CHAPTER 253 Interstitial and Intracavitary Irradiation of Brain Tumors
Brachytherapy is the practice of administering therapeutic radiation by means of a source placed a short distance from or within the body part being treated, as opposed to teletherapy, in which the radiation source is outside the body, several feet away. Brachytherapy attempts to improve the therapeutic ratio by allowing the delivery of high doses of radiation to localized tumor volumes or resection margins without significant irradiation of surrounding normal brain. The first examples of brain tumor brachytherapy used radium as the radiation source. In 1912, Hirsch inserted a radium probe into the sella of an acromegalic woman.1 Two years later, Frazier implanted radium into a malignant glioma tumor bed at the time of a craniotomy.1 The development of new radioisotopes in the late 1940s and early 1950s, coupled with the development of frame-based stereotactic systems, brought about renewed interest in brain tumor brachytherapy.
Two types of brain tumor brachytherapy quickly emerged: intracavitary, or placement of a radiation source in cystic lesions in which the target is a thin rim of neuroepithelial tissue in the cyst wall, and interstitial, or placement of a radiation source in solid tissue, either inoperable tumors or the margins of resection cavities after subtotal or total tumor resection. Intracavitary brachytherapy was performed initially for craniopharyngioma in 1950 by Leksell, who used a phosphorus 32 (32P) sodium phosphate solution to treat a 40-year-old man with recurrent cystic craniopharyngioma.2 Since then, intracavitary brachytherapy has been used mainly outside North America.
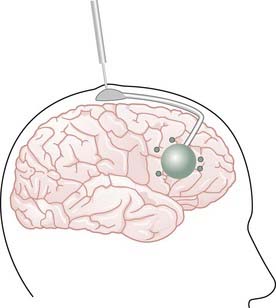
Within North America, there has been extensive experience with interstitial brachytherapy in the form of iodine 125 (125I) implants. These 125I implants began as high-activity temporary implants in the form of stereotactically placed catheters containing small titanium capsules or “seeds” within which reside resin balls containing the radioactive isotope. These catheters had to be removed to prevent toxicity from the radiation, with removal accomplished in a bedside or outpatient procedure involving a small scalp incision followed by catheter removal and suturing of the scalp. Although high-activity temporary implants deliver higher doses in shorter periods, there has been a transition over the past decade toward low-activity permanent implants that deliver radiation over a longer period. Low-activity permanent implants are free individual titanium capsules 4.5 mm long and 0.8 mm in diameter that are placed in the walls of the resection cavity; within the capsules reside the 0.67-mCi 125I radioactive sources (Fig. 253-1). Low-activity permanent implants may be associated with a lower rate of reoperation for radiation necrosis. A report in which low-activity permanent 125I implants were used to treat metastases found that 2 of 26 (8%) patients required reoperation for radiation necrosis3 as opposed to 1 of 6 (17%) in another report using temporary high-activity 125I implants to treat metastases4 (Table 253-1).4–13 Similarly, low-activity permanent 125I implants are associated with a slightly lower rate of reoperation for radiation necrosis than are high-activity temporary 125I implants when used to treat primary and recurrent glioblastoma (Table 253-1).
FIGURE 253-1 Illustration of low-activity, permanent iodine 125 seeds, which are close to the size of a grain of rice.
TABLE 253-1 Comparison of Temporary High-Activity and Permanent Low-Activity 125I Seeds with Temporary High-Activity 125I GliaSite Implants
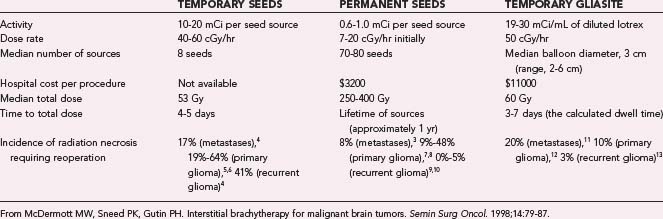
Early studies identified limited homogeneity and reproducibility with low-activity permanent 125I seeds, specifically, (1) inhomogeneous distribution of the radiation dose within a patient’s resection cavity because of spatial asymmetry in the location of the seeds and (2) patient-to-patient heterogeneity, which makes efficacy studies difficult because of an inability to reproduce the spatial distribution of seeds from patient to patient. Seeking to overcome these limitations, Proxima Therapeutics (Alpharetta, GA) returned to high-activity temporary 125I implants when they designed GliaSite, a balloon catheter system that is implanted at the time of tumor craniotomy. The distal end of the balloon lies in the tumor cavity, whereas the proximal injection port lies under the scalp and is filled with 125I-containing solution a few days after the craniotomy. Once the brachytherapy is completed, the patient undergoes an outpatient procedure to remove the implant. Although GliaSite provides better homogeneity within the resection cavity than permanent low-activity 125I seeds do, the dose distribution outside the cavity can be nonconformal with GliaSite because the GliaSite dose distribution is always a sphere whereas the cavity shape varies, which means that targeted positioning of 125I seeds may enable more uniform dose distribution outside the cavity than possible with GliaSite. The clinical significance of the homogeneous intracavitary dose distribution of GliaSite versus the potentially more homogeneous extracavitary dose distribution of 125I seeds remains to be determined. The safety of GliaSite was proved in a study of 21 patients with recurrent glioblastoma published in 2003.14 The radiation necrosis associated with the original catheter-based high-activity 125I implants occurred to a comparable extent when treating metastases with GliaSite but was not as common when treating primary or recurrent glioblastoma with GliaSite (see Table 253-1); however, this observation will require confirmation in larger series. Although 125I low-activity seeds can be applied to tumor of any size and are relatively inexpensive ($3200 is the cost of a typical implant), GliaSite is limited to tumors less than 6 cm in diameter because of limitations in balloon size and can cost $11,000 for a typical implant (see Table 253-1). Since the year 2000, most brachytherapy studies have involved low-activity permanent 125I seeds or high-activity temporary 125I GliaSite implants, with catheters containing high-activity seeds falling out of favor. The biggest alternative to these brachytherapy techniques for brain tumors has been the increased availability and use of radiosurgery. This chapter reviews the radiobiology of brachytherapy and its success in treating various brain tumors, including how it compares with radiosurgery.
Radiobiology
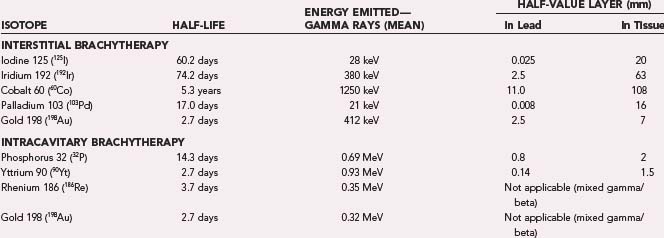
The characteristics of the common isotopes used for brachytherapy are listed in Table 253-2. Intracavitary treatment tends to use low-energy β-emitting sources such as 32P or yttrium 90 (90Y), with which most of the radiation dose is deposited in the first few millimeters, perfect when the source is in the center of a cyst with a thin wall that is the target. Pathologic specimens from patients treated in this manner demonstrate that the epithelial lining cells of the cyst wall are destroyed and replaced largely by fibrous tissue.15 By comparing the activity of cyst fluid aspirated a certain number of days after instillation of the isotope with isotope activity measured on an isotope scintillation scan, Fig and colleagues showed that the larger the cyst, the higher the actual dose to the cyst wall.16
The type of ionizing radiation used, the total activity of the sources, and the dose rate are important determinants of the biologic effect of any radiation dose. In general, the lower the dose rate, the smaller the biologic effect of any given dose, particularly over the range of 0.01 to 1 Gy/min.1,17 Dose rates from interstitial brachytherapy are usually less than 0.6 Gy/hr, much lower than the 1.8 to 2 Gy/min exhibited by conventional linear accelerators. However, there are four distinct advantages of continuous low-dose irradiation. The first therapeutic advantage of continuous low-dose irradiation that occurs with interstitial brachytherapy relates to differences in repair of sublethal radiation-induced damage between tumor and normal tissue, with normal tissue being more efficient in this regard.18 However, the higher the dose rate, the lower the total dose at which normal tissue DNA damage is observed, thereby lessening the difference in repair of radiation-induced DNA damage between tumor and normal tissue.19 A second therapeutic advantage of continuous irradiation is that it allows time for cells in the radiation-resistant G1 phase of the cell cycle to enter the more sensitive G2 and M phases while radiation is still being delivered. A third therapeutic advantage of continuous low-dose irradiation is that hypoxic cells, traditionally regarded as being resistant to conventional radiation, are relatively more sensitive to continuous low-dose irradiation.20 A fourth therapeutic advantage of continuous low-dose irradiation is that despite using a lower dose, the dose of brachytherapy is still sufficient to inhibit mitosis but is present for up to six cell cycles whereas conventional radiotherapy is present for only one cell cycle and uses a dose 2 to 3 times that needed to inhibit mitosis.
Implantation Technique
Interstitial Brachytherapy
High-Activity Implants
Originally, stereotactic catheters were used to place high-activity temporary implants, but this technique has since been replaced by GliaSite implantation. After craniotomy for maximal resection, a 2-, 3-, or 4-cm GliaSite balloon catheter device is placed in the resection cavity, with the neurosurgeon selecting the size closest to but smaller than the diameter of the resection cavity. The GliaSite device is filled with saline and iodinated contrast material, and the catheter access port is secured to the cranium (Fig. 253-2). After closure in the usual method, gadolinium-enhanced magnetic resonance imaging (MRI) is performed for confirmation of catheter placement and dosimetric planning. In the radiation oncology clinic 2 to 6 weeks after surgery, the radiation oncologist removes a volume of iodinated contrast material equal to the planned injection volume of Iotrex solution and then loads the GliaSite balloon with the solution. The dwell time necessary to deliver the prescribed total dose is calculated by integrating the dose rate at the prescription point with the 60.2-day half-life of 125I. Some, but not all institutions admit the patient to the hospital during the dwell time. Patients also receive a regimen of potassium iodide to block any uptake of 125I by the thyroid in the unlikely event of balloon failure. The balloon catheter must be removed by the neurosurgeon in an outpatient procedure within 29 days of completing the radiation treatment.
Low-Activity Implants
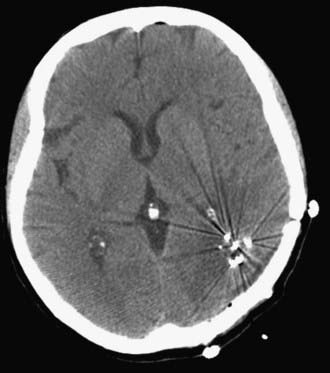
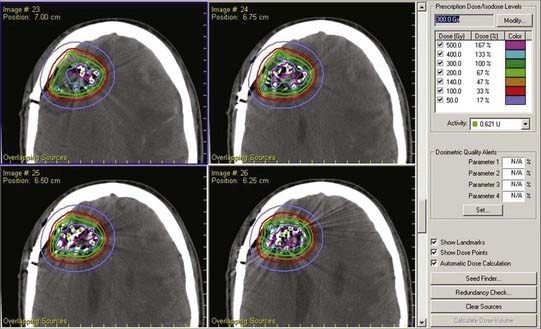
Preoperative imaging is used to determine the volume of the resection cavity that will need to be implanted after surgery. After the surgeon has communicated to the radiation oncologist the anticipated extent of resection, the radiation oncologist estimates the number of seeds and total activity required to deliver the desired dose over the lifetime of the permanent sources, assuming 1-cm2 spacing on the walls of the resection cavity. An image-guided craniotomy for resection is then performed, after which the resection wall must exhibit no signs of bleeding without any covering of hemostatic agents such as Surgicel. The surgeon and radiation oncologist then don radioprotective garments, and the surgeon places individual sterile 125I seeds on or in the walls of the resection cavity at 1-cm2 spacing. The radiation oncologist and surgeon count the number of sources. The surgeon can use fibrin glue to secure the seeds in position. Once the patient’s skin is closed, metered readings of radiation output are taken 1 m from each side of the patient’s head. Computed tomography (CT) can be performed postoperatively to visualize the seeds in the resection cavity (Fig. 253-3), and the radiation oncologist can generate a diagram of dose/isodose levels overlaid on the postoperative CT scan (Fig. 253-4).
Intracavitary Brachytherapy
Either the patient is placed in a stereotactic frame and cranial imaging is performed, or for a frameless approach, Mayfield pins are used and preoperative MRI is registered with an image guidance system. Cyst volume is calculated from the preoperative images. The amount of 32P, the only isotope approved for intracavitary brachytherapy in the United States, needed to deliver 200 Gy to the cyst wall can then be calculated with a method described elsewhere.21 A bur hole is placed over a safe entry point identified by the surgeon, and the cyst is then stereotactically punctured with a needle guided to a target within the cyst by standard frame-based or frameless methods. The central stylet is removed, and a volume of cyst fluid is removed and measured. The 32P is injected, and any remaining isotope is then flushed out of the needle with a volume of sterile saline equal to the volume of cyst fluid removed minus the volume of 32P already injected. The needle and syringes used are collected by the radiation technologist, and the wound is closed in the usual manner.
Brachytherapy for Primary Glioblastoma
High-activity temporary 125I catheter implants remain the only brachytherapy modality to have reached phase III randomized clinical trials, and in two trials they were used in patients with newly diagnosed glioblastoma. The first published trial, conducted by a group in Toronto, randomized 140 patients from 1986 to 1996.22 The second published trial was the Brain Tumor Cooperative Group trial of the National Institutes of Health in the United States, and it randomized 299 patients from 1987 to 1994.23 In both trials, the brachytherapy arm received temporary, stereotactically placed 125I implants delivering 60 Gy of radiation. Neither trial, however, showed a survival benefit associated with brachytherapy.22,23 Another randomized phase II clinical trial of high-activity 125I catheter implants at the University of California at San Francisco (UCSF) randomized patients to undergo interstitial, stereotactically delivered hyperthermia as an adjuvant treatment after 125I catheter implantation. This technique was based on laboratory studies showing that hyperthermia inhibits repair of radiation-induced DNA damage, is particularly toxic against cells in the S phase of the cell cycle and against nutrient-deprived low-pH hypoxic cells, which typically resist radiation, and induces tumor reoxygenation, thereby increasing radiosensitivity.5 From 1990 to 1995, 79 patients were randomized to receive high-activity 125I catheter implants delivering 60 Gy of radiation with or without interstitial hyperthermia for 30 minutes before and after brachytherapy, with temperature elevated to 42.5°C to 50°C in tumor tissue without exceeding 44°C in normal tissue.24 In 68 patients who actually underwent brachytherapy, median survival times were 85 weeks for patients randomized to receive hyperthermia versus 76 weeks for those randomized to not receive hyperthermia, and 2-year survival probabilities were 31% for hyperthermia patients versus 15% for patients not receiving hyperthermia (P = .02), a finding indicative of a potential role for interstitial hyperthermia as a supplement to brachytherapy.24
In 2000, Koot and colleagues retrospectively compared their results in treating newly diagnosed glioblastoma with maximal surgical resection followed by low-activity 125I seed implantation in 45 patients and temporary high-activity iridium 192 (192Ir) wire implantation in 21 patients.7 Both groups also received external beam radiation. Median survival was 13 months in patients receiving 125I-based brachytherapy and 16 months in patients receiving 192Ir temporary implants, an insignificant difference.7 The 33% reoperation rate (mostly for radiation necrosis) was significantly higher with 192Ir temporary implants than the 9% reoperation rate with 125I implants.7 In 2007, the results of low-activity 125I seeds and GliaSite implantation for newly diagnosed glioma were released. Welsh and coauthors reported a retrospective eight-institution analysis of 20 patients who underwent craniotomy for maximal surgical debulking, followed by GliaSite brachytherapy to a median dose of 50 Gy and then external beam radiotherapy to a median dose of 60 Gy.12 Average survival was 11.4 months, 3 months longer than historical controls according to their Radiation Therapy Oncology Group recursive partitioning analysis class.12 There was a 10% incidence of reoperation for radiation necrosis in this series.18 Also in 2007, Chen and associates from the UCSF group reported a 3-year experience in which 21 patients in a phase I protocol underwent gross total resection of newly diagnosed glioblastoma followed by the implantation of permanent 125I seeds (400-Gy median dose measured 5 mm radially outward from the resection cavity) and then postoperative hyperfractionated radiotherapy to 60 Gy (1.0 Gy twice daily 5 days per week).8 In this study there were 12 reoperations, 11 of which found radiation necrosis without tumor.8 Median survival was 114 weeks, not significantly improved over historical controls undergoing gross total resection, chemotherapy, and radiotherapy at the same institution.8
Thus, brachytherapy of any type has yet to produce better results than surgery followed by temozolomide chemotherapy and external beam radiation therapy, the standard of care for newly diagnosed glioblastoma since a randomized trial published in 2005 reported a median survival of 14.6 months.25 As a result, particularly with the University of Toronto and the Brain Tumor Cooperative Group phase III clinical trials described earlier failing to show a therapeutic benefit,22,23 brachytherapy has fallen out of favor for newly diagnosed glioblastoma.
Brachytherapy for Recurrent Glioblastoma
In 2004, Larson and coworkers reported the UCSF experience in treating recurrent glioblastoma in 38 patients with low-activity permanent 125I seeds implanted at the time of craniotomy for gross total resection.26 The median brachytherapy dose 5 mm outside the resection cavity was 300 Gy, and median survival was 52 weeks with brachytherapy.26 There have been two studies of GliaSite implantation for recurrent glioblastoma. In 2005, Chan and coauthors reported the results in 24 patients with recurrent glioblastoma treated by craniotomy and GliaSite implantation to a mean dose of 53.1 Gy; median survival was 9.1 months.27 A year later, Gabayan and associates reported the cumulative experience of 10 institutions in treating recurrent glioblastoma with craniotomy and GliaSite implantation to a median dose rate of 52.3 Gy/hr13; median survival was 35.9 weeks, similar to the results of Chan and colleagues. Although these two GliaSite studies produced shorter survival than the Larson study of low-activity permanent 125I seeds, the UCSF study was limited to tumors that underwent gross total resection, which probably contributed to their longer survival.
Based on studies showing pharmacologic synergy between radiation and chemotherapy in treating glioblastoma, with chemotherapy enhancing the amount of radiation-induced double-strand DNA damage,28 Darakchiev and coworkers in 2008 treated 34 patients with recurrent glioblastoma by combining localized implanted adjuvant therapy consisting of carmustine wafers (Gliadel) with permanent, low-activity 125I seeds.29 Median survival was 69 weeks, and there was a 24% incidence of radiation necrosis. The authors reported that survival in their patients compared favorably with historical controls but that the incidence of radiation necrosis exceeded that reported previously with either treatment alone.29
Brachytherapy for Low-Grade Astrocytoma
European centers have been the primary locations where permanent implants have been studied for low-grade astrocytoma. The results must be compared with the natural history of low-grade astrocytoma, which is slower growing than malignant glioma, and a true analysis therefore requires long-term follow-up. Ostertag and Kreth reported several studies of a large population of patients with low-grade tumors treated by interstitial brachytherapy using permanent and temporary 125I implants. The initial study was published in 1992 and included 430 patients with low-grade glial neoplasms treated by biopsy and interstitial 125I temporary (36%) and permanent (64%) implants as the primary therapy.30 Five-year survival rates were 77% for pilocytic astrocytoma, 65% for grade 2 astrocytoma, 80% for oligoastrocytoma, and 58% for oligodendroglioma. No patients in that series required reoperation for radiation necrosis.30 Fourteen years later, in 2006, the same group presented a more long-term follow-up of 239 of these patients.31 Overall 5-, 10-, and 15-year survival rates were 56%, 37%, and 26%, and the malignant transformation rates at these time points were 33%, 54%, and 67%, respectively.31 There was no leveling off of Kaplan-Meier curves, thus suggesting that long-term tumor stabilization with the use of brachytherapy for low-grade glioma is, unfortunately, rare.31
Brachytherapy for Craniopharyngiomas
Some studies have suggested a benefit of using intracavitary brachytherapy for craniopharyngiomas. The target tissue is the cyst wall, which is only millimeters thick. The low energy and short depth of tissue penetration of several β-emitting radioisotopes (see Table 253-2) allow safe delivery of large doses of radiation to the walls of these tumor cysts. Pathologic specimens from patients treated in this manner demonstrate that the epithelial lining cells are destroyed and replaced largely by fibrous tissue. A retrospective review of stereotactically applied intracystic yttrium 90 in 60 patients with recurrent cystic craniopharyngioma over a 30-year period revealed a 79% initial decrease in cyst volume and mean survival of 9.4 years.32 The rate of symptomatic complications (usually from chiasmatic compression) attributable to brachytherapy was 5.8%, and there was a 1.6% rate of internal carotid artery injury.32 Hasegawa and colleagues treated 49 patients with cystic craniopharyngiomas (25 primary and 24 recurrent) by stereotactic 32P intracavitary brachytherapy, with the radiation dose to the cyst wall ranging from 189 to 250 Gy. The survival rate was 90% at 5 years and 70% at 10 years.33 There was delayed visual deterioration in 23%, improved visual function in 48%, and new hormonal dysfunction in 29% of patients. Tumor cyst control rates were 70% at 10 years after diagnosis.33 Thus, although effective, intracavitary brachytherapy for cystic craniopharyngioma has such a level of toxicity that it should be reserved for cysts that have a significant mass effect and recur after multiple surgeries.
Brachytherapy for Metastases
Newly diagnosed and solitary metastases that recur after surgery and external beam irradiation are among the most common tumor types treated with brachytherapy, particularly metastases larger than 3 cm that are not amenable to radiosurgery and are at greater risk for recurrence after surgical resection. Given the long-term toxicity associated with whole-brain radiotherapy, brachytherapy has been investigated for single brain metastases as an alternative to whole-brain radiotherapy after surgical resection of single metastases. Schulder and coauthors reported the use of permanent low-activity 125I seeds instead of postoperative whole-brain irradiation in 13 patients who had undergone gross total resection of recurrent single brain metastases too large for radiosurgery (3 to 6 cm in diameter).34 Implant doses ranged from 43 to 132 Gy, with a mean of 83 Gy.34 Median survival was 9 months.34 Bogart and colleagues reported the use of low-activity permanent 125I seeds after resection without whole-brain irradiation in 15 patients with solitary brain metastases from primary non–small cell lung cancer.35 Median survival was 14 months, with failure of local control in the brain in 5 patients, all of whom had original metastatic tumors larger than 2.5 cm in diameter (2 exhibited recurrence at the site of the original metastasis, 2 had brain metastases at other sites, and multiple brain metastases at the original site and elsewhere in the brain developed in 1).35 In a retrospective review from two institutions (1997 to 2003), 26 patients underwent gross total resection of single metastases followed by implantation of 125I seeds. Median survival was 17.8 months; distant brain metastases developed in 3 of the 26 patients and radiation necrosis requiring reoperation in 2 of the 26.3 Around the same time, in a prospective phase II multi-institution trial, 54 patients underwent GliaSite brachytherapy at a dose of 60 Gy and depth of 1 cm after complete surgical resection. The local control rate was 82% to 87%, with a median survival of 40 weeks.11 All four of these studies of brachytherapy for single metastases have produced results that are similar to those reported with resection plus whole-brain radiation therapy, but a true comparison will be possible only with large randomized trials.
Brachytherapy for Meningiomas
Recurrent atypical and malignant meningiomas are difficult to treat successfully, with chemotherapy being unsuccessful to date, radiosurgery being limited to smaller tumors, and reoperation providing incomplete tumor control and limited prolongation of survival. Kumar and colleagues treated 15 patients with primary and recurrent skull base meningiomas (detailed pathologic information was not provided) with permanent low-activity 125I implants delivering doses ranging from 100 to 500 Gy.36 At a median follow-up of 29 months, the authors reported a remarkable complete radiographic response rate of 73%, partial responses in the remaining patients, and no early or late delayed complications.36 Ware and associates reported the UCSF experience in treating 17 recurrent malignant meningiomas and 4 recurrent atypical meningiomas with permanent 125I low-activity seeds having a median total activity of 20 mCi.37 The median time to progression was 11.6 months and median survival was 2.4 years after brachytherapy for malignant meningioma.37 However, 33% of the patients had complications requiring surgical intervention: radiation necrosis (27%), wound breakdown (27%), or both. The high incidence of wound breakdown underscores the importance of meticulous technique in brachytherapy cases.34
Brachytherapy for Pediatric Brain Tumors
Three groups reported the results of brachytherapy with temporary high-activity implants for pediatric brain tumors. In 1996, Sneed and coworkers reported the results of long-term follow-up of 28 pediatric patients treated at UCSF with high-activity implants between 1980 and 1991.38 There were 15 glioblastomas, 10 low-grade gliomas, 2 choroid plexus carcinomas, and 1 rhabdomyosarcoma. All patients underwent external beam radiation therapy, and brachytherapy doses ranged from 31.2 to 83.8 Gy. The reoperation rate was 79% a median of 10 months after the brachytherapy implant, and radiation necrosis with or without tumor was identified in 67% of patients at reoperation, a problem reported in other studies of high-activity 125I implants.38 All the glioblastoma patients died, and the median follow-up for the 13 living patients was 8.9 years.38 In 1998, Chuba and coauthors reported the results of treating 28 patients with permanent 125I implants, 10 of which involved stereotactic implantation into brainstem gliomas.39 No complications related to catheter placement occurred. Brachytherapy was carried out after completion of standard external beam irradiation. The planned implant dose was 82.9 Gy at 4 cGy/hr. Half the patients died 7 to 9 months after diagnosis, and 4 remained alive at a median of 10 months after diagnosis. In 2001, Rostomily and associates reported the results of placing permanent low-activity 125I seeds in 6 patients 2 to 14 years of age with residual tumor after surgery. Two of these patients had supratentorial primitive neuroectodermal tumors, 1 had medulloblastoma, 1 had malignant ependymoma, 1 had glioblastoma, and 1 had pleomorphic xanthoastrocytoma. A total of 11 to 126 seeds were implanted, which resulted in doses of 160 to 218 Gy at a depth of 5 mm from the implanted resection bed. Two patients had lasting tumor control, with 1 being alive at 390 weeks and another dying of remote recurrence at 366 weeks.40 These findings suggest that brachytherapy may be safe and effective enough in pediatric brain tumor patients to warrant further study in larger clinical trials.
Bogart JA, Ungureanu C, Shihadeh E, et al. Resection and permanent I-125 brachytherapy without whole brain irradiation for solitary brain metastasis from non–small cell lung carcinoma. J Neurooncol. 1999;44:53-57.
Chan TA, Weingart JD, Parisi M, et al. Treatment of recurrent glioblastoma multiforme with GliaSite brachytherapy. Int J Radiat Oncol Biol Phys. 2005;62:1133-1139.
Chen AM, Chang S, Pouliot J, et al. Phase I trial of gross total resection, permanent iodine-125 brachytherapy, and hyperfractionated radiotherapy for newly diagnosed glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2007;69:825-830.
Chuba PJ, Zamarano L, Hamre M, et al. Permanent I-125 brain stem implants in children. Childs Nerv Syst. 1998;14:570-577.
Dagnew E, Kanski J, McDermott MW, et al. Management of newly diagnosed single brain metastasis using resection and permanent iodine-125 seeds without initial whole-brain radiotherapy: a two institution experience. Neurosurg Focus. 2007;22(3):E3.
Darakchiev BJ, Albright RE, Breneman JC, et al. Safety and efficacy of permanent iodine-125 seed implants and carmustine wafers in patients with recurrent glioblastoma multiforme. J Neurosurg. 2008;108:236-242.
Gabayan AJ, Green SB, Sanan A, et al. GliaSite brachytherapy for treatment of recurrent malignant gliomas: a retrospective multi-institutional analysis. Neurosurgery. 2006;58:701-709.
Gaspar LE, Zamorano LJ, Shamsa F, et al. Permanent 125iodine implants for recurrent malignant gliomas. Int J Radiat Oncol Biol Phys. 1999;43:977-982.
Hasegawa T, Kondziolka D, Hadjipanayis CG, et al. Management of cystic craniopharyngiomas with phosphorus-32 intracavitary irradiation. Neurosurgery. 2004;54:813-820.
Julow J, Backlund EO, Lanyi F, et al. Long-term results and late complications after intracavitary yttrium-90 colloid irradiation of recurrent cystic craniopharyngiomas. Neurosurgery. 2007;61:288-295.
Koot RW, Maarouf M, Hulshof MC, et al. Brachytherapy: results of two different therapy strategies for patients with primary glioblastoma multiforme. Cancer. 2000;88:2796-2802.
Kreth FW, Faist M, Grau S, et al. Interstitial 125I radiosurgery of supratentorial de novo WHO grade 2 astrocytoma and oligoastrocytoma in adults: long-term results and prognostic factors. Cancer. 2006;106:1372-1381.
Laperriere NJ, Leung PM, McKenzie S, et al. Randomized study of brachytherapy in the initial management of patients with malignant astrocytoma. Int J Radiat Oncol Biol Phys. 1998;41:1005-1011.
Larson DA, Suplica JM, Chang SM, et al. Permanent iodine 125 brachytherapy in patients with progressive or recurrent glioblastoma multiforme. Neuro Oncol. 2004;6:119-126.
McDermott MW, Sneed PK, Gutin PH. Interstitial brachytherapy for malignant brain tumors. Semin Surg Oncol. 1998;14:79-87.
Patel et al Patel S, Breneman JC, Warnick RE, et al. Permanent iodine-125 interstitial implants for the treatment of recurrent glioblastoma multiforme. Neurosurgery. 2000;46:1123-1128.
Rogers LR, Rock JP, Sills AK, et al. Results of a phase II trial of the GliaSite radiation therapy system for the treatment of newly diagnosed, resected single brain metastases. J Neurosurg. 2006;105:375-384.
Rostomily RC, Halligan J, Geyer R, et al. Permanent low-activity (125)I seed placement for the treatment of pediatric brain tumors: preliminary experience. Pediatr Neurosurg. 2001;34:198-205.
Schulder M, Black PM, Shrieve DC, et al. Permanent low-activity iodine-125 implants for cerebral metastases. J Neurooncol. 1997;33:213-221.
Selker RG, Shapiro WR, Burger P, et al. The Brain Tumor Cooperative Group NIH Trial 87-01: a randomized comparison of surgery, external radiotherapy, and carmustine versus surgery, interstitial radiotherapy boost, external radiation therapy, and carmustine. Neurosurgery. 2002;51:343-355.
Sneed PK, Russo C, Scharfen CO, et al. Long-term follow-up after high-activity 125I brachytherapy for pediatric brain tumors. Pediatr Neurosurg. 1996;24:314-322.
Sneed PK, Stauffer PR, McDermott MW, et al. Survival benefit of hyperthermia in a prospective randomized trial of brachytherapy boost +/− hyperthermia for glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 1998;40:287-295.
Tatter SB, Shaw EG, Rosenblum ML, et al. An inflatable balloon catheter and liquid 125I radiation source (GliaSite Radiation Therapy System) for treatment of recurrent malignant glioma: multicenter safety and feasibility trial. J Neurosurg. 2003;99:297-303.
Ware ML, Larson DA, Sneed PK, et al. Surgical resection and permanent brachytherapy for recurrent atypical and malignant meningioma. Neurosurgery. 2004;54:55-63.
Welsh J, Sanan A, Gabayan AJ, et al. GliaSite brachytherapy boost as part of initial treatment of glioblastoma multiforme: a retrospective multi-institutional pilot study. Int J Radiat Oncol Biol Phys. 2007;68:159-165.
1 Bernstein M, Gutin PH. Interstitial irradiation of brain tumors: a review. Neurosurgery. 1981;9:741-750.
2 Leksell L. The stereotaxic method and radiosurgery of the brain. Acta Chir Scand. 1951;102:316-319.
3 Dagnew E, Kanski J, McDermott MW, et al. Management of newly diagnosed single brain metastasis using resection and permanent iodine-125 seeds without initial whole-brain radiotherapy: a two institution experience. Neurosurg Focus. 2007;22(3):E3.
4 Sneed PK, Stauffer PR, Gutin PH, et al. Interstitial irradiation and hyperthermia for the treatment of recurrent malignant brain tumors. Neurosurgery. 1991;28:206-215.
5 Wust P, Hildebrandt B, Sreenivasa G, et al. Hyperthermia in combined treatment of cancer. Lancet Oncol. 2002;3:487-497.
6 Wen PY, Alexander E3rd, Black PM, et al. Long term results of stereotactic brachytherapy used in the initial treatment of patients with glioblastomas. Cancer. 1994;73:3029-3036.
7 Koot RW, Maarouf M, Hulshof MC, et al. Brachytherapy: results of two different therapy strategies for patients with primary glioblastoma multiforme. Cancer. 2000;88:2796-2802.
8 Chen AM, Chang S, Pouliot J, et al. Phase I trial of gross total resection, permanent iodine-125 brachytherapy, and hyperfractionated radiotherapy for newly diagnosed glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2007;69:825-830.
9 Gaspar LE, Zamorano LJ, Shamsa F, et al. Permanent 125iodine implants for recurrent malignant gliomas. Int J Radiat Oncol Biol Phys. 1999;43:977-982.
10 Patel S, Breneman JC, Warnick RE, et al. Permanent iodine-125 interstitial implants for the treatment of recurrent glioblastoma multiforme. Neurosurgery. 2000;46:1123-1128.
11 Rogers LR, Rock JP, Sills AK, et al. Results of a phase II trial of the GliaSite radiation therapy system for the treatment of newly diagnosed, resected single brain metastases. J Neurosurg. 2006;105:375-384.
12 Welsh J, Sanan A, Gabayan AJ, et al. GliaSite brachytherapy boost as part of initial treatment of glioblastoma multiforme: a retrospective multi-institutional pilot study. Int J Radiat Oncol Biol Phys. 2007;68:159-165.
13 Gabayan AJ, Green SB, Sanan A, et al. GliaSite brachytherapy for treatment of recurrent malignant gliomas: a retrospective multi-institutional analysis. Neurosurgery. 2006;58:701-709.
14 Tatter SB, Shaw EG, Rosenblum ML, et al. An inflatable balloon catheter and liquid 125I radiation source (GliaSite Radiation Therapy System) for treatment of recurrent malignant glioma: multicenter safety and feasibility trial. J Neurosurg. 2003;99:297-303.
15 Szeifert GT, Julow J, Slowik F, et al. Pathological changes in cystic craniopharyngiomas following intracavital 90yttrium treatment. Acta Neurochir (Wien). 1990;102:14-18.
16 Fig LM, Shapiro B, Taren J. Distribution of [32P]-chromic phosphate colloid in cystic brain tumors. Stereotact Funct Neurosurg. 1992;59:166-168.
17 Ling CC, Chui CS. Stereotactic treatment of brain tumors with radioactive implants or external photon beams: radiobiophysical aspects. Radiother Oncol. 1993;26:11-18.
18 Fowler JF. Why shorter half-times of repair lead to greater damage in pulsed brachytherapy. Int J Radiat Oncol Biol Phys. 1993;26:353-356.
19 Kim JH, Alfieri AA, Rosenblum M, et al. Low dose rate radiotherapy for transplantable gliosarcoma in the rat brain. J Neurooncol. 1990;9:9-15.
20 Ling CC, Spiro IJ, Mitchell J, et al. The variation of OER with dose rate. Int J Radiat Oncol Biol Phys. 1985;11:1367-1373.
21 Kobayashi T, Kageyama N, Ohara K. Internal irradiation for cystic craniopharyngioma. J Neurosurg. 1981;55:896-903.
22 Laperriere NJ, Leung PM, McKenzie S, et al. Randomized study of brachytherapy in the initial management of patients with malignant astrocytoma. Int J Radiat Oncol Biol Phys. 1998;41:1005-1011.
23 Selker RG, Shapiro WR, Burger P, et al. The Brain Tumor Cooperative Group NIH Trial 87-01: a randomized comparison of surgery, external radiotherapy, and carmustine versus surgery, interstitial radiotherapy boost, external radiation therapy, and carmustine. Neurosurgery. 2002;51:343-355.
24 Sneed PK, Stauffer PR, McDermott MW, et al. Survival benefit of hyperthermia in a prospective randomized trial of brachytherapy boost +/− hyperthermia for glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 1998;40:287-295.
25 Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987-996.
26 Larson DA, Suplica JM, Chang SM, et al. Permanent iodine 125 brachytherapy in patients with progressive or recurrent glioblastoma multiforme. Neuro Oncol. 2004;6:119-126.
27 Chan TA, Weingart JD, Parisi M, et al. Treatment of recurrent glioblastoma multiforme with GliaSite brachytherapy. Int J Radiat Oncol Biol Phys. 2005;62:1133-1139.
28 Chakravarti A, Erkkinen MG, Nestler U, et al. Temozolomide-mediated radiation enhancement in glioblastoma: a report on underlying mechanisms. Clin Cancer Res. 2006;12:4738-4746.
29 Darakchiev BJ, Albright RE, Breneman JC, et al. Safety and efficacy of permanent iodine-125 seed implants and carmustine wafers in patients with recurrent glioblastoma multiforme. J Neurosurg. 2008;108:236-242.
30 Ostertag CB, Kreth FW. Iodine-125 interstitial irradiation for cerebral gliomas. Acta Neurochir (Wien). 1992;119:53-61.
31 Kreth FW, Faist M, Grau S, et al. Interstitial 125I radiosurgery of supratentorial de novo WHO Grade 2 astrocytoma and oligoastrocytoma in adults: long-term results and prognostic factors. Cancer. 2006;106:1372-1381.
32 Julow J, Backlund EO, Lanyi F, et al. Long-term results and late complications after intracavitary yttrium-90 colloid irradiation of recurrent cystic craniopharyngiomas. Neurosurgery. 2007;61:288-295.
33 Hasegawa T, Kondziolka D, Hadjipanayis CG, et al. Management of cystic craniopharyngiomas with phosphorus-32 intracavitary irradiation. Neurosurgery. 2004;54:813-820.
34 Schulder M, Black PM, Shrieve DC, et al. Permanent low-activity iodine-125 implants for cerebral metastases. J Neurooncol. 1997;33:213-221.
35 Bogart JA, Ungureanu C, Shihadeh E, et al. Resection and permanent I-125 brachytherapy without whole brain irradiation for solitary brain metastasis from non–small cell lung carcinoma. J Neurooncol. 1999;44:53-57.
36 Kumar PP, Patil AA, Syh HW, et al. Role of brachytherapy in the management of the skull base meningioma. Treatment of skull base meningiomas. Cancer. 1993;71:3726-3731.
37 Ware ML, Larson DA, Sneed PK, et al. Surgical resection and permanent brachytherapy for recurrent atypical and malignant meningioma. Neurosurgery. 2004;54:55-63.
38 Sneed PK, Russo C, Scharfen CO, et al. Long-term follow-up after high-activity 125I brachytherapy for pediatric brain tumors. Pediatr Neurosurg. 1996;24:314-322.
39 Chuba PJ, Zamarano L, Hamre M, et al. Permanent I-125 brain stem implants in children. Childs Nerv Syst. 1998;14:570-577.
40 Rostomily RC, Halligan J, Geyer R, et al. Permanent low-activity (125)I seed placement for the treatment of pediatric brain tumors: preliminary experience. Pediatr Neurosurg. 2001;34:198-205.