Interpretation of Medication Labels and Orders
• Describe components of a prescription
• Interpret prescriptions and medication orders
• Interpret labels found on medication containers
• Use and understand units of measurement found in pharmacology
What Does a Prescription Indicate?
Figure 6-1 provides short descriptions of the parts of a prescription. The five major components are the superscription, inscription, signa (Sig), subscription and signature of the health care professional. Line A of Figure 6-1 is the preprinted name of the physician or group of physicians, the address, and phone number. Line B includes the patient’s name, address, date of the prescription, and the patient’s age if the patient is a child. Dating the prescription is important because prescriptions must be filled within 12 months of writing except with controlled substances or scheduled medications, and the refilling of prescriptions is dependent on the date. Line C is the superscription or the symbol  , meaning “recipe” or “take thou,” from Latin. The inscription, which specifies the name of the medication, its strength, and quantity of drug to be dispensed, appears on line D. If the medication must be compounded or prepared, the ingredients would appear in this location. Line E is the “Sig,” or signa, giving directions for taking the medication. The subscription, line F, tells the pharmacist the drug form, as well as how the medication is be taken. The physician’s signature appears on line G, and the number of allowed refills on Line H. Finally, if the prescription is for a controlled medication, the physician must place his or her U. S. Drug Enforcement Administration (DEA) number either under or beside the signature.
, meaning “recipe” or “take thou,” from Latin. The inscription, which specifies the name of the medication, its strength, and quantity of drug to be dispensed, appears on line D. If the medication must be compounded or prepared, the ingredients would appear in this location. Line E is the “Sig,” or signa, giving directions for taking the medication. The subscription, line F, tells the pharmacist the drug form, as well as how the medication is be taken. The physician’s signature appears on line G, and the number of allowed refills on Line H. Finally, if the prescription is for a controlled medication, the physician must place his or her U. S. Drug Enforcement Administration (DEA) number either under or beside the signature.
Abbreviations are often used in the writing of prescriptions. Only standard abbreviations should be used. Remember that a prescription is a legal document that could appear in a court case. In those instances, “local” or nonstandard abbreviations might become an area of concern for possible misinterpretation. Abbreviations are actually medical shorthand that is used to write clear and concise orders that will be used among health care professionals. For orders to be correctly written and interpreted, the health care professional and the person dispensing the medication must understand the meaning of each abbreviation used. For commonly accepted abbreviations, see the table on the inside front cover and Chapter 1.
What Is a Medication Order?
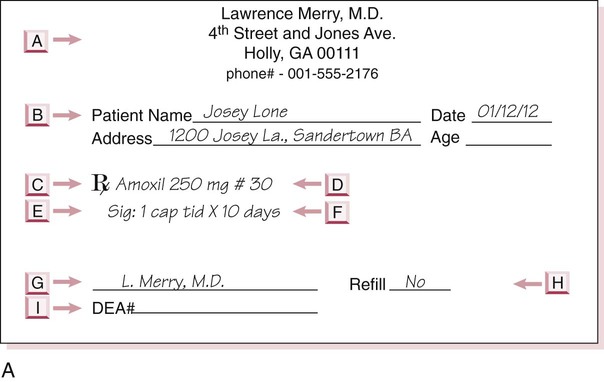
A medication order is a method of providing the same information as found on a prescription given to the patient, but is used in an inpatient or physician’s office environment (Figure 6-2). An exception with the medication order is that the order is written for either the number of doses or for the specific length of time for the medicine to be taken. With the medication order, the health care professional is told what drug or drugs should be administered, the strength of the medication, and the frequency to be taken. This order is a means of providing drugs at correct frequency in an inpatient setting. The medication order has six parts: date; patient name, which may appear on the patient record; medication name; dose or medication strength/form; route, time, and frequency of administration; and signature of the prescribing professional. Medication orders may be verbally communicated, but for legal purposes, each order should be transcribed onto a written form by the health care professional who accepts the order and the order must be countersigned by the health care professional who communicated the order and is licensed to prescribe medications in the state of practice.
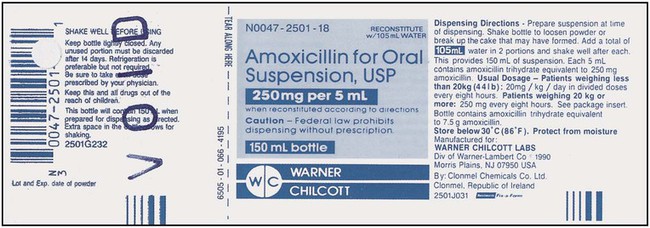
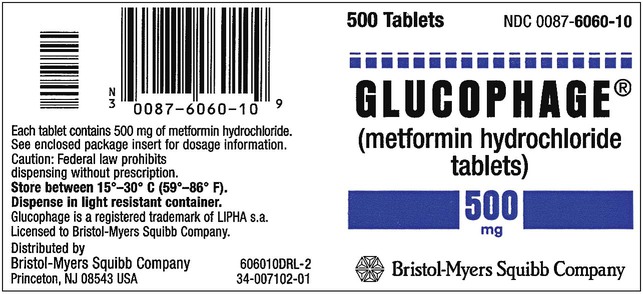
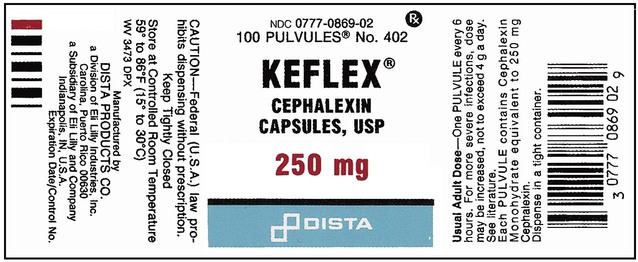
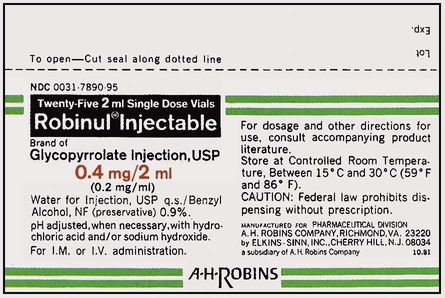
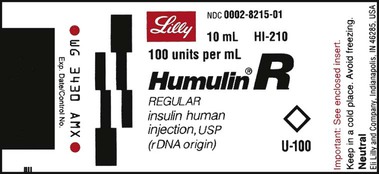
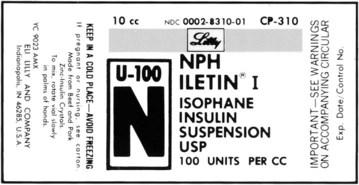
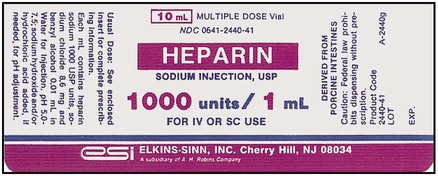
What Does a Medication Label Indicate?
• The generic name is the official name that has been given to a medication when it is accepted by the FDA and is the name found in the U.S. Pharmacopeia-National Formulary (USP-NF).
• The trade or brand name or the marketed name is the name assigned by the manufacturer for a given product. This name begins with a capital letter, such as Valium. Registered trade or brand medications are marked with ® to show that the name is registered.
• The National Drug Code (NDC) number. This usually appears just above the drug name at the top of the label. The NDC identifies the manufacturer, the product, and the size of the container in which the medication is packed.
• The dosage strength, or the amount of active ingredient found in the medication per dosage form, found in the container, such as milligrams (mg), units (u), grams (g), milliliters (mL), grains (gr), or milliequivalents (mEq).
• The total quantity of medication as packed by the manufacturer.
• The dosage form of the medication.
• The name of the company manufacturing the medication.
• Special instructions for mixing or compounding the medicine if indicated by the manufacturer.
• Storage requirements of the medication because of environmental factors.
• Lot and batch numbers or control number of the medication that can be used for identification if the medication is recalled.
Review of Rules
Interpreting Medication Labels and Orders
• Always read the entire label or prescription/order before making decisions concerning the medication to be administered.
• If you are unfamiliar with the medication, read information concerning the medicine before dispensing.
• The drug label will give the total amount of medication in the package, whether this is in solid doses such as tablets or capsules or in liquid medication such as the total volume in the container.
• Solid medications are usually ordered in the weight of medication per tablet. The label will read in milligrams, grams, grains, or other solid measurements per drug form.
• Liquid medications will be found in the strength (weight) in a volume (or the amount of solvent) of medication such as mg/mL.
• The generic name for the medication will be written in lower case letters, whereas the trade or proprietary name will begin with a capital letter and may be followed by ®.
• If there is a question about the medication ordered, always ask the pharmacist to verify the medication before beginning the process of preparing the medication for dispensing.
• Always read medication labels three times to ensure correctness of the dispensed medication to the medication order/prescription: (1) when removing stock medication from the storage shelf; (2) before preparing medication; and (3) before returning stock medication back to the shelf, if applicable.


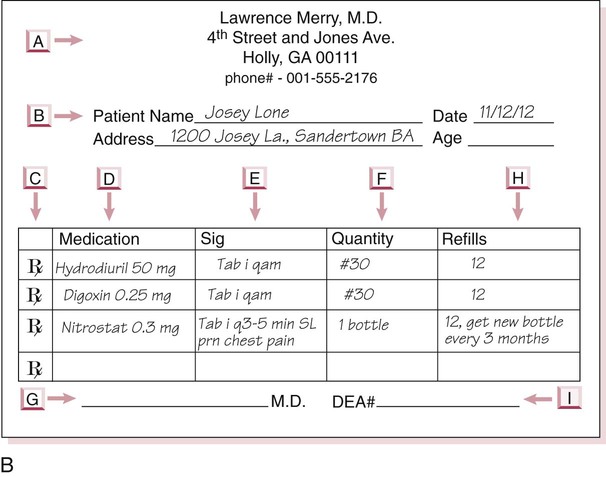
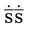 po q4h prn itching
po q4h prn itching

























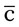 am meal
am meal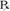 ), are at the level of highest quality possible and are the exact medications that have been ordered by the health care professional.
), are at the level of highest quality possible and are the exact medications that have been ordered by the health care professional.






















 ii po q4h until all medication is taken*
ii po q4h until all medication is taken*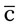 meals and hs
meals and hs 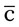 snack
snack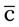 pulse ↑60
pulse ↑60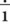 po q4-6h prn nasal congestion
po q4-6h prn nasal congestion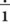 po bid
po bid