Intensive insulin therapy
1. What is intensive insulin therapy?
Intensive insulin therapy (IIT) is the use of multiple daily injections (MDIs) of insulin (both long-acting and rapid-acting formulations) or an insulin pump in an effort to mimic the normal secretion of insulin by the pancreas. IIT may also be referred to as physiologic, multiple-component, or basal-bolus insulin therapy. IIT is only one aspect of comprehensive, intensive diabetes therapy to achieve tight glycemic control. IIT is complex because it requires multiple injections or pump boluses per day in addition to routine monitoring and collaborative decision making.
2. List critical components of intensive therapy.
 Frequent self-monitored blood glucose (SMBG) measurements
Frequent self-monitored blood glucose (SMBG) measurements
 Defined and individualized target blood glucose (BG) levels
Defined and individualized target blood glucose (BG) levels
 Use of SMBG data and glucose patterns to meet treatment goals
Use of SMBG data and glucose patterns to meet treatment goals
 Dose modifications according to the individual’s response to therapy
Dose modifications according to the individual’s response to therapy
 Understanding of diet composition, specifically carbohydrate content
Understanding of diet composition, specifically carbohydrate content
 Careful balance of food intake, activity, and insulin dosage
Careful balance of food intake, activity, and insulin dosage
 Use of carbohydrate-to-insulin ratios according to food intake
Use of carbohydrate-to-insulin ratios according to food intake
 Use of correction factors (CFs) for the adjustment of insulin according to glucose levels
Use of correction factors (CFs) for the adjustment of insulin according to glucose levels
 Patient education and motivation, and ongoing interaction between patient and health care team
Patient education and motivation, and ongoing interaction between patient and health care team
3. Summarize studies evaluating optimal glycemic control to decrease chronic diabetes complications.
The Diabetes Control and Complications Trial (DCCT), evaluating patients with recent-onset type 1 diabetes, showed that improved glycemic control (hemoglobin A1C [HbA1C] < 7%) significantly reduced rates of microvascular complications, including progression of retinopathy, nephropathy, and neuropathy, but increased rates of hypoglycemia. Intensive insulin therapy was a key part of achieving glycemic control in the DCCT. The Kumamoto Study and the United Kingdom Prospective Diabetes Study (UKPDS) confirmed that improved glycemic control (HbA1C < 7%) was associated with significantly reduced rates of microvascular complications in patients with recent-onset type 2 diabetes. The long-term extensions of the DCCT and the UKPDS showed significant reductions in cardiovascular complications with good glycemic control and demonstrated that the microvascular benefits of good glycemic control persisted for decades. Later studies in patients with more advanced type 2 diabetes (Action to Control Cardiovascular Risk in Diabetes [ACCORD] trial, Action in Diabetes and Vascular Disease [ADVANCE] trial, and VA Diabetes Trial [VADT]) failed to show that more aggressive glycemic targets (HbA1C < 6.0%-6.5%) reduced cardiovascular complications, and one study showed an increase in mortality. Rates of hypoglycemia with more aggressive glucose control were significant in all three trials.
4. Which patients are candidates for IIT?
All people with diabetes should be considered potential candidates for IIT. However, the degree of therapy intensification must be based on each patient’s personal situation and abilities. Patient characteristics that predict greater success with IIT include motivation, willingness to perform frequent SMBG (up to 6-10 times/day) measurements and record results, time to spend with a diabetes educator, the ability to recognize and treat hypoglycemia, sick day planning, and a supportive network of family or friends. In addition, implementation of IIT requires a cohesive diabetes team that is available for frequent interaction and discussion about results of monitoring, insulin adjustments, and other issues.
5. What are the risks of intensive insulin therapy?
Hypoglycemia and weight gain are the most common adverse effects of insulin therapy. IIT in the DCCT resulted in a three-fold increased risk of severe hypoglycemia in comparison with conventional treatment (62 episodes per 100 patient-years of therapy). Since the completion of the DCCT, newer rapid-acting and long-acting insulin analogs have been developed that are associated with less hypoglycemia than the short-acting and intermediate-acting human insulin products used in the trial. Frequent episodes of hypoglycemia can lead to loss of clinical warning symptoms (e.g., palpitations, sweating, hunger) with hypoglycemia (known as hypoglycemia unawareness). A unique risk of pump therapy is diabetic ketoacidosis because pump malfunctions or infusion site problems can interrupt insulin delivery. Finally, IIT requires time and commitment from the patient and may have negative psychosocial and economic implications.
6. Explain the difference between basal and bolus insulin coverage.
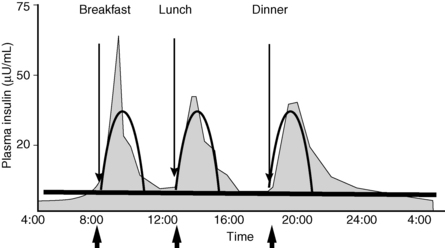
Intensive insulin therapy attempts to mimic normal insulin secretion, which includes continuous basal coverage in addition to bursts of insulin to regulate the rise in glucose after food intake (Fig. 3-1). Basal insulin secretion suppresses hepatic glucose production to control blood glucose levels in the fasting state and premeal periods. Normal basal insulin secretion from the pancreas varies slightly throughout the day, responding to changes in activity, blood glucose levels, and regulatory hormones. Basal coverage is usually accomplished with injections of long-acting insulin preparations or with the basal infusion function on the insulin pump. Bolus insulin doses consist of two components, the nutritional dose (the amount of insulin required to manage glucose excursions following meals) and the correction dose (the amount of insulin required to reduce a high glucose level detected before a meal). Bolus coverage is accomplished by injections of rapid-acting or short-acting insulin preparations or with use of the bolus function on the insulin pump. Physiologic insulin secretion requirements are approximately 50% basal and 50% bolus.
7. How are basal and bolus insulins used with an MDI regimen?
A long-acting insulin is injected either once or twice daily to provide the basal insulin portion of an MDI regimen, which is approximately 50% of a patient’s total daily dose. Ideally, basal insulin should cover background insulin needs only, independent of food intake. A rapid-acting or short-acting insulin is injected before meals to provide the bolus insulin portion of an MDI regimen (see Fig. 3-1). Rapid-acting insulin is preferred because of the rapid onset and short duration of action. A patient can adjust each bolus dose to match the carbohydrate intake and to correct for high glucose levels before the meal, whereas the basal dose remains constant from day to day. Premixed “biphasic” insulin preparations combine either a rapid-acting insulin analog or regular human insulin with a crystalline protaminated form of the analog or NPH (neutral protamine Hagedorn) human insulin in an attempt to imitate basal and bolus therapies with fewer injections.
8. What are the currently available insulin preparations?
TABLE 3-1.
THE PHARMACODYNAMICS OF INSULIN PREPARATIONS
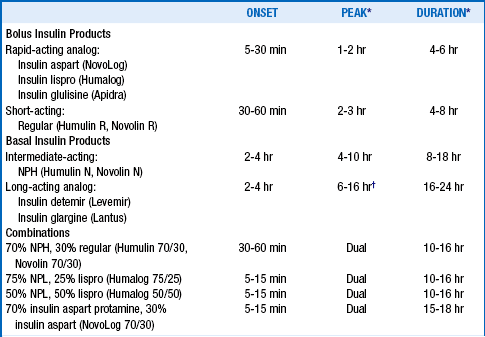
NPH, neutral protamine Hagedorn; NPL, neutral protamine lispro suspension.
*The peak and duration of insulin action are variable, depending on injection site, duration of diabetes, renal function, smoking status, and other factors.
†Insulin glargine is considered “peakless” although it has exhibited peak effects during comparative testing.
9. Describe the pharmacodynamics of insulin preparations.
10. When should bolus insulin be taken?
 Five to 10 minutes before meals and snacks when BG is in the normal range (70-130 mg/dL)
Five to 10 minutes before meals and snacks when BG is in the normal range (70-130 mg/dL)
 Fifteen to 30 minutes before meals if the premeal BG is higher than 130 mg/dL (Correctional bolus insulin [CF] is added to meal insulin when the BG is elevated.)
Fifteen to 30 minutes before meals if the premeal BG is higher than 130 mg/dL (Correctional bolus insulin [CF] is added to meal insulin when the BG is elevated.)
 Immediately after eating, if gastroparesis or an intercurrent illness is present
Immediately after eating, if gastroparesis or an intercurrent illness is present
 Upon arrival of food, if the patient is unfamiliar with meal size, content, or timing (i.e., in a restaurant or hospital)
Upon arrival of food, if the patient is unfamiliar with meal size, content, or timing (i.e., in a restaurant or hospital)
11. When should basal insulin be taken?
 Insulin glargine or detemir should be taken at bedtime if a dawn phenomenon is present or at any consistent time, approximately every 24 hours. (Insulin glargine or detemir cannot be mixed with other insulins.)
Insulin glargine or detemir should be taken at bedtime if a dawn phenomenon is present or at any consistent time, approximately every 24 hours. (Insulin glargine or detemir cannot be mixed with other insulins.)
 If nocturnal hypoglycemia results from taking a full dose of glargine or detemir at bedtime, an option would be to split the dose so that 50% is taken in the morning and the other 50% is taken in the evening, approximately 12 hours apart.
If nocturnal hypoglycemia results from taking a full dose of glargine or detemir at bedtime, an option would be to split the dose so that 50% is taken in the morning and the other 50% is taken in the evening, approximately 12 hours apart.
 NPH insulin is given in the morning and at bedtime to avoid nocturnal hypoglycemia.
NPH insulin is given in the morning and at bedtime to avoid nocturnal hypoglycemia.
An insulin pump is a small, lightweight, portable, battery-operated device that is either attached directly to the body (patch pump) or worn on clothing or a belt like a pager (traditional pump). A traditional pump is composed of a pump reservoir (which holds a 2- to 3-day supply of rapid-acting or short-acting insulin) connected to an infusion set, which ends in a cannula that is inserted into the skin and changed every 2 to 3 days. A patch pump is tubing free and consists of a disposal reservoir that attaches directly to the body with self-adhesive backing and a built-in infusion set in the device for insertion into the subcutaneous tissue. The patch pump is controlled by a handheld personal digital assistant. Insulin is delivered through either system in microliter amounts continuously over 24 hours. The user is responsible for setting basal rates and determining bolus doses, depending on the meal ingested and the SMBG results. Currently, six companies offer insulin pumps in the United States; several other pumps are in development. Each pump has special features and functions that are unique and help with the flexibility of pump use. To learn more about each of these pumps, contact the companies listed in Table 3-2.
TABLE 3-2.
CURRENTLY AVAILABLE INSULIN PUMPS
| COMPANY | INSULIN PUMP | WEBSITE |
| Roche Insulin Delivery Systems | ACCU-CHEK Spirit | Accu-chekinsulinpumps.com |
| Sooil Development | Dana Diabecare IIS | Sooilusa.com |
| Medtronic Diabetes | MiniMed Paradigm Real-Time Revel | Minimed.com |
| Insulet Corporation | OmniPod | MyOmniPod.com |
| Animas Corporation | OneTouch Ping | Animas.com |
| Tandem Diabetes Care | t:slim | Tandemdiabetes.com |
13. What are the patient’s responsibilities before insulin pump therapy can be initiated?
 Commit at least 2 to 3 months to pump initiation, including multiple meetings with the diabetes team before, during, and after the pump is initiated.
Commit at least 2 to 3 months to pump initiation, including multiple meetings with the diabetes team before, during, and after the pump is initiated.
 Demonstrate the ability to monitor BG values at least 4 to 10 times per day; keep logs of BG readings, insulin doses, and food consumed; and communicate information to the team.
Demonstrate the ability to monitor BG values at least 4 to 10 times per day; keep logs of BG readings, insulin doses, and food consumed; and communicate information to the team.
 Review pump training materials and practice pump functions at least 2 or 3 times before wearing the pump.
Review pump training materials and practice pump functions at least 2 or 3 times before wearing the pump.
 Be willing to test basal rates or agree to wear a continuous glucose monitoring (CGM) system or device to ensure that basal rates are set appropriately.
Be willing to test basal rates or agree to wear a continuous glucose monitoring (CGM) system or device to ensure that basal rates are set appropriately.
14. Describe the benefits of insulin pump therapy.
Currently, insulin pump therapy is the dosing strategy that most closely mimics physiologic insulin secretion. Benefits include better, more precise glucose control with less glycemic variability, a reduction in frequency and severity of hypoglycemia, ability to adjust basal rates throughout the day (Fig. 3-2), ability to extend bolus dose durations to better cover high-fat meals (see Fig. 3-2), improved flexibility of lifestyle, ability to administer small amounts of insulin (as little as 0.025 units), protection from overcorrection by tracking of active insulin, and ability to integrate with continuous glucose monitor technology.
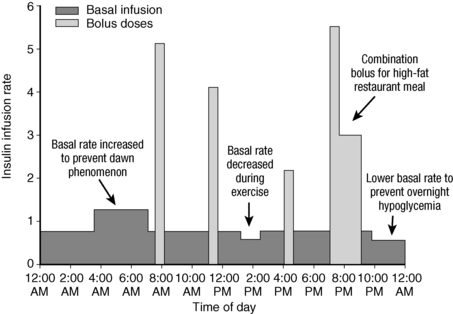
Figure 3-2. Insulin delivery pattern with pump therapy.
15. Describe the limitations of insulin pump therapy.
The cost of an insulin pump and supplies is higher than that of an MDI regimen. The device must be worn 24 hours a day, and optimal use requires a highly motivated, competent patient and a higher level of training. A strong support system from a diabetes team is beneficial. Other limitations include infusion-site infections and risks of diabetic ketoacidosis (DKA) if insulin delivery is interrupted.
Currently, there are three glucose-sensing, or continuous glucose monitoring (CGM), devices available for purchase—the Guardian Real-Time by Medtronic Diabetes (Northridge, CA), the MiniMed Paradigm Real-Time Revel by Medtronic Diabetes, and the SEVEN PLUS by DexCom (San Diego, CA). The sensing system consists of a monitor that collects the data and a sensor that is placed temporarily under the skin, generating an electrical signal that is proportional to the amount of glucose present in the interstitial fluid. The interstitial values are calibrated with finger-stick readings that must be entered into the system at least three times per day. These devices provide values every 5 minutes within a range of 40 to 400 mg/dL that are available to the wearer and feature alarms that sound if the values fall out of the target ranges programmed. Because the systems measure interstitial fluid glucose versus blood glucose (from the finger-stick readings) and lag behind changing glucose values by approximately 20 minutes, the sensor values cannot be used to determine bolus amounts. However, sensor information can be helpful for following blood glucose trends and patterns as well as for picking up on unexpected hypoglycemia, especially nocturnal episodes. Currently insurance coverage for these devices is limited.
17. Define carbohydrate counting. How is it used with IIT?
Currently, carbohydrate counting is considered the “gold standard” for estimation of mealtime insulin doses. Carbohydrate counting is a tool used to match bolus insulin doses to food intake because carbohydrates have the greatest effect on BG levels. The peak of bolus insulin analogs should match the peak of BG following carbohydrate digestion and absorption (≈1-3 hours, depending on the fat and fiber content of the meal).
18. List common foods that contain dietary carbohydrates.
 Starch: cereals, grains, beans, bread, rice, pasta, and starchy vegetables
Starch: cereals, grains, beans, bread, rice, pasta, and starchy vegetables
 Sugar: lactose (milk and yogurt), fructose (fruit, juice, and honey), and sucrose (table sugar and desserts)
Sugar: lactose (milk and yogurt), fructose (fruit, juice, and honey), and sucrose (table sugar and desserts)
 Fiber: cellulose and hemicellulose, lignins, gums, or pectins found in fruits, vegetables, legumes, and whole grains
Fiber: cellulose and hemicellulose, lignins, gums, or pectins found in fruits, vegetables, legumes, and whole grains
19. How are carbohydrates counted?
Calculating the number of carbohydrates may initially require measuring and weighing commonly eaten foods. Nutrition labels on food packages state the number of grams of carbohydrates based on the serving size. Carbohydrate reference books are available at bookstores or through the American Dietetic Association (http://www.eatright.org) or the American Diabetes Association (ADA; http://www .diabetes.org). Software programs are available for personal digital assistants (PDAs) or online. Many restaurant chains provide nutrition brochures.
20. Explain the carbohydrate-to-insulin (C:I) ratio.
The C:I ratio is used to estimate how many grams of carbohydrate each unit of rapid-acting insulin will cover (e.g., 20:1 = 20 g of carbohydrate consumed requires 1 unit of meal insulin).
21. How do you determine an initial C:I ratio?
Ratios are based on a patient’s total daily dose (TDD) of insulin, which usually indicates his or her sensitivity to insulin. An MDI regimen of basal insulin and premeal injections of rapid-acting insulin must be previously (or concurrently) implemented before a C:I ratio is established. A person must be taught to count carbohydrates before using a C:I ratio safely. Determine the C:I ratio as follows:
1. Add up the patient’s TDD of insulin with current therapy.
2. Consider the patient’s HbA1C value (ADA target is < 7%), frequency of hypoglycemia, and comorbidities.
3. The initial C:I is estimated by dividing 550 by the TDD. Example: 550 ÷30 units = 18. The C:I ratio is 18:1.
In clinical practice, the constant in the C:I formula may range from 350 to 550. The initial calculated C:I must then be adjusted on the basis of each patient’s records and is therefore only a starting point.
22. Give an example of an initial C:I ratio when changing to basal and bolus insulins.
 40 units of Humulin 70/30 premixed insulin in the morning
40 units of Humulin 70/30 premixed insulin in the morning
 17 units of Humulin 70/30 premixed insulin before the evening meal
17 units of Humulin 70/30 premixed insulin before the evening meal
 TDD = 57 units (HbA1C 8.5% with 2-3 nocturnal hypoglycemic episodes per week)
TDD = 57 units (HbA1C 8.5% with 2-3 nocturnal hypoglycemic episodes per week)
In this example, 1 unit of rapid-acting insulin will be given for every 10 g of carbohydrate eaten.
23. How do you adjust the C:I ratio once the initial ratio has been established?
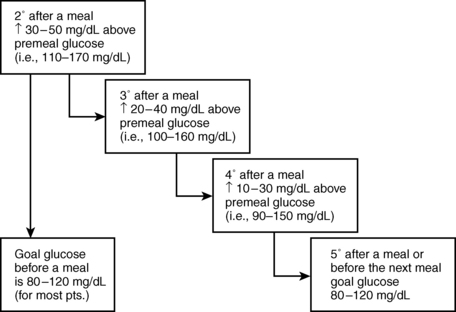
Fine-tuning of a C:I ratio is based on BG records before meals and 2 hours after meals. The desired premeal BG is 70 to 130 mg/dL for most patients using IIT. A C:I ratio is correct if the BG increases by approximately 30 to 50 mg/dL over the premeal value at the 2-hour postprandial reading and returns to the range of 70 to 130 mg/dL by about 5 hours after the bolus insulin is given (Fig. 3-3). If 2-hour postprandial BG increases exceed 50 mg/dL, the C:I ratio should be adjusted and testing repeated, with further adjustments until the desired excursion is consistently achieved.
24. What are common causes of high BG?
25. What are other factors to consider in troubleshooting high BG readings?
 Dawn phenomenon: A rise in BG occurs in predawn hours because of increased growth hormone and cortisol production.
Dawn phenomenon: A rise in BG occurs in predawn hours because of increased growth hormone and cortisol production.
 Bad insulin: High BG occurs when insulin denatures, which can be the result of (1) exposure to moderate to extreme temperatures or agitation, (2) use beyond the expiration date, or (3) use of a vial for more than 1 month.
Bad insulin: High BG occurs when insulin denatures, which can be the result of (1) exposure to moderate to extreme temperatures or agitation, (2) use beyond the expiration date, or (3) use of a vial for more than 1 month.
 Insulin pump or infusion set technical problems: settings programmed incorrectly; battery depleted; pump malfunction; tubing incorrectly primed; air bubbles in the tubing; dislodged, bent, or kinked cannula; occlusion at infusion site; infusion set in place for longer than 72 hours.
Insulin pump or infusion set technical problems: settings programmed incorrectly; battery depleted; pump malfunction; tubing incorrectly primed; air bubbles in the tubing; dislodged, bent, or kinked cannula; occlusion at infusion site; infusion set in place for longer than 72 hours.
26. What causes high postprandial BG readings that are difficult to explain?
 Coffee (caffeine): A rise in BG after drinking coffee (including drinking it black, without cream or sugar) is seen in many patients’ records and is likely due to increases in epinephrine or free fatty acid mobilization and subsequent worsening insulin resistance.
Coffee (caffeine): A rise in BG after drinking coffee (including drinking it black, without cream or sugar) is seen in many patients’ records and is likely due to increases in epinephrine or free fatty acid mobilization and subsequent worsening insulin resistance.
 Cereal: A rise in BG is seen by patients consuming cereal, which requires a lower C:I (more insulin) and may be related to the glycemic index of most cereals combined with greater insulin resistance in the morning.
Cereal: A rise in BG is seen by patients consuming cereal, which requires a lower C:I (more insulin) and may be related to the glycemic index of most cereals combined with greater insulin resistance in the morning.
 Food on the fingers: High BG readings occur from residual food or dextrose on fingers during testing (patient must wash hands or wipe off the first drop of blood).
Food on the fingers: High BG readings occur from residual food or dextrose on fingers during testing (patient must wash hands or wipe off the first drop of blood).
 Restaurant meals: Chinese food, Mexican food, pizza, and fried foods are high in fat and may require more insulin because of insulin resistance. A delay in digestion following a high-fat meal may require a split or extended bolus dose.
Restaurant meals: Chinese food, Mexican food, pizza, and fried foods are high in fat and may require more insulin because of insulin resistance. A delay in digestion following a high-fat meal may require a split or extended bolus dose.
27. How is correctional insulin added for high BG before meals?
Correctional or supplemental insulin (high-BG CF) is used to reduce a high BG detected before meals. A high-BG CF is the expected amount by which one unit of insulin will decrease the BG under normal circumstances. It is determined using a formula based on the person’s insulin sensitivity. The initial CF is estimated by dividing 1650 by the patient’s TDD. In clinical practice, the constant in the CF formula may range from 1500 to 1800. The initial calculated CF must then be adjusted on the basis of each patient’s records and is therefore only a starting point.
28. Give an example of determining an initial CF.
 The patient uses 17 units of insulin glargine at noon and 5 units insulin lispro before each meal.
The patient uses 17 units of insulin glargine at noon and 5 units insulin lispro before each meal.
 TDD = 32 units (HbA1C of 7.2% with 1-2 hypoglycemic episodes per week)
TDD = 32 units (HbA1C of 7.2% with 1-2 hypoglycemic episodes per week)
In this example, 1 unit of rapid-acting insulin will lower the BG about 50 mg/dL. Therefore, 1 extra unit will be taken (in addition to the meal insulin dose) for each 50 mg/dL by which the premeal BG exceeds the premeal goal of 100 mg/dL.
29. Give an example of C:I and CF usage.
To determine the amount of insulin needed before a meal, start by calculating the amount of bolus insulin needed to cover the meal:
 Meal consists of 80 grams of carbohydrates.
Meal consists of 80 grams of carbohydrates.
 Calculation: 80 ÷ 20 = 4 units of insulin
Calculation: 80 ÷ 20 = 4 units of insulin
Next, determine the amount of correctional insulin needed. If the BG is out of the target range before a meal, subtract the goal BG (100 mg/dL) from the actual BG, and divide by the CF.
 Calculation: 220 − 100 mg/dL = 120 mg/dL above target
Calculation: 220 − 100 mg/dL = 120 mg/dL above target
 Calculation: 120 (mg/dL) ÷ 60 = 2 units of insulin
Calculation: 120 (mg/dL) ÷ 60 = 2 units of insulin
In this example, the patient should take 6 units of bolus insulin before the meal, 4 units to cover the carbohydrates in the meal and 2 units to return the premeal high BG to the target range.
 It is recommended that correction boluses for high BG be taken before meals or at least 5 hours after the last bolus because of the duration of action of the bolus insulin analogs.
It is recommended that correction boluses for high BG be taken before meals or at least 5 hours after the last bolus because of the duration of action of the bolus insulin analogs.
 Hypoglycemia may occur from the accumulation of active insulin if BG corrections are performed too frequently.
Hypoglycemia may occur from the accumulation of active insulin if BG corrections are performed too frequently.
 A CF bolus is more effective if it is taken 15 to 30 minutes before eating. This time frame allows the insulin to begin working before the BG rises further because of the meal.
A CF bolus is more effective if it is taken 15 to 30 minutes before eating. This time frame allows the insulin to begin working before the BG rises further because of the meal.
31. What can be done for a high postprandial BG reading?
 If a postprandial BG is dangerously high (i.e., > 300 mg/dL) or a patient insists on making high BG corrections less than 5 hours since the last bolus or during the night, he or she should be instructed in how to take a partial correction for safety.
If a postprandial BG is dangerously high (i.e., > 300 mg/dL) or a patient insists on making high BG corrections less than 5 hours since the last bolus or during the night, he or she should be instructed in how to take a partial correction for safety.
 A target level of 150 mg/dL (expected BG level 2 hours postprandial) rather than a target BG of 100 mg/dL is used in the correction calculation between meals.
A target level of 150 mg/dL (expected BG level 2 hours postprandial) rather than a target BG of 100 mg/dL is used in the correction calculation between meals.
 Using half of the usual premeal CF to calculate the bolus needed to lower the BG to the target level is safest between meals because of the active insulin still present from the prior bolus.
Using half of the usual premeal CF to calculate the bolus needed to lower the BG to the target level is safest between meals because of the active insulin still present from the prior bolus.
32. Provide an example to calculate a half-CF bolus.
 BG 2 hours after dinner = 300 mg/dL
BG 2 hours after dinner = 300 mg/dL
 “Expected” BG 2 hours after dinner = ≈130 to 150 mg/dL
“Expected” BG 2 hours after dinner = ≈130 to 150 mg/dL
 Calculation: 300 −150 mg/dL = 150 mg/dL above target
Calculation: 300 −150 mg/dL = 150 mg/dL above target
 Calculation: 150 ÷ 60 = 2.5 units (full CF bolus)
Calculation: 150 ÷ 60 = 2.5 units (full CF bolus)
 The premeal insulin is still active for about 3 more hours; therefore, use half the CF bolus.
The premeal insulin is still active for about 3 more hours; therefore, use half the CF bolus.
 Calculation of half CF bolus: 2.5 (units) ÷ 2 = 1.3 units
Calculation of half CF bolus: 2.5 (units) ÷ 2 = 1.3 units
In this example, 1.3 units with an insulin pump or 1 unit with a syringe or insulin pen should be given 2 hours after the meal to bring the postprandial BG into the target range. BG should be rechecked within 2 hours to avoid a severely low glucose reading.
33. Calculate an initial basal rate for insulin pump therapy.
 An established C:I ratio and CF with MDI therapy are critical for a smooth transition to pump therapy because the same C:I and CF will be used with the pump.
An established C:I ratio and CF with MDI therapy are critical for a smooth transition to pump therapy because the same C:I and CF will be used with the pump.
 To calculate an initial basal rate, reduce the patient’s current TDD of insulin with MDI therapy by 25% (or other appropriate reduction, depending on current hemoglobin A1C level and number of hypoglycemic episodes) to estimate the new TDD to be used with the pump.
To calculate an initial basal rate, reduce the patient’s current TDD of insulin with MDI therapy by 25% (or other appropriate reduction, depending on current hemoglobin A1C level and number of hypoglycemic episodes) to estimate the new TDD to be used with the pump.
 Use 50% of the reduced TDD as the total basal dose to be given over 24 hours.
Use 50% of the reduced TDD as the total basal dose to be given over 24 hours.
 Start with one basal rate for 24 hours (divide the total basal dose by 24). [Initial basal rate per hour = (TDD × 0.75) ÷ (2 × 24).]
Start with one basal rate for 24 hours (divide the total basal dose by 24). [Initial basal rate per hour = (TDD × 0.75) ÷ (2 × 24).]
 The remaining 50% will be used as bolus doses for meals on the basis of carbohydrate counting, using the established C:I ratio and CF.
The remaining 50% will be used as bolus doses for meals on the basis of carbohydrate counting, using the established C:I ratio and CF.
34. Calculate an example of an initial basal rate for insulin pump therapy.
1. Current TDD of insulin is 50 units. 25% reduction of TDD = 37.5 units.
2. 50% of reduced TDD = 37.5 ÷ 2 = 18.75 units as total basal insulin.
3. Total basal insulin = 18.75 ÷ 24 = 0.78 U/hr
In this example, the initial basal rate is 0.8 U/hr. Basal rate adjustments will then be made on the basis of testing and recording BG of profiles throughout the day.
35. When are nighttime basal rate adjustments made?
Nighttime basal rates should be adjusted before the daytime basal rates are verified. Testing is typically performed during the first week of insulin pump therapy. Be aware that patients transitioning from glargine (Lantus) or detemir (Lavema) insulin may have overlapping insulin coverage, causing hypoglycemia during the first week. Testing is then repeated if a significant weight change occurs, if an exercise routine is begun or altered, after hormonal changes (i.e., puberty, menopause), or as needed.
36. List recommendations to follow during the nighttime basal rate verification process.
 Assess basal rate accuracy on three nights.
Assess basal rate accuracy on three nights.
 Eat evening meal early, preferably before 5 pm (or begin the test period ≈5 hours after eating). Take the usual bolus for dinner and the correction if needed.
Eat evening meal early, preferably before 5 pm (or begin the test period ≈5 hours after eating). Take the usual bolus for dinner and the correction if needed.
 Patients who typically eat high-fat meals or are unsure of their carbohydrate counting skills should choose a meal that they frequently eat or one for which they are confident about the carbohydrate amount.
Patients who typically eat high-fat meals or are unsure of their carbohydrate counting skills should choose a meal that they frequently eat or one for which they are confident about the carbohydrate amount.
 Avoid meals with more than 15 to 20 g of fat, 10 g of fiber, and alcohol on testing nights.
Avoid meals with more than 15 to 20 g of fat, 10 g of fiber, and alcohol on testing nights.
 Avoid any food or insulin boluses after the evening meal.
Avoid any food or insulin boluses after the evening meal.
 Avoid exercise other than typical activity on test evenings.
Avoid exercise other than typical activity on test evenings.
 Monitor BG before and 2 hours after the evening meal, at 9 pm, 12 midnight, 3 am, and 6 am, and before breakfast.
Monitor BG before and 2 hours after the evening meal, at 9 pm, 12 midnight, 3 am, and 6 am, and before breakfast.
 Stop the test if BG is less than 70 mg/dL or greater than 250 mg/dL during the basal test, and treat the abnormal BG.
Stop the test if BG is less than 70 mg/dL or greater than 250 mg/dL during the basal test, and treat the abnormal BG.
37. How are nighttime basal rate adjustments made?
 If BG levels change by more than 20 to 30 mg/dL during overnight monitoring, adjust the basal rate for the next night by 0.1 U/hr, starting 1 to 3 hours before the BG change was seen.
If BG levels change by more than 20 to 30 mg/dL during overnight monitoring, adjust the basal rate for the next night by 0.1 U/hr, starting 1 to 3 hours before the BG change was seen.
 Changes are made until the fasting BG in the morning is within the target range (70-130 mg/dL) and within 30 mg/dL of the bedtime BG.
Changes are made until the fasting BG in the morning is within the target range (70-130 mg/dL) and within 30 mg/dL of the bedtime BG.
 Daytime basal rates are verified next, usually 1 to 2 weeks after pump initiation or as necessary.
Daytime basal rates are verified next, usually 1 to 2 weeks after pump initiation or as necessary.
38. Describe the procedure for making daytime basal rate adjustments.
 Have patients skip breakfast and check their BG levels every hour from 7 am to 12 noon to verify the morning basal rate.
Have patients skip breakfast and check their BG levels every hour from 7 am to 12 noon to verify the morning basal rate.
 If BG levels change by more than 20 to 30 mg/dL during this time, adjust the basal rate for the next day by 0.1 U/hr, starting 1 to 3 hours before the glucose change was seen.
If BG levels change by more than 20 to 30 mg/dL during this time, adjust the basal rate for the next day by 0.1 U/hr, starting 1 to 3 hours before the glucose change was seen.
 After the morning basal rate is set, have patients skip their other meals (on separate days) and follow the same monitoring and adjustment procedures to confirm the afternoon and evening basal rate(s).
After the morning basal rate is set, have patients skip their other meals (on separate days) and follow the same monitoring and adjustment procedures to confirm the afternoon and evening basal rate(s).
39. What is the recommended treatment of hypoglycemia?
Dextrose should be taken for a BG less than 70 mg/dL. The patient should take 15 g of a quick-acting carbohydrate: glucose tablets or gel or dextrose-based candy (SweetTARTS, Smarties, Spree). The patient should wait 15 minutes and test BG again. If the second BG is less than 70 mg/dL, additional dextrose should be taken.
40. Why does rebound hyperglycemia occur after hypoglycemia?
 Overtreatment with an inappropriate amount of carbohydrate may occur.
Overtreatment with an inappropriate amount of carbohydrate may occur.
 No treatment (i.e., sleeping through a low glucose episode) may result in counterregulatory hormone release and increased hepatic glycogenolysis.
No treatment (i.e., sleeping through a low glucose episode) may result in counterregulatory hormone release and increased hepatic glycogenolysis.
 Treatment with a food that contains fat delays digestion and absorption, thereby prolonging hypoglycemia and causing counterregulatory hormone release with subsequent hepatic glycogenolysis.
Treatment with a food that contains fat delays digestion and absorption, thereby prolonging hypoglycemia and causing counterregulatory hormone release with subsequent hepatic glycogenolysis.
41. Discuss the use of glucagon to treat severe hypoglycemia.
All patients using MDI or pump therapy should be given a glucagon emergency kit prescription and a demonstration. Glucagon is used to raise BG in a person who is unable to swallow. This inability may occur as a result of either a seizure or unconsciousness. Family members should receive instruction on administering the glucagon, and the patient should be able to demonstrate the procedure to a third party (coworker or neighbor).
, American Diabetes Association. Standards of Medical Care in Diabetes–2012. Diabetes Care. 2012;35(Suppl 1):S11–S63.
Aschner, P, Horton, E, Leiter, A, et al, Practical steps to improving the management of type 1 diabetes. recommendations from the Global Partnership for Effective Diabetes Management. Int J Clin Pract 2010;64:305–315.
Davidson, PC, Hebblewhite, HR, Steed, RD, Bode, BW, Analysis of guidelines for basal-bolus insulin dosing. basal insulin, correction factor, and carbohydrate-to-insulin ratio. Endocr Pract 2008;14:1095–1101.
DeWitt, DE, Hirsch, IB. Outpatient insulin therapy in type 1 and type 2 diabetes mellitus. JAMA. 2003;289:2254–2264.
, Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986.
Duckworth, W, Abraira, C, Moritz, T, et al, VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129–139.
Heinemann, L, Insulin pump therapy. what is the evidence for using different types of boluses for coverage of prandial insulin requirements. J Diabetes Sci Technol 2009;3:1490–1500.
Nathan, DM, Cleary, PA, Backlund, JY, et al, Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–2653.
Ohkubo, Y, Kishikawa, H, Araki, E, et al, Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus. a randomized prospective 6-year study. Diabetes Res Clin Pract 1995;28:103–117.
Patel, A, MacMahon, S, Chalmers, J, et al, ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–2572.
Pickup, JC. Insulin-pump therapy for type 1 diabetes mellitus. N Engl J Med. 2012;366:1616–1624.
Skyler, JS, Bergenstal, R, Bonow, RO, et al, Intensive glycemic control and the prevention of cardiovascular events. implications of the ACCORD, ADVANCE, and VA Diabetes Trials. Diabetes Care 2009;32:187–192.
, The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–2559.
, UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853.
, UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352:854–865.
White, RD. Insulin pump therapy (continuous subcutaneous insulin infusion). Primary Care: Clinics in Office Practice. 2007;34:845–871.
Wolfsdorf, JI. Intensive Diabetes Management, ed 5. Alexandria: American Diabetes Association, Inc.; 2012.