Intensive Care of the Cancer Patient
METABOLIC AND ENDOCRINE COMPLICATIONS
CARDIAC COMPLICATIONS IN CANCER PATIENTS
PULMONARY COMPLICATIONS IN CANCER PATIENTS
Lymphangitic Tumor Involvement
Treatment-Induced Pulmonary Dysfunction
INFECTIOUS COMPLICATIONS IN CANCER PATIENTS
NEUROLOGIC COMPLICATIONS IN CANCER PATIENTS
GASTROINTESTINAL COMPLICATIONS IN CANCER PATIENTS
GENITOURINARY COMPLICATIONS IN CANCER PATIENTS
HEMATOLOGIC COMPLICATIONS IN CANCER PATIENTS
SPECIAL CONSIDERATIONS IN BONE MARROW TRANSPLANTATION
The annual estimated incidence of new invasive cancers in the United States in 2012 exceeds 1.6 million, with greater than 570,000 deaths.1 Long-term remissions and control of advanced cancer are being achieved with targeted therapy, and new immunotherapy agents in many malignancies.2–6 We are entering an era of “personalized” oncologic care in which treatments are prescribed based on the profile of mutated or overexpressed genes in the tumor specimen. For the treatment of metastatic malignancies, enhanced success has come from the ability to deliver chemotherapy, radiation therapy, immunotherapy, or combination regimens with increased dose intensity. Progress in supportive care and intensive care medicine has allowed oncologists to treat their patients aggressively and support them despite the toxicities inherent in dose-intense treatment modalities. A greater understanding of the mechanisms for these toxicities has also improved care and patient outcomes.
Metabolic and Endocrine Complications
Endocrine syndromes associated with malignancies have been described for many years and some clinically significant endocrinopathies are induced by immunotherapy agents, such as ipilimumab, currently used in the treatment of advanced melanoma.4,7 These problems may manifest as solitary laboratory derangements, such as hypercalcemia or hyperphosphatemia, or can present as clinical syndromes, such as Cushing syndrome in small cell lung cancer. Metabolic disorders can also arise as a consequence of cancer treatment. This is most often seen with chemotherapy for rapidly growing tumors such as leukemias or lymphomas. Abrupt changes in metabolic variables have also been observed after interleukin 2 (IL-2)-based immunotherapy and the rapid in vivo expansion of lymphocytes.8 The most common of these clinical entities are tumor lysis syndrome (TLS), hypercalcemia, oncogenic osteomalacia, syndrome of inappropriate secretion of antidiuretic hormone (SIADH), adrenal failure, pheochromocytoma, tumor-induced hypoglycemia, and chemotherapy-induced metabolic disturbances.
Tumor Lysis Syndrome
Case reports of metabolic and electrolyte abnormalities after chemotherapy for rapidly growing tumors such as Burkitt’s lymphoma and leukemias were first published in the 1950s.9 Cadman and his colleagues in the 1970s proposed a mechanism that linked these metabolic observations.10 More recently, TLS has been observed in patients with solid tumors and has been observed in patients receiving immunotherapy, such as IL-2, sunitinib, imatinib, and rituximab.11–14 TLS after treatment for solid tumors is relatively rare.
TLS is characterized by hyperuricemia, hyperkalemia, hyperphosphatemia, and hypocalcemia.10 Electrolyte abnormalities can appear as soon as 6 hours after chemotherapy administration and can persist for 5 to 7 days after treatment. The hyperuricemia comes from the massive release of intracellular nucleic acids and their metabolism by xanthine oxidase into uric acid. Urate crystals can form in the renal collecting ducts and result in oliguric and anuric renal failure. Similarly, potassium and phosphate are released from lysing tumor cells, and renal excretion of these intracellular ions is impaired by hyperuricemia. Serum calcium levels drop from ectopic calcium deposition; this becomes more likely as the calcium-phosphorus product increases. Calcium deposition is favored by a calcium-phosphorus product greater than 60 mg2/dL2 and becomes severe when the product is more than 75 mg2/dL2. The clinical manifestations of TLS depend on which electrolyte derangement predominates. Tetany, confusion, hypotension, dysrhythmias, and sudden death have been reported with TLS. The most effective management approach for this syndrome is to anticipate its occurrence and intervene prospectively. Patients at greatest risk for TLS are those with a diagnosis of a rapidly growing lymphoma or leukemia with high blast counts, and pretreatment levels of lactate dehydrogenase greater than 1500 U/dL and uric acid greater than 10 mg/dL. Pretreatment azotemia is also a poor prognostic sign. Azotemia may be exacerbated by uric acid nephropathy, which is more common with uric acid levels exceeding 20 mg/dL. It is unlikely that TLS will occur in patients at risk who do not develop metabolic changes within 48 hours after receiving chemotherapy. Guidelines for prophylaxis and treatment of TLS are given in Box 80.1. It is important to note that rasburicase poses an oxidative stress and can induce hemolytic anemia in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency.
Hypercalcemia
Hypercalcemia is the most common metabolic abnormality occurring in cancer patients. Approximately 10% to 20% of all cancer patients have hypercalcemia at some point in their course. The clinical symptoms of hypercalcemia are nonspecific and include lethargy, confusion, nausea, and anorexia. Often the clinical symptoms in cancer patients may be subtle because the onset of hypercalcemia is gradual. The mechanism that underlies all cases of cancer-related hypercalcemia is increased calcium resorption from bone due to enhanced osteoclast activity mediated through receptor activator for nuclear factor κB ligand (RANKL).15 This resorption increase can be due to local action of tumor in bone or to the production of bone-resorbing hormones and cytokines by tumor cells remote from bone. Normally, increased circulating calcium results in decreased parathyroid hormone (PTH) production. When PTH levels decrease, bone resorption and renal tubular reabsorption of calcium decline. Low PTH levels cause a decrease in vitamin D production; thus, gut absorption of calcium is lowered. Although PTH levels are suppressed in cancer patients with hypercalcemia, the destructive action of tumor deposits in bone or the action of tumor-produced hormones on bone maintains high calcium resorption rates. This is accomplished through osteoclast activation and proliferation from factors produced by the tumor, such as interleukin 1 (IL-1), tumor necrosis factor (TNF), prostaglandin E2, granulocyte-macrophage colony-stimulating factor (GM-CSF), transforming growth factor-α, platelet-derived growth factor, and PTH-related peptides.16–20 Malignancies that commonly cause hypercalcemia include multiple myeloma, breast carcinoma, epidermoid lung carcinoma, and renal cell carcinoma. Hypercalcemia in lymphoma and leukemia is probably not associated with PTH-related peptide, but rather with the overproduction of activated vitamin D.20,21
Management for any symptomatic hypercalcemic patient should begin with intravenous hydration, which may increase renal blood flow and enhance calciuresis.22 Renal excretion of calcium can be enhanced with furosemide diuresis, although no randomized trials exist to support its use in hypercalcemia. These measures should be viewed as temporizing steps until definitive treatment has been implemented. The bisphosphonates zoledronic acid, pamidronate, alendronate, etidronate, and clodronate have been shown to be highly effective in the long-term treatment of hypercalcemia of malignancy. These agents work by binding to the hydroxyapatite in bone and preventing calcium resorption, although they may also have much more complicated effects on the cell cycle and bone turnover.23,24 A commonly used bisphosphonate regimen is a single dose of pamidronate (60-90 mg intravenously over 2 to 4 hours) or zoledronic acid (4 mg intravenously over 15 minutes). Doses may be repeated in 3 to 4 days if the calcium does not decline. In addition, therapy directed at controlling the tumor should be implemented. RANKL inhibitors are now available for clinical use (denosumab) that inhibit osteoclast activity induced by malignancy and may be more potent in inhibiting bone resorption compared to bisphosphonates.25,26 Gallium nitrate can be tried in patients with hypercalcemia unresponsive to bisphosphonates.27 Calcitonin, glucocorticoids, or mithramycin can also be tried in patients unresponsive to first-line therapies, although these therapies are no longer commonly used owing to potential renal injury. Dialysis may be necessary if renal compromise is severe. Treatment recommendations are summarized in Table 80.1.
Table 80.1
Agents Used for the Management of Hypercalcemia
| Drug | Dosage |
| Pamidronate | 90 mg IV over 2 hours |
| Zoledronic acid | 4 mg IV over 15 minutes |
| Gallium nitrate | 200 mg/m2 by continuous infusion for 5 days |
| Calcitonin | 400 IU SQ every 8 hours |
| Mithramycin | 25 µg/kg IV once or twice per week |
Adrenal Failure
Adrenal failure has also been observed in patients receiving ipilimumab, an antibody that blocks the effects of an inhibitory protein in T cells known as CTLA-4 (cytotoxic T-lymphocyte antigen 4) and is used in the treatment of advanced melanoma.4 Hypoadrenalism from ipilimumab is usually secondary to panhypopituitarism induced by T-cell–mediated hypophysitis.28 These patients can present with headache and visual changes from pituitary swelling in addition to the clinical findings of hypoadrenalism as reviewed earlier. Evaluation of these patients should include a contrast-enhanced brain magnetic resonance imaging (MRI), and measurement of serum levels of cortisol, adrenocorticotropic hormone (ACTH), and thyroid-stimulating hormone (TSH).
Pheochromocytoma
Pheochromocytoma is most commonly associated with the multiple endocrine neoplasia syndrome. The clinical features of this tumor are related to episodic catecholamine release and include hypertension, severe headache, cardiac dysrhythmias, pallor, perspiration, and rarely, hypotension. Patients can also present with a multisystem crisis characterized by encephalopathy, hyperpyrexia, and hemodynamic instability.29 Diagnosis is made by measuring urinary catecholamine metabolites. An elevated vanillylmandelic acid is accurate approximately 90% of the time in making the diagnosis.30 Patients with borderline catecholamine levels can often be diagnosed with the clonidine suppression test.31 Localization of pheochromocytomas can be difficult because the tumors can arise anywhere between the base of the brain and the scrotum and can be multicentric. MRI and computed tomography (CT) are helpful in visualizing adrenal abnormalities. Nuclear medicine studies with m-[111I]iodobenzylguanidine (MIBG) can be used if the CT scan is negative. MIBG scans are sensitive and specific for detecting ectopic adrenal medullary tissue.32 Positron emission tomography (PET) imaging and diffusion-weighted MRI can detect sites of disease not apparent on CT or MIBG and can aid in surgical planning.33–35 Surgical extirpation of the tumor is the only effective treatment. Preoperative control of catechol secretion is necessary and can be attained with long- or short-acting α-adrenergic blockade (phenoxybenzamine 10 mg orally two or three times daily or doxazosin 2-16 mg orally daily). A comparison of preoperative management strategies using long- versus short-acting α-antagonists showed no difference in long-term outcome after surgery, although the incidence of intraoperative hypertension was greater with short-acting medications like doxazosin, terazosin, and prazosin.36 Tachycardia can be controlled with beta blockers, but these should be started only after phenoxybenzamine. Patients in hypertensive crisis can be managed with α-methyltyrosine or calcium channel blockers such as nifedipine or nicardipine.37–39
Tumor-Induced Hypoglycemia
Functional endocrine tumors can give rise to a variety of clinical syndromes. Most of these problems can be managed outside the ICU; however, tumor-induced hypoglycemia can cause serious consequences including coma, seizures, and focal neurologic deficits. A number of different mechanisms can give rise to hypoglycemia. Autonomous insulin production is most commonly associated with islet cell tumors, whereas production of insulin-like growth factors (IGF-1 or IGF-2) is seen with non–islet cell tumors.40 Slow-growing mesenchymal tumors such as leiomyosarcoma, mesothelioma, and fibrosarcoma are the most common non–islet cell tumors that cause hypoglycemia.
Treatment should be focused on control of the tumor. Insulinomas are often benign and can be cured by surgical removal. For unresectable malignancies, hypoglycemic episodes can often be reduced with supportive measures such as dietary modification with frequent meals. Insulinomas may respond to diazoxide, an inhibitor of insulin secretion. Glucagon infusions may be beneficial in some patients.41
Chemotherapy-Induced Metabolic Disturbances
A number of chemotherapy drugs can cause potentially severe electrolyte disturbances. Cyclophosphamide is associated with hyponatremia from SIADH. Vinca alkaloids such as vinorelbine and vinblastine also cause SIADH. Cisplatin and carboplatin can cause renal tubular defects resulting in hypokalemia and hypomagnesemia, which can be severe enough to require intravenous replacement. Mithramycin lowers serum calcium by a mechanism that is thought to involve inhibition of the effect of PTH on osteoclasts. Although mithramycin can be used for the treatment of hypercalcemia, it can also cause hypocalcemia in patients with normal serum calcium. Cetuximab, a humanized murine antibody directed against the epidermal growth factor receptor (EGFR) and used to treat colon carcinoma and head and neck cancer is associated with severe and symptomatic hypomagnesemia from inappropriate urinary excretion.42 Cetuximab may interact with EGFR in the loop of Henle blocking resorption of magnesium and causing secondary hypokalemia and hypocalcemia. Abiraterone, a CYP17 inhibitor of androgen biosynthesis used in men with advanced prostate cancer, also increases adrenal mineralocorticoid synthesis resulting in clinically significant hypokalemia and decreases glucocorticoid synthesis necessitating concurrent administration of prednisone. Everolimus, an oral inhibitor of the mammalian target of rapamycin (mTOR), used in the management of advanced renal cancer and neuroendocrine tumors, can induce severe hyperglycemia requiring insulin.43,44 The mechanism through which mTOR inhibitors cause hyperglycemia is not fully understood but may involve decreased insulin secretion, direct toxicity to pancreatic β-cells, or impaired suppression of hepatic glucose production.45
Cardiac Complications in Cancer Patients
Superior Vena Cava Syndrome
Obstruction of blood flow through the superior vena cava (SVC) can be caused by fibrosis, thrombosis, external compression, or invasion of the vessel by tumor. SVC syndrome can also be caused by thrombus secondary to a central venous access device, which is now a common fixture of oncologic care. Malignancies that involve the mediastinum, such as lung carcinoma and lymphoma, are the most common causes of this syndrome. Facial and upper extremity edema, facial plethora, headache, and tachypnea are the most common clinical presentations. Collateral venous channels may be found on the chest or abdomen. Death from SVC syndrome is rare, but life-threatening respiratory compromise and elevated intracranial pressure can occur. Therapy for SVC syndrome depends on the underlying malignancy; thus, a biopsy is mandatory for optimal management of these patients. If lymphoma or small cell lung carcinoma is the cause of SVC syndrome, initiation of the appropriate chemotherapy regimen can rapidly shrink the mediastinal mass and is the treatment of choice. For tumors not responsive to chemotherapy, radiation therapy given with high initial fractions (3 to 4 Gy/day) can provide symptomatic relief in more than 80% of patients.46 Thrombolysis has only been studied in catheter-associated SVC syndrome and is effective in this setting.47 Endovascular stents can restore patency of the SVC in approximately 50% of patients and can result in significant palliation.48 Improvement is often evident within 72 hours and the patency of occluded endovascular stents can sometimes be restored with angioplasty.
Cardiac Tamponade
Although primary or metastatic tumors of the heart can decrease cardiac output by impairing ventricular outflow, the most frequent causes of cardiac tamponade in cancer patients are metastatic tumors of the breast and lung, and melanoma, lymphomas, and leukemias. Tamponade may occur through either encasement of the heart by tumor or production of a malignant pericardial effusion. The clinical manifestations of tamponade include decreased exercise tolerance, shortness of breath, and cough. Voltage may be decreased on ECG with a pulsus alternans pattern present. Muffled heart tones, a pericardial rub, or an increased paradoxical pulse (i.e., decrease in systolic blood pressure on inspiration exceeds 10 mm Hg) may be present on physical examination. Echocardiography is extremely useful in confirming the diagnosis of tamponade if it is suspected on physical examination. Diastolic collapse of the right atrium or right ventricle on echocardiogram is an indicator of hemodynamic compromise.49,50 Swan-Ganz catheterization may be helpful to confirm the presence of significant tamponade.
Pericardiocentesis for relief of tamponade is indicated emergently when echocardiographic or clinical evidence of hemodynamic compromise is present. Intravenous infusions of normal saline at high flow rates (100 to 500 mL/hour) may be required to support the patient until a drainage procedure is performed. Although rapid reversal of cardiac filling problems can be accomplished by this procedure, a long-term solution is required. Creation of a pericardial window can prevent the reaccumulation of fluid in more than 90% of patients.51 Sclerosing agents such as tetracycline and bleomycin have also been used to prevent reaccumulation of pericardial fluid.52 Sclerosants may be instilled into the pericardial space after adequate drainage has been accomplished and appear to have a success rate comparable to pericardial window procedures. Some centers also perform pericardial windows using video-assisted thoracoscopy, but there have been no prospective randomized studies showing superior clinical outcomes for subxiphoid versus video-assisted thoracoscopy approaches.53
Treatment-Induced Cardiac Dysfunction
A number of chemotherapy medicines have cardiac toxicities that can be life threatening. Cumulative doses of doxorubicin greater than 450 mg/m2 are associated with an increased risk for congestive heart failure (CHF). Heart damage from this drug is thought to be from an iron-dependent generation of free radicals, which secondarily cause oxidative damage to lipid membranes and intracellular organelles.52 This toxicity can present acutely or months after drug administration. It is more prevalent in older patients and those with a history of coronary artery disease, hypertension, tobacco abuse, or chest radiation therapy. Initial management with diuretics, digoxin, and angiotensin blockers is usually of benefit, but heart failure can be progressive. Liposomal encapsulation of doxorubicin or the use of dexrazoxane to prevent oxygen-derived free radical formation, may diminish the cardiac toxicities of this agent.54–56 Other anthracyclines, such as mitoxantrone and epirubicin, may have a lower incidence of CHF. Furthermore, weekly low-dose boluses or continuous-infusion methods of doxorubicin administration appear to reduce the incidence of clinically significant heart damage. Although anthracycline-induced cardiac damage has generally been considered irreversible, some studies suggest that some improvement in cardiac function may occur with aggressive medical management.57 Paclitaxel, which is commonly used in ovarian, breast, and lung carcinomas, is associated with bradydysrhythmias.57,58 Ventricular tachycardia, myocardial infarction, and cardiac ischemia have also been reported. Cyclophosphamide, which is commonly used in breast cancer, lymphoma, and stem cell transplant conditioning regimens, is associated with sporadic instances of CHF, which may be severe and occurs within a few days of cyclophosphamide administration, especially at high doses. Hemorrhagic myocarditis with myonecrosis was seen on autopsy specimens from these patients. These events appear unrelated to cumulative dose or method of administration. CHF from ifosfamide has also been reported.59 CHF is usually seen approximately 2 weeks after high doses of the drug and appears more frequently in patients with concurrent renal insufficiency. Medical management successfully reverses the heart failure in most patients. CHF is also associated with trastuzumab, an anti-HER2 (human epidermal growth factor receptor 2) antibody used commonly in the management of certain forms of breast carcinoma. The incidence of CHF after trastuzumab in a large randomized trial was between 3% and 4%, and was more common in patients with antecedent cardiac disease, older patients, and those having diminished ejection fraction (EF) after anthracycline-containing chemotherapy.60 Most patients with trastuzumab-induced cardiac dysfunction have improved symptoms with appropriate medical management for CHF and discontinuation of trastuzumab.
Serial echocardiography studies have been used to assess cardiac toxicities in patients receiving chemotherapy for many years, but subtle alterations in myocardial function can be missed with standard assessment of EF. A decrease in longitudinal strain assessed by Doppler measurements of tissue velocity at baseline and repeated at 3 months after starting anthracyclines or trastuzumab is a more sensitive predictor of cardiac dysfunction than EF61,62 and may be useful in identifying patients in need of medical management before significant symptoms occur.
Radiation therapy delivered to the chest for the treatment of Hodgkin’s disease, lung malignancies, breast cancer, or other neoplasms can result in a number of cardiac toxicities, including radiation pericarditis with tamponade, myocardial fibrosis, and premature coronary artery disease. The toxic effects of radiation therapy are secondary to microvessel fibrosis and may take up to 20 years to appear.63 After mantle-field radiation therapy, the risk of fatal myocardial infarction is more than three times greater than in age-matched control subjects, although mantle-fields are rarely used currently to treat patients with lymphoma. Nervertheless, it is difficult to treat the mediastinum without also treating the heart.
Pulmonary Complications in Cancer Patients
Lymphangitic Tumor Involvement
Interstitial lung processes in cancer patients may be due to a variety of infectious insults but can also be caused by direct lymphangitic spread of the tumor. The symptoms of lymphangitic involvement are nonspecific and include dyspnea, nonproductive cough, and hypoxemia. Pulmonary hypertension and cor pulmonale can also be present. The diagnosis can be established by video-assisted thoracoscopic biopsy or transbronchial biopsy. Pulmonary microvascular cytologic specimens obtained with a wedged pulmonary artery catheter may be a less invasive way to make the diagnosis of lymphangitic carcinomatosis.64 The prognosis of this condition is generally poor, with a life expectancy of 1 to 6 months. Appropriate systemic treatment should be implemented when the site of the primary malignancy is diagnosed.
Treatment-Induced Pulmonary Dysfunction
A number of chemotherapy agents and radiation therapy can cause pneumonitis leading to chronic pulmonary fibrosis. The chemotherapeutic agents most likely to cause this problem are bleomycin and mitomycin, but other alkylators, nitrosoureas, antimetabolites, gemcitabine, taxanes, and vinca alkaloids can cause pulmonary dysfunction. Inhibitors of the mTOR pathway such as everolimus and temsirolimus used in the treatment of advanced renal cancer can also induce severe pneumonitis.44,65 Erlotinib, an inhibitor of the phosphorylation of EGFR used in the treatment of advanced lung cancer, can also induce severe and irreversible pneumonitis.66 The underlying mechanisms for lung injury induced by these agents are not fully understood, but likely involves oxygen-derived free radical toxicity67 and dysregulation of leukocyte apoptosis regulated by TNF-receptor family members. TRAIL (TNF-related apoptosis-inducing ligand) has shown promise in preclinical models of bleomycin lung injury and may also have antitumor properties.68 We advocate that cancer patients in need of supplemental oxygen should receive the lowest possible fractional concentration of oxygen that produces a hemoglobin oxygen saturation of greater than 90%. Irreversible lung damage can occur if excessive oxygen is administered to patients receiving bleomycin or radiation therapy. Clinical assessment is crucial to patient management because there are no sensitive or accurate tests to predict the onset or course of bleomycin-induced pulmonary toxicity. The resting diffusion capacity has been used, but is suboptimal to follow patients.69 Treatment recommendations are based on the recognition of three distinct clinical entities:
1. Patients who have radiographic changes but no symptoms do not require treatment.
2. Glucocorticoids are used for managing pneumonitis induced by chemotherapy, mTOR and EGFR inhibitors, and radiation when fever, cough, shortness of breath, and pulmonary infiltrates are present. The mechanism of glucocorticoid action may involve reducing inflammation and microvessel damage through inhibition of leukotriene synthesis, inducing granulocyte demargination from endothelial cells, and direct toxicity to lymphocytes.
3. There is no effective treatment for patients with chronic pulmonary fibrosis, cor pulmonale, or pulmonary hypertension from chemotherapy or radiation therapy.
Diffuse Interstitial Pneumonitis
The differential diagnosis of diffuse pulmonary infiltrates in cancer patients is large. Infectious causes include bacterial, viral, fungal, and protozoal pathogens. Noninfectious causes for diffuse pulmonary infiltrates are neoplasm, autoimmune disease, cardiac failure, leukostasis, pulmonary hemorrhage, and radiation- or chemotherapy-induced pneumonitis. Making a diagnosis on clinical grounds is difficult because the radiographic and physical examination findings are virtually indistinguishable among these diverse causative entities. Performing an open-lung biopsy is often the only way to confirm a diagnosis; however, empiric treatment may result in equally good patient outcomes. A randomized study compared immediate open-lung biopsy followed by therapy directed at the diagnosis versus empiric antibiotics alone without biopsy to treat diffuse pulmonary infiltrates in cancer patients.70 The antibiotic regimen included trimethoprim-sulfamethoxazole (20 mg/kg/day intravenously) and erythromycin (30 mg/kg/day intravenously, divided into four daily doses). A broad-spectrum antibiotic was added if the patient was neutropenic at the time of diagnosis. There was no significant difference in the outcome for these patients; however, those who received an open lung biopsy had a greater complication rate. Empiric antibiotics are appropriate initial management for diffuse interstitial infiltrates, but patients who do not improve after 4 days of empiric therapy should receive open-lung biopsy.
The decision to place a patient with a cancer diagnosis on ventilator support is often controversial for medical staff and families. It is generally recognized that such patients have a poor prognosis, with a mortality rate approaching 80%. A large multicenter trial prospectively examined prognostic variables for cancer patients requiring ventilatory support.71 Factors having a statistically significant negative influence on survival were a diagnosis of leukemia, allogeneic stem cell transplantation, progressive cancer, cardiac dysrhythmia, presence of disseminated intravascular coagulation (DIC), and need for vasopressor support. Prior surgery with curative intent was protective and probably relates to a selection bias for patients with physiologic reserve sufficient to tolerate surgery. Although this model is similar to other prognostic models used in the ICU setting, it differs in its emphasis on cancer-specific factors. There also appears to be a positive association between recovery from neutropenia, pneumonia, and acute respiratory distress syndrome (ARDS) requiring mechanical ventilation and an inverse relation to survival in patients with hematologic malignancy.72 The mortality rate of patients with ARDS during neutropenic recovery was 86.8% versus 51.5% in patients without ARDS in this single institution study. In general, the assessment of the potential reversibility of the organ dysfunction is the critical variable. Most iatrogenic toxicities are reversible, and patients having toxicity from treatment can generally be supported to recovery. Similarly, organ dysfunction from treatable malignancies should be assumed to be reversible. However, when more than three organ systems are failing, the chances of recovery are very small. The decision to place a cancer patient on ventilator support is highly individualized, but such models can assist in counseling families about level-of-care issues.
Hemoptysis
Hemoptysis can be a presenting sign of cancer, especially endobronchial lesions of non–small cell lung cancer. Patients presenting with significant hemoptysis and airway compromise can be palliated with a variety of bronchoscopic techniques including argon or neodymium:yttrium-aluminum-garnet (Nd:YAG) laser, photodynamic therapy, stent placement, endoluminal brachytherapy, or combinations of these techniques.73 External beam radiation, pulmonary artery embolization, and blood pressure control can also be effective in controlling hemoptysis.
Bevacizumab, a monoclonal antibody against vascular endothelial growth factor, has been used with chemotherapy agents to increase overall response and time-to-progression in lung cancer. Bevacizumab can also cause significant and life-threatening hemoptysis; especially in patients with squamous cell histologic lung cancer.74 The mechanism of hemoptysis after bevacizumab is collapse of the tumor vasculature resulting in tumor cavitation in proximity to major blood vessels. Because cancer outcomes can be improved with bevacizumab, we endorse an aggressive approach in supporting patients who experience hemoptysis as a result of this or other vascular-targeting agents.
Infectious Complications in Cancer Patients
Pancytopenia is perhaps the most common sequela of dose-intense chemotherapy regimens. Fever in the setting of neutropenia in conjunction with bacteremia is a life-threatening complication of many chemotherapy regimens, whether given with adjuvant, palliative, or curative intent. The microbial pathogens that infect neutropenic patients have changed over time. Previously, Pseudomonas aeruginosa was one of the most common organisms in this setting, but staphylococci, streptococci, and vancomycin-resistant organisms have become increasingly prevalent.75 Improved broad-spectrum antibiotics and hematopoietic growth factors have greatly improved the outcome for febrile neutropenic patients,76,77 many of whom can be managed easily without intensive care interventions. This section focuses on septic shock and the infections that commonly occur as complications of cancer treatment. The infections that arise as a consequence of the severe immune compromise after bone marrow transplant are covered separately.
Neurologic Complications in Cancer Patients
Spinal Cord Compression
The tumors that most often cause spinal cord compression from epidural metastases or bony destruction are carcinomas of the lung, breast, and prostate and multiple myeloma. The level of the spinal cord involvement determines the clinical neurologic deficit. Cervical cord compression can cause quadriplegia or respiratory arrest; thoracic, paraplegia, and lumbar involvement can give rise to loss of bladder and bowel function. If the problem is detected when local or radicular pain is the only symptom, treatment with glucocorticoids, radiation therapy using conventional or conformal techniques, or laminectomy can be highly effective. If the patient presents with a neurologic deficit, such as the inability to walk, the chance of significant improvement is less than 10%.78 MRI is the diagnostic test of choice for diagnosing epidural metastases. Glucocorticoids should be started emergently for patients who present with myelopathy (dexamethasone 10 mg followed by 4 mg every 6 hours) and imaging obtained as soon as possible. The maximal effect of dexamethasone on alleviating symptoms may not be achieved at total doses of 24 mg/day. If clinical improvement from glucocorticoids is suboptimal, doubling the dose each day up to a maximum of 200 mg/day total dose may improve symptom control until definitive therapy is implemented.79 The standard of care for cord compression had been glucocorticoids and radiation; however, a randomized trial has shown benefit for decompressive surgery followed by radiation.80 Eighty-four percent of patients randomized to receive surgery and radiation maintained the ability to walk compared to 57% in the radiation alone group. Better palliation of pain and the duration of ambulation were maintained better in the surgery group. A meta-analysis of 1595 articles describing 2495 patients supports better functional improvement and pain control using surgery with or without postsurgical radiation in the management of malignant spinal cord compression.81
Brain Metastases and Hemorrhage
Tumor metastatic to the brain may be a localized problem, amenable to surgical resection with good results.82 Patients who present with significant peritumoral edema and increased intracranial pressure may suffer brain herniation if acute measures are not taken. Therapy includes high-dose glucocorticoids (dexamethasone 10 mg, followed by 4 mg intravenously every 6 hours), intubation and mechanical hyperventilation to maintain partial pressure of carbon dioxide in arterial blood (PaCO2) between 25 and 30 mm Hg, and mannitol diuresis (1.0 to 1.5 g/kg intravenously as a 20% solution). Patients who do not respond may benefit from higher glucocorticoid doses (dexamethasone 25 to 50 mg every 6 hours). After stabilizing the patient with these acute interventions, definitive radiation therapy or surgery can be started. Prophylactic anticonvulsants are often administered; however, this intervention has scant supportive data and should be held in most patients until a seizure has occurred.83,84 Frontal lobe tumor deposits and brain metastases from melanoma are two situations in which prophylactic antiseizure medication should be considered. Newer radiation techniques such as stereotactic or gamma knife radiosurgery can also be effective in controlling or eradicating brain metastatic disease, although this modality is more appropriate for patients with good functional status and a total volume of metastatic disease less than 12 cm3.85
Dose-intensive therapy for acute leukemia is associated with prolonged thrombocytopenia and the possibility of intracranial hemorrhage. It was previously thought that such events were highly associated with platelet counts less than 20,000 cells/µL86; however, the threshold for platelet transfusion used in most medical centers is now 10,000/µL, based on a randomized trial that showed no difference in patient outcome with the more stringent threshold.87 Other events, such as sepsis and fever, contribute to the likelihood of bleeding with thrombocytopenia. Patients with solid tumors are also less likely than those with leukemia to bleed as a consequence of thrombocytopenia. In cancer patients with suspected intracranial bleeding and thrombocytopenia, the platelet count should be maintained above 50,000 cells/µL. The best strategy to maintain adequate hemostatic function with platelet transfusion support is controversial because platelet kinetics are complex.88,89 We advocate frequent dosing or continuous infusion of platelets in actively bleeding thrombocytopenic patients who have poor increments in platelet count after transfusion resulting from platelet sensitization. The blood bank should identify human leukocyte antigen (HLA)-matched platelet donors for such patients and maintain an adequate supply of the HLA-matched platelets. Single-donor platelets are often more effective than pooled platelets in this setting.
Uncontrolled Seizures
In 15% to 30% of patients who develop brain metastases, the initial sign is a generalized seizure.90 Metabolic disturbances, such as hyponatremia from SIADH, may also cause seizures in cancer patients. Acute control can be obtained with intravenous diazepam (5 mg intravenously every 5 to 10 minutes up to 30 mg). Standard measures to protect the airway, prevent aspiration, and avoid limb injury should also be implemented. After acute control has been attained, phenytoin should be started (15 mg/kg intravenously at a maximum rate of 50 mg/minute, then maintenance doses of 300 mg/day). Phenytoin up-regulates the P-450 system in the liver, which may accelerate the metabolism of certain chemotherapy agents, such as paclitaxel and docetaxel.91,92 Levetiracetam (1000 mg daily) is also highly effective in the acute and chronic management of seizures induced by brain malignancy.93 Antiseizure agents that do not influence P-450 cytochromes, like gabapentin, should be used in patients requiring taxane-based chemotherapy. Serum levels of phenytoin and levetiracetam should be monitored.
Seizures, coma, and other neurologic complications can occur with a variety of chemotherapeutic and biologic agents. Most of these toxicities improve with cessation of the causative agent and supportive care. Ifosfamide-induced neurotoxicity has a unique mechanism related to changes in mitochondrial fatty acid oxidation and the accumulation of glutaric acid metabolites.94 Treatment with methylene blue (200-300 mg orally or intravenously daily), an electron-accepting drug, can reverse and prevent neurologic toxicity during ifosfamide infusion.95
Gastrointestinal Complications in Cancer Patients
Bowel injury is a common cause of morbidity and death in cancer patients. Lymphomatous bowel involvement is relatively common in acquired immunodeficiency syndrome (AIDS)–lymphoma patients96 and can be a site of metastatic disease in a variety of solid tumors including ovarian cancer, melanoma, and renal cancer. Management of these problems can often be accomplished with meticulous standard care; however, intensive support may be needed for emergent surgical or medical conditions arising from gastrointestinal complications.
Tumor-Induced Emergencies
For most tumor types, obstruction may be related to a localized lesion that is readily amenable to surgical correction. In women with ovarian cancer, the obstruction is often related to loss of peristalsis in long segments of bowel because of diffuse wall invasion by malignancy. Little can be done with surgical intervention in such circumstances. Improvement hinges on the availability of effective chemotherapy. Bowel obstruction or perforation can occur from primary or metastatic tumors. If the patient presents with peritoneal signs of an acute abdomen, then emergent exploratory surgery is indicated.97 For less clear-cut presentations, abdominal radiographs, CT scans, and endoscopy may be helpful. These patients can be managed initially with bowel rest, nasogastric suction, and anaerobic antibiotic coverage until a diagnosis is made. Somatostatin can provide palliation in some patients, probably by increasing intestinal water resorption.98
Chemotherapy-Induced Gastrointestinal Dysfunction
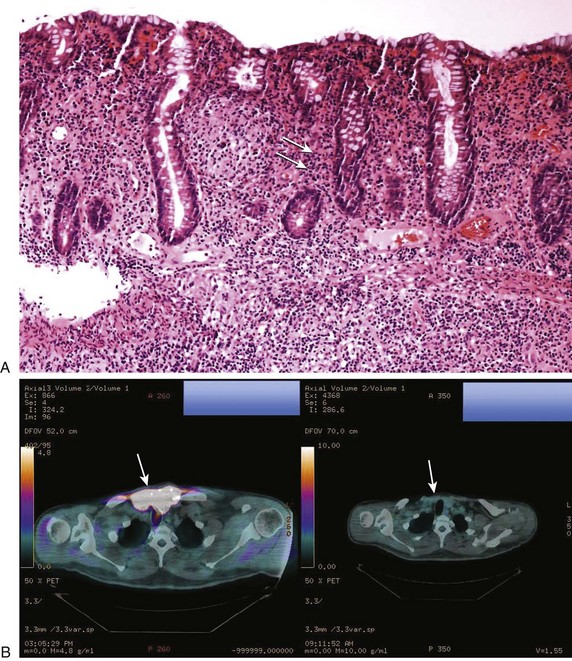
Colitis can occur in up to 40% of patients who have received ipilimumab of whom 5% experience severe symptoms including high-output diarrhea and intestinal perforation.4,99 Neutrophilic and lymphocytic infiltrates are evident on biopsy consistent with the idea that ipilimumab elicits autoimmune tissue damage by breaking self-tolerance. Intervention early when symptoms are mild or moderate (e.g., 4-6 stools per day over baseline) is key to avoiding severe or life-threatening colitis. Initial management is to withhold ipilimumab and administer loperamide. If symptoms persist, then a course of glucocorticoids should be started (e.g., prednisone 0.5 mg/kg/day). For patients who have seven or more stools per day, abdominal pain, melena, or hematochezia, then glucocorticoids (e.g., prednisone 1-2 mg/kg/day) should begin promptly with a slow taper when symptoms resolve. Endoscopic evaluation may be helpful. Emergent surgery for perforation is appropriate. TNF inhibitors (e.g., infliximab) can be effective in managing diarrhea from ipilimumab unresponsive to glucocorticoids.99 Figure 80.1A and B depicts the biopsy findings in ipilimumab colitis and an associated tumor response.
A particularly difficult management problem is the severe constipation that can accompany the use of opioid analgesics in patients with advanced cancer. Prophylactic measures are very important, including the use of stool softeners and osmotic laxatives and maintaining patient activity as much as possible. Even with optimal prophylaxis, results are often unsatisfactory. Methylnaltrexone, a µ-opioid receptor antagonist that does not cross the blood-brain barrier, can significantly improve constipation, usually within 4 hours of dosing, without reducing the analgesic effect of narcotics.100
Gastrointestinal Lymphomas
Lymphoma presenting as a gastric or intestinal mass is common in AIDS patients and in patients with B-cell lymphomas arising in mucosal-associated lymphatic tissue (MALT) secondary to Helicobacter pylori infection. This situation warrants extreme caution because perforation of an abdominal viscus is a potentially life-threatening complication of potentially curative chemotherapy. Because perforation is a major concern in these patients, initial surgical resection is advocated by some authors.101,102 Others have noted low rates of abdominal catastrophe in patients treated with chemotherapy and radiation therapy alone.103 Data from patients with gastric MALT lymphoma treated with antibiotics or radiation therapy suggest that perforation is rare in patients achieving a tumor response.104 Patients with extensive gastric involvement by lymphoma should start chemotherapy in the hospital with surgical consultation to monitor for possible perforation or obstruction. Perforation is more common in patients with small intestinal involvement with aggressive histologic lymphomas compared to gastric or colon involvement by lymphoma.
Genitourinary Complications in Cancer Patients
Tumor-Induced Genitourinary Dysfunction
Obstructive uropathy resulting in hydronephrosis can occur at the bladder outlet or anywhere along the path of the ureter. Bladder outlet problems are most commonly caused by local tumor invasion from prostate, bladder, rectosigmoid, cervical, or ovarian neoplasms. Metastatic deposits from gastric, breast, or pancreatic malignancies can also obstruct the bladder outlet. Tumors that arise from retroperitoneal structures or metastasize to the retroperitoneum can cause ureteral obstruction. Examples of tumors that commonly have a retroperitoneal presentation are Hodgkin’s and non-Hodgkin’s lymphoma, germ cell tumors that metastasize to retroperitoneal lymph nodes, and axial sarcomas. Primary tumors of the ureter can also cause obstruction. If the obstruction and the resulting hydronephrosis are of short duration, percutaneous drainage and decompression are recommended. If there is a concurrent infection, decompression is mandatory and constitutes an oncologic emergency. After a period of 48 to 72 hours, a ureteral stent can be placed using the anterograde or retrograde approach. Furosemide-renal scanning can be used to confirm the presence of kidney function if there is a question about the reversibility of the functional impairment of the obstructed kidney.105 After stenting, appropriate radiation therapy, chemotherapy, or hormonal treatment should be implemented for control of the malignancy.
Chemotherapy-Induced Genitourinary Complications
Hemorrhagic cystitis can be caused by acrolein, a metabolite of cyclophosphamide and ifosfamide. This problem can usually be prevented with adequate hydration, bladder irrigation, or the use of thiol-based chemoprotectants, such as mesna. Mesna administration is mandatory for ifosfamide treatment. If the hemorrhagic cystitis is severe and unresponsive to supportive measures and saline bladder irrigation, formalin bladder instillation can be performed under general anesthesia.106 A 1% formalin solution should be used, but bladder fibrosis and strictures may occur despite this low concentration. Care must be taken to avoid reflux of the formalin up the ureters. Urinary diversion and cystectomy may be required for uncontrolled bleeding.
Methotrexate, an antifolate chemotherapy agent, can precipitate in renal tubules and cause acute tubular necrosis if adequate hydration and urinary alkalinization are not achieved before therapy. If renal toxicity occurs, leucovorin rescue should be started, based on serum levels of methotrexate (Table 80.2 provides details of methotrexate toxicity prevention or reversal). Intravenous fluids containing bicarbonate, furosemide, and mannitol may be helpful in preventing oliguric renal failure.
Hematologic Complications in Cancer Patients
Hyperleukocytosis
Large numbers of leukemic blasts may be present in acute leukemias or in the late stages of chronic leukemias. When the number of circulating myeloblasts is greater than 100,000 cells/µL, the viscosity of the blood increases because white blood cells are much less deformable than red blood cells. Patients with chronic lymphocytic leukemia can tolerate higher circulating numbers of malignant cells (e.g., 100,000-300,000 cells/µL) without consequence. In acute leukemia, the blasts may invade and weaken the vessel wall, leading to hemorrhage. Hyperleukocytosis chiefly affects the microvasculature in the lungs and the central nervous system. Symptoms can range from mild shortness of breath and blurred vision to pulmonary congestion, hypoxia, intracranial hemorrhage, and TLS (tumor lysis syndrome). Rapid institution of leukapheresis can often decrease leukocyte counts by 20% to 50%.107 Although the improvement may be transient, chemotherapy treatments for the underlying leukemia can be accomplished with greater safety, and perhaps a lesser degree of TLS.
All-trans-retinoic acid (ATRA), used in the treatment of acute promyelocytic leukemia (APL), induces differentiation of leukemic cells.108 ATRA is associated with a leukocytosis syndrome characterized by fever, dyspnea, and interstitial lung infiltrates on chest radiograph, which can progress to ARDS.109 ARDS may be secondary to accumulation of differentiated leukemic blasts and their release of cytokines, such as IL-2, in the lung. Leukapheresis is ineffective in this setting; however, the early implementation of glucocorticoids is beneficial (dexamethasone 10-20 mg/day in divided doses).
Disseminated Intravascular Coagulation
DIC can be associated with a variety of solid tumors, including carcinoma of the prostate, lung, breast, and gastrointestinal tract, and melanomas. However, DIC is the hallmark of the clinical presentation for APL. The leukemic blasts in APL manufacture procoagulants, which are released into the circulation, particularly after cytotoxic chemotherapy.110 The use of ATRA for treating APL has lessened the severity of DIC in this illness, but a new complication has been added (discussed in the previous paragraph). To manage DIC in APL, serial determinations of fibrin split products and fibrinogen levels should be made. Replacement of fibrinogen can be accomplished with cryoprecipitate (1 bag/2 kg of body weight initially, followed by 1 bag/10-15 kg of body weight daily). If DIC worsens after cryoprecipitate, intravenous heparin can be started but should be used cautiously. Antifibrinolytic agents, such as epsilon-aminocaproic acid, should be avoided because they can block the normal dissolution of thrombi and increase organ damage.
Biologic Therapy
Interleukin 2
IL-2-induced capillary leak is the chief underlying cause for most of its end-organ toxicity. The capillary leak is associated with endothelial relaxation caused by nitric oxide.111,112 Clinical data also support the notion that nitrate levels are greatly elevated in patients treated with IL-2.113 In addition, the adhesion of activated lymphocytes to vascular endothelium after IL-2 administration has been shown to cause vascular leak in a rabbit model.114
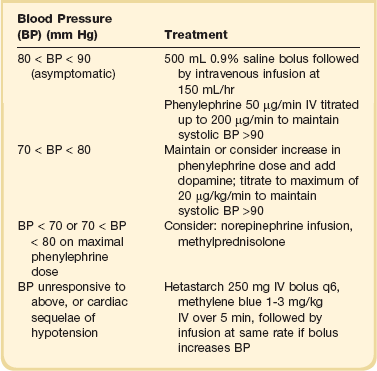
The most common serious manifestation of IL-2–induced capillary leak is hypotension with essentially the same hemodynamic findings of warm shock115; thus, effective support for these patients requires the use of parenteral α-adrenergic agonists, such as phenylephrine or dopamine.116,117 Recommendations for treating cytokine-induced hypotension are given in Table 80.3. Fluid administration, though of transient benefit, often exacerbates the pulmonary capillary leak seen with IL-2. IL-2 infusions can be continued, despite hypotension, with appropriate pressor management. In our clinical experience, phenylephrine doses of up to 500 µg/minute can be tolerated to reverse IL-2–induced hypotension in this selected group of patients who have normal cardiopulmonary function before treatment. IL-2 doses are held until capillary leak has improved sufficiently to warrant a decrease in phenylephrine to less than 100 µg/minute and can be resumed thereafter in the absence of other dose-limiting toxicities.
Interleukin 2 Pulmonary Capillary Leak
The clinical manifestations of IL-2 pulmonary toxicity often do not correlate with the severity of radiologic findings. Between 70% and 80% of patients receiving IL-2 have some radiographic abnormality, which may consist of pleural effusions, diffuse infiltrates, or focal infiltrates.118,119
Because clinically significant pulmonary capillary leak cannot be anticipated by radiographic findings, relatively minor symptoms such as tachypnea need to be carefully evaluated in patients receiving IL-2. Pulse oximetry is sometimes helpful but can be falsely low in patients concurrently receiving phenylephrine for IL-2–induced hypotension; therefore, an arterial blood gas may be required for these patients. Hypoxemia should be treated with oxygen supplementation, which can be delivered initially via nasal cannula, Venturi mask, or positive-pressure airway ventilation. Worsening hypoxemia may sometimes respond to diuresis; however, intubation may be required in some patients. A key to the ventilatory management of IL-2 lung toxicity is to reverse the pulmonary edema through positive end-expiratory pressure and enhancement of renal function. Although high partial pressures of oxygen may be required in the initial treatment of these patients, prolonged oxygen exposure may exacerbate IL-2 toxicity.116 For this reason, rapid titration of oxygen to maintain arterial partial pressure oxygen greater than or equal to 60 mm Hg is recommended. If diffuse pulmonary infiltrates worsen and an ARDS-like syndrome develops, parenteral glucocorticoids should be administered. The beneficial effect of glucocorticoids may be mediated by the lysis of activated lymphocytes that have been stimulated by IL-2.120
Interleukin 2 Renal Dysfunction
Hypotension and the systemic capillary leak associated with IL-2 also create renal and liver toxicities. The renal dysfunction typically consists of oliguria and prerenal azotemia with elevated blood urea nitrogen and creatinine.121,122 Creatinine levels greater than 6.0 mg/dL or 530 µmol/L are often tolerated without modifying IL-2 doses because recovery is rapid after IL-2 treatment is completed without hemodialysis. The associated oliguria mandates meticulous fluid management because pulmonary toxicity may be exacerbated by fluid overload. Furosemide, or other nonthiazide diuretics, can be tried but are infrequently effective and may exacerbate IL-2–related hypotension.
Cardiac Rhythm Disturbances and Myocardial Infarction
IL-2 is associated with a variety of cardiac problems, including nonspecific ST-segment or T-wave changes, dysrhythmias (most commonly supraventricular tachycardia, but ventricular tachycardia can also occur), myocarditis, pericarditis, and myocardial ischemia or infarction. The rhythm disturbances and inflammatory conditions may be secondary to lymphocyte infiltration of the myocardium or pericardium.123 Cardiac toxicity (with the exception of sinus tachycardia) should be treated initially by holding or discontinuing IL-2 doses. Supraventricular tachycardia may respond to adenosine (12 mg by rapid intravenous bolus) and is our agent of choice when IL-2–induced hypotension is present. If hypotension is present and adenosine is ineffective, intravenous diltiazem at doses between 0.15 and 0.45 mg/kg should be considered (as long as the QRS complex is not prolonged, >0.12 second) because the incidence of hypotension appears to be less than with other calcium channel blockers.124 Amiodarone is useful in treating atrial fibrillation that occurs in approximately 5% of patients receiving IL-2. After sinus rhythm is reestablished, amiodarone can be continued (200-400 mg orally daily) to prevent or lessen the incidence of atrial fibrillation and IL-2 can generally be continued. Other cardiovascular events should be managed with the same medical and intensive care interventions used for any other acute cardiac patient.
Immune-Mediated Toxicities from T-Cell–Directed Therapy
The toxicities of ipilimumab have been extensively reviewed125 and include rash, diarrhea, colitis with perforation, endocrinopathies, hepatocellular injury, fatigue, and pyrexia. The toxicities are presumed due to T-cell activation induced by CTLA-4 blockade by ipilimumab, although the precise mechanism is not well understood. Of these toxicities, immune-mediated colitis has the greatest potential to induce critical illness, as discussed earlier.
Special Considerations in Bone Marrow Transplantation
Acute and Chronic Graft-Versus-Host Disease
Graft-versus-host (GVH) disease is most commonly seen in the setting of allogeneic transplants but can be observed after syngeneic and autologous transplants. It is well documented that GVH disease can reduce recurrence rates in leukemia126; it also lowers overall survival after transplant. The prerequisites for GVH disease are that the graft must include a population of immunologically competent T cells, the graft recipient must be unable to destroy these cells, and tissue antigens must be present in the recipient that are not present in the donor.127 Studies of the immunobiology of GVH disease suggest that dysregulation of T-cell subsets (CD4+, CD25+, Foxp3+, Treg, and TH17) are instrumental in the clinical manifestations of GVH disease and represent potential therapeutic targets.128,129
The clinical manifestations of GVH include skin rash (maculopapular or diffusely erythematous), liver function abnormalities, and gastrointestinal symptoms including diarrhea, nausea, vomiting, and ileus.126 These toxicities are graded (I to IV) on a semiquantitative basis. Grade I or II GVH disease has relatively little morbidity, but grade IV GVH disease carries a 100% mortality rate. Treatment of established GVH disease requires suppression of the immune system. The drugs used most commonly to suppress GVH disease are glucocorticoids, cyclosporine, tacrolimus, and methotrexate, which are usually used in combination. Gamma globulin infusions may also be beneficial in the prophylaxis of GVH disease.130 The mechanism of action of gamma globulin is unknown but may involve binding to Fc receptors, which may prevent T cells from recognizing target tissues.
Veno-occlusive Disease
Hepatic veno-occlusive disease (VOD) is a clinical syndrome characterized by weight gain (fluid), tender hepatomegaly, and elevations of hepatocellular enzymes and bilirubin. The syndrome is the result of endothelial toxicity from high-dose chemotherapy, and results in a local hypercoagulable state with tissue factor synthesis, down-regulation of thrombomodulin, and release of von Willebrand factor with the resultant formation of blood clots in hepatic veins.131 The incidence of VOD has diminished with the prophylactic use of heparin.132 Agents used in the treatment of established VOD include tissue plasminogen activator,133 antithrombin III,134 antioxidant therapy,135 and transjugular intrahepatic portosystemic stent-shunt.136 Clinical trials using a polydisperse oligonucleotide known as defibrotide137 are showing activity and defibrotide may be helpful in both the prevention and treatment of VOD. Intensive support is often needed for patients with VOD. Despite aggressive treatment, the mortality rate for severe established VOD approaches 100%.138 Patients with milder disease may recover with supportive measures. Early recognition and intervention are key factors influencing outcome.
Infectious Complications of Stem Cell Transplantation
Different facets of immune function return at varying intervals after bone marrow transplant.139 Integumentary and mucosal barriers are disrupted in the period immediately after myeloablative chemotherapy. For this reason, aerobic bacteria and Candida organisms are the most likely pathogens early after transplant. There are decreased neutrophil numbers for the first 2 to 4 weeks after transplant, although the use of colony-stimulating factors shortens the neutrophil recovery period. Until neutrophil numbers increase, herpes simplex virus, Candida, Aspergillus, and bacterial infections are common. It should be noted that the full chemotactic function of neutrophils does not return for 100 days after transplant. Cellular and humoral immunity defects persist for 1 to 3 months after transplant and may lengthen if GVH disease occurs. Fungal and cytomegalovirus (CMV) infections predominate in this period.140,141 From 3 months to 1 year after transplant, T cells remain dysfunctional and disordered immunoregulation may occur. Varicella zoster, hepatitis C, Pneumocystis jiroveci pneumonia, and pneumococcal pneumonia are the most common infections until T-cell function has recovered. Immune deficits may be moderated by antigen-specific immunity conferred by the transplanted marrow and the use of gamma globulin infusions, which are now routinely given after allogeneic transplant. Antibiotic recommendations in bone marrow transplant are similar to those for other febrile neutropenic cancer patients. Individual recommendations should take into account the frequency of specific microorganisms isolated at a given institution. Trimethoprim-sulfamethoxazole and fluoroquinolone antibiotics (e.g., ciprofloxacin) are routinely used for the prophylaxis of Pneumocystis organisms and gram-positive infections, respectively. Invasive fungal infections can be a significant cause of morbidity and death after transplant, but voraconazole prophylaxis or secondary therapy can be effective. CMV responds poorly to single-agent therapy, but the combination of ganciclovir and gamma globulin infusions improves outcome in these patients. Death rates from CMV pneumonitis approached 80% before use of the combination regimen. The use of CMV-negative donors and the elimination of CMV-contaminated leukocytes from platelet and red blood cell transfusions with leukocyte filters have reduced the mortality rate from CMV infection.
There has been ongoing work in using cytotoxic T-lymphocyte (CTL) clones to treat specific opportunistic infections including those from CMV, Epstein-Barr virus (EBV), and adenovirus.142–144 Augmentation of antiviral activity was conferred with these infusions in most patients.145 This strategy may prove useful in reducing the number of life-threatening infections in bone marrow transplant, human immunodeficiency virus, and other immunodeficiency states.142
Code Status and Intensive Care in Cancer Patients
The high cost of intensive care and the demands placed on these precious resources by all medical and surgical subspecialties mandate that critical care interventions be meted out wisely. Because cost containment has become imperative in medicine practice and health care reform, certain poor prognosis illnesses, including cancer, are perceived by the public and often by medical staff as relative contraindications to cardiopulmonary resuscitation (CPR) or intensive care interventions.146 Although several studies have shown that CPR in patients with cancer diagnoses has a low success rate (less than 10%),147,148 the success rate of CPR in noncancer diagnoses is less than 5%.149 Hospital utilization studies do not support the notion that a disproportionate amount of special care resources are used in terminally ill cancer patients.150 The central determination in each case should hinge on the answer to a single question: “Is it likely that these abnormalities are reversible?” For nearly all iatrogenic toxicities, the answer is generally yes. For patients with curable tumor types, every effort should be made to support the patient until the long-term prognosis of the underlying tumor can be ascertained. Given that nearly 60% of individuals with a cancer diagnosis are cured of their disease, intensive care interventions are justified.
When viewed in the context of other underlying medical conditions, a cancer diagnosis is neither medically nor ethically a contraindication to intensive care management. The wishes of the individual and the family are central in establishing an appropriate level of care for any patient. In order to better define the level of care desired by individuals with serious or terminal illnesses, the state of Oregon originated a program known as POLST (Physician Orders for Life-Sustaining Treatment), which is now being implemented in at least 15 other states.151 In cancer patients, the compliance of physicians with documented care preferences is generally greater than 90%, but the timing and extent of this documentation vary.152 Public awareness of these issues is increasing, and many cancer patients now have such directives, which include living wills, do-not-resuscitate orders, and POLST. These directives can be helpful to the physician and the patient’s family, although they sometimes complicate medical management in clinical situations in which reversible conditions exist but for which care might require violating a directive.153 Patient education and strong support from social work and pastoral and legal services can assist the physician in arriving at the appropriate level of care for each individual.
References
1. Siegel, R, Naishadham, D, Jemal, A. Cancer statistics, 2012. CA Cancer J Clin. 2012; 62:10–29.
2. Atkins, MB, Lotze, MT, Dutcher, JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: Analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999; 17:2105–2116.
3. Fisher, RI, Rosenberg, SA, Sznol, M, et al. High-dose aldesleukin in renal cell carcinoma: Long-term survival update. Cancer J Sci Am. 1997; 3(Suppl 1):S70–S72.
4. Hodi, FS, O’Day, SJ, McDermott, DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010; 363:711–723.
5. Robert, N, Leyland-Jones, B, Asmar, L, et al. Randomized phase III study of trastuzumab, paclitaxel, and carboplatin compared with trastuzumab and paclitaxel in women with HER-2-overexpressing metastatic breast cancer. J Clin Oncol. 2006; 24:2786–2792.
6. Chapman, PB, Hauschild, A, Robert, C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011; 364:2507–2516.
7. Weber, JS, O’Day, S, Urba, W, et al. Phase I/II study of ipilimumab for patients with metastatic melanoma. J Clin Oncol. 2008; 26:5950–5956.
8. Curti, BD, Longo, DL, Ochoa, AC, et al. Treatment of cancer patients with ex vivo anti-CD3-activated killer cells and interleukin-2. J Clin Oncol. 1993; 11:652–660.
9. Holland, JF, Sharpe, W, Mamrod, LM, et al. Urate excretion in patients with acute leukemia. J Natl Cancer Inst. 1959; 23:1097–1105.
10. Cadman, EC, Lunberg, WB, Bertino, JR. Hyperphosphatemia and hypocalcemia accompanying rapid cell lysis in a patient with Burkitt’s lymphoma and Burkitt cell leukemia. Am J Med. 1977; 62:283–290.
11. Castro, MP, VanAuken, J, Spencer-Cisek, P, et al. Acute tumor lysis syndrome associated with concurrent biochemotherapy of metastatic melanoma: A case report and review of the literature. Cancer. 1999; 85:1055–1059.
12. Yang, H, Rosove, MH, Figlin, RA. Tumor lysis syndrome occurring after the administration of rituximab in lymphoproliferative disorders: High-grade non-Hodgkin’s lymphoma and chronic lymphocytic leukemia. Am J Hematol. 1999; 62:247–250.
13. Saylor, PJ, Reid, TR. Tumor lysis syndrome after treatment of a gastrointestinal stromal tumor with the oral tyrosine kinase inhibitor sunitinib. J Clin Oncol. 2007; 25:3544–3546.
14. Keane, C, Henden, A, Bird, R. Catastrophic tumour lysis syndrome following single dose of imatinib. Eur J Haematol. 2009; 82:244–245.
15. Lacey, DL, Timms, E, Tan, HL, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998; 93:165–176.
16. Dewhirst, FE, Stashenko, PP, Mole, JE, et al. Purification and partial sequence of human osteoclast-activating factor: Identity with interleukin 1 beta. J Immunol. 1985; 135:2562–2568.
17. Stashenko, P, Dewhirst, FE, Peros, WJ, et al. Synergistic interactions between interleukin 1, tumor necrosis factor, and lymphotoxin in bone resorption. J Immunol. 1987; 138:1464–1468.
18. Stern, PH, Krieger, NS, Nissenson, RA, et al. Human transforming growth factor-alpha stimulates bone resorption in vitro. J Clin Invest. 1985; 76:2016–2019.
19. Seyberth, HW, Segre, GV, Morgan, JL, et al. Prostaglandins as mediators of hypercalcemia associated with certain types of cancer. N Engl J Med. 1975; 293:1278–1283.
20. Wysolmerski, JJ, Broadus, AE. Hypercalcemia of malignancy: The central role of parathyroid hormone-related protein. Annu Rev Med. 1994; 45:189–200.
21. Fetchick, DA, Bertolini, DR, Sarin, PS, et al. Production of 1,25-dihydroxyvitamin D3 by human T cell lymphotrophic virus-I-transformed lymphocytes. J Clin Invest. 1986; 78:592–596.
22. Blythe, WB, Gitelman, HJ, Welt, LG. Effect of expansion of the extracellular space on the rate of urinary excretion of calcium. Am J Physiol. 1968; 214:52–57.
23. Mundy, GR, Yoneda, T. Bisphosphonates as anticancer drugs. N Engl J Med. 1998; 339:398–400.
24. Lipton, A. Improving progression-free and overall survival in patients with cancer: A potential role for bisphosphonates. Expert Opin Pharmacother. 2011; 12:749–762.
25. Morony, S, Warmington, K, Adamu, S, et al. The inhibition of RANKL causes greater suppression of bone resorption and hypercalcemia compared with bisphosphonates in two models of humoral hypercalcemia of malignancy. Endocrinology. 2005; 146:3235–3243.
26. Stopeck, AT, Lipton, A, Body, JJ, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: A randomized, double-blind study. J Clin Oncol. 2010; 28:5132–5139.
27. Bertheault-Cvitkovic, F, Tubiana-Hulin, M, Chevalier, B. Gallium nitrate (GN) vs pamidronate (APD) for acute control of cancer-related hypercalcemia (CRH): Interim results of a randomized, double-blind, multi-national study. Proc Annu Meet Am Soc Clin Oncol. 1995; 14:A369.
28. Yang, JC, Hughes, M, Kammula, U, et al. Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J Immunother. 2007; 30:825–830.
29. Newell, KA, Prinz, RA, Pickleman, J, et al. Pheochromocytoma multisystem crisis. A surgical emergency. Arch Surg. 1988; 123:956–959.
30. Sheps, SG, Jiang, NS, Klee, GG, et al. Recent developments in the diagnosis and treatment of pheochromocytoma. Mayo Clin Proc. 1990; 65:88–95.
31. Bravo, EL, Tarazi, RC, Fouad, FM, et al. Clonidine-suppression test: A useful aid in the diagnosis of pheochromocytoma. N Engl J Med. 1981; 305:623–626.
32. Shapiro, B, Copp, JE, Sisson, JC, et al. Iodine-131 metaiodobenzylguanidine for the locating of suspected pheochromocytoma: Experience in 400 cases. J Nucl Med. 1985; 26:576–585.
33. Mann, GN, Link, JM, Pham, P, et al. [11C]metahydroxyephedrine and [18F]fluorodeoxyglucose positron emission tomography improve clinical decision making in suspected pheochromocytoma. Ann Surg Oncol. 2006; 13:187–197.
34. Havekes, B, Lai, EW, Corssmit, EP, et al. Detection and treatment of pheochromocytomas and paragangliomas: Current standing of MIBG scintigraphy and future role of PET imaging. Q J Nucl Med Mol Imaging. 2008; 52:419–429.
35. Takano, A, Oriuchi, N, Tsushima, Y, et al. Detection of metastatic lesions from malignant pheochromocytoma and paraganglioma with diffusion-weighted magnetic resonance imaging: Comparison with 18F-FDG positron emission tomography and 123I-MIBG scintigraphy. Ann Nucl Med. 2008; 22:395–401.
36. Weingarten, TN, Cata, JP, O’Hara, JF, et al. Comparison of two preoperative medical management strategies for laparoscopic resection of pheochromocytoma. Urology. 2010; 76:508, e6-11.
37. Chimori, K, Miyazaki, S, Nakajima, T, et al. Preoperative management of pheochromocytoma with the calcium-antagonist nifedipine. Clin Ther. 1985; 7:372–379.
38. Perry, RR, Keiser, HR, Norton, JA, et al. Surgical management of pheochromocytoma with the use of metyrosine. Ann Surg. 1990; 212:621–628.
39. Proye, C, Thevenin, D, Cecat, P, et al. Exclusive use of calcium channel blockers in preoperative and intraoperative control of pheochromocytomas: Hemodynamics and free catecholamine assays in ten consecutive patients. Surgery. 1989; 106:1149–1154.
40. Daughaday, WH, Emanuele, MA, Brooks, MH, et al. Synthesis and secretion of insulin-like growth factor II by a leiomyosarcoma with associated hypoglycemia. N Engl J Med. 1988; 319:1434–1440.
41. Samaan, NA, Pham, FK, Sellin, RV, et al. Successful treatment of hypoglycemia using glucagon in a patient with an extrapancreatic tumor. Ann Intern Med. 1990; 113:404–406.
42. Schrag, D, Chung, KY, Flombaum, C, et al. Cetuximab therapy and symptomatic hypomagnesemia. J Natl Cancer Inst. 2005; 97:1221–1224.
43. Yao, JC, Shah, MH, Ito, T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011; 364:514–523.
44. Motzer, RJ, Escudier, B, Oudard, S, et al. Efficacy of everolimus in advanced renal cell carcinoma: A double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008; 372:449–456.
45. Johnston, O, Rose, CL, Webster, AC, et al. Sirolimus is associated with new-onset diabetes in kidney transplant recipients. J Am Soc Nephrol. 2008; 19:1411–1418.
46. Armstrong, BA, Perez, CA, Simpson, JR, et al. Role of irradiation in the management of superior vena cava syndrome. Int J Radiat Oncol Biol Phys. 1987; 13:531–539.
47. Gray, BH, Olin, JW, Graor, RA, et al. Safety and efficacy of thrombolytic therapy for superior vena cava syndrome. Chest. 1991; 99:54–59.
48. Lanciego, C, Pangua, C, Chacon, JI, et al. Endovascular stenting as the first step in the overall management of malignant superior vena cava syndrome. Am J Roentgenol. 2009; 193:549–558.
49. Chandraratna, PA. Echocardiography and Doppler ultrasound in the evaluation of pericardial disease. Circulation. 1991; 84:1303–1310.
50. Hutchison, SJ, Smalling, RG, Albornoz, M, et al. Comparison of transthoracic and transesophageal echocardiography in clinically overt or suspected pericardial heart disease. Am J Cardiol. 1994; 74:962–965.
51. Sugimoto, JT, Little, AG, Ferguson, MK, et al. Pericardial window: Mechanisms of efficacy. Ann Thorac Surg. 1990; 50:442–445.
52. Myers, CE, McGuire, WP, Liss, RH, et al. Adriamycin: The role of lipid peroxidation in cardiac toxicity and tumor response. Science. 1977; 197:165–167.
53. O’Brien, PK, Kucharczuk, JC, Marshall, MB, et al. Comparative study of subxiphoid versus video-thoracoscopic pericardial “window. Ann Thorac Surg. 2005; 80:2013–2019.
54. Speyer, JL, Green, MD, Sanger, J, et al. A prospective randomized trial of ICRF-187 for prevention of cumulative doxorubicin-induced cardiac toxicity in women with breast cancer. Cancer Treat Rev. 1990; 17:161–163.
55. Hortobagyi, GN, Frye, D, Buzdar, AU, et al. Decreased cardiac toxicity of doxorubicin administered by continuous intravenous infusion in combination chemotherapy for metastatic breast carcinoma. Cancer. 1989; 63:37–45.
56. Swain, SM, Whaley, FS, Gerber, MC, et al. Cardioprotection with dexrazoxane for doxorubicin-containing therapy in advanced breast cancer. J Clin Oncol. 1997; 15:1318–1332.
57. Moreb, JS, Oblon, DJ. Outcome of clinical congestive heart failure induced by anthracycline chemotherapy. Cancer. 1992; 70:2637–2641.
58. Rowinsky, EK, McGuire, WP, Guarnieri, T, et al. Cardiac disturbances during the administration of taxol. J Clin Oncol. 1991; 9:1704–1712.
59. Quezado, ZM, Wilson, WH, Cunnion, RE, et al. High-dose ifosfamide is associated with severe, reversible cardiac dysfunction. Ann Intern Med. 1993; 118:31–36.
60. Tan-Chiu, E, Yothers, G, Romond, E, et al. Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2-overexpressing breast cancer: NSABP B-31. J Clin Oncol. 2005; 23:7811–7819.
61. Sawaya, H, Sebag, IA, Plana, JC, et al. Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am J Cardiol. 2011; 107:1375–1380.
62. Fallah-Rad, N, Walker, JR, Wassef, A, et al. The utility of cardiac biomarkers, tissue velocity and strain imaging, and cardiac magnetic resonance imaging in predicting early left ventricular dysfunction in patients with human epidermal growth factor receptor II-positive breast cancer treated with adjuvant trastuzumab therapy. J Am Coll Cardiol. 2011; 57:2263–2270.
63. Adamson, IY, Bowden, DH. Endothelial injury and repair in radiation-induced pulmonary fibrosis. Am J Pathol. 1983; 112:224–230.
64. Masson, RG, Krikorian, J, Lukl, P, et al. Pulmonary microvascular cytology in the diagnosis of lymphangitic carcinomatosis. N Engl J Med. 1989; 321:71–76.
65. Motzer, RJ, Hudes, GR, Curti, BD, et al. Phase I/II trial of temsirolimus combined with interferon alfa for advanced renal cell carcinoma. J Clin Oncol. 2007; 25:3958–3964.
66. Yoneda, KY, Shelton, DK, Beckett, LA, et al. Independent review of interstitial lung disease associated with death in TRIBUTE (paclitaxel and carboplatin with or without concurrent erlotinib) in advanced non-small cell lung cancer. J Thorac Oncol. 2007; 2:537–543.
67. Cooper, JA, Jr., White, DA, Matthay, RA. Drug-induced pulmonary disease. Part 1: Cytotoxic drugs. Am Rev Respir Dis. 1986; 133:321–340.
68. McGrath, EE, Lawrie, A, Marriott, HM, et al. Deficiency of tumour necrosis factor-related apoptosis-inducing ligand exacerbates lung injury and fibrosis. Thorax. 2012; 67(9):796–803.
69. McKeage, MJ, Evans, BD, Atkinson, C, et al. Carbon monoxide diffusing capacity is a poor predictor of clinically significant bleomycin lung. New Zealand Clinical Oncology Group. J Clin Oncol. 1990; 8:779–783.
70. Browne, MJ, Potter, D, Gress, J, et al. A randomized trial of open lung biopsy versus empiric antimicrobial therapy in cancer patients with diffuse pulmonary infiltrates. J Clin Oncol. 1990; 8:222–229.
71. Groeger, JS, White, P, Jr., Nierman, DM, et al. Outcome for cancer patients requiring mechanical ventilation. J Clin Oncol. 1999; 17:991–997.
72. Rhee, CK, Kang, JY, Kim, YH, et al. Risk factors for acute respiratory distress syndrome during neutropenia recovery in patients with hematologic malignancies. Crit Care. 2009; 13:R173.
73. Santos, RS, Raftopoulos, Y, Keenan, RJ, et al. Bronchoscopic palliation of primary lung cancer: Single or multimodality therapy? Surg Endosc. 2004; 18:931–936.
74. Johnson, DH, Fehrenbacher, L, Novotny, WF, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2004; 22:2184–2191.
75. Hughes, WT, Armstrong, D, Bodey, GP, et al. 1997 guidelines for the use of antimicrobial agents in neutropenic patients with unexplained fever. Infectious Diseases Society of America. Clin Infect Dis. 1997; 25:551–573.
76. Freifeld, A, Marchigiani, D, Walsh, T, et al. A double-blind comparison of empirical oral and intravenous antibiotic therapy for low-risk febrile patients with neutropenia during cancer chemotherapy. N Engl J Med. 1999; 341:305–311.
77. Hartmann, LC, Tschetter, LK, Habermann, TM, et al. Granulocyte colony-stimulating factor in severe chemotherapy-induced afebrile neutropenia. N Engl J Med. 1997; 336:1776–1780.
78. Gilbert, RW, Kim, JH, Posner, JB. Epidural spinal cord compression from metastatic tumor: Diagnosis and treatment. Ann Neurol. 1978; 3:40–51.
79. Renaudin, J, Fewer, D, Wilson, CB, et al. Dose dependency of Decadron in patients with partially excised brain tumors. J Neurosurg. 1973; 39:302–305.
80. Patchell, RA, Tibbs, PA, Regine, WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: A randomised trial. Lancet. 2005; 366:643–648.
81. Kim, JM, Losina, E, Bono, CM, et al. Clinical outcome of metastatic spinal cord compression treated with surgical excision +/- radiation versus radiation therapy alone: A systematic review of literature. Spine. 2012; 37(1):78–84.
82. Patchell, RA, Tibbs, PA, Walsh, JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990; 322:494–500.
83. Cohen, N, Strauss, G, Lew, R, et al. Should prophylactic anticonvulsants be administered to patients with newly-diagnosed cerebral metastases? A retrospective analysis. J Clin Oncol. 1988; 6:1621–1624.
84. Mikkelsen, T, Paleologos, NA, Robinson, PD, et al. The role of prophylactic anticonvulsants in the management of brain metastases: A systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010; 96:97–102.
85. Seung, SK, Sneed, PK, McDermott, MW, et al. Gamma knife radiosurgery for malignant melanoma brain metastases. Cancer J Sci Am. 1998; 4:103–109.
86. Beutler, E. Platelet transfusions: The 20,000/microL trigger. Blood. 1993; 81:1411–1413.
87. Rebulla, P, Finazzi, G, Marangoni, F, et al. The threshold for prophylactic platelet transfusions in adults with acute myeloid leukemia. Gruppo Italiano Malattie Ematologiche Maligne dell’Adulto. N Engl J Med. 1997; 337:1870–1875.
88. Harker, LA, Roskos, L, Cheung, E. Effective and efficient platelet transfusion strategies that maintain hemostatic protection. Transfusion. 1998; 38:619–621.
89. Hersh, JK, Hom, EG, Brecher, ME. Mathematical modeling of platelet survival with implications for optimal transfusion practice in the chronically platelet transfusion-dependent patient. Transfusion. 1998; 38:637–644.
90. Posner, JB. Management of central nervous system metastases. Semin Oncol. 1977; 4:81–91.
91. Cresteil, T, Monsarrat, B, Alvinerie, P, et al. Taxol metabolism by human liver microsomes: Identification of cytochrome P450 isozymes involved in its biotransformation. Cancer Res. 1994; 54:386–392.
92. Royer, I, Monsarrat, B, Sonnier, M, et al. Metabolism of docetaxel by human cytochromes P450: Interactions with paclitaxel and other antineoplastic drugs. Cancer Res. 1996; 56:58–65.
93. Usery, JB, Michael, LM, 2nd., Sills, AK, et al. A prospective evaluation and literature review of levetiracetam use in patients with brain tumors and seizures. J Neurooncol. 2010; 99:251–260.
94. Kupfer, A, Aeschlimann, C, Wermuth, B, et al. Prophylaxis and reversal of ifosfamide encephalopathy with methylene-blue. Lancet. 1994; 343:763–764.
95. Pelgrims, J, De Vos, F, Van den Brande, J, et al. Methylene blue in the treatment and prevention of ifosfamide-induced encephalopathy: Report of 12 cases and a review of the literature. Br J Cancer. 2000; 82:291–294.
96. Kaplan, LD, Abrams, DI, Feigal, E, et al. AIDS-associated non-Hodgkin’s lymphoma in San Francisco. JAMA. 1989; 261:719–724.
97. Stellato, TA, Shenk, RR. Gastrointestinal emergencies in the oncology patient. Semin Oncol. 1989; 16:521–531.
98. Mulvihill, SJ, Pappas, TN, Fonkalsrud, EW, et al. The effect of somatostatin on experimental intestinal obstruction. Ann Surg. 1988; 207:169–173.
99. Beck, KE, Blansfield, JA, Tran, KQ, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006; 24:2283–2289.
100. Thomas, J, Karver, S, Cooney, GA, et al. Methylnaltrexone for opioid-induced constipation in advanced illness. N Engl J Med. 2008; 358:2332–2343.
101. Paulson, S, Sheehan, RG, Stone, MJ, et al. Large cell lymphomas of the stomach: Improved prognosis with complete resection of all intrinsic gastrointestinal disease. J Clin Oncol. 1983; 1:263–269.
102. Fleming, ID, Mitchell, S, Dilawari, RA. The role of surgery in the management of gastric lymphoma. Cancer. 1982; 49:1135–1141.
103. Rosen, CB, van Heerden, JA, Martin, JK, Jr., et al. Is an aggressive surgical approach to the patient with gastric lymphoma warranted? Ann Surg. 1987; 205:634–640.
104. Bayerdorffer, E, Neubauer, A, Rudolph, B, et al. Regression of primary gastric lymphoma of mucosa-associated lymphoid tissue type after cure of Helicobacter pylori infection. MALT Lymphoma Study Group. Lancet. 1995; 345:1591–1594.
105. Mesrobian, HG, Perry, JR. Radionuclide diuresis pyelography. J Urol. 1991; 146:601–604.
106. Brown, RB. A method of management of inoperable carcinoma of the bladder. Br J Urol. 1968; 40:489.
107. Cuttner, J, Holland, JF, Norton, L, et al. Therapeutic leukapheresis for hyperleukocytosis in acute myelocytic leukemia. Med Pediatr Oncol. 1983; 11:76–78.
108. Degos, L, Dombret, H, Chomienne, C, et al. All-trans-retinoic acid as a differentiating agent in the treatment of acute promyelocytic leukemia. Blood. 1995; 85:2643–2653.
109. Frankel, SR, Eardley, A, Lauwers, G, et al. The “retinoic acid syndrome” in acute promyelocytic leukemia. Ann Intern Med. 1992; 117:292–296.
110. Warrell, RP, Jr., de The, H, Wang, ZY, et al. Acute promyelocytic leukemia. N Engl J Med. 1993; 329:177–189.
111. Kilbourn, RG, Griffith, OW. Overproduction of nitric oxide in cytokine-mediated and septic shock. J Natl Cancer Inst. 1992; 84:827–831.
112. Kilbourn, RG, Belloni, P. Endothelial cell production of nitrogen oxides in response to interferon gamma in combination with tumor necrosis factor, interleukin-1, or endotoxin. J Natl Cancer Inst. 1990; 82:772–776.
113. Ochoa, JB, Curti, B, Peitzman, AB, et al. Increased circulating nitrogen oxides after human tumor immunotherapy: Correlation with toxic hemodynamic changes. J Natl Cancer Inst. 1992; 84:864–867.
114. Ohkubo, C, Bigos, D, Jain, RK. Interleukin 2 induced leukocyte adhesion to the normal and tumor microvascular endothelium in vivo and its inhibition by dextran sulfate: Implications for vascular leak syndrome. Cancer Res. 1991; 51:1561–1563.
115. Lee, RE, Lotze, MT, Skibber, JM, et al. Cardiorespiratory effects of immunotherapy with interleukin-2. J Clin Oncol. 1989; 7:7–20.
116. Margolin, KA, Rayner, AA, Hawkins, MJ, et al. Interleukin-2 and lymphokine-activated killer cell therapy of solid tumors: Analysis of toxicity and management guidelines. J Clin Oncol. 1989; 7:486–498.
117. Schwartzentruber, DJ. Guidelines for the safe administration of high-dose interleukin-2. J Immunother. 2001; 24:287–293.
118. Saxon, RR, Klein, JS, Bar, MH, et al. Pathogenesis of pulmonary edema during interleukin-2 therapy: Correlation of chest radiographic and clinical findings in 54 patients. Am J Roentgenol. 1991; 156:281–285.
119. Vogelzang, PJ, Bloom, SM, Mier, JW, et al. Chest roentgenographic abnormalities in IL-2 recipients. Incidence and correlation with clinical parameters. Chest. 1992; 101:746–752.
120. Crabtree, GR, Gillis, S, Smith, KA, et al. Mechanisms of glucocorticoid-induced immunosuppression: Inhibitory effects on expression of Fc receptors and production of T-cell growth factor. J Steroid Biochem. 1980; 12:445–449.
121. Kozeny, GA, Nicolas, JD, Creekmore, S, et al. Effects of interleukin-2 immunotherapy on renal function. J Clin Oncol. 1988; 6:1170–1176.
122. Guleria, AS, Yang, JC, Topalian, SL, et al. Renal dysfunction associated with the administration of high-dose interleukin-2 in 199 consecutive patients with metastatic melanoma or renal carcinoma. J Clin Oncol. 1994; 12:2714–2722.
123. Kragel, AH, Travis, WD, Feinberg, L, et al. Pathologic findings associated with interleukin-2-based immunotherapy for cancer: A postmortem study of 19 patients. Hum Pathol. 1990; 21:493–502.
124. Dougherty, AH, Jackman, WM, Naccarelli, GV, et al. Acute conversion of paroxysmal supraventricular tachycardia with intravenous diltiazem. IV Diltiazem Study Group. Am J Cardiol. 1992; 70:587–592.
125. Hoos, A, Ibrahim, R, Korman, A, et al. Development of ipilimumab: Contribution to a new paradigm for cancer immunotherapy. Semin Oncol. 2010; 37:533–546.
126. Deeg, HJ. Prophylaxis and treatment of acute graft-versus-host disease: Current state, implications of new immunopharmacologic compounds and future strategies to prevent and treat acute GVHD in high-risk patients. Bone Marrow Transplant. 1994; 14(Suppl 4):S56–S60.
127. Billingham, RE. The biology of graft-versus-host reactions. Harvey Lect. 1966; 62:21–78.
128. Yi, T, Chen, Y, Wang, L, et al. Reciprocal differentiation and tissue-specific pathogenesis of Th1, Th2, and Th17 cells in graft-versus-host disease. Blood. 2009; 114:3101–3112.
129. Koreth, J, Matsuoka, K, Kim, HT, et al. Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med. 2011; 365:2055–2066.
130. Sullivan, KM, Kopecky, KJ, Jocom, J, et al. Immunomodulatory and antimicrobial efficacy of intravenous immunoglobulin in bone marrow transplantation. N Engl J Med. 1990; 323:705–712.
131. Bearman, SI. The syndrome of hepatic veno-occlusive disease after marrow transplantation. Blood. 1995; 85:3005–3020.
132. Attal, M, Huguet, F, Rubie, H, et al. Prevention of hepatic veno-occlusive disease after bone marrow transplantation by continuous infusion of low-dose heparin: A prospective, randomized trial. Blood. 1992; 79:2834–2840.
133. Bearman, SI, Lee, JL, Baron, AE, et al. Treatment of hepatic venocclusive disease with recombinant human tissue plasminogen activator and heparin in 42 marrow transplant patients. Blood. 1997; 89:1501–1506.
134. Morris, JD, Harris, RE, Hashmi, R, et al. Antithrombin-III for the treatment of chemotherapy-induced organ dysfunction following bone marrow transplantation. Bone Marrow Transplant. 1997; 20:871–878.
135. Nattakom, TV, Charlton, A, Wilmore, DW. Use of vitamin E and glutamine in the successful treatment of severe veno-occlusive disease following bone marrow transplantation. Nutr Clin Pract. 1995; 10:16–18.
136. Levy, V, Azoulay, D, Rio, B, et al. Successful treatment of severe hepatic veno-occlusive disease after allogeneic bone marrow transplantation by transjugular intrahepatic portosystemic stent-shunt (TIPS). Bone Marrow Transplant. 1996; 18:443–445.
137. Corbacioglu, S, Cesaro, S, Faraci, M, et al. Defibrotide for prophylaxis of hepatic veno-occlusive disease in paediatric haemopoietic stem-cell transplantation: An open-label, phase 3, randomised controlled trial. Lancet. 2012; 379:1301–1309.
138. McDonald, GB, Hinds, MS, Fisher, LD, et al. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: A cohort study of 355 patients. Ann Intern Med. 1993; 118:255–267.
139. Lum, LG. Recapitulation of immune ontogeny: A vital component for the success of bone marrow transplantation. Cancer Treat Res. 1990; 50:27–54.
140. Wingard, JR. Fungal infections after bone marrow transplant. Biol Blood Marrow Transplant. 1999; 5:55–68.
141. Wingard, JR. Viral infections in leukemia and bone marrow transplant patients. Leuk Lymphoma. 1993; 11(Suppl 2):115–125.
142. Greenberg, PD, Finch, RJ, Gavin, MA, et al. Genetic modification of T-cell clones for therapy of human viral and malignant diseases. Cancer J Sci Am. 1998; 4(Suppl 1):S100–S105.
143. Leen, AM, Christin, A, Myers, GD, et al. Cytotoxic T lymphocyte therapy with donor T cells prevents and treats adenovirus and Epstein-Barr virus infections after haploidentical and matched unrelated stem cell transplantation. Blood. 2009; 114:4283–4292.
144. Bao, L, Cowan, MJ, Dunham, K, et al. Adoptive immunotherapy with CMV-specific cytotoxic T lymphocytes for stem cell transplant patients with refractory CMV infections. J Immunother. 2012; 35:293–298.
145. Walter, EA, Greenberg, PD, Gilbert, MJ, et al. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995; 333:1038–1044.
146. Lawrence, VA, Clark, GM. Cancer and resuscitation. Does the diagnosis affect the decision? Arch Intern Med. 1987; 147:1637–1640.
147. Vitelli, CE, Cooper, K, Rogatko, A, et al. Cardiopulmonary resuscitation and the patient with cancer. J Clin Oncol. 1991; 9:111–115.
148. Schapira, DV, Studnicki, J, Bradham, DD, et al. Intensive care, survival, and expense of treating critically ill cancer patients. JAMA. 1993; 269:783–786.
149. Gray, WA, Capone, RJ, Most, AS. Unsuccessful emergency medical resuscitation—Are continued efforts in the emergency department justified? N Engl J Med. 1991; 325:1393–1398.
150. Studnicki, J, Schapira, DV, Straumfjord, JV, et al. A national profile of the use of intensive care by Medicare patients with cancer. Cancer. 1994; 74:2366–2373.
151. Tolle, SW, Tilden, VP, Nelson, CA, et al. A prospective study of the efficacy of the physician order form for life-sustaining treatment. J Am Geriatr Soc. 1998; 46:1097–1102.
152. Ahluwalia, SC, Chuang, FL, Antonio, AL, et al. Documentation and discussion of preferences for care among patients with advanced cancer. J Oncol Pract. 2012; 7:361–366.
153. Ewer, MS, Taubert, JK. Advance directives in the intensive care unit of a tertiary cancer center. Cancer. 1995; 76:1268–1274.