Chapter 191 Interventional Nonoperative Management of Neck and Back Pain
Pain and disability from spine pathology are usually benign, temporally limited occurrences that may be accompanied by anxiety and hypervigilance on the part of the sufferer. Occasionally, symptoms of pain are caused by tumor, infection, or trauma, requiring aggressive and often morbid interventions. On the other hand, most acute episodes of spinal pain improve substantially within 90 days.1,2 When the complaint of spinal pain is accompanied by minor/mild neurologic deficit and ample evidence of degenerative change, the answer may become confounded by contradictory evidence. In such cases, interventional procedures are increasingly utilized to relieve symptoms, ameliorate disability, and reduce surgical risk.3,4 This chapter presents an overview of available interventional spine procedures, their indications, and the best available evidence for efficacy within a continuum of clinical care.
Conservative Care Progression
When discussing treatment options with patients in acute distress, it is important to keep in mind the first tenet of Western medicine: “first, do no harm.” This is best accomplished by following a progression of conservative care that first presents strategies that provide the least risk with the highest likelihood of success. In the rush to ameliorate pain, experienced physicians occasionally forget that any intervention should have patient function as the primary goal and pain relief as a laudable, but secondary, goal (Fig. 191-1). Moreover, it has been shown that people will accept a degree of chronic pain if they are able to participate in desired activities.5 The decoupling of symptoms and radiologic/anatomic findings can lead similarly well-trained professionals to vastly different opinions. In the end, the patient is best served when the treatment modality with the least risk is applied to relieve a given pathology. Following the “do not harm” tenet, efficacious, timely applied spinal injections may allow enough time for the body to heal and return to function without the need for further invasive procedures.
Overview of Selective Spinal Procedures
As stated previously, selective spine procedures are being used with increasing frequency to manage acute and chronic pain syndromes.3 The implied societal benefit of a greater number of procedures is more robustly measurable functional improvements. To the contrary, literature states that increasing expenditures for interventional and surgical procedures have not translated into improved health status for spinal pain sufferers.6,7 Moreover, disability from spine-related pain is increasing in the United States.8 Overutilization of procedures is a problem that undermines the fundamental doctor-patient relationship as well as the collegial doctor-doctor relationship. Failure to live up to the promise of increased function leads to a rapid erosion of the trust that underlies everything physicians do. Therefore, this chapter follows the principles of Edward Benzel, MD, when discussing and describing indications and evidence for selective spine procedures. These principles enable increasing trust: Wisdom has foremost importance, but should be combined with evidence, and statements of risk/efficacy should be based on what one would do to their own mother, spouse, or child.9
The pain generator, the Holy Grail of clinical and diagnostic medicine, is often illusory in spinal pain patients. Multiple studies by Boden, Wiesel, Mayer, Rainville, and Carragee10–14 have shown the discordance between pain and anatomic or physiologic abnormalities. Given that pain is as much a cognitive as it is a nocioceptive phenomenon, if pain response is the sole outcome measure, the selection bias will always trend toward failure. This chapter, therefore, attempts to utilize evidence that has a functional component (sparse in spine literature) accompanying the usual visual analogue scale (VAS) when discussing the various procedures available to spine surgeons to improve their outcomes.
One must also understand the masquerade, or the concept of pain referral. Often a lumbar issue can masquerade as or coincide with a hip problem.15,16 This concept applies to the neck and shoulder as well as to various nerve entrapment syndromes in the upper or lower extremity.17 Acumen to unlock the masquerade is found in the office and not in the operating suite. Therefore, a skilled interventionist who performs a thorough physical examination can accrue good internally valid, reproducible diagnostic information to keep a surgeon from making a hasty, unwise decision.
The inflammatory basis for pain’s relation to the degenerative changes in spinal structures and diarthrodial joints is an additional concept to critically consider. Although concepts of legal indemnity and political policy lag behind, there is substantial evidence that degenerative changes to intervertebral discs, cartilage, and joints begin with inflammatory changes and not with mechanical stress/injury (cumulative or otherwise). This subtle concept is important for two reasons. First, the radiologic appearance of the structure is less important than its functional range and strength, which may recover through a combination of aggressive treatment of acute-phase inflammation with maintenance of functional range of motion over the same time. Second and more importantly, physician advocacy of inactivity to prevent further injury undermines the ultimate benefit of any proposed intervention. Specifically, patients who have been advised by physicians that activity (not inflammation) causes injury have higher pain scores and trends to disability.18 Physician advice that worsens the cognitive aspect of pain begin a self-fulfilling pain/disability cycle that rarely ends well.
Radicular Nerve Pain and Therapeutic versus Diagnostic Injections
Radiculopathy is a pathologic process affecting a spinal nerve root in which sensory and/or motor symptoms present distant from the pathology. Whether the process is mechanical/compressive, inflammatory, or both remains poorly elucidated. What is clear is that the sodium-channel blocking effects of local amide or ester anesthetics provide relief from pain but do not improve any functional deficits. Due to the pharmacokinetics of existing local anesthetics, this benefit is temporary but can (when applied discreetly and correctly) provide excellent diagnostic information. Synthetic glucocorticoids act to suppress production of inflammatory mediators, stabilize membrane irritability, and enhance macrophage demargination to the site of injury. Due to the complexity of the interactions, there is often a delay before the benefit from locally or remotely administered glucocorticoid is perceived by the patient.19 Depending on the nature of the nerve irritation/injury, the benefit of the glucocorticoid can often be long-lived. A tapering dose of medication that up-regulates systemic circulating glucocorticoid is still in common use for suspected radiculopathy; however, rapid steroid metabolism and intolerable systemic side effects occasionally make systemic use impractical or too short-lived for functional improvement. For this patient, treatment targeted to the pathology is often beneficial.
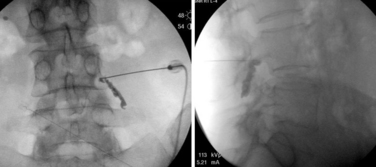
Epidural steroid injection for radiculopathy was first described in the United States in 1960 in the Cleveland Clinic Quarterly and was expanded upon thereafter.20,21 In the years since that time, its efficacy has been enhanced by use of imaging guidance as well as training programs that ensure a physician’s safe delivery of medication closest to the site of pathology. The intent of delivery of glucocorticoid medication to the site of pathology is the deposition of fat-soluble steroid in a nearby plane of epidural fat. Glucocorticoid steroids will release from this adipose tissue in a prolonged fashion to reduce pain by down-regulating pro-anti-inflammatory, acute-phase chemical mediators from inflamed/reactive tissue. Broadly, the three ways of accessing the epidural fat are through the sacral hiatus (caudal injection), between the bony lamina and traversing the ligamentum flavum (interlaminar injection), and through the neural foramina near the bifurcation of the nerve root from the spinal cord or dural sac (transforaminal injection). Each method has its utility, with research showing a trend toward greater efficacy, fewer systemic effects, and increased cost utility when the injection delivers medicine closest to the pathologic process responsible for pain and functional inhibition. In general, all of these types of injections should be assisted by imaging guidance. The studied rate of misapplication of medication with resultant complications varies from 22% to 69%. Therefore, an interventionist’s failure to use imaging guidance should be a red flag to the referring physician (Fig. 191-2).
Caudal Epidural Steroid Injection
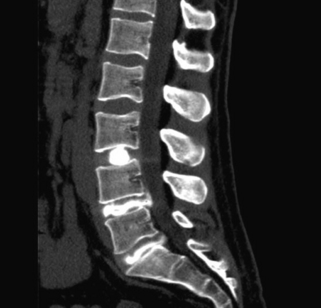
The starting needle placement for a caudal injection can be guided by palpable anatomic landmarks or by imaging. With this in mind, there is a greater than 25% rate of needle-tip misadventure without imaging guidance.22 The procedure is fairly simple, with a blunt-tipped needle directed through the sacral hiatus assisted by a simple two-picture confirmation in anteroposterior and lateral views. The needle is advanced along the dorsal bony elements of the sacrum to approximately the S3-4 space. The thecal sac typically ends around S2, but may extend as low as S4. Nonionic contrast material is used to confirm that the needle is placed within the epidural space and outside the thecal sac. Violation of the dura mater results in a spinal headache. Injection of a steroid and anesthetic solution into the caudal epidural space may result in urine retention. Additionally, delivery of the medicine through the caudal approach is often remote from the location of pathology. Finally, the concentration of corticosteroid is significantly less well distributed in the sacral epidural fat, with a concentration decrement of tenfold or more of the injectate reaching the site of pathology (Fig. 191-3).
Interlaminar Epidural Steroid Injection
The steps for getting good results from interlaminar epidural steroid injections are similar to those for other techniques. First, the physician should plan the procedure by taking a history, performing a thorough physical examination, and correlating that information to appropriate imaging. If the predominant complaint is axial back pain, the chances of a medium or long-term, nonplacebo, beneficial result from depot of steroid in the epidural space is low. Alternatively, when the pain presentation is predominantly in the limb and subjective complaints correlate with discreet pathology on radiology, the chance of injection benefit and avoiding surgery is high.23
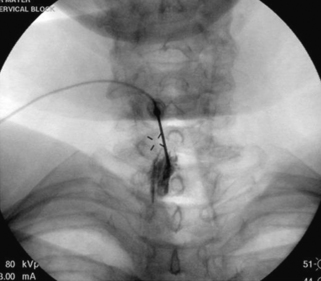
Once planning is complete, the procedure is straightforward. Fluoroscopic guidance facilitates the localization of the desired level in the lumbar, thoracic, or cervical spine. The skin overlying the interspace between the spinous processes is anesthetized, and most commonly a blunt-tipped needle is passed through the skin and directed midline. The needle is advanced using a combination of direct, fluoroscopic visualization and “loss of resistance” technique with a syringe containing water or air. Skillful completion of these techniques is important in reducing the incidence of spinal headache by dural puncture. A sound understanding of anatomy is vital for this procedure, because the spinous processes are oriented differently in the cervical, thoracic, and lumbar spinal divisions. Additionally, the thickness of ligaments, depth of lateral recesses, and location of vascular anatomy changes over the course of the spine. To obtain an efficacious result, medication needs to be placed in the epidural space only. To ensure proper placement, it is strongly recommended that a form of nonionic contrast be used to verify placement once loss of resistance is encountered. Frequently, even in the most experienced hands, a false loss of resistance may be encountered between the interspinous ligament and the ligamentum flavum or, more problematically, tissue may become lodged in the bevel of the needle, resulting in a failure to perceive loss of resistance. This failure often results in dural puncture and in the contrast flow pattern appearing identical to a myelogram. Finally, knowledge of anatomy allows one to understand that the epidural space is not uniform in shape but is pyramidal in cross section, meaning that if the needle tip is directed away from midline, the likelihood of dural puncture increases in proportion to the decreased epidural space laterally. McGoldrick expanded on the cryomicrotome dissection work of Hogan to show that failure of ligamentum fusion in the dorsal midline occurs in the cervical and high thoracic space in 51% to 74% of dissections.24,25 Additionally, controversy persists about whether a “fat pad” exists dorsally above the dura mater at a level above C7.24 The lack of landmarks for traditional loss of resistance technique make dural puncture in the cervical and high thoracic spine more likely. The consequence of dural puncture with intrathecal infusion of anesthetic can range from slow-onset respiratory depression (~30 minutes) to seizure to cardiovascular collapse.26
Finally, Cluff et al. noted a disturbing trend: that only 39% of academic institutions used fluoroscopy for epidural injections, whereas 74% of private practices used fluoroscopy.27 In light of the compelling data that only 26% of blind attempts (without fluoroscopic guidance) by experienced anesthesiologists placed medication at the site of pathology, this trend may account for the jaundiced view held by many referrers with regard to the efficacy of injections.28 This jaundiced view is seen at its most extreme in the oft-cited study by Valat that, in attempting to demonstrate the efficacy of epidural steroid injection versus placebo merely showed that blind epidural steroid injection is no better than placebo even when delivered three times.29 In conclusion, the prosaic use of the interlaminar technique has bred contempt that can only be improved if referring physicians demand excellence; one may consider the oft-quoted Edward Benzel aphorism: “A fool with a tool is still a fool.”
Transforaminal Epidural Steroid Injection
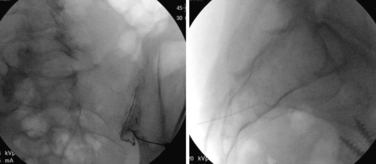
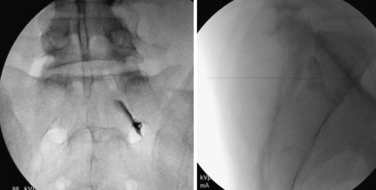
The transforaminal epidural steroid injection technique (Fig. 191-4) has been used with increasing favor owing to beliefs around the selectivity, proximity (to the pathology), and longevity of benefit with this technique. These assumptions have proven difficult to substantiate in the literature. Riew et al. published two very persuasive studies that were shrewdly designed to eliminate the winner’s bias seen in many studies examining injection versus surgery.30 The authors took all patients who were scheduled for surgery and randomized them to receive a transforaminal injection of either anesthetic alone or anesthetic plus steroid. Of those receiving steroid via transforaminal delivery, 71% elected to not have surgery at end points ranging from 13 to 27 months. This was a statistically significant difference compared to the anesthetic-alone group, in which only 33% elected not to have surgery by the end point (p < .004). This finding held at 5-year analysis, with 3 of 4 patients requiring late surgery (between 27 and 73 months) with progression of symptomatic spinal stenosis.23
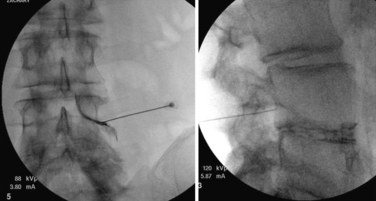
FIGURE 191-4 Right L4 transforaminal epidural steroid injection: anteroposterior (left) and lateral (right).
Recently, there has been a proliferation of expert opinion about the risks associated with cervical epidural steroid injections in general and cervical transforaminal injections specifically. The main concerns originate from case reports of catastrophic complications, including death, persistent vegetative state, stroke, spinal cord infarction, and high anesthesia-related cardiorespiratory collapse. For the most part, the culprit is injection of medication into the arterial circulation supplying the brain or spinal cord. Several safeguards exist and should be known by a fellowship-trained interventionalist—including use of contrast enhancement to confirm needle-tip placement, use of “live” fluoroscopic visualization to confirm arterial uptake, use of test-dose anesthetic medication, and use of digital subtraction technology. Each of these recommendations has its benefits; however, the most convincing evidence to recommend modifying current practice comes from an animal study that demonstratied zero infarction rate with administration of nonparticulate steroid in two different formulations; 100% of the particulate steroid group suffered neurologic damage that correlated with hypoxic and ischemic damage.31
In conclusion, epidural steroid injections show benefit when performed for the correct reason (radicular, not axial, pain) and when medicine is delivered to the correct location (using fluoroscopic guidance and contrast confirmation). These injections have been shown to help reduce surgical rate, allow for earlier return to function, and save $12,666 per responder (in 1999 dollars).30,32,33 Therefore, referring physicians should insist on the highest clinician acumen and the highest-quality training for proper delivery of medication to the site of pathology.
Joint-Mediated Pain
The joints of the spine include a three-part joint that combines the disc and a matched pair of dorsal joints at each spinal segment colloquially called the facet joints. Additionally, the cervical spine has accessory joints that aid with shear forces; these are named after the anatomist Luschka, or are commonly called the uncovertebral joints. A separate joint comprises the skull, first vertebra, dens, and second vertebra and allows for complex head movement in relation to the neck and the rest of the body. Finally, a pair of complex joints that transmit/disperse rotational force applied through the pelvis are called the sacroiliac joints. All of these joints are diarthrodial joints of the spine, and each one can produce pain. Briefly we discuss the role of injection procedures in diagnosis and treatment of pain syndromes involving the various spinal joints.
Facet Joints of Cervical, Thoracic, and Lumbar Spine
The facet joints are paired diarthrodial joints on the dorsal aspect of the vertebral bodies. These joints provide stability and are a key component to ensuring accommodation of a neutral zone in the three-joint complex model of the vertebral motion segment. As early as 1911, Goldthwait postulated that the facet joints, specifically the zygapophyseal joints (Z joints) of the lumbar spine, could be a potential pain generator in the spine.34 Anatomic studies have verified the presence of nociceptive structures and mechanoreceptors in the capsule and synovial folds of the facet joints.35–37 Additionally, Mooney and Hirsch et al. identified the zygapophyseal joint as a source of pseudoradicular pain in both a control hypertonic saline group and in study patients.38–40 And why wouldn’t high-stress joint complexes be a potential source of pain? It stands to reason that like the knee, the shoulder, or any diarthrodial joint, the facet joint is subject to the ravages of time, inflammation, and apoptosis, giving it the ability to act as a pain generator in the spine.
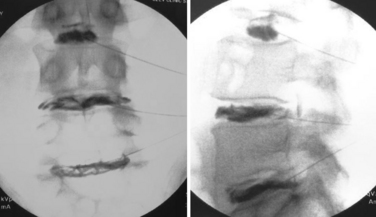
Statistical methodology varies, but epidemiologic studies note that facet-mediated pain may account for between 4%41 and 45%42 of chronic spine pain.43 The large variance in these statistics may be due to study inclusion criteria and the lack of adherence to exacting standards. Although proof for the existence of facet joint–mediated pain is debated, the ubiquity of facet joint arthropathy is undeniable. Cadaveric studies have shown facet joint arthropathy to be present in 100% of cadaveric spines over 60 years old.44 Although clues to facet-mediated pain may be obtained from a thorough history, physical examination findings, and imaging, no pathognomonic test/maneuver exists. Facet joint pain is currently diagnosed by a preponderance of evidence because we lack a single test for facet pain that combines high sensitivity and specificity. The lack of any test with a high positive prognostic value is likely due to known compensatory mechanisms that are associated with joint pain/inflammation, such as muscle spasm, altered movement mechanics, and pain referral patterns. Facet joints present more of the prerequisites to be a pain generator than pain associated with the more amorphous construct of discogenic pain. Facet joints have a robust nerve supply and are capable of causing pain similar to that seen in peripheral joints, as in overuse injuries elsewhere in the body. Articular joints possess a known pattern of disease/injury susceptibility, and their pain can be ameliorated using well-described diagnostic techniques with acceptable reliability and validity (Fig. 191-5).45
Unfortunately, today there is no “gold standard” criterion to confirm with high positive predictive value the presence of facet-mediated pain. The accepted standard combines clinical judgment with a diagnostic or therapeutic facet joint injection. Early studies performed by Hirsch et al. and Mooney not only supported the existence of facet joint pain but also seemed to show improvement when injections of anesthetic or glucocorticoids were combined with exercise. Similar results were seen with provocative injections to the medial branch nerves that supply the facet joints.46 Though prone to operator error, these studies show that wise use of facet joint injections can serve dual diagnostic and therapeutic goals in returning patients to function. Currently two techniques are employed to block pain from facet joints: intra-articular injection of the facet joint capsule and blockade of the medial branch nerves transmitting pain signals from the joint to the spinal cord/brain. Both techniques have been shown to possess variable, operator-dependent accuracy/efficacy when combined with appropriate clinical judgment.47,48
The early standard for the diagnosis of facet joint pain was to seek mitigation of pain with a single facet joint injection. However, this methodology produced an unacceptably high false-positive rate of up to 38%,49 meaning results showed poor prognostic value. Coupled with a known/accepted positive placebo response rate of up to 32% with many interventional procedures, this result significantly reduced the validity of this treatment protocol.50 It has therefore been advocated that a reliable control be employed in the form of the double-block protocol, in which anesthetics of varying durations of effect are administered on subsequent injections and the patient maintains two accurate postinjection pain diaries. If the duration of pain relief is concordant with the half-life of the diagnostic anesthetic, the injection is considered diagnostic for facet pain. In addition, many of the best-performed postoperative studies advocate the use of a cut-off score of 75%, 80%,51 or even 100%52 pain relief to indicate a positive response, as opposed to the earlier practices of 50% pain relief.51–54 Furthermore, the importance of outcome measures cannot be stressed enough. As was alluded to earlier in this chapter, the focus of diagnostic and therapeutic injections should be functional improvement rather than mitigation on VAS scores. Therefore, evaluation with validated functional measures such as the Pain Disability Questionaire (PDQ), Pain Disability Index (PDI), Euro-Qual, SF-18, or even the old standard Oswestry Disability Index should be utilized when interpreting the effectiveness of any of the interventional procedures discussed in this chapter.
Much debate has arisen regarding the selectivity of intra-articular injection versus medial branch blocks. Cadaveric studies by Dreyfuss et al. suggest that volume control is important in limiting spread of local anesthetic to nearby structures to avoid anesthetizing other nociceptive structures and providing a false-positive result by exerting pain relief and functional benefit to structures other than the facet joint.53 This research has led many clinicians to follow the “less is more” axiom and to use less than 1 mL of anesthetic for their diagnostic blockade. This small aliquot of anesthetic medication seems to have the best postprocedure positive predictive value, correlating to prospective successful outcomes.51
Understanding the myriad of variables that complicate all clinical research, many functional outcome studies have been performed with questionable enrollment criteria. Meta-analyses utilizing stringent compliance with Agency for Healthcare Research and Quality (AHRQ) and Quality Assessment of Diagnostic Accuracy Studies (QUADAS) criteria for evaluation of diagnostic tests suggest that there is strong evidence that controlled diagnostic facet joint blocks establish a diagnosis of facet joint pain in chronic cervical and lumbar spinal pain and moderate evidence for diagnosing thoracic facet joint pain.55–57 A review by Boswell et al. and another by van Tulder have supported the diagnostic efficacy of facet joint blockade.58,59 Some reviews still conclude that the multifactorial nature of degenerative joint-associated pain means that there is insufficient evidence to support the use of facet joint injections to diagnose or treat facet joint pain.7,60
As with epidural injections, it is important that the reader note that the use of fluoroscopy or CT imaging guidance is critical to success when performing any diagnostic spinal procedure. Significant care should be taken when selecting an interventionist to perform diagnostic injections on patients who you may ultimately be taking to surgery. These procedures cannot be performed with any degree of accuracy without the use of imaging to verify appropriate needle placement and ensure patient safety. Most interventional societies also advocate the additional use of nonionic contrast to verify placement of injectate. This has additional benefit if the interventionalist and/or the referring physician need to review the procedure at a later date. In preparation for an injection, prior review of available advanced imaging studies (CT or MRI) is important to understand the three-dimensional anatomy of the target joints. This is especially true with intra-articular facet joint injections because there can be considerable variability in joint morphology among individuals. The C arm is usually aligned in a slightly oblique fashion to afford the physician access to a portion of the facet joint capsule. The facet joint capsule can be accessed at various locations along the joint line or at the superior or inferior recess. The amount of subcutaneous anesthetic required to keep a particular patient comfortable varies from practitioner to practitioner and from patient to patient. Overinjection of large amounts of anesthetic into the subcutaneous structures and paraspinal musculature has been criticized as a likely contributor to the false-positive rates seen in some studies.61 Yet another reason to follow the “less is more” dictum, with use of a 25G needle smaller amounts of subcutaneous anesthetic are required. The needle is advanced under fluoroscopic guidance and is often felt piercing the joint and capsule. Confirmation of intra-articular needle placement is made with a small amount of contrast material. The resulting arthrogram will typically reveal retained contrast material in the superior and inferior capsular recesses of the facet joint. At times, a well-demarcated plane of contrast will be seen between the two articular surfaces. Fluoroscopic confirmation is best made in at least two planes, preferably 90 degrees from each other. As stated previously, retention of fluoroscopic images can be critical for future evaluation of the efficacy of the injection and for quality control by the referring surgeon. Once intra-articular flow of contrast is confirmed, a small amount of anesthetic or a solution of anesthetic and long-acting glucocorticoid is injected into the joint before the needle is removed. Typically the amount of this solution is less than 1.25 mL.
An additional confounding issue surrounds the routine use of moderate or deep sedation by anesthesiologists during interventional procedures; this may have unintended consequences and can worsen the predictive value of these procedures. Manchikanti et al. studied the effects of IV midazolam and/or fentanyl during cervical and lumbar facet joint injections and showed that with strict criteria the effects of sedation may be minimized to a 10% false-positive rate.61
We do not advocate consideration of medial branch nerve radiofrequency ablation (or rhizotomy) unless suspicion of facet joint–mediated pain is supported by clear clinical suspicion, imaging evidence, and two successful diagnostic blocks. This literature-supported opinion has been brought into question by a recent paper by Cohen et al., who contend that the cost of blocks to increase the positive predictive value exceeds the cost utility from pain relief and increased function seen when one proceeds straight to radiofrequency rhizotomy of medical branches.62 First described by Shealy, this procedure denervates the neural pathways (medial branch nerves) that supply the afferent pain information from the facet joint to the central nervous system.63 This procedure has been shown to offer longer relief than intra-articular facet joint injections and medial branch blocks.51 The consistent locations of the medial branch nerves in the lumbar and cervical spine, reported by many anatomists, make these excellent locations for dorsal rami radiofrequency rhizotomy. However, one must recall the redundant nature of the medial branch innervation of the facet joints. Namely, each joint receives innervation from two different dorsal rami levels. For example, the L4-5 facet joint receives its innervation from the L3 medial branch from above and the L4 medial branch at that level. The innervation of the cervical facet joints is somewhat different. The cervical facet joint is innervated by the medial branch nerve of that level and the level below. The reason for this variance lies in the presence of the C8 nerve, which innervates the T1-2 facet joint in conjunction with the T1 medial branch. This consistency of location explains the favorable success rates with cervical and lumbar medial branch blocks and radiofrequency procedures. On the other hand, the location of the thoracic medial branch nerves is known to be quite variable along the length of the thoracic transverse process, which makes effective lesioning of these nerves quite challenging technically. There is ongoing debate as to the most effective techniques to effectively denervate the thoracic facet joints. Directing the radiofrequency rhizotomy probe to its target is performed in much the same manner as in the medial branch block procedure. The major difference is considerations of cross-sectional area in placing a maximal amount of the uninsulated tip of the special insulated radiofrequency needle in the area where the nerve resides. Care is taken to avoid adjacent structures such as nerve roots and deep muscles that were partially lesioned and can cause a new and persistent pain.64 Again, placement of these needles is performed using fluoroscopy and radiopaque contrast. A lesion is created using either a continuous or pulsed radiofrequency apparatus at 80 to 90 degrees for 80 to 90 seconds. Some practitioners repeat this procedure with slight adjustment to the location of the active tip to create several lesions along the same nerve.
A review by Slipman et al. reports moderately strong evidence (level III) in accordance with the guidelines described by the AHCPR that radiofrequency ablation of the medial branch nerves is an effective treatment for facet joint pain.65 Other reviews have supported the presence of only moderately weak evidence or conflicting evidence regarding the efficacy of a radiofrequency rhizotomy of medial branch nerves for facet-mediated pain.66–68 The goal again is increased function and avoidance of the surgical alternative, which is often a fusion. Many continue to argue that the studies reviewed showed poor efficacy due to the authors’ failure to employ the double-block criteria that are advocated to identify patients with facet joint–related pain. Therefore, many studies that refute the efficacy of radiofrequency rhizotomy of the medial branch nerves for facet joint pain may have not been treating facet joint pain. In a study by Dreyfuss et al. that employed strict standards and double-block techniques, 60% of patients experienced at least a 90% pain reduction, and 87% of patients experienced at least a 60% reduction in pain after a radiofrequency ablation procedure for low back pain, lasting at 12-month follow-up.51 Patient safety and efficacy of procedure should remain stronger considerations than cost despite ongoing pressure from insurers. It is the authors’ opinion that double-block protocol to determine the injection location and pain generator remains the standard of care.
Despite the continuing debate regarding the efficacy of these procedures, there is a consensus that facet joint injections, medial branch blocks, and radiofrequency ablation are safe, reliable, and minimally invasive options to diagnose and treat patients with facet joint pain (Fig. 191-6).
Sacroiliac Joint Injections
The sacroiliac (SI) joint is a well-accepted source of low back, upper buttock, and leg pain.69 Much like the facet joint, the SI joint is a true diarthodial joint, receiving its innervation via nerve branches from the lumbar and sacral nerve roots.70 Debate still remains as to whether the innervation of the SI joint arises primarily from the sacral dorsal rami71 or from a combination of dorsal and ventral rami.72 Studies support the presence of neural structures and mechanoreceptors within the SI joint capsule and intra-articularly.71–73 SI joint pain has been attributed to numerous pathologic processes, including autoimmune inflammation (e.g., spondyloarthropathies), trauma, pregnancy, SI joint dysfunction, and chronic degenerative changes. Studies on asymptomatic individuals who underwent provocative SI joint injections have defined the common pain referral patterns of this joint.69,74
The diagnosis of SI joint pain has historically been accomplished through examination findings and pain elicited with provocative maneuvers. Some physicians have advocated a battery of maneuvers, including Gaenslen’s, Patrick’s, and Gillet’s maneuvers, as well as distraction, compression, and thigh thrust tests, as a fairly reliable method to determine the presence or absence of SI joint pain.75 Others suggest a simpler approach of having the patient point to the area of greatest pain. If the patient points just medial to the dorsal rostral iliac spine, there is a high likelihood of SI joint pain; this simple but effective test is known colloquially as the Fortin finger test76
The efficacy of imaging studies to accurately diagnose SI joint pain has been shown to be poor. Despite some reviews that suggest certain physical examination findings or tests as effective in localizing SI joint nociception, SI joint pain remains bereft of a diagnostic “gold standard.” Unfortunately, SI joint anesthetic injection has been held by some to be a “pyrite” standard.7,77 Although there are significant limitations with regard to specificity, many physicians believe this procedure provides the best method of differentiating a clinical suspicion of SI joint pathology from other etiologies of low back pain.78
If patients have failed a conservative treatment course that includes medications, physical modalities, stretching, core strengthening, and/or an “SI belt,” diagnostic/therapeutic SI joint injection remains a reasonable consideration. Reproducing a patient’s pain by distention of the suspect SI joint or mitigating the pain with SI joint injection clearly helps to define a problem isolated to this complex diarthrodial joint, which is innervated in a more redundant/complex fashion than many other diarthrodial joints. Diagnosis of SI joint pain through SI joint injection serves two purposes: (1) to diagnose and treat SI joint pain and (2) to rule out other structures as the possible etiology for back pain. The latter may prevent unnecessary interventions or surgery to nearby structures (i.e., hip and spine).
McKenzie-Brown et al. evaluated the specificity and validity of SI joint injections and found there to be moderate evidence to support this procedure using AHRQ, Cochrane Review Group, and QUADAS criteria for diagnostic studies.79,80 The authors also concluded that the evidence for moderate to long-term therapeutic value from SI joint injections and/or radiofrequency denervation of nerves innervating the SI joint was limited.
Sympathetic Blockade
The sympathetic nervous system plays an important role in certain chronic pain states that can be relieved by sympathetic blockade.81 The pathways and mechanisms by which the sympathetic nervous system facilitates a persistently painful pathologic state remain poorly understood. Techniques for lumbar sympathetic and cervical sympathetic (stellate ganglion) blockade are primarily used to diagnose and occasionally assist in treatment of sympathetically mediated complex regional pain syndrome (CRPS).
The stellate ganglion block remains a commonly performed injection for pain management, although the injection should be referred to as a lower cervical or upper thoracic sympathetic block because the stellate ganglion is present in only about 80% of the population.82 The conditions commonly treated with this block include CRPS of the upper limb, Raynaud phenomenon, arterial embolism, postherpetic neuralgia, phantom limb pain, and stump pain. The stellate ganglion is formed by the fusion of the inferior cervical ganglion and the first thoracic ganglion. The stellate ganglion is approached ventrally by palpating the landmark at the lateral aspect of the ventral C6 vertebral body (known as the Chaussignac tubercle). This landmark can occasionally be seen radiographically. When performing this procedure, a combination of palpatory and fluoroscopic guidance is helpful. One must manually retract the carotid artery while palpating the Chaussignac tubercle to avoid inadvertent vascular puncture. Fluoroscopic guidance helps one to avoid advancing the needle past the C6 tubercle where the vertebral artery lies. Once the needle is positioned correctly, it is withdrawn slightly from the periostium; contrast is injected and should be observed spreading evenly in a rostral-caudal fashion (as with the lumbar sympathetic chain). Failure to observe spread of contrast in both the cranial and caudal directions may indicate intramuscular injection. The needle should be repositioned so a wispy, rostral-caudal spread is visualized. A small test dose of anesthetic is usually administered; barring any untoward effects, the remainder of the solution (up to 8 mL) is injected. The total injectate needed depends on the desired block. Sympathetic block of the ganglion can be easily documented with the presence of Horner syndrome: miosis, ptosis, enophthalmos, conjunctival injection, nasal congestion, and facial anhidrosis. Successful sympathetic blockade to the upper limb can be determined by venous engorgement, psychogalvanic reflex, positive sweat test, and rise in skin temperature. Complications from this procedure have included tracheal trauma, injury to the brachial plexus, injury to the pleura and lung, diaphragmatic paralysis, intraspinal injection, and seizures from intravascular injection (Figs. 191-7 and 191-8).82
Discogenic Pain
For much of the latter half of the 20th century, provocative discography and a host of scoring criteria were the mainstays of algorithmic decisions for or against fusion surgery.83,84 In 2006, Carragee et al. presented the first of a series of high-level, prospective collected evidence showing high false-positive rates, poor specificity, and false sensitivity and questioning the safety of discography’s role in confirming discogenic pain.85,86 The final coffin nails appeared with data from multiple prospective studies showing outcomes of lumbar fusion remaining poor even when discography was utilized to supplement the clinical decision making. These outcomes look especially poor when outcomes such as return to work are employed (rather than the usual soft outcomes such as VAS scores alone or combined with Oswestry scores).87–90 The system of remuneration continues to shift toward evidence-based recommendations. Both the Official Disability Guidelines (ODG) and American College of Occupational and Environmental Medicine (ACOEM) guidelines now recommend against discography. Does the continuing poor outcome data for lumbar fusions (as opposed to much better outcome data for cervical fusions) mean that the diagnostic test is a poor test?91 Alternatively, does poor lumbar fusion data mean that lumbar fusion is an overutilized procedure,4 while saying nothing about the positive predictive value of discography? These questions remain unanswered. As it stands in 2011, the raw numbers of discographies requested by surgeons has fallen in the authors’ practice by more than 90%. The seeming final nail in discography’s coffin is an as-yet unpublished paper presented at a national meeting that purports to show 10-year data with advanced radiologic visualized degenerative changes to the normal, control-level disc used in prior discography tests.92 Should this paper pass peer review, showing that discography is both inaccurate at improving outcomes and has the additional component of harm, causing degeneration and eventual symptomatic herniation in otherwise healthy discs, that would likely eliminate the use of discography as a reasonable test.
An emerging technology that remains under study is anesthetic discography. The principle is quite simple: irritate (either mechanically or chemically) the disc of interest (the assumed source of nociception). If concordant pain is reproduced, infuse the disc percutaneously with anesthetic to see if the pain resolves. At the moment, one product is available in the United States, but prospective data remain unconvincing that use of this diagnostic test will improve results on hard outcomes such as return to work.93
Heat-modulated disruption of the “degenerative cascade” is the stand-alone mechanism of action. The thought of “annealing” the proteoglycan structure of the nucleus via thermocoagulation seems to be a dead end. Placing a straight radiofrequency probe in the center of the disc nucleus and creating lesions at varying duration and varying temperatures has failed to show benefit despite the initial hope/hype. Only two studies have emerged, with neither showing any significant improvement in discogenic back pain.94,95
Another “orphan” intradiscal procedure that enjoyed the limelight as the original minimally invasive procedure is chemonucleolysis. This procedure involves the injection of chymopapain (a derivative of the papaya fruit) into the nucleus pulposus to dissolve nuclear material. Additionally, the chemical digestion of the nuclear material often causes a robust inflammatory response that occasionally resulted in interbody fusion of the treated level. Although the risks of severe complications from this procedure are relatively rare, the inability to control spread of the chymopapain injectate through anular fissures likely results in irritation of nearby neural, arthrodial, and vascular structures. Although still performed by some physicians, this procedure has fallen out of favor in light of lack of supporting evidence of its effectiveness and the pain patients experienced in the first 6 to 8 weeks postprocedure.
One prominent and briefly popular method focused on modulating pain nerve innervation of the anulus by heating this area of the disc. This modality, known as intradiscal electrothermal therapy, or IDET, had initial strength of evidence to receive a category I Current Procedural Terminology (CPT) code. One prospective trial demonstrating the procedure’s effectiveness won a national “best paper” award from The Spine Journal96 This procedure utilizes a percutaneous catheter that allows deployment of a 5-cm active tip wire. The wire is wound along the curved, dorsal aspect of the nucleus–anulus junction.94,97 Once in place, the wire is heated to 90°C. The resulting effect is believed to promote thermal coagulation of the anular nerve endings, remodeling of the degenerating anulus, and possibly structural reinforcement near weak areas of the anulus, spilling nuclear material. In their review, Derby et al. proposed several mechanisms by which heat may reduce disc-related pain, including changes in disc biomechanics, anular contraction, thermally induced healing response, sealing of anular tears, and anular denervation. After a thorough biomechanical analysis, the authors concluded that the mechanism of pain relief remains unclear.98 Early studies by Saal and Saal demonstrated 24-month outcomes with mean improvement in VAS of 3.16 points, improved sitting times on average by 52.7 minutes, mean improvements in SF-36 of 31.33 points, and at least a 7-point improvement on a bodily pain scale in 78% of subjects.99
Although IDET has been the most frequently performed anuloplasty procedure in recent times, much debate remains regarding the efficacy of the procedure and its mechanism of pain relief. The two available randomized, prospectively collected, sham-control studies have had conflicting results and conclusions. Pauza’s award-winning paper demonstrated significantly better improvement in pain severity (36% vs. 17%, P = .045) and back function (35% vs. 12%, P < .05) among patients treated with the IDET procedure compared to patients randomly assigned to sham control.96 Freeman et al., on the other hand, demonstrated failure to improve at any of the outcome end points in either the treatment or sham subjects. Numerous issues have been raised regarding the conflicting results. The issues mostly center around aspects of patient selection, including severity of degenerative disc changes, patient comorbidities, duration of pain, and lack of any response to treatment (in the Freeman paper), which seems to contradict the accepted internal control for the placebo.100 Andersson et al. compared 18 IDET studies and 33 published studies of spine fusion for treatment of discogenic pain, which seemed to show comparable outcomes with regard to pain severity and quality of life.101 However, complications were far more common in the fusion studies compared to the IDET studies.
Two other percutaneous anuloplasty procedures are currently being reported: percutaneous intradiscal radiofrequency thermocoagulation and biacuplasty. The first procedure involves the placement of a unipolar radiofrequency probe into the nucleus–anulus junction. The literature regarding the success of this procedure to date has been equivocal; no significant improvements have been shown in patients suffering from discogenic pain. Kapural et al. published a study regarding the efficacy of unipolar anuloplasty compared to IDET and found that at 12-month follow-up, those who underwent IDET experienced significantly better improvement and significantly greater reductions in pain severity (81% vs. 33%, P = .001).102 Biacuplasty is a newer version of the same anuloplasty procedure that utilizes two radiofrequency probes introduced into the dorsal anulus that create a radiofrequency lesion across the dorsal anulus. Six-month follow-up data from this procedure have reported improvements in several pain assessment scales (e.g., VAS) without associated improvement in functional scales. Further prospective, randomized studies are clearly needed given the mixed record with IDET.103
Other minimally invasive intradiscal procedures focus on reduction of disc pressure by removal of disc material. These procedures employ various devices that use either mechanical means or thermal energy to remove disc material. One procedure, known as nucleoplasty, utilizes a radiofrequency probe to create small channels in the nuclear material to attempt to decrease the nuclear volume. Based on U.S. Preventive Services Task Force (USPSTF) criteria, Manchikanti et al. maintain that level II-3 evidence exists that favors an indication that nucleoplasty decreases lower extremity pain due to disc herniation.104 However, evidence of efficacy is lacking for relief of back pain. Techniques to manually remove disc material go by several names. One, the automated percutaneous lumbar discectomy device, utilizes a modified vitrectomy instrument with a reciprocating suction-cutter to aspirate, cut, circulate, and remove fluid and extruded disc material. Although initial studies were promising, a subsequent randomized, controlled study shows a 29% success rate; this evidence has caused this procedure to fall out of favor.105 Another mechanical device utilizes a high-RPM Archimedes screw to remove nuclear material through the cannula of the device. Level III evidence (by AHRQ criteria) was cited to support relief of pain, with functional data notably missing from the review.106 Finally, percutaneous laser discectomy utilizes a high-powered visible-spectrum laser to vaporize nuclear material, thereby reducing intradiscal pressure. Recent review for this procedure purports to show level II-2 evidence of efficacy based on USPSTF criteria.107
The success of these procedures and published evidence relies largely on patient selection. The goal is to harness/enhance the body’s natural, self-healing processes, but the evidence of true mechanism of action for functional improvement remains sparse for these techniques. Enrollment criteria for all of the aforementioned safety procedures state that the disc space should be preserved. Loss of more than 50% of the disc space excluded patients from most studies. Unfortunately, patients with concomitant pain, functional loss, and disc degenerative changes still represent the majority of our patient population. Does this fact make many of the studies of efficacy nongeneralizable? Patients studied also had normal neurologic examination results, imaging supporting/concordant with pain at the degenerative disc levels, positive disc pain on provocative discography, and lack of significant confounding psychosocial issues. Does this represent the standard patient? Although these procedures are performed percutaneously, they still have a substantial risk profile. Complications from these procedures have included both aseptic and septic spondylodiscitis, bleeding, nerve root injury, vascular injury, sigmoid artery injury, worsening pain, and failure of the mechanical device requiring surgical removal. Contraindications to these procedures include chronic radiculopathy, large fragmented or sequestered disc fragments, equivocal results from diagnostic discography, severe spinal canal stenosis, uncontrolled coagulopathy and bleeding disorders, cauda equina syndrome, and infection.
Diagnostic Tests
In the authors’ experience spine injection procedures are used for diagnostic as well as therapeutic purposes. Multiple studies have shown that abnormalities seen on MRI or CT do not necessarily correlate with symptoms.10,12,108,109 Moreover, even a first episode of pain may not correspond to new MRI pathology.110 With these complicating factors, injections serve a purpose to improve surgical outcomes. Several authors of recent meta-analyses have opined that evidence is insufficient to recommend diagnostic injections.111,112 As discussed in the facet joint portion of this chapter, the relevance of a diagnostic test lies in the acceptance of false-positive and false-negative rates. Diagnostic injections are used frequently in clinical practice, and many surgeons find them clinically helpful. Most physicians understand that no test is perfect. The crux of a test’s utility lies in the rigor of the person interpreting the test. Therefore, if there is incentive to shade diagnostic results, the test loses all value in the eyes of the public. The lack of rigor in so-called meta-analyses does not allow for good recommendations regarding positive predictive value.111 Instead, diagnostic and prognostic tests should be evaluated in prospectively controlled trials with hard outcomes.112
Yeom et al. elegantly demonstrated with blocks of affected and unaffected nerve roots that the diagnostic predictive value of selective nerve blocks approaches 80% when the best practice principles are adopted.113 These tests, like virtually all diagnostic tests in medicine, should be used in conjunction with other information to maximize benefit for the patient.114,115 In this chapter, we have discussed reliability issues surrounding diagnostic techniques for facet joint pain, SI joint pain, articular hip pain, and articular shoulder pain, as well as the maelstrom of controversy surrounding the diagnosis of discogenic pain. The arguments are not repeated here, with the exception of stating that if this information improves surgical outcomes and prevents even one catastrophic failure, it probably has shown its worth (Fig. 191-9).
Intrathecal Drug Delivery
Since the late 1980s, the use of intrathecal analgesia for the treatment of severe chronic cancer pain and neuropathic and chronic nonmalignant pain has been growing in popularity. Delivery of intrathecal analgesics is not a new concept. As early as 1885, Leonard Corning reported on intrathecal anesthetic administration.116 Intrathecal morphine administration in the treatment of cancer pain was reported in 1979 by Wang et al.117
Over the past 20 years, continuous infusion of intrathecal baclofen via a subcutaneously implanted pump has become a powerful tool in the management of spasticity in various neurologic conditions. Baclofen (Lioresal), the p-chlorophenyl derivative of GABA, possesses poor lipid solubility and therefore crosses the blood-brain barrier poorly. The resulting positive evidence, safe management, and increased function for spinal cord–injured patients demonstrates a safety profile that allowed pumps to be applied for narcotic infusions.118 Originally introduced as a treatment modality for patients suffering severe pain from malignancy, intrathecal administration of morphine directly to the site of therapeutic action provided better analgesia at a lower dose with fewer adverse effects than oral medications. Morphine is the current gold standard in intrathecally delivered pain medications and is the only opiate approved by the Food and Drug Administration for spinal administration. Because the typical life expectancy of the pump outlasts the typical life expectancy of the patient, this offers a pain management modality that has been deemed very effective. Strong disagreement still surrounds the use of intrathecal morphine pumps for chronic nonmalignant pain. Although administration of opiate medications is common practice in treating chronic nonmalignant pain, the systemic adverse effects of these medications often limit their use.119 In light of longer life expectancy in patients with nonmalignant pain, these patients are much more likely to experience the adverse physiologic and psychosocial effects of long-term opiate therapy. Early studies suggested that intrathecal delivery of morphine was effective in about 60% of patients with chronic pain of nonmalignant origin.120 Neuraxial morphine administration has been found to be safe and efficacious in the short term in palliating chronic, intractable malignant, and nonmalignant pain.120–122 Despite its relative safety, adverse effects from intrathecal morphine include nausea, vomiting, drowsiness, pruritus, weakness, diaphoresis, urine retention, respiratory depression, weight gain, impotence, amenorrhea, hypothyroidism, paranoia, nystagmus, polyarthralgia, myoclonus, peripheral edema, and failure to sustain benefit.119,123
Another rare side effect that has been receiving much attention in recent years is the development of granulomatous tissue near the tip of the infusion catheter. Although reported symptomatic cases are still relatively rare, this complication has resulted in some significant neurologic sequela. There is much debate as to the cause of these granulomas, but their development is believed to be related to the concentration of morphine administered.124 Although morphine is the most common medication administered through the intrathecal route, other medications, such as fentanyl, sufentanil, clonidine, and bupivacaine, have been employed as well. Due to each drug’s pharmacokinetics and lipophilic or lipophobic characteristics, each has its own set of positive and negative qualities. Newer medications, including N-type calcium channel blockers, have shown a high side effect profile with relatively modest benefits over opioid infusion.
Neuromodulation
Spinal cord stimulation has been evolving as a treatment modality since the 1970s. Advances in technology around spinal cord stimulators or dorsal column stimulation have tracked with good short-term and medium-term evidence of efficacy. Since the 1960s, this technology has been considered a clinical application of Melzack and Wall’s “gate-control theory.”125 First reports of spinal cord stimulation clinical use in patients were published in 1975.126 Over the years, this modality has been applied to the treatment of peripheral ischemia, cardiac ischemia, complex regional pain syndrome, diabetic neuropathy, postherpetic neuralgia, phantom limb pain, postamputation stump pain, and chronic pain resulting from postlaminectomy syndrome. The exact mechanism of action underlying the sustained efficacy of spinal cord stimulation is unknown.
The sympatholytic effect is still postulated to be the major underlying property behind the success of spinal cord stimulation. While recent research around the plasticity of the neuron-axis has called into question some of the original theories, spinal cord stimulation has recently gained greater acceptance in the treatment of both neuropathic pain and axial spine pain resulting from chronic radiculopathy and postlaminectomy syndrome.127 In light of its growing clinical significance and advancements in stimulator and electrode design, an entire chapter of this text has been dedicated to the discussion of this technology and its applications. The rigor of recent research and protocols for reasonable use has allowed for evidence-based acceptance of neurostimulation and the inclusion in guidelines as an adjunct to return patients to function (Figs. 191-10 and 191-11).
Complications
The most common risk both in terms of frequency of occurrence and potentially catastrophic effects (as with surgery) is bleeding inside the spinal canal. This risk is minimized by avoiding patients with bleeding diathesis, avoiding patients actively using anticoagulation, knowing the location of the vascular anatomy under fluoroscopy/CT, and utilizing nonionic contrast dye to avoid misadventure.
Carragee E., Alamin T., Cheng I., et al. Are first-time episodes of serious LBP associated with new MRI findings? Spine J. 2006;6(6):624-635.
Dreyfuss P., Halbrook B., Pauza K., et al. Efficacy and validity of radiofrequency neurotomy for chronic lumbar zygapophysial joint pain. Spine. 2000;25:1270-1277.
Mooney V., Robertson J. The facet syndrome. Clin Orthop Relat Res 115. 1976:149-156.
Okubadejo G.O., Talcott M.R., Schmidt R.E., et al. Perils of intravascular methylprednisolone injection into the vertebral artery. An animal study. J Bone Joint Surg [Am]. 2008;90(9):1932-1938.
Rainville J., Pransky G., Indahl A., Mayer E.K. The physician as disability advisor for patients with musculoskeletal complaints. Spine. 2005;30(22):2579-2584.
Riew K.D., Yin Y., Gilula L., et al. The effect of nerve-root injections on the need for operative treatment of lumbar radicular pain. A prospective, randomized, controlled, double-blind study. J Bone Joint Surg [Am]. 2000;82(11):1589-1593.
Rubinstein S.M., van Tulder M. A best-evidence review of diagnostic procedures for neck and low-back pain. Best Pract Res Clin Rheumatol. 2008;22:471-482.
Tachihara H., Sekiguchi M., Kikuchi S., Konno S. Do corticosteroids produce additional benefit in nerve root infiltration for lumbar disc herniation? Spine. 2008;33(7):743-747.
1. Coste J., Delecoeuillerie G., Cohen de Lara A., et al. Clinical course and prognostic factors in acute low back pain: an inception cohort study in primary care practice. BMJ. 1994;308(6928):577-580.
2. Shaw W.S., Pransky G., Fitzgerald T.E. Early prognosis for low back disability: intervention strategies for health care providers. Disabil Rehabil. 2001;23(18):815-828.
3. Friedly J., Chan L., Deyo R. Increases in lumbosacral injections in the Medicare population. Spine. 2007;32:1754-6170.
4. Deyo R.A., Gray D.T., Kreuter W., et al. United States trends in lumbar fusion surgery for degenerative conditions. Spine. 2005;30:1441-1445.
5. McGeary D., Mayer T., Gatchel R. High pain ratings predict treatment failure in chronic occupational musculoskeletal disorders. J Bone Joint Surg [Am]. 2006;88:317-325.
6. Martin B.I., Deyo R.A., Mirza S.K., et al. Expenditures and health status among adults with back and neck problems. JAMA. 2008;299:656-664.
7. Chou R., Atlas S.J., Stanos S.P., Rosenquist R.W. Nonsurgical interventional therapies for low back pain: a review of the evidence for an American Pain Society Clinical Practice Guideline. Spine. 2009;34(10):1078-1093.
8. Bureau of Labor Statistics. Workplace injuries and illnesses in 1996 (USLD publication No. 97–453). Washington, DC: US Department of Labor; 1997.
9. Benzel E.C. Defining collective experience: when does wisdom take precedence? Proceedings of the Congress of Neurological Surgeons, Orlando, FL, 2008. Clin Neurosurg. 2009;56:48-53.
10. Wiesel S.W., Tsourmas N., Feffer H.L., et al. A study of computer-assisted tomography: the incidence of positive CAT scans in an asymptomatic group of patients. Spine. 1984;9(6):549-551.
11. Mayer T., Gatchel R., Kishino N., et al. Objective assessment of spine function following industrial injury. A prospective study with comparison group and one-year follow-up. Spine. 1985;10:482-493.
12. Boden S.D., Davis D.O., Dina T.S., et al. Abnormal magnetic resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg [Am]. 1990;72:403-408.
13. Rainville J., Sobel J.B., Hartigan C., Wright A. The effect of compensation involvement on the reporting of pain and disability by patients referred for rehabilitation of chronic low back pain. Spine. 1997;22(17):2016-2024.
14. Carragee E.J., Han M.Y., Suen P.W., Kim D. Clinical outcomes after lumbar discectomy for sciatica: the effects of fragment type and anular competence. J Bone Joint Surg [Am]. 2003;85:102-108.
15. Offierski C.M., Macnab I. Hip-spine syndrome. Spine. 1983;8(3):316.
16. Fogel G.R., Esses S.I. Hip spine syndrome: management of coexisting radiculopathy and arthritis of the lower extremity. Spine J. 2003;3(3):238-241.
17. Hviid Andersen J., Kaergaard A., Frost P., et al. Physical, psychosocial, and individual risk factors for neck/shoulder pain with pressure tenderness in the muscles among workers performing monotonous, repetitive work. Spine. 2002;27(6):660-667.
18. Rainville J., Pransky G., Indahl A., Mayer E.K. The physician as disability advisor for patients with musculoskeletal complaints. Spine. 2005;30(22):2579-2584.
19. Tachihara H., Sekiguchi M., Kikuchi S., Konno S. Do corticosteroids produce additional benefit in nerve root infiltration for lumbar disc herniation? Spine. 2008;33(7):743-747.
20. Goebert H.W., Jallo S.J., Gardner W.J., et al. Sciatica: treatment with epidural injections of procaine and hydrocortisone. Cleve Clin Q. 1960;27:191-197.
21. Goebert H.W., Jallo S.J., Gardner W.J., Wasmuth C.E. Painful radiculopathy treated with epidural injections of procaine and hydrocortisone acetate: results in 113 patients. Anesth Analg. 1961;40:130-134.
22. Stitz M.Y., Sommer H.M. Accuracy of blind versus fluoroscopically guided caudal epidural injection. Spine. 1999;24(13):1371-1376.
23. Riew K.D., Park J.B., Cho Y.S., et al. Nerve root blocks in the treatment of lumbar radicular pain. A minimum 5-year follow-up. J Bone Joint Surg [Am]. 2006;88(8):1722-1725.
24. Hogan Q.H. Epidural anatomy examined by cryomicrotome section. Influence of age, vertebral level, and disease. Reg Anesth. 1996;21(5):395-406.
25. McGoldrick K.E. Cervical and high thoracic ligamentum flavum frequently fails to fuse in the midline. Surv Anesth. 2004;48(3):151.
26. Abbasi A., Malhotra G., Malanga G., et al. Complications of interlaminar cervical epidural steroid injections: a review of the literature. Spine. 2007;32(19):2144-2151.
27. Cluff R., Mehio A.K., Cohen S.P., et al. The technical aspects of epidural steroid injections: a national survey. Anesth Analg. 2002;95(2):403-408.
28. Fredman B., Nun M.B., Zohar E., et al. Epidural steroids for treating “failed back surgery syndrome”: is fluoroscopy really necessary? Anesth Analg. 1999;88:367-372.
29. Valat J.P., Giraudeau B., Rozenberg S., et al. Epidural corticosteroid injections for sciatica: a randomised, double blind, controlled clinical trial. Ann Rheum Dis. 2003;62(7):639-643.
30. Riew K.D., Yin Y., Gilula L., et al. The effect of nerve-root injections on the need for operative treatment of lumbar radicular pain. A prospective, randomized, controlled, double-blind study. J Bone Joint Surg [Am]. 2000;82(11):1589-1593.
31. Okubadejo G.O., Talcott M.R., Schmidt R.E., et al. Perils of intravascular methylprednisolone injection into the vertebral artery. An animal study. J Bone Joint Surg [Am]. 2008;90(9):1932-1938.
32. Karppinen J., Ohinmaa A., Malmivaara A., et al. Cost effectiveness of periradicular infiltration for sciatica: subgroup analysis of a randomized controlled trial. Spine. 2001;26(23):2587-2595.
33. Schaufele M.K., Hatch L., Jones W. Interlaminar versus transforaminal epidural injections for the treatment of symptomatic lumbar intervertebral disc herniations. Pain Physician. 2006;9(4):361-366.
34. Goldthwait J.E. The lumbosacral articulation: an explanation of many cases of lumbago, sciatica and paraplegia. Boston Med Surg J. 1911;164:365-372.
35. McLain R.F., Pickar J.G. Mechanoreceptor endings in human thoracic and lumbar facet joints. Spine. 1998;23:168-173.
36. Giles L.G.F., Taylor J.R. Human zygapophyseal joint capsule and synovial fold innervation. Br J Rheumatol. 1987;26:93-98.
37. Gronblad M., Weinstein J.N., Santavirta S. Immunohistochemical observations on spinal tissue innervation. Acta Orthop Scand. 1991;62:614-622.
38. Hirsch D., Inglemark B., Miller M. The anatomical basis for low back pain. Acta Orthop Scand. 1963;33:1-17.
39. Mooney V., Robertson J. The facet syndrome. Clin Orthop Relat Res. 1976;115:149-156.
40. Mooney V. The syndromes of low back disease. Orthop Clin North Am. 1983;14(3):505-515.
41. Schwarzer A.C., Aprill C., Derby R., et al. Clinical features of patients with pain stemming from the lumbar zygapophysial joints. Is the lumbar facet syndrome a clinical entity? Spine. 1994;19:1132-1137.
42. Manchikanti L., Pampati V.S., Pakanati R.R., et al. Prevalence of facet joint pain in chronic low back pain. Pain Physician. 1999;2:59-64.
43. Schwarzer A.C., Aprill C.N., Derby R., et al. The relative contributions of the disc and zygapophyseal joint in chronic low back pain. Spine. 1994;19:801-806.
44. Eubanks J.D., Lee M.J., Cassinelli E., Ahn N.U. Prevalence of lumbar facet arthrosis and its relationship to age, sex, and race: an anatomic study of cadaveric specimens. Spine. 2007;32(19):2058-2062.
45. Bogduk N. Low back pain: clinical anatomy of lumbar spine and sacrum, ed 4. New York: Ch Bogduk urchill Livingstone; 2005. pp 183–216
46. Marks R.C., Houston T., Thulbourne T. Facet joint injection and facet nerve block: a randomised comparison in 86 patients with chronic low back pain. Pain. 1992;49:325-328.
47. Nash T.P. Facet joints—intra-articular steroids or nerve block? Pain Clinic. 1989;3:77-82.
48. Revel M., Poiraudeau S., Auleley G.R., et al. Capacity of the clinical picture to characterize low back pain relieved by facet joint anesthesia: proposed criteria to identify patients with painful facet joints. Spine. 1998;23:1972-1977.
49. Schwarzer A.C., Aprill C.N., Derby R., et al. The false-positive rate of uncontrolled diagnostic blocks of the lumbar zygapophysial joints. Pain. 1994;58:195-200.
50. Schwarzer A.C., Wang S.C., Bogduk N., et al. Prevalence and clinical features of lumbar zygapophysial joint pain: a study in an Australian population with chronic low back pain. Ann Rheum Dis. 1995;54:100-106.
51. Dreyfuss P., Halbrook B., Pauza K., et al. Efficacy and validity of radiofrequency neurotomy for chronic lumbar zygapophysial joint pain. Spine. 2000;25:1270-1277.
52. Lord S.M., Barnsley L., Wallis B.J., et al. Percutaneous radio-frequency neurotomy for chronic cervical zygapophyseal-joint pain. N Engl J Med. 1996;335(23):1721-1726.
53. Dreyfuss P., Schwarzer A.C., Lau P., Bogduk N. Specificity of lumbar medial branch and L5 dorsal ramus blocks: a computed tomography study. Spine. 1997;22:895-902.
54. Revel M., Poiraudeau S., Auleley G.R., et al. Capacity of the clinical picture to characterize low back pain relieved by facet joint anesthesia: proposed criteria to identify patients with painful facet joints. Spine. 1998;23:1972-1977.
55. Sehgal N., Dunbar E.E., Shah R.V., Colson J. Systematic review of diagnostic utility of facet (zygapophysial) joint injections in chronic spinal pain: an update. Pain Physician. 2007;10:213-228.
56. West S., King V., Carey T., et al. Systems to rate the strength of scientific evidence. Evidence Report/Technology Assessment No. 47. April 2002. University of North Carolina: Agency for Healthcare Research and Quality, AHRQ Publication No. 02-E016
57. Whiting P., Rutjes A., Reitsma J., Bossuyt P., Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. 2003;3:25.
58. Boswell M.V., Singh V., Staats P.S., Hirsch J.A. Accuracy of precision diagnostic blocks in the diagnosis of chronic spinal pain of facet or zygapophysial joint origin: a systematic review. Pain Physician. 2003;6:449-456.
59. Rubinstein S.M., van Tulder M. A best-evidence review of diagnostic procedures for neck and low-back pain. Best Pract Res Clin Rheumatol. 2008;22:471-482.
60. Ackerman W.E., Munir M.A., Zhang J.M., Ghaleb A. Are diagnostic lumbar facet injections influenced by pain of muscular origin? Pain Pract. 2004;4:286-291.
61. Manchikanti L., Pampati V., Damron K.S., et al. The effect of sedation on diagnostic validity of facet joint nerve blocks: an evaluation to assess similarities in population with involvement in cervical and lumbar regions. Pain Physician. 2006;9:47-52.
62. Cohen S.P., Williams K.A., Kurihara C., et al. Multicenter, randomized, comparative cost-effectiveness study comparing 0, 1, and 2 diagnostic medial branch (facet joint nerve) block treatment paradigms before lumbar facet radiofrequency denervation. Anesthesiology. 2010;113(2):395-405.
63. Shealy C.N. Percutaneous radiofrequency denervation of spinal facets. Treatment for chronic back pain and sciatica. J Neurosurg. 1975;43(4):448-451.
64. Kornick C., Kramarich S.S., Lamer T.J., et al. Complications of lumbar facet radiofrequency denervation. Spine. 2004;29:1352-1354.
65. Slipman C.W., Bhat A.L., Gilchrist R.V., et al. A critical review of the evidence for the use of zygapophysial injections and radiofrequency denervation in the treatment of low back pain. Spine J. 2003;3(4):310-316.
66. Bigos S.J., Boyer O.R., Braen G.R., et al. Clinical practice guideline number 4: acute low back problems in adults, AHCPR publication 95–0642. Rockville, MD: Agency for Health Care Policy and Research, Public Health Service, US Department of Health and Human Services; December 1994.
67. Geurts J.W., van Wijk R.M., Stolker R.J., et al. Efficacy of radiofrequency procedures for the treatment of spinal pain: a systematic review of randomized clinical trials. Reg Anesth Pain Med. 2001;26:394-400.
68. Niemisto L., Kalso E., Malmivaara A., et al. Radiofrequency denervation for neck and back pain: a systematic review within the framework of the Cochrane collaboration back review group. Spine. 2003;28:1877-1888.
69. Slipman C.W., Jackson H.B., Lipetz J.S., et al. Sacroiliac joint pain referral zones. Arch Phys Med Rehabil. 2000;81:334-338.
70. Holm S., Indahl A., Solomonow M. Sensorimotor control of the spine. J Electromyogr Kinesiol. 2002;12:219-234.
71. Grob K.R., Neuhuber W.L., Kissling R. Die Innervation des Sacroiliacalgelenkes beim Menschen. Z Rheumatol. 1995;54:117-122.
72. Dreyfuss P., Henning T., Malladi N., et al. The ability of multi-site, multi-depth sacral lateral branch blocks to anesthetize the sacroiliac joint complex. Pain Med. 2009;10(4):679-688.
73. Vilensky J.A., O’Connor B.L., Fortin J.D., et al. Histologic analysis of neural elements in the human sacroiliac joint. Spine. 2002;27:1202-1207.
74. Fortin J.D., Dwyer A.P., West S., Pier J. Sacroiliac joint: pain referral maps upon applying a new injection/arthrography technique. Part I: asymptomatic volunteers. Spine. 1994;19:1475-1482.
75. van der Wurff P., Buijs E.J., Groen G.J. A multi-test regimen of pain provocation tests as an aid to reduce unnecessary minimally invasive sacroiliac joint procedures. Arch Phys Med Rehabil. 2006;87(1):10-14.
76. Fortin J.D., Falco F.J. The Fortin finger test: an indicator of sacroiliac pain. Am J Orthop. 1997;26(7):477-480.
77. Berthelot J.M., Labat J.J., Le Goff B., et al. Provocative sacroiliac joint maneuvers and sacroiliac joint block are unreliable for diagnosing sacroiliac joint pain. Joint Bone Spine. 2006;73(1):17-23.
78. Zelle B.A., Gruen G.S., Brown S., et al. Sacroiliac joint dysfunction: evaluation and management. Clin J Pain. 2005;21(5):446-455.
79. McKenzie-Brown A.M., Shah R.V., Sehgal N., Everett C.R. A systematic review of sacroiliac joint interventions. Pain Physician. 2005;8:115-126.
80. Hansen H.C., McKenzie-Brown A.M., Cohen S.P. Sacroiliac joint interventions: a systematic review. Pain Physician. 2007;10:165-184.
81. Loh L., Nathan P.W. Painful peripheral states and sympathetic blocks. J Neurol Neurosurg Psychiatry. 1978;41:664-667.
82. Raj P.P. Stellate ganglion block. In: Waldman S.D., Winne A.P., editors. Interventional pain management. Philadelphia: Dannemiller Education, WB Saunders; 1996:138-141.
83. Sachs B.L., Vanharanta H., Spivey M.A., et al. Dallas discogram description. A new classification of CT/discography in low-back disorders. Spine. 1987;12(3):287-294.
84. Vanharanta H., Sachs B.L., Ohnmeiss D.D., et al. Pain provocation and disc deterioration by age: a CT/discography study in a low-back pain population. Spine. 1989;14:420-423.
85. Carragee E.J., Lincoln T., Parmar V.S., et al. A gold standard evaluation of the “discogenic pain” diagnosis as determined by provocative discography. Spine. 2006;31:2115-2123.
86. Carragee E.J., Tanner C.M., Khurana S., et al. The rates of false-positive lumbar discography in select patients without low back symptoms. Spine. 2000;25:1373-1380.
87. DeBerard M.S., Masters K.S., Colledge A.L., et al. Outcomes of posterolateral lumbar fusion in Utah patients receiving workers’ compensation: a retrospective cohort study. Spine. 2001;26:738-746.
88. Madan S., Gundanna M., Harley J.M., et al. Does provocative discography screening of discogenic back pain improve surgical outcome? J Spinal Disord Tech. 2002;15:245-251.
89. Chou R., Loeser J.D., Owens D.K., et al. American Pain Society Low Back Pain Guideline Panel. Interventional therapies, surgery, and interdisciplinary rehabilitation for low back pain: an evidence-based clinical practice guideline from the American Pain Society. Spine. 2009;34(10):1066-1077.
90. Nguyen T.H., Randolph D.C., Talmage J., et al. Long-term outcomes of lumbar fusion among workers’ compensation subjects: a historical cohort study. Spine. 2011;36(4):320-331.
91. Carragee E.J., Alamin T.F., Miller J.L., et al. Discographic, MRI and psychosocial determinants of low back pain disability and remission: a prospective study in subjects with benign persistent back pain. Spine J. 2005;5:24-35.
92. Carragee E.J., Don A.S., Hurwitz E.L., et al. 2009 ISSLS prize winner: does discography cause accelerated progression of degeneration changes in the lumbar disc: a 10-year matched cohort study. Spine (Phila Pa 1976). 2009;34:2338-2345.
93. Ohtori S., Kinoshita T., Yamashita M., et al. Results of surgery for discogenic low back pain: a randomized study using discography versus discoblock for diagnosis. Spine. 2009;34(13):1345-1348.
94. Barendse G.A., van Den Berg S.G., Kessels A.H., et al. Randomized controlled trial of percutaneous intradiscal radiofrequency thermocoagulation for chronic discogenic back pain: lack of effect from a 90-second 70 C lesion. Spine. 2001;26:287-292.
95. Ercelen O., Bulutcu E., Oktenoglu T., et al. Radiofrequency lesioning using two different time modalities for the treatment of lumbar discogenic pain: a randomized trial. Spine. 2003;28:1922-1927.
96. Pauza K.J., Howell S., Dreyfuss P., et al. A randomized, placebo controlled trial of intradiscal electrothermal therapy for the treatment of discogenic low back pain. Spine J. 2004;4:27-35.
97. Saal J.S., Saal J.A. Management of chronic discogenic low back pain with a thermal intradiscal catheter. A preliminary report. Spine. 2000;25:382-388.
98. Derby R., Baker R.M., Lee C.H., Anderson P.A. Evidence-informed management of chronic low back pain with intradiscal electrothermal therapy. Spine J. 2008;8:80-95.
99. Saal J.A., Saal J.S. Intradiscal electrothermal treatment for chronic discogenic low back pain. A prospective outcome study with a minimum 1-year follow-up. Spine. 2000;25:2622-2627.
100. Freeman B.J., Fraser R.D., Cain C.M., et al. A randomized, doubleblind, controlled trial: intradiscal electrothermal therapy versus placebo for the treatment of chronic discogenic low back pain. Spine. 2005;30:2369-2377.
101. Andersson G.B., Mekhail N.A., Block J.E. Treatment of intractable discogenic low back pain. A systematic review of spinal fusion and intradiscal electrothermal therapy (IDET). Pain Physician. 2006;9:237-248.
102. Kapural L., Hayek S., Malak O., et al. Intradiscal thermal annuloplasty versus intradiscal radiofrequency ablation for the treatment of discogenic pain: a prospective matched control trial. Pain Med. 2005;6:425-431.
103. Kapural L., Ng A., Dalton J., et al. Intervertebral disc biacuplasty for the treatment of lumbar discogenic pain: results of a 6-month follow-up. Pain Med. 2008;9(1):60-67.
104. Manchikanti L., Derby R., Benyamin R.M., et al. A systematic review of mechanical lumbar disc decompression with nucleoplasty. Pain Physician. 2009;12(3):561-572.
105. Chatterjee S., Foy P.M., Findlay G.F. Report of a controlled clinical trial comparing automated percutaneous lumbar discectomy and microdiscectomy in the treatment of contained lumbar disc herniation. Spine. 1995;20:734-738.
106. Singh V., Benyamin R.M., Datta S., et al. Systematic review of percutaneous lumbar mechanical disc decompression utilizing Dekompressor. Pain Physician. 2009;12(3):589-599.
107. Singh V., Manchikanti L., Benyamin R.M., et al. Percutaneous lumbar laser disc decompression: a systematic review of current evidence. Pain Physician. 2009;12(3):573-588.
108. Boos N., Semmer N., Elfering A., et al. Natural history of individuals with asymptomatic disc abnormalities in magnetic resonance imaging: predictors of low back pain-related medical consultation and work incapacity. Spine. 2000;25:1484-1492.
109. Schenk P., Laubli T., Hodler J., et al. Magnetic resonance imaging of the lumbar spine: findings in female subjects from administrative and nursing professions. Spine. 2006;31(23):2701-2706.
110. Carragee E., Alamin T., Cheng I., et al. Are first-time episodes of serious LBP associated with new MRI findings? Spine J. 2006;6(6):624-635.
111. Chou R., Loeser J.D., Owens D.K., et al. Interventional therapies, surgery, and interdisciplinary rehabilitation for low back pain: an evidence-based clinical practice guideline from the American Pain Society. Spine. 2009;34(10):1066-1077.
112. Leonardi M., Pfirrmann C., Boos N. Injection studies in spinal disorders. Clin Orthop Relat Res. 2006;443:168-182.
113. Yeom J.S., Lee J.W., Park K.W., et al. Value of diagnostic lumbar selective nerve root block: a prospective controlled study. Am J Neuroradiol. 2008;29(5):1017-1023.
114. Saal J.S. General principles of diagnostic testing as related to painful lumbar spine disorders: a critical appraisal of current diagnostic techniques. Spine. 2002;27:2538-2545.
115. Sasso R.C., Macadaeg K., Nordmann D., et al. Selective nerve root injections can predict surgical outcome for lumbar and cervical radiculopathy: comparison to magnetic resonance imaging. J Spinal Disord Tech. 2005;18:471-478.
116. Corning J.L. Spinal analgesia and local medication of the cord. NY Med J. 1885;42:483-485.
117. Wang J.K., Nauss L.A., Thomas J.E. Pain relief by intrathecally applied morphine in man. Anesthesiology. 1979;50:149-151.
118. Taricco M., Pagliacci M.C., Telaro E., Adone R. Pharmacological interventions for spasticity following spinal cord injury: results of a Cochrane systemic review. Eur Medicophysica. 2006;42:5-15.
119. Dougherty P.M., Staats P.S. Intrathecal drug therapy for chronic pain: from basic science to clinical practice. Anesthesiology. 1999;91:1891-1918.
120. Hassenbusch S.J., Stanton-Hicks M., Covington E.C., et al. Long-term intraspinal infusions of narcotics and treatment of neuropathic pain. J Pain Symptom Manage. 1995;10:527-543.
121. Portenoy R.K. Opioid therapy for chronic nonmalignant pain: current status. In: Fields H.L., Liebeskind J.C., editors. Progress in pain research and management. Seattle: IASP Press; 1994:247-287.
122. Wallace M.S. Treatment options for refractory pain: the role of intrathecal therapy. Neurology. 2002;59(suppl):18-24.
123. Penn R.D. Intrathecal medication delivery. Neurosurg Clin North Am. 2003;14:381-387.
124. Miele V.J., Price K.O., Bloomfield S., et al. A review of intrathecal morphine therapy related granulomas. Eur J Pain. 2006;10:251-261.
125. Melzack R., Wall P.D. Pain mechanisms: a new theory. Science. 1965;150(3699):971-979.
126. Shealy C.N. Percutaneous radiofrequency denervation of spinal facets. J Neurosurg. 1975;43:448-451.
127. North R.B., Kidd D.H., Petrucci L., et al. Spinal cord stimulation electrode design: a prospective, randomized, controlled trial comparing percutaneous with laminectomy electrodes: part II—clinical outcomes. Neurosurgery. 2005;57:990-996.