Chapter 99 Interventional Device Therapy in Atrial Fibrillation
Thromboembolism is the most feared complication in patients with permanent atrial fibrillation (AF).1 The risk of embolism may vary from less than 1% to 20%, depending on the underlying disease. Obviously, patients with previous strokes have the highest risk of embolism. Trans-esophageal echocardiographic (TEE) studies show that most thrombi are located in the left atrial appendage (LAA) and that only a few thrombi originate in the left atrium itself.2,3 Although prospective and randomized studies show that oral anticoagulation (OAC) reduces the risk of thromboembolism significantly, patients often may not receive OAC because of bleeding complications, increased bleeding risk, allergies to warfarin derivates, or fear of complications.4,5 Occluding the LAA is another way to reduce the risk of thromboembolism. Hence, the LAA is often occluded or removed in patients undergoing cardiac surgery with concomitant AF. Surgical obliteration of the LAA in patients with a high risk for thromboembolism is a well-established procedure to eliminate this predilection site for the development of thrombi.6–8
In 2001, an alternative procedure was introduced for patients with contraindications for OAC, that is, the interventional occlusion of the LAA.9–11 This chapter provides information on the indication, technique, safety, results, imaging, and potential future applications of percutaneous LAA occlusion.
Indication
Oral anticoagulation is contraindicated for at least an estimated 30% of patients with AF. Furthermore, it has been shown that contraindications for OAC are particularly common in older patients.5 Interventional occlusion of the LAA is suggested for patients with a high risk of embolism and a contraindication for OAC. Often the so-called CHADS2 score is used for defining the risk of embolism.
Devices and Implantation Techniques
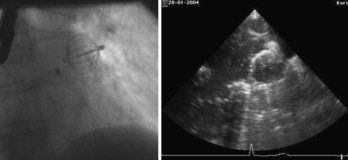
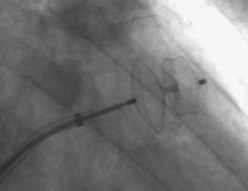
The first device for the interventional occlusion of the LAA was introduced by Lesh and co-workers (animal studies).9 The device was called PLAATO (percutaneous left atrial appendage transcatheter occlusion). It consists of a self-expanding, balloon-shaped nitinol cage with an expanded polytetrafluoroethylene (ePTFE) membrane with three rows of anchors stabilizing the device in the appendage (Figure 99-1). The membrane covers the atrial surface of the device, whereas the opposite surface is not covered, which allows secondary thrombosis of the lumen by the device. The morphology of the LAA is evaluated by angiography and simultaneous TEE. The maximal diameter of the LAA orifice is determined by both methods and a mean diameter obtained. A 20% to 30% oversized occlusion device is selected. The device is introduced via a 14-F femoral vein sheath and advanced to the LAA after trans-septal puncture of the atrial septum. Then, the device is expanded and the position is controlled using both angiography and TEE (Figure 99-2). The mean size of the devices used is 29 mm. After verifying the optimal position and adequate sizing of the device, it is released and the final position assessed by repeat angiography.
A similar device, called Watchman device, was introduced by Atritech (Plymouth, MN) (Figure 99-3).12 It is similar to the PLAATO device and consists of a self-expanding nickel-titanium frame structure with external fixation barbs and a permeable polyester fabric cover (sizes ranging from 21 to 33 mm). The procedure of implanting the device is similar to that of the implantation of the PLAATO device.
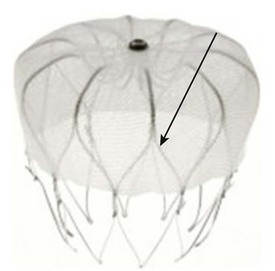
FIGURE 99-3 Watchman device (Atritech) with a self-expanding nitinol frame structure with external fixation barbs.
Meier and coworkers published a study on the successful occlusion of the LAA using an Amplatzer device, which was not designed uniquely for this purpose.13 In December 2008, AGA Medical (Plymouth, MN) received the European Conformite Europeene mark of approval for an LAA occlusion device that is different from the previously described ones. It is called a cardiac plug and is designed to achieve optimal occlusion of the LAA; it consists of a distal lobe and disc that is designed to cover the orifice of the LAA (Figure 99-4). The diameter of the lobe ranges from 16 to 30 mm, and the corresponding disc diameter ranges from 20 to 36 mm.
Safety and Results
In various studies, data about the safety and success of LAA occlusion were collected for both the PLAATO device (Figure 99-5) and the Watchman device.12,14
The researchers evaluating the PLAATO device showed that the procedure is feasible and may be performed within 2 hours.13 Detailed results concerning 111 patients with attempted LAA occlusion were published in 2005.13 The procedure was successful in 97% of the patients. Migration of the device or formation of mobile thrombi on the surface of the device were not observed during follow-up 10 months later. One of the patients in the study group died because of procedural complications. Pericardiocentesis was performed in three patients for cardiac tamponade. In addition, one patient developed left-sided hemothorax. Hence, relevant procedural complications were not uncommon. However, they occurred less frequently with increasing experience of the operator.
Two patients had a stroke, and two patients experienced transient ischemic attacks during the observation period. No thrombi formed on the device in any of the patients. Another study followed up the patients closely via echocardiography and showed that the implant was covered with a neointima after 6 months and that the device did not alter pulmonary vein flow.11 Furthermore, no significant atrial septal defects were detected after the puncture of the intra-atrial septum with an F-14 sheath.15 Because of the high rate of complications, a randomized study comparing anticoagulation and device therapy was requested. Such a study has not been performed yet, and the company has halted distribution of the device. Long-term follow-up data have not systematically been published yet.16–18
Similar experiences were reported for the Watchman device from Atritech. Sick et al provided information on 75 patients who had the device implanted.12 A failure rate of 10% was reported for implantation of the device. Two patients experienced device embolization. The devices were successfully retrieved percutaneously in both cases. Two cardiac tamponades and one air embolism were reported as well. One device had a wire fracture and needed to be explanted surgically. In four patients, flat thrombi had formed on the atrial surface of the device by the time of the 6-month echocardiographic follow-up. Two neurologic complications occurred during the follow-up period. To assess how effectively strokes and bleeding events are reduced in patients with AF by occluding the LAA with the Watchman device, the prospective Embolic Protection in Patients with Atrial Fibrillation (PROTECT-AF) study was performed in patients with nonvalvular AF and a CHADS2 score greater than 1.19 It is a key study for evaluating the concept of LAA occlusion for the prevention of thromboembolism in patients with AF, and hence the study will be discussed in detail in this chapter.
Other studies on LAA occlusion included far fewer patients. Meier et al provided information on the use of the Amplatzer device for occluding the LAA in 16 patients.13 One patient had an embolization of the device. All other patients had no serious complications.
Other devices are currently being assessed in animal models. Fumoto et al reported on the successful implantation of a third-generation atrial exclusion device in 14 dogs.20 Jayakar et al investigated a tissue welding technology to obliterate the LAA.21
Importantly, long-term data or data from randomized prospective trials are not available yet.
Imaging
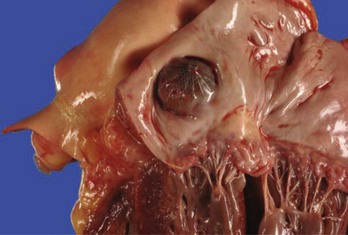
Imaging of the left atrium and the LAA is very important for planning and performing LAA occlusion. In addition, potential thrombi in the LAA need to be excluded. The anatomies of the left atrium and the LAA are complex and varies considerably as shown by anatomic studies.22,23 The LAA orifice is usually oval in shape and averages 17.4 mm.23 Adjacent structures are the left upper PV (11 mm) and the mitral valve (10.7 mm). Histologic examination of the LAA shows that the wall is very thin in places, which explains the high risk of perforation during the procedure. Hence, visual guidance of the procedure is of extreme importance.
Multiple-plane TEE has been shown to exclude left atrial thrombi very accurately and should be performed in all patients considered for LAA occlusion.24–27 Both TEE and intracardiac echocardiography may be used to guide the procedure for the following purposes28,29:
Computed tomography (CT) has been used for analyzing the size and morphology of the LAA. However, measurements vary considerably between TEE and CT.30 Hence, further studies are needed to determine whether CT may be used alternatively for device sizing prior to the procedure.
Summary
Multiple reports have shown that LAA occlusion is technically feasible. However, a considerable risk in employing the procedure still exists.31 Potentially, increased operator experience, better patient selection, and improvement of technology will lead to a decrease in the complication rate. Hence, patient selection for the procedure is very important. Importantly, the randomized PROTECT-AF study demonstrated the non-inferiority of LAA occlusion with the Watchman device in preventing stroke compared with treatment with warfarin. Nevertheless, ideal candidates for LAA occlusion have contraindications for long-term OAC, or these patients may refuse to take OAC. In any case, the risk and benefits have to be carefully weighted.
Modern anticoagulants are currently being developed and tested clinically. These medications may have fewer side effects and a lower bleeding rate than do the current oral anticoagulants, as recently shown in the Randomized Evaluation of Long-Term Anticoagulation Therapy (RELY) study.32 Therefore, clinicians will have to observe these developments carefully and decide accordingly.
In conclusion, LAA occlusion devices are an important alternative to OAC in patients with AF.
Key References
Agmon Y, Khandheria BK, Gentile F, Seward JB. Echocardiographic assessment of the left atrial appendage. J Am Coll Cardiol. 1999;34:1867-1877.
Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg. 1996;61:755-759.
Meier B, Palacios I, Windecker S, et al. Transcatheter left atrial appendage occlusion with Amplatzer devices to obviate anticoagulation in patients with atrial fibrillation. Catheter Cardiovasc Interv. 2003;60(3):417-422.
Nakai T, Lesh MD, Gerstenfeld EP, et al. Percutaneous Left Atrial Appendage Occlusion (PLAATO) for preventing cardioembolism: First experience in canine model. Circulation. 2002;105:2217-2222.
Omran H, Hardung D, Schmidt H, et al. Mechanical occlusion of the left atrial appendage. J Cardiovasc Electrophysiol. 2003;14(9 Suppl):S56-S59.
Omran H, Jung W, Rabahieh R, et al. Echocardiographic predictors of maintenance of sinus rhythm after internal atrial defibrillation. Am J Cardiol. 1998;81:1446-1449.
Omran H, Jung W, Rabahieh R, et al. Imaging of thrombi and assessment of left atrial appendage function: A prospective study comparing transthoracic and transoesophageal echocardiography. Heart. 1999;81:192-198.
Ostermayer SH, Reisman M, Kramer PH, et al. Percutaneous left atrial appendage transcatheter occlusion (PLAATO system) to prevent stroke in high-risk patients with non-rheumatic atrial fibrillation: Results from the international multi-center feasibility trials. J Am Coll Cardiol. 2005;46(1):9-14.
Poller L, Jespersen J, Ibrahim S. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(27):2673-2674.
Sick PB, Schuler G, Hauptmann KE, et al. Initial worldwide experience with the WATCHMAN left atrial appendage system for stroke prevention in atrial fibrillation. J Am Coll Cardiol. 2007;49(13):1490-1495.
Sievert H, Lesh MD, Trepels T, et al. Percutaneous Left Atrial Appendage Transcatheter Occlusion to prevent stroke in high-risk patients with atrial fibrillation. Circulation. 2002;105:1887-1889.
Stöllberger C, Schneider B, Finsterer J. Serious complications from dislocation of a Watchman left atrial appendage occluder. J Cardiovasc Electrophysiol. 2007;18(8):880-881.
Stroke Prevention in Atrial Fibrillation Investigators. Adjusted-dose warfarin versus low-intensity, fixed dose warfarin plus aspirin for high-risk patients with atrial fibrillation: Stroke Prevention in Atrial Fibrillation III randomized clinical trial. Lancet. 1996;348:633-638.
Sudlow M, Thomson R, Thwaites B, et al. Prevalence of atrial fibrillation and eligibility for anticoagulants in the community. Lancet. 1998;352:1167-1171.
1 Albers GW, Dalen JE, Laupacis A, et al. Antithrombotic therapy in atrial fibrillation. Chest. 2001;119:194S-206S.
2 Mügge A, Kühn H, Nikutta P, et al. Assessment of left atrial appendage function by biplane transesophageal echocardiography in patients with nonrheumatic atrial fibrillation: Identification of a subgroup of patients at increased embolic risk. J Am Coll Cardiol. 1994;23:599-607.
3 Fatkin D, Kelly RP, Fenely MP. Relations between left atrial appendage blood flow velocity, spontaneous echocardiographic contrast and thromboembolic risk in vivo. J Am Coll Cardiol. 1994;23:961-969.
4 Stroke Prevention in Atrial Fibrillation Investigators. Adjusted-dose warfarin versus low-intensity, fixed dose warfarin plus aspirin for high-risk patients with atrial fibrillation: Stroke Prevention in Atrial Fibrillation III randomized clinical trial. Lancet. 1996;348:633-638.
5 Sudlow M, Thomson R, Thwaites B, et al. Prevalence of atrial fibrillation and eligibility for anticoagulants in the community. Lancet. 1998;352:1167-1171.
6 Johnson WD, Ganjoo AK, Stone CD, et al. The left atrial appendage: Our most lethal human attachment: Surgical implications. Eur J Cardiothorac Surg. 2000;17:718-722.
7 Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg. 1996;61:755-759.
8 Healey JS, Crystal E, Lamy A, et al. Left Atrial Appendage Occlusion Study (LAAOS): Results of a randomized controlled pilot study of left atrial appendage occlusion during coronary bypass surgery in patients at risk for stroke. Am Heart J. 2005;150(2):288-293.
9 Nakai T, Lesh MD, Gerstenfeld EP, et al. Percutaneous Left Atrial Appendage Occlusion (PLAATO) for preventing cardioembolism: First experience in canine model. Circulation. 2002;105:2217-2222.
10 Sievert H, Lesh MD, Trepels T, et al. Percutaneous left atrial appendage transcatheter occlusion to prevent stroke in high-risk patients with atrial fibrillation. Circulation. 2002;105:1887-1889.
11 Omran H, Hardung D, Schmidt H, et al. Mechanical occlusion of the left atrial appendage. J Cardiovasc Electrophysiol. 2003;14(9 Suppl):S56-S59.
12 Sick PB, Schuler G, Hauptmann KE, et al. Initial worldwide experience with the WATCHMAN left atrial appendage system for stroke prevention in atrial fibrillation. J Am Coll Cardiol. 2007;49(13):1490-1495.
13 Meier B, Palacios I, Windecker S, et al. Transcatheter left atrial appendage occlusion with Amplatzer devices to obviate anticoagulation in patients with atrial fibrillation. Catheter Cardiovasc Interv. 2003;60(3):417-422.
14 Ostermayer SH, Reisman M, Kramer PH, et al. Percutaneous left atrial appendage transcatheter occlusion (PLAATO system) to prevent stroke in high-risk patients with non-rheumatic atrial fibrillation: Results from the international multi-center feasibility trials. J Am Coll Cardiol. 2005;46(1):9-14.
15 Hanna IR, Kolm P, Martin R, et al. Left atrial structure and function after percutaneous left atrial appendage transcatheter occlusion (PLAATO): Six-month echocardiographic follow-up. J Am Coll Cardiol. 2004;43(10):1868-1872.
16 Onalan O, Crystal E. Left atrial appendage exclusion for stroke prevention in patients with nonrheumatic atrial fibrillation. Stroke. 2007;38(2 Suppl):624-630.
17 Bayard YL, Ostermayer SH, Hein R, et al. Percutaneous devices for stroke prevention. Cardiovasc Revasc Med. 2007;8(3):216-225.
18 El-Chami MF, Grow P, Eilen D, et al. Clinical outcomes three years after PLAATO implantation. Catheter Cardiovasc Interv. 2007;69(5):704-707.
19 Holmes DR, Reddy VY, Tri ZG, et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: A randomized non-inferiority trial. Lancet. 2009;374:534-542.
20 Fumoto H, Gillinov AM, Ootaki Y, et al. A novel device for left atrial appendage exclusion: The third-generation atrial exclusion device. J Thorac Cardiovasc Surg. 2008;136(4):1019-1027.
21 Jayakar D, Gozo F, Gomez E, Carlos C. Use of tissue welding technology to obliterate left atrial appendage—novel use of ligature. Interact Cardiovasc Thorac Surg. 2005;4(4):372-373.
22 Agmon Y, Khandheria BK, Gentile F, Seward JB. Echocardiographic Assessment of the left atrial appendage. J Am Coll Cardiol. 1999;34:1867-1877.
23 Su P, McCarthy KP, Ho SY. Occluding the left atrial appendage: Anatomical considerations. Heart. 2008;94(9):1113-1116.
24 Omran H, Jung W, Rabahieh R, et al. Imaging of thrombi and assessment of left atrial appendage function: A prospective study comparing transthoracic and transoesophageal echocardiography. Heart. 1999;81:192-198.
25 Omran H, Jung W, Rabahieh R, et al. Echocardiographic predictors of maintenance of sinus rhythm after internal atrial defibrillation. Am J Cardiol. 1998;81:1446-1449.
26 Yao S, Meisner JS, Factor SM, et al. Assessment of left atrial appendage structure and function by transesophageal echocardiography: A review. Echocardiography. 1998;15:243-255.
27 Mráz T, Neuzil P, Mandysová E, et al. Role of echocardiography in percutaneous occlusion of the left atrial appendage. Echocardiography. 2007;24(4):401-404.
28 Jorgensen J, Palmer S, Kalogeropoulos A, et al. Implantation of left atrial appendage occlusion devices and complex appendage anatomy: The importance of transesophageal echocardiography. Echocardiography. 2007;24(2):159-161.
29 Ho IC, Neuzil P, Mraz T, et al. Heart Rhythm. Use of intracardiac echocardiography to guide implantation of a left atrial appendage occlusion device (PLAATO). Heart Rhythm. 2007;4(5):567-571.
30 Budge LP, Shaffer KM, Moorman JR, et al. Analysis of in vivo left atrial appendage morphology in patients with atrial fibrillation: A direct comparison of transesophageal echocardiography, planar cardiac CT, and segmented three-dimensional cardiac CT. J Interv Card Electrophysiol. 2008;23(2):87-93.
31 Stöllberger C, Schneider B, Finsterer J. Serious complications from dislocation of a Watchman left atrial appendage occluder. J Cardiovasc Electrophysiol. 2007;18(8):880-881.
32 Poller L, Jespersen J, Ibrahim S. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(27):2673-2674.