Chapter 98 Interstitial and LINAC-Radiosurgery for Brain Metastases∗
Brain metastases are diagnosed in approximately 20% to 40% of patients with neoplastic diseases1–3 and thereby represent the most common intracranial malignancy, with lung, breast, and renal cancers, and melanoma as the most frequent primary tumors.4,5 The incidence of brain metastatic lesions appears to be increasing, possibly due to an aging population, improved neuroimaging techniques, and more efficient treatment of the systemic disease, leading to a growing group of patients presenting multiple lesions within the central nervous system at the time of diagnosis. Brain metastatic tumors are predominantly encountered supratentorially within the cerebral hemispheres, followed by cerebellar and brain stem localization.6 Despite the significant improvement of the management of intracranial metastases in the last decades, the overall prognosis remains relatively poor. Thorough assessment of the individual prognosis is, therefore, required to offer the best possible care while avoiding unnecessary and possibly debilitating treatment. The extensive analysis of various demographic and clinical variables, including age, performance status (determined as on the Karnofsky performance scale, or KPS), type of primary tumor, number of cerebral metastases, and activity of the extracranial disease, based on Radiation Therapy Oncology Group (RTOG) trials7 using recursive partitioning analysis (RPA) led to the identification of prognostic groups (RPA groups 1-3 in modification of Lutterbach et al.;8 for details, see Table 98-1), with age below 65 years, a KPS score of at least 70, and no extracranial disease, as well as a single metastatic tumor, as the most important positive predictive factors concerning favorable outcome.
Table 98-1 Stratification of Prognostic Factors by RPA Class
| Median Survival (months) | |
|---|---|
| RPA Class 1 | |
| RPA Class 2 | |
| RPA Class 3 | |
| KPS < 70 | 2.3 |
Modified from Kaal et al. 2005.5
SRS is based on focusing multiple, high-dose, ionizing radiation beams using stereotactic guidance on an intracranial target. It converges with the pioneering work of Leksell and Larsson,9 inventors of the first gamma knife (GK) unit at the Karolinska Institute in Stockholm in 1967.10 However, the growing importance of the SRS techniques has greatly benefited from the development of novel visualization methods—computed tomography (CT) and magnetic resonance imaging (MRI) based—that allow precise planning and safe execution of radiosurgical treatment. Classical radiosurgery requires application of either photon or charged particles radiation (usually proton beam radiation) using multiple cobalt-60 sources (GK), linear accelerators (LINACs) (XKnife or CyberKnife) or cyclotrons. The original definition of SRS has been significantly extended by application of ionized radiation.11 Therefore, besides traditional SRS techniques based on external radiation sources, more invasive radiosurgical methods like iodine-125 (I-125) seeds implantation and the Photon Radiosurgery System for interstitial application of radiation in the case of metastatic brain lesions are discussed in this chapter.
Radiosurgical Techniques
Noninvasive radiosurgical treatment modalities consist of percutaneous or external irradiation using photon radiation technology (GK and LINACs). Current GK units use 201 cobalt-60 sources enclosed within a hemispheric vault, leading to the emission of gamma ray energy converged at the isocenter and allowing high-precision treatment of an intracranial target. The aperture of collimators differs from 4 to 18 mm, so the diameter of the radiation beam can be adapted to the size of the lesion. Due to high radiation activity (up to 300 TBq) the propagation of the dose reaches 2 Gy/min by 80 cm from the radiation source. However, during the therapy of irregular lesions, multiple spherical isocenters have to be superimposed to allow conformal treatment, resulting in anatomic regions that receive much higher radiation dosage than the marginal dose. GK technology is limited exclusively to the treatment of intracranial lesions and high cervical lesions (when GK perfexion is available).
Another important technique of radiosurgical brain metastases therapy is interstitial radiosurgery (brachytherapy), allowing direct intratumoral application of low-energy ionizing radiation (0.5-10 cGy/min). Following stereotactic serial biopsy that provides the confirmation of the histopathologic diagnosis, single or multiple radioactive sources are temporarily implanted, allowing optimal dose distribution (e.g., I-125, iridium-192, and gold-198).12 I-125 seeds (0.8 × 4.5 mm titanium cylinder) are fixed in a Teflon catheter, sterilized, and inserted into the center of the lesion, where they are left for 3 to 4 weeks (delivering a dose of 60 Gy to the tumor margins). The steep radial dose falloff is inversely proportional to the square of the distance from the radiation source, which provides minimal exposure to radiation of the healthy surrounding brain tissue.
At the beginning of 1990s, Photoelectron (Lexington, Waltham, MA) developed the Photon Radiosurgery System. It is a battery-powered, miniature x-ray generator capable of delivering low-energy radiation (soft x-rays) directly to small brain lesions in a single therapeutic session. Thus, it combines the direct dose application of brachytherapy with the advantages of short-time exposure typical for external radiosurgical techniques (GK and LINAC).13 Brief characteristics of the physical parameters of the radiosurgical procedures discussed in this chapter are summarized in Table 98-2.
Linac-Based Stereotactic Radiosurgery
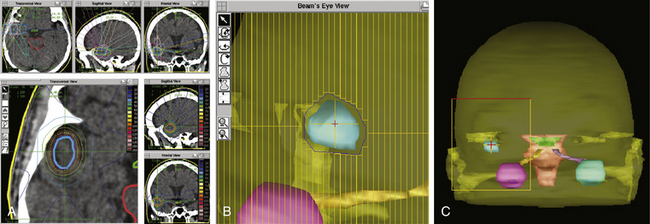
Due to the pioneer work of Betti and Derechinsky,14 Colombo et al.,15 Winston and Lutz,16 and others in the 1980s, precision and stability of LINAC radiosurgery systems was dramatically enhanced and became comparable to GK units. Nowadays, several techniques exist for LINAC systems that can be used to achieve an exact and highly conformal dose distribution. Target dose distribution can be adjusted by varying the collimator size, eliminating undesirable arcs, manipulating arc angles, using multiple isocenters, and differentially weighting the isocenters.17 A highly conformal dose distribution can be achieved by generating nonspherical beam shapes that are conformal to the beam’s eye view of the tumor with a multileaf collimator (Fig. 98-1A-C). Comparison of a modern LINAC radiosurgery system with GK units showed no differences in efficiency and safety.18,19 The most important difference between these two systems is the number of metastases that can be irradiated in a single session. In GK units, up to 25 brain metastases can be treated simultaneously when the lesions are diffusely scattered and the total tumor volume is less than 15 to 30 cm320 In contrast, LINAC radiosurgery systems can irradiate a maximum of 3 to 4 metastases as the multiple intersecting radiation arcs may cause a hot spot outside the target volume within normal brain tissue.21
Brain metastases are ideal targets for SRS, because these tumors are usually pseudospherical and show, in contrast to glioma, a noninfiltrative growth pattern with a sharp delineation to normal brain tissue. In addition, brain metastases occur often multifocally and have a diameter of less than 3.5 cm at the time of diagnosis. In Freiburg, we usually treat a maximum of three metastases during one session, and the prescribed dose for metastases with a diameter below 2.5 cm is 20 Gy calculated on the 80% isodose surrounding the outer tumor margin. Metastases with a diameter greater than 2.5 cm are treated with 18 Gy to prevent radiation necrosis. With nonspherical, irregularly shaped tumor margins, the application of one isocenter is not adequate to spare healthy brain tissue, especially when the metastasis is located within eloquent areas (e.g., the central region or brain stem). In these cases, we apply techniques like the use of multiple isocenters or a micromultileaf collimator to achieve a highly conformal target volume. Nataf et al.22 showed the importance of an exact dose application to the target volume by comparison of patients with a 2-mm margin to patients without this additional radiation volume. In contrast to Nöel et al.,23 who achieved improved local control with a 1-mm margin, the addition of a 2-mm margin resulted in more severe parenchymal complications, no increase in the 1- and 2-year local control rates, and no statistical significant difference in median overall survival (classic radiosurgery of 11.3 months vs. 2-mm margin radiosurgery of 19 months, p = 0.34).
For a solitary metastasis, one prospective24 and four retrospective studies25–28 compared stereotactic radiosurgery (SRS) with open tumor resection. Because they generally found no difference in overall survival, open surgery and radiosurgery are not concurrent but complementary methods in the treatment of brain metastases. Therefore, indication for SRS versus open surgery is based on individual aspects of the patients.
Clinical Condition of the Patient
Patients with a pronounced comorbidity and a reduced general condition are usually treated by SRS as long as the patient is not threatened by the mass effect or surrounding edema of the lesion. In these patients, the possibility of not interrupting chemotherapy while treating brain metastases adds another reason in favor of SRS.
Prognosis
According to Lutterbach et al.,8 prognosis of patients with up to three cerebral metastases treated by LINAC radiosurgery can be predicted accurately with the RPA classification. Median overall survival for patients is 13.4, 9.3, and 1.5 months in RPA classes 1, 2, and 3, respectively. Due to the poor prognosis of RPA class 3, patients with a KPS score below 70 should only be treated with SRS when their age is under 65 and have no signs of extracerebral tumor progression. These two factors are independent risk factors associated with an improved prognosis in RPA class 3 patients.
In summary, SRS achieves local tumor control rates at 1 year between 80% and 90%. Brain metastases from radioresistant tumors (renal cell carcinoma, melanoma, and sarcoma) may also benefit from SRS.29 Complication rates associated with SRS are low. However, the risk of radiation necrosis increases with larger tumor volumes and prior radiation therapy. Other risk factors for neurologic complications after SRS are the location in eloquent brain areas, SRS doses higher than 15 Gy, and progression of the primary cancer.30
Interstitial Radiosurgery with I-125 Seeds
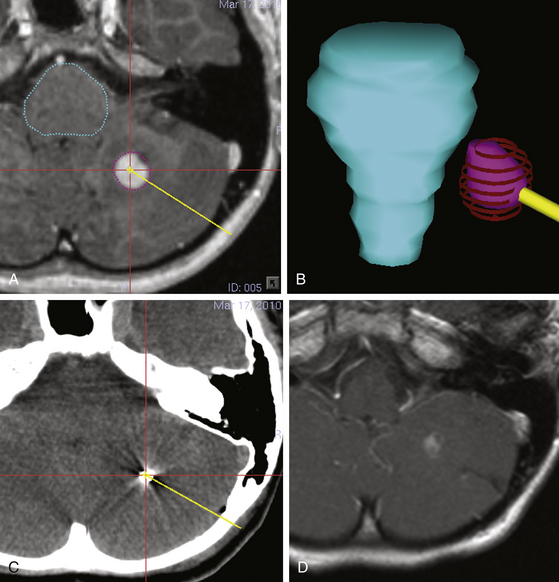
In early procedures, permanent implants made from thin flexible wires of iridium-192 that could be cut to any length were implanted in a stereotactic procedure. Iridium-192 has a half-life of 73.83 days and a mean energy of 380 keV. Nowadays, mostly I-125 seeds are used, which are sized 4.5 × 0.8 mm with a half-life of 59.43 days and deliver lower photon energy of 27.4, 31.4, and 35.5 keV (Fig. 98-2A-D). These seeds contain I-125 adsorbed onto a radiopaque silver rod hermetically encapsulated in a welded titanium capsule. Typical radioactivities range from 1 to 25 mCi.
Absorbed Dose Rates
In our center, patients are proposed a stereotactic biopsy prior to radiosurgery to confirm the histopathologic diagnosis and to exclude misinterpretations of benign or inflammatory lesions if one of the following conditions is given: (1) missing extracranial proof of a primary tumor, (2) low probability for brain metastases of a known primary tumor (e.g., prostate carcinoma), (3) an interval longer than 3 years between the initial diagnosis of a primary tumor and a late cerebral lesion that is suspected to be a metastatic recurrence of this tumor, or (4) the presence of multiple and different primary tumors.
Treatment Planning
The stereotactic biopsy is done by a small skin incision and a 5-mm bur hole and typically following the tract of the seed catheter. The planned target position is retained by fluoroscopy, preferably in two plains, using a probe. The sterile seed within the catheter is exactly implanted at the planned target position under fluoroscopy control. The seed catheter is fixed in the bur hole using either fibrin foam or bone cement and a radiopaque hemoclip. The skin is closed by a suture. If high precision is required, the exact positioning of the seed can be controlled by a native CT scan and a fusion with the treatment plan. The radiation emission is measured and documented at distances of 0, 20, and 100 cm to the body’s surface according to legal rules. If applicable, the patient is instructed to avoid long-lasting and close contact (less than 1 m) with pregnant women and children. The patient is dismissed the second day after seed implantation. We propose autoclaving of the seeds and using a single-shot antibiosis during the seed implantation. A possible reactive brain edema during the implantation time can be treated best with oral administration of a low-dose steroid such as dexamethasone.
Discussion
The annual incidence of intracranial metastases is about 8.3 per 100,000 population31 and is present in up to 24% of cancer patients based on autopsy studies.32 Approximately one third of the patients with cerebral metastases are candidates for microsurgical resection. The other patients are amenable to nonsurgical treatment options due to their medical comorbidities precluding surgery, multiple metastases (roughly 50%-63% of the patients have multiple lesions at initial presentation),1,6 or location in surgically inaccessible regions.33 Brain metastases usually are almost spherical and small; unlike the glioma, they have a clear border to the surrounding brain tissue. Therefore, they are regarded as perfect targets for SRS, provided they are causing only minimal mass effect (less than 1 cm of midline shift).
The number of brain metastases that can be treated in one session is still an issue of debate. In most centers, including ours, it is common to treat up to three lesions in one radiosurgery session. In 2006, the American Society for Therapeutic Radiology and Oncology, the American Association of Neurological Surgeons, and the Congress of Neurological Surgeons jointly agreed to define SRS in a way that includes both traditional single-dose SRS and multidose SRS for up to five doses (two to five doses).34,35
Both LINAC and GK radiosurgery have been used for the treatment of these lesions. There were no survival differences using either LINAC or GK radiosurgery, according to the RTOG 95-08 trial.19 The CyberKnife, which is a recently commercially available LINAC-based system, combines a robotic arm with an image guidance system to track the patient’s position, thereby obviating the need for application of a stereotactic frame.
SRS can be used alone or with surgical resection or WBRT.
SRS Alone Versus WBRT+SRS
One prospective randomized, controlled trial (RCT) (class I evidence) with a companion paper has evaluated superior local control and an improved KPS alone versus WBRT+SRS for the initial management of patients with solid metastatic brain tumors.36,37 There was no difference between study groups for median survival (8.0 vs. 7.5 months), one year local control rate (72.5% vs. 88.7%), neurologic cause of death, 1-year KPS score, acute or late neurotoxicity. The 1-year chance of recurrence locally (27.5% vs. 11.3%), at a distant site (63.7% vs. 41.5%), or anywhere in the brain (76.4% vs. 46.8%) was significantly greater for SRS alone.
WBRT Alone Versus WBRT+SRS
There are two prospective RCTs (class I evidence) on the topic of WBRT alone versus WBRT+SRS. The first is a RTOG multicenter trial published by Andrews et al. in 2004.19 This trial showed significantly better survival for patients with single metastatic tumors, superior local control and an improved KPS score for patients with one to three metastatic tumors treated with WBRT+SRS. A shortcoming of this study is that there was no follow-up neuroimaging review on 43% of the patients, and tumors greater than 3 cm had been included, which are known to be less favorable for SRS. Therefore, no conclusion can be assured.
The second RCT is from a Pittsburgh group and was published by Kondziolka et al. in 1999.38 This study was stopped at the 60% accrual point due to an overwhelming positive tumor control difference at interim analysis. There was a significantly better local control rate measured in terms of local failure at 1 year (8% vs. 100%) and median time to recurrence/progression at the original site (36 vs. 6 months) for patients treated with WBRT+SRS.
Surgical Resection+WBRT Versus WBRT+SRS
Both surgical resection+WBRT and WBRT+SRS represent effective treatment strategies, resulting in relatively equal survival times. SRS has not been evaluated for larger lesions (greater than 3 cm) or those with significant mass effect (greater than 1-cm midline shift).
SRS Alone Versus WBRT Alone
Moriarty et al.39 presented factors related to decreased survival and failure of local tumor control in uni- and multivariate analysis of their series. The only independent factors decreasing survival were ongoing systemic disease and age over 60 years. Failure of local tumor control was associated with infratentorial location, tumor volume greater than 3 cm3, and recurrent lesions. Neither histology (radiosensitive vs. radioresistant lesions) nor number of lesions had an impact on survival or local tumor control rates.
American Society for Radiation Oncology. Stereotactic radiosurgery definition. Int J Radiat Oncol Biol Phys. 2007;67:1280.
Andrews D.W., Scott C.B., Sperduto P.W., et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 1995-2008 randomised trial. Lancet. 2004;363:1665-1672.
Aoyama H., Shirato H., Tago M., et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs. stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295:2483-2491.
Aoyama H., Tago M., Kato N., et al. Neurocognitive function of patients with brain metastasis who received either whole brain radiotherapy plus stereotactic radiosurgery or radiosurgery alone. Int J Radiat Oncol Biol Phys. 2007;68:1388-1395.
Barnett G.H., Linskey M.E., Adler J.R., et al. Stereotactic radiosurgery—an organized neurosurgery-sanctioned definition. J Neurosurg. 2007;106:1-5.
Bindal A.K., Bindal R.K., Hess K.R., et al. Surgery versus radiosurgery in the treatment of brain metastasis. J Neurosurg. 1996;84:748-754.
Gaspar L.E., Scott C., Murray K., Curran W. Validation of the RTOG recursive partitioning analysis (RPA) classification for brain metastases. Int J Radiat Oncol Biol Phys. 2000;47:1001-1006.
Kaal E.C., Niel C.G., Vecht C.J. Therapeutic management of brain metastasis. Lancet Neurol. 2005;4:289-298.
Kondziolka D., Patel A., Lunsford L.D., et al. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys. 1999;45:427-434.
Lutterbach J., Cyron D., Henne K., Ostertag C.B. Radiosurgery followed by planned observation in patients with one to three brain metastases. Neurosurgery. 2003;52:1066-1073.
Moriarty T.M., et al. Radiosurgery. Basel: Karger; 1996.
Muacevic A., Kreth F.W., Horstmann G.A., et al. Surgery and radiotherapy compared with gamma knife radiosurgery in the treatment of solitary cerebral metastases of small diameter. J Neurosurg. 1999;91:35-43.
Muacevic A., Wowra B., Siefert A., et al. Microsurgery plus whole brain irradiation versus gamma knife surgery alone for treatment of single metastases to the brain: a randomized controlled multicentre phase III trial. J Neurooncol. 2008;87:299-307.
Muller-Riemenschneider F., Bockelbrink A., Ernst I., et al. Stereotactic radiosurgery for the treatment of brain metastases. Radiother Oncol. 2009;91:67-74.
Nataf F., Schlienger M., Liu Z., et al. Radiosurgery with or without a 2-mm margin for 93 single brain metastases. Int J Radiat Oncol Biol Phys. 2008;70:766-772.
Nöel G., Simon J.M., Valery C.A., et al. Radiosurgery for brain metastasis: impact of CTV on local control. Radiother Oncol. 2003;68:15-21.
O’Neill B.P., Iturria N.J., Link M.J., et al. A comparison of surgical resection and stereotactic radiosurgery in the treatment of solitary brain metastases. Int J Radiat Oncol Biol Phys. 2003;55:1169-1176.
Ostertag C.B. [Stereotaxic radiosurgery]. Nervenarzt. 1994;65:660-669.
Ostertag C.B. Brachytherapy—interstitial implant radiosurgery. Acta Neurochir Suppl (Wien). 1993;58:79-84.
Pantazis G., Trippel M., Birg W., et al. Stereotactic interstitial radiosurgery with the Photon Radiosurgery System (PRS) for metastatic brain tumors: a prospective single-center clinical trial. Int J Radiat Oncol Biol Phys. 2009;75:1392-1400.
Schoggl A., Kitz K., Reddy M., et al. Defining the role of stereotactic radiosurgery versus microsurgery in the treatment of single brain metastases. Acta Neurochir (Wien). 2000;142:621-626.
Serizawa T. Radiosurgery for metastatic brain tumors. Int J Clin Oncol. 2009;14:289-298.
Serizawa T., Saeki N., Higuchi Y., et al. Gamma knife surgery for brain metastases: indications for and limitations of a local treatment protocol. Acta Neurochir (Wien). 2005;147:721-726.
Sneed P.K., Suh J.H., Goetsch S.J., et al. A multi-institutional review of radiosurgery alone vs. radiosurgery with whole brain radiotherapy as the initial management of brain metastases. Int J Radiat Oncol Biol Phys. 2002;53:519-526.
Williams B.J., Suki D., Fox B.D., et al. Stereotactic radiosurgery for metastatic brain tumors: a comprehensive review of complications. J Neurosurg. 2009;111:439-448.
1. Gavrilovic I.T., Posner J.B. Brain metastases: epidemiology and pathophysiology. J Neurooncol. 2005;75:5-14.
2. Muller-Riemenschneider F., Bockelbrink A., Ernst I., et al. Stereotactic radiosurgery for the treatment of brain metastases. Radiother Oncol. 2009;91:67-74.
3. Smith M.L., Lee J.Y. Stereotactic radiosurgery in the management of brain metastasis. Neurosurg Focus. 2007;22:E5.
4. Schouten L.J., Rutten J., Huveneers H.A., Twijnstra A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer. 2002;94:2698-2705.
5. Kaal E.C., Niel C.G., Vecht C.J. Therapeutic management of brain metastasis. Lancet Neurol. 2005;4:289-298.
6. Delattre J.Y., Krol G., Thaler H.T., Posner J.B. Distribution of brain metastases. Arch Neurol. 1988;45:741-744.
7. Gaspar L.E., Scott C., Murray K., Curran W. Validation of the RTOG recursive partitioning analysis (RPA) classification for brain metastases. Int J Radiat Oncol Biol Phys. 2000;47:1001-1006.
8. Lutterbach J., Cyron D., Henne K., Ostertag C.B. Radiosurgery followed by planned observation in patients with one to three brain metastases. Neurosurgery. 2003;52:1066-1073.
9. Leksell L. The stereotaxic method and radiosurgery of the brain. Acta Chir Scand. 1951;102:316-319.
10. Niranjan A., Lunsford L.D. Radiosurgery: where we were, are, and may be in the third millennium. Neurosurgery. 2000;46:531-543.
11. Ostertag C.B. [Stereotaxic radiosurgery]. Nervenarzt. 1994;65:660-669.
12. Ostertag C.B. Brachytherapy—interstitial implant radiosurgery. Acta Neurochir Suppl (Wien). 1993;58:79-84.
13. Pantazis G., Trippel M., Birg W., et al. Stereotactic interstitial radiosurgery with the Photon Radiosurgery System (PRS) for metastatic brain tumors: a prospective single-center clinical trial. Int J Radiat Oncol Biol Phys. 2009;75:1392-1400.
14. Betti O.O., Derechinsky V.E. Hyperselective encephalic irradiation with a linear accelerator. Acta Neurochir Suppl. 1984;33:385-390.
15. Colombo F., Benedetti A., Pozza F., et al. External stereotactic irradiation by linear accelerator. Neurosurgery. 1985;16:154-160.
16. Winston K.R., Lutz W. Linear accelerator as a neurosurgical tool for stereotactic radiosurgery. Neurosurgery. 1988;22:454-464.
17. Friedman W.A., Buatti J.M., Bova F.J., Mendenhall W.M. LINAC RadioSurgery: A Practical Guide. Berlin: Springer Verlag; 1998.
18. Sneed P.K., Suh J.H., Goetsch S.J., et al. A multi-institutional review of radiosurgery alone vs. radiosurgery with whole brain radiotherapy as the initial management of brain metastases. Int J Radiat Oncol Biol Phys. 2002;53:519-526.
19. Andrews D.W., Scott C.B., Sperduto P.W., et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 1995-2008 randomised trial. Lancet. 2004;363:1665-1672.
20. Serizawa T., Saeki N., Higuchi Y., et al. Gamma knife surgery for brain metastases: indications for and limitations of a local treatment protocol. Acta Neurochir (Wien). 2005;147:721-726.
21. Serizawa T. Radiosurgery for metastatic brain tumors. Int J Clin Oncol. 2009;14:289-298.
22. Nataf F., Schlienger M., Liu Z., et al. Radiosurgery with or without a 2-mm margin for 93 single brain metastases. Int J Radiat Oncol Biol Phys. 2008;70:766-772.
23. Nöel G., Simon J.M., Valery C.A., et al. Radiosurgery for brain metastasis: impact of CTV on local control. Radiother Oncol. 2003;68:15-21.
24. Muacevic A., Wowra B., Siefert A., et al. Microsurgery plus whole brain irradiation versus gamma knife surgery alone for treatment of single metastases to the brain: a randomized controlled multicentre phase III trial. J Neurooncol. 2008;87:299-307.
25. Muacevic A., Kreth F.W., Horstmann G.A., et al. Surgery and radiotherapy compared with gamma knife radiosurgery in the treatment of solitary cerebral metastases of small diameter. J Neurosurg. 1999;91:35-43.
26. Schoggl A., Kitz K., Reddy M., et al. Defining the role of stereotactic radiosurgery versus microsurgery in the treatment of single brain metastases. Acta Neurochir (Wien). 2000;142:621-626.
27. O’Neill B.P., Iturria N.J., Link M.J., et al. A comparison of surgical resection and stereotactic radiosurgery in the treatment of solitary brain metastases. Int J Radiat Oncol Biol Phys. 2003;55:1169-1176.
28. Bindal A.K., Bindal R.K., Hess K.R., et al. Surgery versus radiosurgery in the treatment of brain metastasis. J Neurosurg. 1996;84:748-754.
29. Soffietti R., Ruda R., Trevisan E. Brain metastases: current management and new developments. Curr Opin Oncol. 2008;20:676-684.
30. Williams B.J., Suki D., Fox B.D., et al. Stereotactic radiosurgery for metastatic brain tumors: a comprehensive review of complications. J Neurosurg. 2009;111:439-448.
31. Walker A.E., Robins M., Weinfeld F.D. Epidemiology of brain tumors: the national survey of intracranial neoplasms. Neurology. 1985;35:219-226.
32. Posner J.B., Chernik N.L. Intracranial metastases from systemic cancer. Adv Neurol. 1978;19:579-592.
33. Bradley K.A., Mehta M.P. Management of brain metastases. Semin Oncol. 2004;31:693-701.
34. Barnett G.H., Linskey M.E., Adler J.R., et al. Stereotactic radiosurgery—an organized neurosurgery-sanctioned definition. J Neurosurg. 2007;106:1-5.
35. American Society for Radiation Oncology. Stereotactic radiosurgery definition. Int J Radiat Oncol Biol Phys. 2007;67:1280.
36. Aoyama H., Shirato H., Tago M., et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs. stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295:2483-2491.
37. Aoyama H., Tago M., Kato N., et al. Neurocognitive function of patients with brain metastasis who received either whole brain radiotherapy plus stereotactic radiosurgery or radiosurgery alone. Int J Radiat Oncol Biol Phys. 2007;68:1388-1395.
38. Kondziolka D., Patel A., Lunsford L.D., et al. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys. 1999;45:427-434.
39. Moriarty T.M., et al. Radiosurgery. Basel: Karger; 1996.
∗ We dedicate this chapter to our esteemed academic and clinical teacher and mentor Prof. Christoph Ostertag for his 70th birthday. He has influenced and encouraged us through his high academic standards and his deep personal relationship with our patients. His vision is always to approach each target in the shortest and best way and continuously improve our understanding of the human brain, its pathology, and novel treatment modalities.