol. 2008;14:666-74.
141. Keiser J, Utzinger J. Emerging foodborne trematodiasis. Emerg Infect Dis. 2005;11:1507-14.
142. Kim EM, Kim JL, Choi SY, et al. Infection status of freshwater fish with metacercariae of Clonorchis sinensis in Korea. Korean J Parasitol. 2008;46:247-51.
143. Koenigstein RP. Observations on the epidemiology of infections with Clonorchis sinensis. Trans R Soc Trop Med Hyg. 1949;42:503-6.
144. Park DH, Son HY. Images in clinical medicine. Clonorchis sinensis. N Engl J Med. 2008;358:e18.
145. Infection with liver flukes (Opisthorchis viverrini, Opisthorchis felineus and Clonorchis sinensis). IARC Monogr Eval Carcinog Risks Hum. 1994;61:121-75.
146. Gibson JB. Parasites, liver disease and liver cancer. IARC Sci Publ. 1971;1:42-50.
147. Kim YI, Yang DH, Chang KR. Relationship between Clonorchis sinensis infestation and cholangiocarcinoma of the liver in Korea. Seoul J Med. 1974;15:247-53.
148. Chung CS, Lee SK. An epidemiological study of primary liver carcinomas in Busan area with special reference to clonorchiasis. Korean J Pathol. 1976;10:33-46.
149. Parkin DM, Srivatanakul P, Khlat M, et al. Liver cancer in Thailand. I. A case-control study of cholangiocarcinoma. Int J Cancer. 1991;48:323-8.
150. Haswell-Elkins MR, Mairiang E, Mairiang P, et al. Cross-sectional study of Opisthorchis viverrini infection and cholangiocarcinoma in communities within a high-risk area in northeast Thailand. Int J Cancer. 1994;59:505-9.
151. Kim YI. Liver carcinoma and liver fluke infection. Arzneimittelforschung. 1984;34:1121-6.
152. Haswell-Elkins MR, Satarug S, Elkins DB. Opisthorchis viverrini infection in northeast Thailand and its relationship to cholangiocarcinoma. J Gastroenterol Hepatol. 1992;7:538-48.
153. Kammerer WS, Van Der Decker JD, Keith TB, Mott KE. Clonorchiasis in New York City Chinese. Trop Doct. 1977;7:105-6.
154. Schwartz DA. Cholangiocarcinoma associated with liver fluke infection: A preventable source of morbidity in Asian immigrants. Am J Gastroenterol. 1986;81:76-9.
155. Leung JW, Sung JY, Chung SC, Metreweli C. Hepatic clonorchiasis—a study by endoscopic retrograde cholangiopancreatography. Gastrointest Endosc. 1989;35:226-31.
156. Choi D, Hong ST, Lim JH, et al. Sonographic findings of active Clonorchis sinensis infection. J Clin Ultrasound. 2004;32:17-23.
157. Harinasuta T, Pungpak S, Keystone JS. Trematode infections. Opisthorchiasis, clonorchiasis, fascioliasis, and paragonimiasis. Infect Dis Clin North Am. 1993;7:699-716.
158. Reinhard GH, Graf V, Augustin HJ. Chronic fascioliasis with destructive cholangitis. Fortschr Med. 1991;109:737-8.
159. Arjona R, Riancho JA, Aguado JM, et al. Fascioliasis in developed countries: A review of classic and aberrant forms of the disease. Medicine. 1995;74:13-23.
160. Veerappan A, Siegel JH, Podany J, et al. Fasciola hepatica pancreatitis: Endoscopic extraction of live parasites. Gastrointest Endosc. 1991;37:473-5.
161. Hillyer GV, Soler de Galanes M, Rodriguez-Perez J, et al. Use of the Falcon assay screening test—enzyme-linked immunosorbent assay (FAST-ELISA) and the enzyme-linked immunoelectrotransfer blot (EITB) to determine the prevalence of human fascioliasis in the Bolivian Altiplano. Am J Trop Med Hyg. 1992;46:603-9.
162. Apt W, Aguilera X, Vega F, et al. Treatment of human chronic fascioliasis with triclabendazole: Drug efficacy and serologic response. Am J Trop Med Hyg. 1995;52:532-5.
163. Sene M, Bremond P, Herve JP, et al. Comparison of human and murine isolates of Schistosoma mansoni from Richard-Toll, Senegal, by isoelectric focusing. J Helminthol. 1997;71:175-81.
164. Hillyer GV, Soler de Galanes M. Seroepidemiology of schistosomiasis in Puerto Rico: Evidence for vanishing endemicity. Am J Trop Med Hyg. 1999;60:827-30.
165. Kolarova L, Skirnisson K, Horak P. Schistosoma cercariae as the causative agent of swimmer’s itch in Iceland. J Helminthol. 1999;73:215-20.
166. Skelly PJ, Alan WR. Making sense of the schistosome surface. Adv Parasitol. 2006;63:185-284.
167. Fishelson Z. Novel mechanisms of immune evasion by Schistosoma mansoni. Mem Inst Oswaldo Cruz. 1995;90:289-92.
168. Hornstein L, Lederer G, Schechter J, et al. Persistent Schistosoma mansoni infection in Yemeni immigrants to Israel. Isr J Med Sci. 1990;26:386-9.
169. Doenhoff MJ, Hassounah O, Murare H. The schistosome egg granuloma: Immunopathology in the cause of host protection or parasite survival? Trans R Soc Trop Med Hyg. 1986;80:503-14.
170. el-Masry NA, Farid Z, Bassily S, et al. Schistosomal colonic polyposis: Clinical, radiological and parasitological study. J Trop Med Hyg. 1986;89:13-7.
171. Doehring-Schwerdtfeger E, Abdel-Rahim IM, Kardorff R, et al. Ultrasonographical investigation of periportal fibrosis in children with Schistosoma mansoni infection: Reversibility of morbidity twenty-three months after treatment with praziquantel. Am J Trop Med Hyg. 1992;46:409-15.
172. Boisier P, Ramarokoto CE, Ravaoalimalala VE, et al. Reversibility of Schistosoma mansoni–associated morbidity after yearly mass praziquantel therapy: Ultrasonographic assessment. Trans R Soc Trop Med Hyg. 1998;92:451-3.
173. Rocha H, Kirk JW, Hearey CDJr. Prolonged Salmonella bacteremia in patients with Schistosoma mansoni infection. Arch Intern Med. 1971;128:254-7.
174. Martinelli R, Pereira LJ, Brito E, Rocha H. Clinical course of focal segmental glomerulosclerosis associated with hepatosplenic schistosomiasis mansoni. Nephron. 1995;69:131-4.
175. Elliott DE. Schistosomiasis. Pathophysiology, diagnosis, and treatment. Gastroenterol Clin North Am. 1996;25:599-625.
176. Sanguino J, Peixe R, Guerra J, et al. Schistosomiasis and vascular alterations of the colonic mucosa. Hepatogastroenterology. 1993;40:184-7.
177. Abdel-Hafez MA, Bolbol AH. Fibre-optic sigmoidoscopy compared with the Kato technique in diagnosis and evaluation of the intensity of Schistosoma mansoni infection. Trans R Soc Trop Med Hyg. 1992;86:641-3.
178. Tsang VC, Wilkins PP. Immunodiagnosis of schistosomiasis. Immunol Invest. 1997;26:175-88.
179. de Jonge N, Kremsner PG, Krijger FW, et al. Detection of the schistosome circulating cathodic antigen by enzyme immunoassay using biotinylated monoclonal antibodies. Trans R Soc Trop Med Hyg. 1990;84:815-8.
180. van Lieshout L, Panday UG, de Jonge N, et al. Immunodiagnosis of schistosomiasis mansoni in a low endemic area in Surinam by determination of the circulating antigens CAA and CCA. Acta Tropica. 1995;59:19-29.
181. De Clercq D, Sacko M, Vercruysse J, et al. Assessment of cure by detection of circulating antigens in serum and urine, following schistosomiasis mass treatment in two villages of the Office du Niger, Mali. Acta Tropica. 1997;68:339-46.
182. Abdel-Wahab MF, Esmat G, Milad M, et al. Characteristic sonographic pattern of schistosomal hepatic fibrosis. Am J Trop Med Hyg. 1989;40:72-6.
183. De Jesus AR, Miranda DG, Miranda RG, et al. Morbidity associated with Schistosoma mansoni infection determined by ultrasound in an endemic area of Brazil, Caatinga do Moura. Am J Trop Med Hyg. 2000;63:1-4.
184. Richter J, Hatz C, Campagne G, et al. Ultrasound in schistosomiasis: A practical guide to the standardized use of ultrasonography for the assessment of schistosomiasis-related morbidity. Geneva: World Health Organization; 2000.

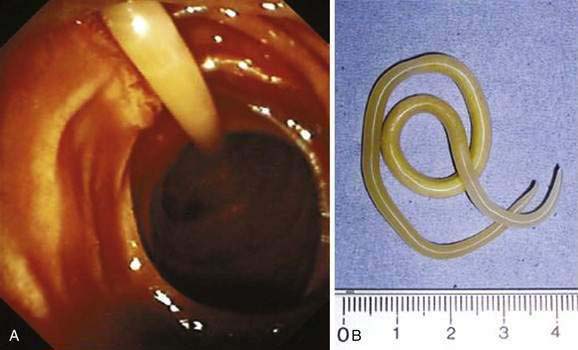
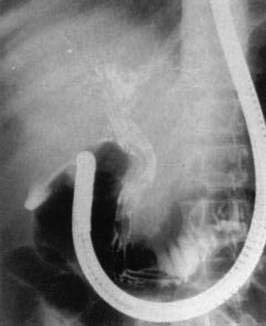
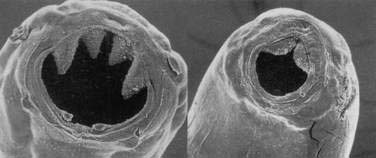
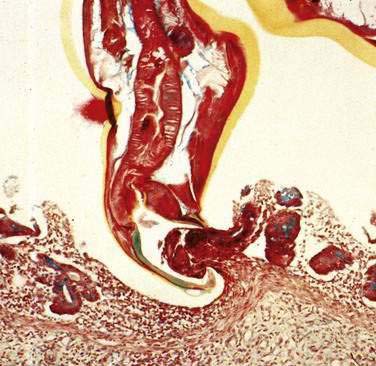
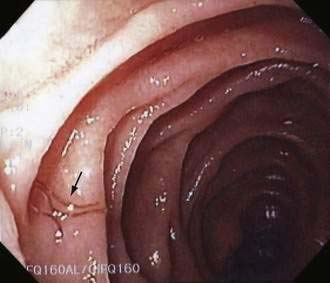

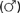 ) and female (
) and female (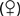 ) whipworms.
) whipworms.