Chapter 11 Interventional Ultrasound
General Principles of Ultrasound-Guided Interventions
Human anatomy is typically more complicated than the remnants of gross anatomy class that most nonsurgical physicians carry around in their heads. Between the surface and the target structures of nerve or muscle, there are not only multiple types of tissues with different ultrasonographic signatures, but also a variety of interposed blood vessels, nerves, and lymphatic structures, some of which are apparent by ultrasound. Furthermore, anatomic relationships vary with patient size, positioning, and bone structure and anatomic variants are common. To that end, the first phase of all ultrasound-guided interventions is preprocedural examination of the area of interest. This allows the clinician to visualize the target tissue and analyze surrounding structures in three dimensions. The diligent examiner uses this time to address three critical questions for needle-based interventions: (1) is the target well delineated; (2) are there anatomic variants or pathologic changes present that might complicate the procedure; and (3) what is the optimal pathway for a needle to reach the target with the least risks of complications and discomfort for the patient? These questions warrant further elaboration.
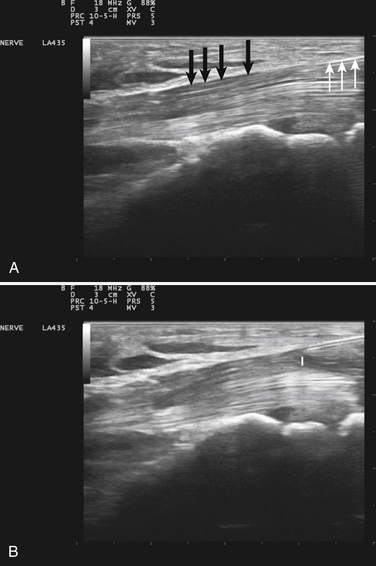
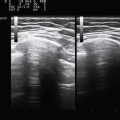
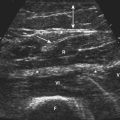
Visualization of the target implies that the examiner can fully image the site of eventual needle placement and the pathway leading up to it. Furthermore, it implies that the needle should be visible during its trajectory, if not throughout its entire passage, at least in areas where the needle is in proximity to other vital structures. If the probe is positioned to obtain a cross-sectional image of an inserted needle, the needle appears as only a single point, and the technique cannot distinguish the tip of the needle from its shaft, except at the point where the needle first appears while advancing it (or disappears while retracting it). The probability of error using this method can be reduced somewhat by needle or probe movement back and forth, observation of local tissue movement by such maneuvers, or the injection of a small test dose of material that might be apparent by ultrasound. More information about the tip of the needle is available by in-plane or an image in the sagittal plane of the needle in which the needle is advanced in-plane with the long axis of the ultrasound transducer (Fig. 11.1 and Videos 11.1, 11.2, and 11.3).
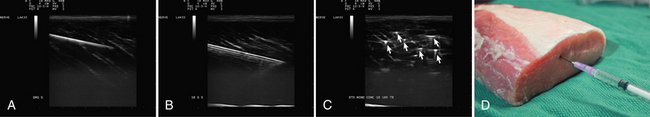
Fig. 11.1 Images of electromyography (EMG) needles in a convenient phantom, an uncooked pork loin. A and B are in-plane views of the needle, with the probe parallel to the direction of the needle. A, A Teflon-coated EMG injection needle, with an uninsulated beveled tip; this characteristic is captured better on this image than on the direct photographs of the needles in Figure 11.2. B, An 18-gauge hypodermic needle with a ring-down–type artifact (this image results from the sound waves reverberating once in the hypodermic compartment before being transmitted back to the transducer). C, Six different needle types in cross section with an out-of-plane view, probe perpendicular to the needle. These are an EMG injection needle, a monopolar EMG needle, a concentric EMG needle, an 18-gauge needle, an 18-gauge needle that has been sandpapered slightly, and the needle from an insulin syringe. Note that the size of the footprint in this image is relatively independent of needle thickness. D, 18-gauge needles show different ring-down artifacts because they are inserted at slightly different angles to the plane of the surface of the phantom.
The presence of pathologic changes and anatomic variants should be apparent on the screening examination. Of course, an informed user should have a heightened suspicion of pathology based on preprocedural clinical assessment of the patient, and should be sufficiently educated on possible anatomic variants so as to be able to deal with the majority of them. This requires specific acquired knowledge of the target area and its surroundings. For example, injectors of the median nerve at the wrist should be aware of common variants such as bifid median nerves and persistent median arteries (see Chapter 5). For those using ultrasound to guide needle biopsy of muscle, an awareness of myopathies associated with particularly echogenic tissue changes (such as inclusion body myopathy) is helpful in that these changes can cause difficulty precisely locating the needle for biopsy.
In evaluating the optimal pathway for passing the needle to the target, the clinician may benefit from having experience with more than one approach to the structure of interest. Anomalous blood vessels, anatomic variants, or scar tissue may present problems amenable to selection of an alternate pathway. The clinician should also be aware of other imaging modalities (computed tomography [CT] or fluoroscopy) that might be available for guiding needle placement should ultrasound prove inadequate, and be ready to refer the patient for such procedures or consider referral for interventions when appropriate. Also compatible with concurrent ultrasound imaging is the use of EMG guidance. EMG guidance needles are monopolar hypodermic needles that are covered with a thin electrical insulator (e.g., Teflon) up to the bare tip, which serves as a recording electrode (Fig. 11.2). As this needle tip is passed through muscle, it generates an injury potential that creates a distinct high-pitched audible sound known as insertional rip on an EMG instrument. This type of needle monitoring is a useful adjunct when injecting tissue near muscles, because insertional activity is unique to muscle and its presence on entering, and absence when leaving muscle provides valuable localizing information about the needle tip. The needle tip can also be used for stimulation, which can help with localization.

Fig. 11.2 Images of the hypodermic needles used in Figure 11.1. A, Left to right: 30-gauge needle, 10-mm length; insulin syringe, 15-mm length; 18-gauge needle, 35-cm length; Teflon-coated electromyography (EMG) injection needle, 35-mm length; and 21-gauge needle, 45-mm length. B, The EMG injection needle (Myoject), compared with a Teca monopolar EMG needle, with a shaft coated to its tip, and a TECA concentric EMG needle, with a bare shaft. All three needles are 35 mm in length. Note the slightly greater thickness of the injection needle. C and D, Zoom images of the tip of the Teflon-coated injection EMG injection needle (Myoject) and monopolar EMG needle shown in B.
Ultrasound Guidance for Steroid Injections for Carpal Tunnel Syndrome
Steroid injections into the carpal tunnel are helpful for treating symptoms of the disorder. In some series a significant percentage of treated patients achieve meaningful results that last for up to 7 years or longer.1 However, like most interventional procedures, it is difficult to acquire an acceptable evidence base because of inconsistent variations in technique, drug dose, concentration, and combinations of drug with local anesthetics, and lack of a standard, in many published studies, to guarantee proper location of the injection. Further complicating the process is the absence of a strong financial interest from pharmaceutical companies to fund such research, because the cost of the drugs used is far too little to justify funding expensive clinical trials. The advent of widely available high-resolution ultrasound helps address the concern about accurate injection placement and makes the technique more available to those with a good working knowledge of the anatomy and familiarity with ultrasound technique. More definitive studies will be welcome.
Because it is likely that many neuromuscular medicine physicians will see and diagnose carpal tunnel syndrome (CTS) in a laboratory setting, it is appropriate to discuss CTS injection techniques in detail. Standard, blind techniques for injecting the carpal tunnel involve identifying either the flexor carpi radialis tendon or palmaris longus tendon at the wrist and inserting a needle adjacent to the tendon and advancing the needle at a 30- to 45-degree angle to the skin toward the median nerve. The injection is made just proximal to the distal wrist crease, and the angle of approach is determined by anticipated location of the median nerve under the flexor retinaculum. Some techniques involve starting 1 cm proximal to the distal wrist crease, others start closer and adjust the needle angle accordingly.2–4 Injuries from blind techniques for steroid injections do occur but are rare,1 perhaps less than 0.1%, a figure much lower than that caused by surgery;1,5 relief begins in just a few days for most patients.2
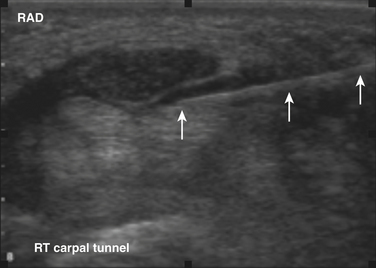
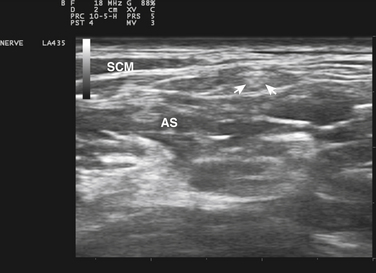
Ultrasound guidance can be used to monitor the procedure, insure proper localization of the injectate, and avoid intraneural injection. Two approaches are commonly used, although there are probably a number of variations that could be considered as well. The nerve can be approached in a fashion similar to the blind techniques. The proximal edge of the transducer can be positioned over the nerve at the distal wrist crease in a sagittal plane. This frees up the area just proximal to the distal wrist crease. Using the tendons as surface landmarks that correspond to their visual location and relationship to the median nerve, a site 1-cm proximal to the distal wrist crease can be selected, the needle placed just ulnar to the palmaris longus tendon, and then advanced at an angle toward the nerve (Fig. 11.3 and Video 11.4).6 With ultrasound the angle of approach does not have to be arbitrary, but can be mapped out visually by rotating the probe slightly toward the advancing needle, where it can be followed to a position just lateral or superficial to the nerve where the injection can be given. The median nerve depth is shallow at this location.
Another approach using ultrasound has been described (Fig. 11.4).7 In this approach the transducer is placed at the wrist over the distal wrist acquiring a standard cross-sectional image of the median nerve at this level. The needle is inserted at the ulnar edge of the distal wrist crease, and advanced underneath the transducer at a shallow depth. It is then guided to pass over the ulnar nerve and artery, enters the carpal tunnel, and approximates the ulnar edge of the median nerve. The authors recommend injecting superiorly first, to slightly separate the nerve from the ceiling of the tunnel formed by the transverse carpal ligament.7 The needle can then be withdrawn slightly and re-advanced to a deeper level, ulnar to the nerve, and additional material injected to surround the nerve, from below, with the injectate. This technique has the advantage of keeping the nerve, tendons, ulnar nerve, ulnar artery, and needle tip in sight at all times.
A variety of parenteral steroid preparations and local anesthetics can be used for intralesional therapy of CTS. The author prefers to use a mixture of 1 mL of 1% lidocaine and 1 mL of triamcinolone acetonide 40 mg/mL. Others use 1 mL of 1% lidocaine mixed with 1 mL of methylprednisolone (40 mg).7 Because the mechanism of action is not yet understood, it is difficult to predict how to best determine optimal dose, concentration, and volume of either the steroid compound, or the local anesthetic. The use of ultrasound guidance should enhance the comfort level of physicians performing this technique and possibly lead to the type of quality clinical studies needed to advance the field.
Ultrasound Guidance for Botulinum Toxin Injections
The Physiology and Pharmacology of Botulinum Toxin
The mechanism of action of the botulinum toxins is that they bind to and disable the interior membrane proteins essential for neurotransmitter release in the nerve terminals at the neuromuscular junction.8–10 The explanation for their prolonged duration of effect is still not well understood, a phenomenon all the more remarkable in view of the minuscule doses used in patients that are on the order of nanograms of active toxin. Perhaps the nerve terminal environment, where the toxin is retained, is relatively free of enzymatic processes that degrade the active toxin.
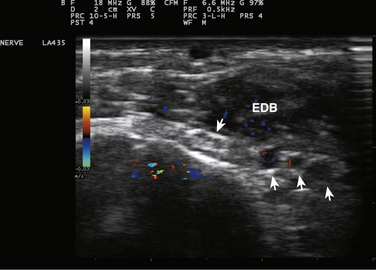
The human extensor digitorum brevis, a largely superfluous muscle for shoe-wearing bipeds, is an ideal muscle for studying the clinical effects of botulinum toxins.11 Injection of 5 units of Botox in this muscle has a profound but slightly delayed effect on muscle function as measured by motor responses evoked by supramaximal stimulation of the peroneal nerve (compound muscle action potentials) or by the mean rectified voltage of surface EMG recorded during maximal voluntary contraction of the extensor digitorum brevis as shown in Figures 11.5 to 11.7. At 100 days, a time when many patients undergo repeat injection for dystonia, the muscle is far from full recovery. In many ways this should not be unexpected. Patients with postoperative surgical pain do not wait for the effects of a single dose of morphine to wear off before asking for the next, so it makes sense that the beneficial effects of the drug should still be present at a time when repeat therapy is requested. Interviews of individuals who recover from accidental botulinum toxin poisoning indicate that they do not experience complete recovery from it for 1 to 2 years.12
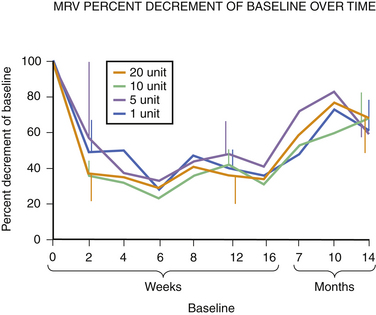
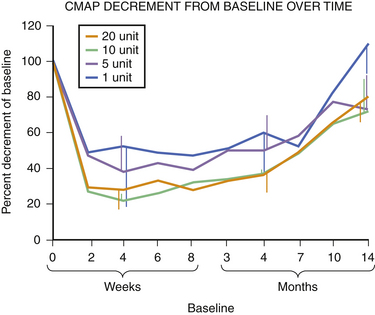
Fig. 11.6 This figure is from the same subjects as in Figure 11.5 and represents the data from full voluntary activation of the extensor digitorum brevis while recording the mean rectified voltage of a 200 ms epoch of data. Again, full recovery has not occurred, this time in any subject, at 14 months. The disparity between the compound muscle action potentials data and the mean rectified voltage data raises the possibility that there may be a central effect of botulinum toxin related to voluntary recruitment; however, the n is too small for definitive conclusions. Claims of longer duration of one botulinum toxin product versus another based on subjective estimates of perceived duration of clinical benefit may need to be tempered in light of more tangible evidence available from quantitative studies of muscle.
Experience in this author’s own laboratory indicates that this may be the case. Following extensor digitorum brevis injections of 1, 5, 10, or 20 units of botulinum toxin, it was found that at 10 months all injected muscles averaged only 70% recovery; even at 14 months, those injected with 5, 10, or 20 units of botulinum toxin were still only 70% of baseline. Only those injected with 1 unit were completely recovered at that point (see Fig. 11.5).
In addition to measurements of electrical activity, the effects of botulinum toxin can also be assessed by loss of muscle bulk. Hamjian and Walker11 showed that Botox injections into the extensor digitorum brevis caused a significant and measurable loss of thickness of this muscle by ultrasound imaging that peaked at 42 days after injections of botulinum toxin. This observation has two substantive implications. The first relates to the fact that muscle hypertrophy contributes to the severity of the symptoms of dystonia. Prior to the advent of botulinum toxin in patients whose symptoms went untreated for years it was common to find that patients complained not only of visible hypertrophy of the sternocleidomastoid muscle (the only muscle with sufficiently distinct contours to be so described), but also of enlargement of their neck size. Women noted that they needed to add links to their necklaces, and men noted that their collar size increased. One of the consequences of constant pulling of the neck muscles was that they became increasingly stronger with the exercise afforded by the involuntary muscle contractions, adding to the disability component of the disorder. Botulinum toxin reverses the hypertrophy, and in fact with repeated injections results in palpable and visible atrophy.13 Anecdotally, some have suggested that not all botulinum toxins cause the same degree of atrophy following injection, speculation that has yet to be validated by clinical data. Ultrasound would be a tool that could easily address this question if clinical observation warranted it. The other implication of this observation is that ultrasound would likely be an effective tool for measuring the efficacy of drugs that reversed atrophy, an observation of potential relevance for disorders such as neuropathy or motor neuron disease where atrophy is a fundamental manifestation of illness.
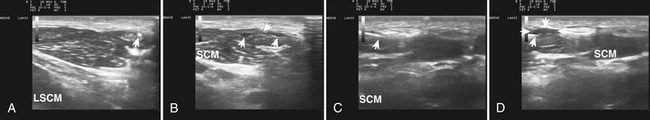
Guiding Botulinum Toxin Injections with Ultrasound
The pioneers of botulinum toxin therapy in the 1980s for neurologic indications in the United States used palpation and surface landmarks for guiding toxin injections. Subsequently, EMG guidance evolved, using Teflon-coated hypodermic recording needles that were insulated with only a bare tip exposed. Blind injections were generally effective, particularly if administered by experienced clinicians, but subsequent studies showed that EMG needle guidance enhanced the effects of such injections with fewer side effects.14 This is not surprising because “blind” injectors often used 10 or 15-mm–long needles that were of inadequate length to reach key target muscles. Whereas superficial muscles such as the sternocleidomastoid and trapezius are within 15 mm of the surface, muscles such as the levator scapulae and semispinalis capitis, are well beyond that, and, depending on the angle of insertion, muscles of intermediate depth, such as the splenius capitis and scalenes, were often out of reach. Muscles of the deep posterior triangle of the neck require needles of 25-mm length to reach them (Fig. 11.8). Even more definitive was a simple yet elegant study by Speelman and Brans.15 They studied patients who came in for routine injections for cervical dystonia, and compared blind needle placement with the EMG machine off, with precise localization provided by turning the EMG machine on. Even for the most superficial easily palpated cervical muscle, the sternocleidomastoid, they found blind needle insertion, even when using needles of adequate length missed the target 15% of the time. The error rate was 50% or higher for deeper muscles such as levator scapulae and semispinalis capitis. Given these findings, it is only prudent to provide trainees with additional aids for ensuring accurate localization of injected botulinum toxin; not to do so risks a lifetime of poor technique and suboptimal results for future patients.
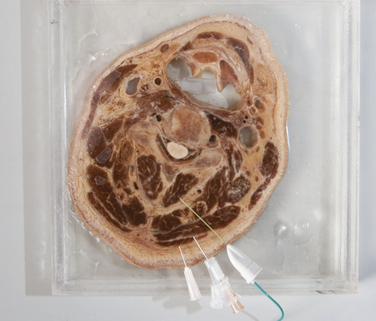
Fig. 11.8 A coronal section of an adult male head preserved in formalin. Superimposed on this image are the needles shown in Figure 11.2. Note that the 10-mm needle is barely long enough to inject trapezius, the 15-mm needle is barely long enough to inject splenius capitis, and that a 35-mm needle is needed to inject deeper muscles such as the semispinalis capitis muscle (which it traverses) or the oblique capitis inferior, which is below the tip of this needle.
Botulinum Toxin for Muscles in Cervical Dystonia
The location of the cervical muscles in dystonia is readily apparent by ultrasound, although sometimes fascial planes between muscles are not as clear in some areas as others. The most superficial muscles include the sternocleidomastoid and trapezius; the next layer includes splenius capitis, scalenes, and levator scapulae; the next layer semispinalis capitis; and then the deep posterior triangle of the neck. More detailed instructions on how to locate the muscles and their appropriate layers are available in other resources.13–19 It should be noted that necks vary considerably in thickness and length, and that although relative relationships to bony landmarks tend to be fairly consistent across patients, depths and angles of insertion to inject cervical muscles are less consistent. Of particular interest are muscles in the deep posterior triangle of the neck, and their smaller counterparts at descending cervical levels, that exert considerable lateral, rotational, and extensor forces on the cervical spine and head. Limiting injections to just the most superficial layers risks poor responses by patients and wasted toxin.
Benefits of Ultrasonography in Cervical Injections for Dystonia
Ultrasonography can be used in two ways to assist with cervical dystonia injections. The first is to outline the general anatomy of cervical muscles, identify focal atrophy or hypertrophy, and to determine the depth of both bony landmarks and target muscles. The errors committed by early blind injectors could have been avoided had they used ultrasonography because it would have clearly identified the depth needed to access deep cervical muscles. Second, ultrasonography prior to the first trial of injections could be useful for identifying target muscles. The hypertrophy associated with untreated cervical dystonia is often asymmetric, and careful study of different layers, with particular comparisons with left and right, could provide data that could help apportion doses of botulinum toxin, with emphasis on those muscles showing the greatest degree of relative hypertrophy. Even in patients with antecollis or retrocollis, there is often a mild asymmetry, but in these cases, absolute rather than relative hypertrophy would likely be more informative. Unfortunately, although some normative data are available for the thickness of extremity and trunk muscles, little is currently available for cervical muscles (other than the sternocleidomastoid),20 and none for toxin-naïve patients with cervical dystonia.
Ultrasound can be used to ensure proper insertion of needles into muscle with direct visualization of the injection (Fig. 11.9 and Video 11.5.) The advantage to this approach is that the location of the actual injectate can be monitored and documented, although for routine injections for dystonia in multiple muscles this step might increase the time required for injections compared with EMG. Patients prefer speed when possible, and ultrasound guidance may increase the time required to complete a series of injections for this disorder. However, for those uncomfortable with using EMG needle guidance, ultrasound is a suitable alternative for correctly localizing injections.
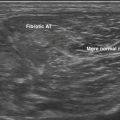
Particularly concerning are physicians who, ignoring kinesiology, base their injections solely on neck posture and location of discomfort. There are occasional patients who develop extensor weakness of the neck and come to the office with a head that tends to be flexed on the chest. The weakness can be the result of myopathy of extensor muscles,21 camptocormia in Parkinson’s disease,22 motor neuron disease, or myasthenia gravis.23,24 In these cases, EMG shows that the disorder is not caused by excessive contraction of the neck flexors, and may demonstrate diagnostic abnormalities in the extensor muscles. There are also cases in which the neck is flexed as a result of structural processes, such as vertebral anomalies or a fibrotic band within the sternocleidomastoid,25,26 in which ultrasound can be helpful in diagnosis; in both of these situations, however, EMG can clarify that there is no pathologic ongoing muscle contraction as seen in dystonia.
Although local pain can be helpful for guiding botulinum toxin injections in nondystonic disorders such as migraine, in cervical dystonia, pain is often a false localizing sign, occurring in muscles that are not hypertrophic from overuse and which are presumably painful from overpowering by their hypertrophic antagonist. In almost all the patients with nondystonic disorders leading to posturing of the head on the neck, there is pain in the posterior portions of the neck. Inexperienced injectors sometimes aggravate the condition by inappropriate injection of neck extensors, and in the process can complicate the correct diagnosis of the disorder, particularly if it involves the motor neuron or neuromuscular junction.
Ultrasound, of course, is also useful for identifying areas of particular risk, such as blood vessels, plexus, and pulmonary structures. For those wishing to inject the longus colli muscle unilaterally in patients with antecollis, ultrasound can be helpful in identifying the muscle and selecting an optimal approach; the author does not do these injections and does not advise that novices consider them. However, for patients with problematic antecollis, unilateral injections of modest amounts of botulinum toxin in this muscle, by anecdotal report, may be helpful and tolerable in some refractory antecollis patients. Bilateral injections are invariably associated with dysphagia. One novel report used fluorodeoxyglucose positron emission tomography to identify overactive muscles in cervical dystonia, including longus colli, and described using CT guidance to inject this muscle.16
Ultrasound Guidance of Botulinum Toxin in Spasticity
There are several alternate techniques for muscle localization in spasticity. First, the digit or joint can be passively moved; if the needle is in the target muscle, it should move with flexion and extension of the relevant joint. It is often helpful to identify the tendon of the target muscle as it appears distally, and to follow it back to the target muscle. Another approach involves EMG needle stimulation.27,28 In this case the same needle used for EMG guidance is connected to a stimulator. A surface electrode is placed over the muscle, and when the needle is in the muscle belly, stimulation can be used to determine when the needle is appropriately located. However, neither of these techniques is completely free of error. Passive movement provides information about the shaft of the needle, but not necessarily its tip, so it cannot guarantee the injection will be deposited in the target muscle. Needle stimulation can also be problematic in that electrical current is widely distributed between the surface electrode and needle tip. If a motor nerve branch to the muscle is between these two structures, a muscle contraction can result even if the tip is not in the target muscle. Because spastic muscles lead to chronic postural changes and muscle atrophy, the optimal position of needle placement suitable for diagnostic EMG studies in relatively healthy patients may not be suitable in patients with spasticity.
Ultrasound can be used to help clarify proper localization of needles either by direct visualization or by scanning the tissue prior to injections to identify the true anatomic depth and course of the muscle. This, coupled with EMG needle guidance or stimulation techniques, may significantly enhance accuracy, particularly if there is just one or two affected muscles. Also, once the anatomy is elucidated, further use of ultrasound (on other muscles, or on the same muscle in subsequent injections) may not be needed. For example, injections into the tibialis posterior may be particularly problematic.29 This muscle is difficult to access. Standard textbooks often recommend an approach posterior to the tibia from the back of the leg. Ultrasound, however, demonstrates the depth of this muscle from the anterolateral surface of the leg. It lies just deep to the interosseous membrane, which is deep to the tibialis anterior muscle. Sometimes a neurovascular bundle runs just below this membrane, but with color Doppler this can be easily recognized and avoided. The depth of the interosseous membrane decreases as one moves from the upper one third of the tibia to its distal end. Some physicians have advocated measuring the distance with ultrasound, and then using a needle stop at a distance that ensures adequate penetration to access the muscle. One must be careful to account for any indentation of the skin by the probe or needle, and be sure that the needle is inserted at the same distal/proximal level at which the measurements were taken. Given the depth of this muscle, the angle of insertion of the needle into the muscle needs to be rather steep (Fig. 11.10).
Not all muscles are surrounded by clearly identifiable fascial planes, even using the highest-resolution ultrasound probes. Sometimes using other references can be helpful. For example, in the forearm, the median nerve helps to define the plane between the superficial and deep flexor muscles. The preferred method for finding the flexor digitorum profundus muscles is to insert the needle anterior to the ulna, about 4 cm distal from the elbow, deep to the location of the median nerve. Blind needle insertions to this muscle through the anterior forearm and flexor digitorum superficialis risk possible needle insertion into the median nerve. If this happens, the patient describes pain and paresthesias in a median distribution (personal experience); although not necessarily associated with injury, this should be avoided when possible. With ultrasound guidance and identification of the course of the median nerve, this approach would be safe. The four superficial flexor tendons have somewhat complex anatomy in the forearm.30 Distally in the wrist, the flexor tendons line up in order for digits 2, 3, 4, and 5 from the radial to ulnar. However, more proximally in the forearm, these tendons superimpose, and the bellies of the flexor digitorum superficialis 2 and 5 muscles tend be more centrally located, with the bellies of flexor digitorum superficialis 3 and 4 muscles being somewhat more proximal and deeper than those of 2 and 5. A short handbook addresses more details of how to best inject muscles for spasticity.31 This includes information about the distribution of endplates within these muscles, which may help get peak effect from the injections, along with illustrative ultrasound images.
Ultrasound-Guided Use of Phenol for Spasticity
Lee and Lee32 elected to evaluate the efficacy of phenol therapy for spasticity using ultrasound guidance. They used ultrasound to guide intraneural injections of phenol, using either predetermined volumes of phenol depending on nerve size, or the arbitrary observation of doubling of nerve cross-sectional area. Using these criteria, almost all injections were successful in their sample of 29 patients, and the average duration of action was 165 days, an outcome clearly superior to botulinum toxin injections at a fraction of the drug cost. The average time required for injection was reported as 15 minutes, which is longer than that routinely used for botulinum toxin injections. There was additional time required for preprocedural ultrasound to localize the injected nerves. Anecdotal evidence suggests that the anatomy of the musculocutaneous nerve in patients with spasticity is sometimes atypical, and that the echogenicity of the biceps muscle may be increased, factors that may make these injections more difficult.
With the recent approval of Botox for the treatment of spasticity, it may be appropriate for there to be further controlled studies with phenol for spasticity because it has the potential for being significantly more cost effective. Phenol has been used for treating other problems with ultrasound, particularly painful neuromas and pseudoneuromas of the foot (Morton’s neuroma).33–35 In a world of limited health-care resources, appropriate distribution of funds for research on the use of this available, inexpensive, and effective agent seems warranted, particularly now that ultrasound can be used to ensure optimal administration of this compound.
Ultrasound-Guided Botulinum Toxin for Sialorrhea
Perhaps more challenging than injections into muscle are injections into the salivary glands.36–38 The parotid and submandibular glands are not composed of excitable tissue, so EMG needle guidance or stimulation is of little use in identifying tip placement within these structures. Such guidance, however, can be of use for ensuring that the needle tip is not in a nearby muscle such as the tongue or strap muscles. Here ultrasound can be used in two ways. First it is helpful in mapping the extent of salivary gland tissue, which can be quite variable. Salivary glands, like other exocrine/endocrine glands such as the thyroid, have a distinctive appearance on ultrasound. The tissue has a ground-glass pattern, quite homogeneous and hyperechoic. It is readily distinguished from the nearby sternocleidomastoid and masseter muscles, which have the classic heterogeneous predominantly hypoechoic appearance of muscle in axial section, and a distinctively different striated pattern in sagittal section. Furthermore, both these structures thicken on voluntary contraction. Second, ultrasound can visualize the needle tip and injectate in the salivary gland tissue.
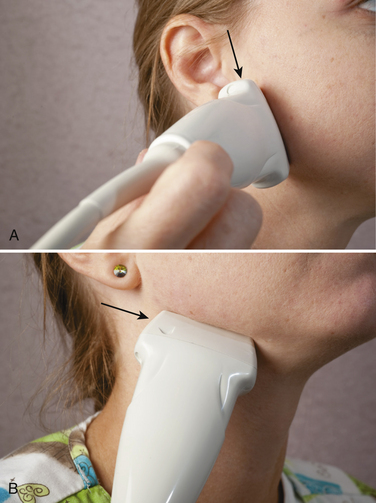
A number of studies have shown how to use ultrasound guidance for botulinum toxin injections into the salivary glands.39–44 The parotid gland is easiest to identify with the probe placed just anterior to the external auditory meatus with the long axis placed parallel to the ear (Fig. 11.11). The probe can be moved anteriorly to identify the masseter muscle, or caudad to identify the caudal extent of the gland. The probe can be moved caudally and anteriorly, just below the angle of the jaw to identify the submandibular gland in cross section (see Fig. 11.11). The author typically injects the salivary glands with a needle inserted along the long axis of the probe for the parotid gland. For the submandibular gland, the probe is held parallel to the length of the mandible and the needle advanced subcutaneously into the belly of the gland. The author prefers to use an EMG-guidance needle, with the audio on, so that if the needle enters muscle, a telltale audio signal results. It can be difficult to maintain the tip of the needle as visible throughout the course of the highly echogenic salivary gland, so this additional layer of safety ensures avoidance of injection into a muscle that could result in subsequent swallowing difficulty. Typically, 30 units of Botox can be injected into one parotid gland, and 20 into one submandibular gland; a typical patient, therefore, who receives bilateral injections can receive a total of 100 units (Fig. 11.12). Botulinum B toxin (Myobloc) has also been used successfully to treat sialorrhea, and in theory, may be even more effective than botulinum toxin, because patients who receive this agent for cervical dystonia frequently note problematic dry mouth more often than those who receive botulinum A toxin. However, because of the acidic pH of Myobloc, these injections may be more painful than those with botulinum A toxin, and for sensitive subjects, particularly children, this could add to the challenge of injections. In the author’s experience, there are occasional patients with cerebral palsy who have elevated pain thresholds, and in such cases, this distinction is irrelevant.
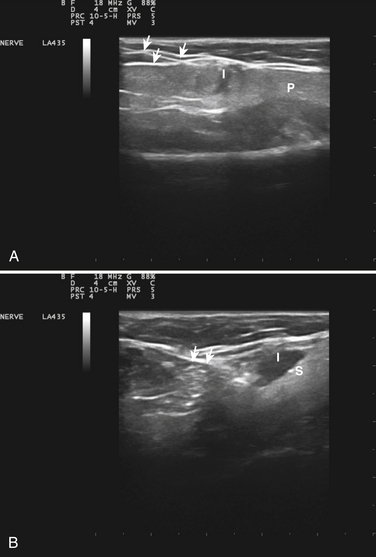
Fig. 11.12 Botulinum toxin injections into the salivary glands. A, An injection of Botox into the parotid gland using the approach shown in Figure 11.11. Note the needle entering the gland in the upper left of the figure (arrows) and the hypoechoic injectate (I); the parotid gland has a hyperechoic appearance (P). B, The clear deposition of injectate (I) into the hyperechoic submandibular gland (S); the injection needle is less well defined but is apparent through part of its course (arrows).
The sublingual glands are typically more difficult to locate,45 and the author does not routinely inject these glands. These can be imaged by ultrasound, either from below the chin or intraorally, below the anterior portion of the voluntarily retracted tongue, so guided injections are possible. However, if the outcome is the control of problematic drooling, it is suggested that the more prominent and larger glands be treated first to see if this will suffice for symptom control. Excessive loss of saliva is associated with problems swallowing and dental caries, so overinjection should be avoided.
Ultrasound Guidance for Regional Anesthesia
Ultrasound guidance has had a profound effect on the administration of local anesthetic blocks over the past decade.46–52 About 25 years ago anesthesiologists first began realizing that ultrasound was helpful for identifying vascular structures as landmarks for local anesthetic injections, but with the evolution and application of high-resolution ultrasonography 13 years ago,53,54 it became apparent that ultrasound could be used to visualize nerve targets and assist in the administration of local anesthetic blocks. Since that time, thousands of practicing anesthesiologists, many of whom previously had only used surface landmarks or needle stimulation to guide local anesthetic blocks, have obtained training and purchased ultrasound equipment so that they now administer the majority of their blocks using ultrasound guidance. Ultrasound guidance is routinely taught in anesthesia training programs as well.
Once the concept of ultrasound guidance took hold, it quickly advanced because the principles were readily adapted to any nerve block. Even in areas where nerves were not able to be easily identified by ultrasound, the fascial planes through which nerves traversed were found to be recognizable, as were blood vessels, and these could be used to assist in guidance. Although local anesthetic complications are rare, they can be problematic, and it was immediately obvious that ultrasound could help avoid intravascular injections and in many circumstances intraneural injections. Furthermore, in a busy operating room setting, ultrasound guidance significantly reduced the time required to achieve adequate nerve block for surgery by increasing the accuracy of these injections.53,55 This was due not only to ensuring proper location of the needle near the desired target, but also by being able to observe the injected local anesthetic and making sure that it surrounded the target nerve(s), to maximize effect. In the process, it was found that anesthesiologists could significantly reduce the total dose of local anesthetic,56,57 thereby reducing the likelihood of the rare, but life-threatening complications of systemic toxicity of local anesthetics, a phenomenon that could be exacerbated by patients who harbored rare defects in metabolizing or excreting these compounds.
The rapid acceptance of ultrasound guidance for injections in the anesthesiology community has significantly outpaced the adoption in the electrodiagnostic community. Both technologies have emerged primarily in the past decade (although the key papers on the diagnostic use of ultrasound for carpal tunnel syndrome predated those in anesthesia by several years). Several reasons for this can be suggested from a close review of the literature, discussion with leaders in the field, and collaborative working relationships with anesthesiologists. The first is the detailed anatomic orientation of most anesthesiologists, particularly regional anesthesiologists. Those expert in clinical neurophysiology tend to focus first on function and then on localization, and then, only in terms of localization in large increments. Anesthesiologists have always considered localization for nerve blocks more refined than that because they need to slide a needle into a defined area, sometimes in close approximation to vital respiratory or vascular structures. Furthermore, their working relationships with surgeons, routine access to surgical visualization of anatomy, and fear of intravascular or intrapleural injections, help to clarify their ability to visualize space in three dimensions. The second is the risk factor of local anesthetic injections and the frequency of anatomic variations. For someone who does 1000 or more regional blocks a year, a complication rate of even modest morbidity, for example, 0.1%, means that once a year the typical anesthesiologist will have to deal with a problematic side effect from his or her procedure, something that most electromyographers do not have to face in a lifetime of practice. The third reason is the demands of training. Because academic medical centers are held to the same, if not higher, standards of practice than non-university hospitals, it behooves them to be leaders in the development of technology that ensures safety—a challenge because of the involvement of trainees in university systems. It is stressful to supervise trainees in the conduct of invasive procedures—particularly those that are blind procedures, and ultrasound enables attending physicians to better guide and educate their trainees in the safe and effective administration of local anesthesia. Fourth, time is more precious in the conduct of most nerve blocks than in electrodiagnosis because a failed block may easily entails an additional delay of 15 to 20 minutes of an entire operating team and the cases that follow. As such, anesthesiologists are highly motivated to adopt technologies that improve time efficiency. Fifth, the competing technology for needle guidance, electrical stimulation, has been fairly limited in its efficacy and accuracy, whereas in clinical neurophysiology, EMG and nerve conduction studies are quite informative, so the impetus to change is less. Sixth, anesthesia training encourages cultivation of diverse approaches and new technologies so the incorporation of ultrasound into clinical practice is a natural evolution of trained readiness and clinical reasoning. Regrettably, the tantalizingly slow pace of the evolution of electrodiagnostic technology has shielded many trainees and EMG training programs from the need to adapt, encouraging rote learning and resistance to change. Nor has the current system of compensation for laboratory-based diagnosis in the United States been helpful because it tends to reward electrodiagnostic practitioners for numbers of individual procedures performed rather than the ability to obtain the most informative result with the greatest time efficiency and least discomfort for patients.
Ultrasound for Biopsy Guidance
Muscle Biopsy
The use of ultrasound for guiding muscle biopsies has been recognized for some time, but it is not commonly used for this purpose.58–61 The small number of case reports on this topic, and the lack of any evidence-based data regarding its utility raise the possibility that the procedure is described more as a novelty rather than a procedure of value for routinely advancing diagnosis. However, for identifying site selection for needle core biopsies, particularly tumors,59 the technique clearly is of benefit. Even in the case of open biopsies, surface markings or wire insertion guided by ultrasound can be used to subsequently guide the surgeon after the skin is opened. For other types of primary muscle diseases, the potential for improving the diagnostic yield of biopsy is as yet unproven; perhaps future studies will help determine when such guidance is most helpful.
Perhaps the most enterprising example of ultrasound-guided muscle biopsy was the use of the technique to diagnose intrauterine Duchenne’s muscular dystrophy.60 Although this particular indication has been outdated by the evolution of subsequent genetic tests, the ability for ultrasound to localize muscle for biopsy in fetal tissue represents a unique technical opportunity for future prenatal diagnostic and therapeutic interventions.
Nerve Biopsy
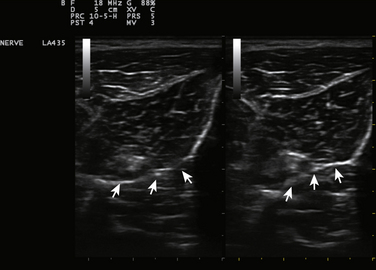
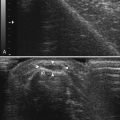
Ultrasound can identify tiny nerves,62 and this may make possible biopsies of nerve fascicles to muscles, or small distal motor nerve branches to smaller distal muscles such as peroneus tertius or extensor digitorum brevis (Fig. 11.13 and see Video 11.3).63 In cases of suspected nerve tumors, or focal nerve swelling, it can identify areas suitable for exploration preoperatively. These sites can be marked for future operative or diagnostic reference by inserting wires, or even echogenic clips so that the ideal locations can be readily identified for biopsy and followed over time for evidence of recurrence if needed. For focal nerve swelling in leprosy, ultrasound is a particularly useful technique for identifying an area suitable for fascicular biopsy and appropriate diagnosis of the disorder.64
Ultrasound for Presurgical Screening
However, at this time there have not been studies performed looking at the outcomes of adopting preoperative ultrasound nerve mapping as a surgical safety measure. Because the likelihood of problematic nerve injury for any given procedure is low, typically less than 1%, to have enough power to detect a statistically significant protective effect of a safety intervention requires large subject populations, perhaps 1000 or more subjects. Studies that serve to provide proof of this principle have been conducted.65,66 The problem of finding ways to collect data and establish outcomes research on this type of approach is similar to that required to establish the value of intraoperative monitoring procedures that serve to prevent rare surgical complications. Perhaps such studies will be forthcoming regarding a variety of different nerves. One factor that should make preoperative ultrasound nerve mapping easier than intraoperative monitoring studies is the ability to map nerves prior to surgery outside of the spatial and temporal requirements of a busy operating room and its tight schedule.
A variety of nerves would be suitable for preoperative mapping. Almost all experienced electromyographers have encountered patients with disabling spinal accessory nerve injuries caused by minor biopsy procedures, typically for lymph nodes, in the posterior cervical triangle. Gruber and Kovacs,67 Kessler and Gray,68 and Bodner and colleagues,69 have shown how easily the accessory nerve can be identified and mapped in normal subjects (Fig. 11.14). A variety of other superficial nerves are also at risk during routine procedures. Many of these nerves, if injured, rarely result in significant motor deficits, so such patients are infrequently seen by electromyographers. However, damage to these nerves can be painful and problematic for affected patients. The palmar branch of the median nerve, subject to injury during surgery for carpal tunnel syndrome, is amenable to ultrasound imaging.66 Superficial branches of the radial and ulnar nerves are at risk during wrist surgery, the ilioinguinal and iliohypogastric nerves are at risk during abdominal wall surgery and hernia repairs, and a variety of other superficial nerves are at risk and can be identified with ultrasound.67,70,71 Even in cases in which the nerve may not be directly visualized, ultrasound can demonstrate the depth and location of the compartments within which they are located, and such efforts preoperatively may help in conducting safe and efficient surgical procedures.
Intraoperative Use of Ultrasound
Ultrasound has been used in elective brain surgery and has robust specificity and sensitivity in differentiating tumor from healthy brain tissue.72,73 Transcranial ultrasound has been used to help guide placement of deep brain-stimulating electrodes into the globus pallidum for the treatment of Parkinson’s disease.74 Ultrasound has also been used intraoperatively to assist with neurosurgical identification and repair of peripheral neuromas and other focal nerve lesions.69 Intraoperative use of this modality is in its infancy, but as ultrasound continues to improve and surgeons become more familiar with the technique, it has the potential to assist in nerve exploration and repair. Extremely high-resolution ultrasound is used routinely to explore ocular pathology, a technique called ultrasound biomicroscopy,75,76 which raises the possibility of unique ways to study nerves in the operative field with considerable magnification.
Other Interventional Applications of Ultrasound
Lumbar Puncture and Epidural Needle Placement
Ultrasound can be used to help with establishing landmarks for lumbar puncture or epidural needle placement. Using ultrasound guidance to identify landmarks led to an increased success rate of the block and increased ease of administration77; other studies have shown similar results.78 Of interest, use of the standard landmark for the L3-4 interspace, the line between the iliac crests, is accurate less than 50% of the time, usually placing the operator above this level.79 In young children, the hyperflexed position used for administering epidural anesthetics tends to elevate the distal termination of the dural sac about one half of one interspace, a finding relevant to avoidance of dural puncture.80 This is another example of the ability of ultrasound, because of its real-time capabilities, to identify positional variations in anatomy not apparent by studies in cadavers. With increased experience it seems likely that ultrasound will be of increasing usefulness in performing lumbar punctures and epidural blocks. The technique is fairly simple and shows promise in making the procedures more efficient and less uncomfortable. The risk from lumbar puncture is extremely low, so it remains to be seen if studies can be designed to show that ultrasound reduces complications of the technique.
Sonoporation, Microbubbles, and Localized Drug Delivery
Ultrasound can increase the permeability of cell membranes (sonoporation) to enhance drug uptake, and in animal models it can be used to alter the permeability of the blood-brain barrier, a phenomenon enhanced by the concomitant use of microbubbles.81,82 This technique can increase the uptake of systemically administered therapy. Of even greater interest is the ability to package therapeutic agents such as drugs or gene delivery material in microbubbles. These can be injected systemically, and focal areas of brain or muscle can be insonated, leading to selective, localized release and enhanced absorption of the targeted materials. Although not used yet in clinical practice, these techniques show considerable promise for addressing two critical therapeutic challenges in neurology: (1) circumvention of the blood-brain barrier and the blood-nerve barrier, and (2) reduction of unwanted systemic side effects of treatment. Higher-energy delivery of ultrasound can be used for carrying out ablative procedures for small renal tumors in humans83 and shows a potential for managing aberrant foci of cardiac excitability and possibly nerve pain.84,85
Conclusion
Ultrasound is in its infancy in terms of guiding routine interventions. The technique is revolutionizing regional anesthesia, and it is likely to be increasingly important in aiding procedures involving therapeutics and diagnostics in neuromuscular medicine. This is a particularly exciting area for neurologists and physiatrists because it provides access to novel ways to not only diagnose but also treat neuromuscular disorders.
1. Bland J.D. Treatment of carpal tunnel syndrome. Muscle Nerve. 2007;36:167-171.
2. White DL, DeMar S, Wiesler E, et al: Median nerve changes following steroid injection for carpal tunnel syndrome, Muscle Nerve. In press
3. Ozturk K., Esenyel C.Z., Sonmez M., et al. Comparison of carpal tunnel injection techniques: a cadaver study. Scand J Plast Reconstr Surg Hand Surg. 2008;42:300-304.
4. MacLennan A., Schimizzi A., Meier K.M., et al. Comparison of needle position proximity to the median nerve in 2 carpal tunnel injection methods: a cadaveric study. J Hand Surg Am. 2009;34:875-879.
5. Peters-Veluthamaningal C., Winters J.C., Groenier K.H. Randomised controlled trial of local corticosteroid injections for carpal tunnel syndrome in general practice. BMC Fam Pract11. 54, 2010.
6. Grassi W., Farina A., Filippucci E., Cervini C. Intralesional therapy in carpal tunnel syndrome: a sonographic-guided approach. Clin Exp Rheumatol. 2002;20:73-76.
7. Smith J., Wisniewski S.J., Finnoff J.T., Payne J.M. Sonographically guided carpal tunnel injections: the ulnar approach. J Ultrasound Med. 2008;27:1485-1490.
8. Dressler D., Benecke R. Pharmacology of therapeutic botulinum toxin preparations. Disabil Rehabil. 2007;29:1761-1768.
9. Grumelli C., Verderio C., Pozzi D., et al. Internalization and mechanism of action of clostridial toxins in neurons. Neurotoxicology. 2005;26:761-767.
10. Dolly O. Synaptic transmission: inhibition of neurotransmitter release by botulinum toxins. Headache. 2003;43(Suppl 1):S16-S24.
11. Hamjian J.A., Walker F.O. Serial neurophysiological studies of intramuscular botulinum: a toxin in humans. Muscle Nerve. 1994;17:1385-1392.
12. Simpson L. Botulinum neurotoxin and tetanus toxin. San Diego: Academic Press Inc; 1989.
13. Walker F.O. Botulinum toxin therapy for cervical dystonia. Phys Med Rehabil Clin North Am. 2003;14:749-766.
14. Comella C.L., Buchman A.S., Tanner C.M., et al. Botulinum toxin injection for spasmodic torticollis: increased magnitude of benefit with electromyographic assistance. Neurology. 1992;42:878-882.
15. Speelman J.D., Brans J.W. Cervical dystonia and botulinum treatment: is electromyographic guidance necessary? Mov Disord. 1995;10:802.
16. Lee I.H., Yoon Y.C., Sung D.H., et al. Initial experience with imaging-guided intramuscular botulinum toxin injection in patients with idiopathic cervical dystonia. AJR Am J Roentgenol. 2009;192:996-1001.
17. Sung D.H., Choi J.Y., Kim D.H., et al. Localization of dystonic muscles with 18F-FDG PET/CT in idiopathic cervical dystonia. J Nucl Med. 2007;48:1790-1795.
18. Lee L.H., Chang W.N., Chang C.S. The finding and evaluation of EMG-guided BOTOX injection in cervical dystonia. Acta Neurol Taiwan. 2004;13:71-76.
19. Comella C.L., Jankovic J., Brin M.F. Use of botulinum toxin type A in the treatment of cervical dystonia. Neurology. 2000;55:S15-S21.
20. Arts I.M., Pillen S., Schelhaas H.J., et al. Normal values for quantitative muscle ultrasonography in adults. Muscle Nerve. 2010;41:32-41.
21. Gaeta M., Mazziotti S., Toscano A., et al. “Dropped-head” syndrome due to isolated myositis of neck extensor muscles: MRI findings. Skeletal Radiol. 2006;35:110-112.
22. Spuler S., Krug H., Klein C., et al. Myopathy causing camptocormia in idiopathic Parkinson’s disease: a multidisciplinary approach. Mov Disord. 2009;15:552-559.
23. Casasnovas C., Povedano M., Jauma S., et al. Musk-antibody positive myasthenia gravis presenting with isolated neck extensor weakness. Neuromuscul Disord. 2007;17:544-546.
24. Spengos K., Vassilopoulou S., Papadimas G., et al. Dropped head syndrome as prominent clinical feature in Musk-positive myasthenia gravis with thymus hyperplasia. Neuromuscul Disord. 2008;18:175-177.
25. Tang S.F., Hsu K.H., Wong A.M., et al. Longitudinal followup study of ultrasonography in congenital muscular torticollis. Clin Orthop Relat Res. 2002:179-185.
26. Swain B. Transaxillary endoscopic release of restricting bands in congenital muscular torticollis: a novel technique. J Plast Reconstr Aesthet Surg. 2007;60:95-98.
27. Kinnett D. Botulinum toxin A injections in children: technique and dosing issues. Am J Phys Med Rehabil. 2004;83:S59-S64.
28. Chin T.Y., Nattrass G.R., Selber P., Graham H.K. Accuracy of intramuscular injection of botulinum toxin A in juvenile cerebral palsy: a comparison between manual needle placement and placement guided by electrical stimulation. J Pediatr Orthop. 2005;25:286-291.
29. Rah D.W., Im S.H., Lee S.C., et al. Needle insertion into the tibiailis posterior: ultrasonographic evaluation of an anterior approach. Arch Phys Med Rehabil. 2010;91:283-287.
30. Bickerton L.E., Agur A.M., Ashby P. Flexor digitorum superficialis: locations of individual muscle bellies for botulinum toxin injections. Muscle Nerve. 1997;20:1041-1043.
31. Koman L.A., Smith B., Papadonikolakis A. Ultrasound injection techniques for upper and lower extremities. Winston-Salem, NC: Wake Forest University School of Medicine; 2010.
32. Lee J., Lee Y.S. Percutaneous chemical nerve block with ultrasound-guided intraneural injection. Eur Radiol. 2008;18:1506-1512.
33. Gruber H., Glodny B., Kopf H., et al. Practical experience with sonographically guided phenol instillation of stump neuroma: predictors of effects, success, and outcome. AJR Am J Roentgenol. 2008;190:1263-1269.
34. Gruber H., Kovacs P., Peer S., et al. Sonographically guided phenol injection in painful stump neuroma. AJR Am J Roentgenol. 2004;182:952-954.
35. Magnan B., Marangon A., Frigo A., Bartolozzi P. Local phenol injection in the treatment of interdigital neuritis of the foot (Morton’s neuroma). Chir Organi Mov. 2005;90:371-377.
36. Thoeny H.C. Imaging of salivary gland tumours. Cancer Imaging. 2007;7:52-62.
37. Boyd Z.T., Goud A.R., Lowe L.H., Shao L. Pediatric salivary gland imaging. Pediatr Radiol. 2009;39:710-722.
38. Wernicke D., Hess H., Gromnica-Ihle E., et al. Ultrasonography of salivary glands: a highly specific imaging procedure for diagnosis of Sjögren’s syndrome. J Rheumatol. 2008;35:285-293.
39. Pena A.H., Cahill A.M., Gonzalez L., et al. Botulinum toxin A injection of salivary glands in children with drooling and chronic aspiration. J Vasc Interv Radiol. 2009;20:368-373.
40. Adav S.S., Lee D.J. Extraction of extracellular polymeric substances from aerobic granule with compact interior structure. J Hazard Mater. 2008;154:1120-1126.
41. Ellies M., Laskawi R., Rohrbach-Volland S., Arglebe C. Up-to-date report of botulinum toxin therapy in patients with drooling caused by different etiologies. J Oral Maxillofac Surg. 2003;61:454-457.
42. Ellies M., Laskawi R., Rohrbach-Volland S., et al. Botulinum toxin to reduce saliva flow: selected indications for ultrasound-guided toxin application into salivary glands. Laryngoscope. 2002;112:82-86.
43. Capaccio P., Torretta S., Osio M., et al. Botulinum toxin therapy: a tempting tool in the management of salivary secretory disorders. Am J Otolaryngol. 2008;29:333-338.
44. Marina M.B., Sani A., Hamzaini A.H., Hamidon B.B. Ultrasound-guided botulinum toxin A injection: an alternative treatment for dribbling. J Laryngol Otol. 2008;122:609-614.
45. Gritzmann N., Rettenbacher T., Hollerweger A., et al. Sonography of the salivary glands. Eur Radiol. 2003;13:964-975.
46. Tsui B., Suresh S. Ultrasound imaging for regional anesthesia in infants, children, and adolescents: a review of current literature and its application in the practice of extremity and trunk blocks. Anesthesiology. 2010;112:473-492.
47. Tsui B.C., Pillay J.J. Evidence-based medicine: assessment of ultrasound imaging for regional anesthesia in infants, children, and adolescents. Reg Anesth Pain Med. 2010;35:S47-S54.
48. Wang A.Z., Gu L., Zhou Q.H., et al. Ultrasound-guided continuous femoral nerve block for analgesia after total knee arthroplasty: catheter perpendicular to the nerve versus catheter parallel to the nerve. Reg Anesth Pain Med. 2010;35:127-131.
49. Kirkpatrick J.D., Sites B.D., Antonakakis J.G. Preliminary experience with a new approach to performing an ultrasound-guided saphenous nerve block in the mid to proximal femur. Reg Anesth Pain Med. 2010;35:222-223.
50. Sites B.D., Chan V.W., Neal J.M., et al. The American Society of Regional Anesthesia and Pain Medicine and the European Society of Regional Anaesthesia and Pain Therapy Joint Committee recommendations for education and training in ultrasound-guided regional anesthesia. Reg Anesth Pain Med. 2010;35:S74-S80.
51. Mariano E.R., Loland V.J., Sandhu N.S., et al. A trainee-based randomized comparison of stimulating interscalene perineural catheters with a new technique using ultrasound guidance alone. J Ultrasound Med. 2010;29:329-336.
52. Salinas F.V. Ultrasound and review of evidence for lower extremity peripheral nerve blocks. Reg Anesth Pain Med. 2010;35:S16-S25.
53. Marhofer P., Schrogendorfer K., Koinig H., et al. Ultrasonographic guidance improves sensory block and onset time of three-in-one blocks. Anesth Analg. 1997;85:854-857.
54. Yang W.T., Chui P.T., Metreweli C. Anatomy of the normal brachial plexus revealed by sonography and the role of sonographic guidance in anesthesia of the brachial plexus. AJR Am J Roentgenol. 1998;171:1631-1636.
55. Williams S.R., Chouinard P., Arcand G., et al. Ultrasound guidance speeds execution and improves the quality of supraclavicular block. Anesth Analg. 2003;97:1518-1523.
56. O’Donnell B.D., Iohom G. An estimation of the minimum effective anesthetic volume of 2% lidocaine in ultrasound-guided axillary brachial plexus block. Anesthesiology. 2009;111:25-29.
57. Sandhu N.S., Bahniwal C.S., Capan L.M. Feasibility of an infraclavicular block with a reduced volume of lidocaine with sonographic guidance. J Ultrasound Med. 2006;25:51-56.
58. O’Sullivan P.J., Gorman G.M., Hardiman O.M., et al. Sonographically guided percutaneous muscle biopsy in diagnosis of neuromuscular disease: a useful alternative to open surgical biopsy. J Ultrasound Med. 2006;25:1-6.
59. Lopez J.I., Del Cura J.L., Zabala R., Bilbao F.J. Usefulness and limitations of ultrasound-guided core biopsy in the diagnosis of musculoskeletal tumours. APMIS. 2005;113:353-360.
60. Kuller J.A., Hoffman E.P., Fries M.H., Golbus M.S. Prenatal diagnosis of Duchenne muscular dystrophy by fetal muscle biopsy. Hum Genet. 1992;90:34-40.
61. Lindequist S., Larsen C., Daa S.H. Ultrasound guided needle biopsy of skeletal muscle in neuromuscular disease. Acta Radiol. 1990;31:411-413.
62. Umans H., Kessler J., de la Lama M., et al. Sonographic assessment of volar digital nerve injury in the context of penetrating trauma. AJR Am J Roentgenol. 2010;194:1310-1313.
63. Antonakakis J.G., Scalzo D.C., Jorgenson A.S., et al. Ultrasound does not improve the success rate of a deep peroneal nerve block at the ankle. Reg Anesth Pain Med. 2010;35:217-221.
64. Lolge S.J., Morani A.C., Chaubal N.G., Khopkar U.S. Sonographically guided nerve biopsy. J Ultrasound Med. 2005;24:1427-1430.
65. Flavin R., Gibney R.G., O’Rourke S.K. A clinical test to avoid sural nerve injuries in percutaneous Achilles tendon repairs. Injury. 2007;38:845-847.
66. Tagliafico A., Pugliese F., Bianchi S., et al. High-resolution sonography of the palmar cutaneous branch of the median nerve. AJR Am J Roentgenol. 2008;191:107-114.
67. Gruber H., Kovacs P. Sonographic anatomy of the peripheral nervous system. In: Peer G., Bodner S., editors. High resolution sonography of the peripheral nervous system. Berlin: Springer; 2003:13-36.
68. Kessler J., Gray A.T. Course of the spinal accessory nerve relative to the brachial plexus. Reg Anesth Pain Med. 2007;32:174-176.
69. Bodner G., Harpf C., Gardetto A., et al. Ultrasonography of the accessory nerve: normal and pathologic findings in cadavers and patients with iatrogenic accessory nerve palsy. J Ultrasound Med. 2002;21:1159-1163.
70. Lange J.F., Wijsmuller A.R., van G.D., et al. Feasibility study of three-nerve-recognizing Lichtenstein procedure for inguinal hernia. Br J Surg. 2009;96:1210-1214.
71. van Ramshorst G.H., Kleinrensink G.J., Hermans J.J., et al. Abdominal wall paresis as a complication of laparoscopic surgery. Hernia. 2009;13:539-543.
72. Lee F.C., Singh H., Nazarian L.N., Ratliff J.K. High-resolution ultrasonography in the diagnosis and intraoperative management of peripheral nerve lesions. J Neurosurg. 2011;114:206-211.
73. Chacko A.G., Kumar N.K., Chacko G., et al. Intraoperative ultrasound in determining the extent of resection of parenchymal brain tumours: a comparative study with computed tomography and histopathology. Acta Neurochir (Wien). 2003;145:743-748.
74. Walter U., Wolters A., Wittstock M., et al. Deep brain stimulation in dystonia: sonographic monitoring of electrode placement into the globus pallidus internus. Mov Disord. 2009;24:1538-1541.
75. Weisbrod D.J., Pavlin C.J., Xu W., Simpson E.R. Long-term follow-up of 42 patients with small ciliary body tumors with ultrasound biomicroscopy. Am J Ophthalmol. 2010;149:616-622.
76. Johansson M., Myredal A., Friberg P., Gan L.M. High-resolution ultrasound showing increased intima and media thickness of the radial artery in patients with end-stage renal disease. Atherosclerosis. 2010;211:159-163.
77. Schlotterbeck H., Schaeffer R., Dow W.A., et al. Ultrasonographic control of the puncture level for lumbar neuraxial block in obstetric anaesthesia. Br J Anaesth. 2008;100:230-234.
78. Nomura J.T., Leech S.J., Shenbagamurthi S., et al. A randomized controlled trial of ultrasound-assisted lumbar puncture. J Ultrasound Med. 2007;26:1341-1348.
79. Locks G.F., Almeida M.C., Pereira A.A. Use of the ultrasound to determine the level of lumbar puncture in pregnant women. Rev Bras Anestesiol. 2010;60:13-19.
80. Koch B.L., Moosbrugger E.A., Egelhoff J.C. Symptomatic spinal epidural collections after lumbar puncture in children. AJNR Am J Neuroradiol. 2007;28:1811-1816.
81. Liang H.D., Tang J., Halliwell M. Sonoporation, drug delivery, and gene therapy. Proc Inst Mech Eng H. 2010;224:343-361.
82. McDannold N. Temporary modulation of vascular barriers with focused ultrasound and microbubbles. J Acoust Soc Am 127. 1939:2010.
83. Ritchie R.W., Leslie T., Phillips R., et al. Extracorporeal high intensity focused ultrasound for renal tumours: a 3-year follow-up. BJU Int. 2010;106:1004-1009.
84. Schopka S., Schmid C., Keyser A., et al. Ablation of atrial fibrillation with the Epicor system: a prospective observational trial to evaluate safety and efficacy and predictors of success. J Cardiothorac Surg. 2010;5:34.
85. Haider N., Mekasha D., Chiravuri S., Wasserman R. Pulsed radiofrequency of the median nerve under ultrasound guidance. Pain Physician. 2007;10:765-770.