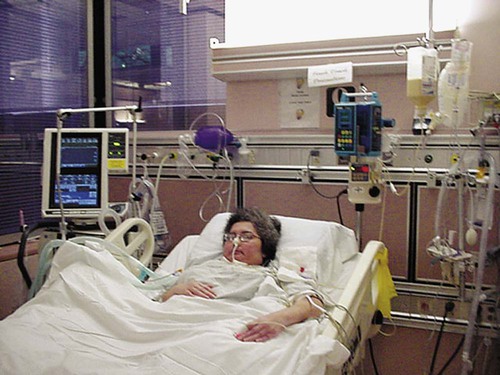
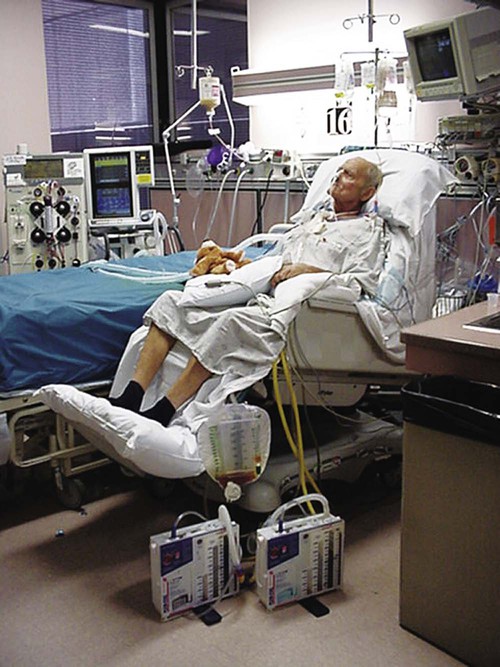
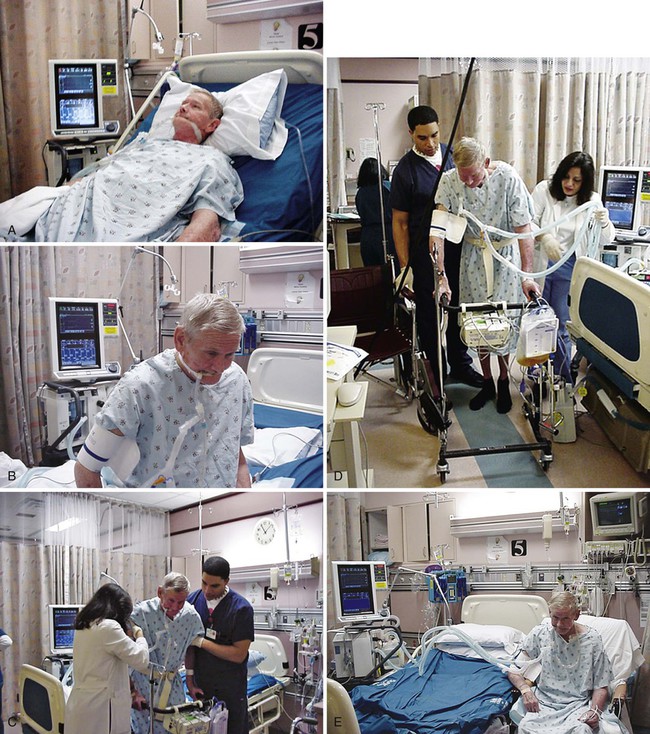
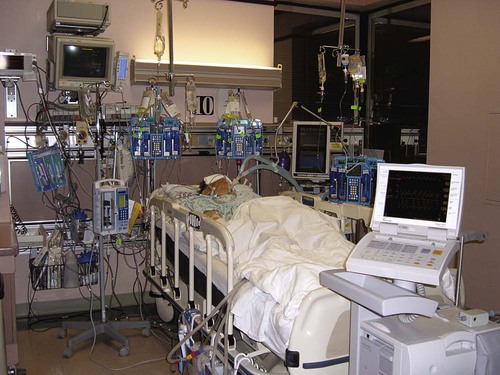
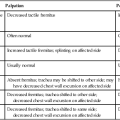
Intensive Care Management of Individuals with Primary Cardiovascular and Pulmonary Dysfunction
The principles of physical therapy management that are described are not treatment prescriptions. Rather, each patient must be assessed and treated individually, taking into consideration all factors that contribute to impaired oxygen transport (i.e., recumbency, restricted mobility, extrinsic factors related to the patient’s care, intrinsic factors related to the patient, and the underlying pathophysiology) (see Chapter 2).
Cardiovascular and Pulmonary Failure
Pathophysiology and Medical Management
Pulmonary failure reflects a gas exchange defect or defect of the ventilatory pump. Table 34-1 shows the classification of pulmonary failure by specific causes and the mechanisms involved. Some common predisposing conditions include primary cardiovascular and pulmonary conditions (e.g., chronic lung disease, overwhelming pneumonia, and myocardial infarction [MI]) and cardiovascular and pulmonary conditions that are secondary to other conditions (e.g., motor neuron diseases, spinal cord injury, stroke, and muscular dystrophy) (see Chapter 35). The oxygen and carbon dioxide tensions that have been used to define failure are variable because they depend on factors such as premorbid status, general health, age, prior blood gas profile, and the time frame for the development of failure. Arterial blood gases and pH are essential in the assessment of cardiovascular and pulmonary failure, which is usually diagnosed when the PaO2 falls below 50 to 60 mm Hg and the PaCO2 rises above 50 mm Hg.1
Table 34-1
Classification of Respiratory Failure by Cause and Mechanism
| Origin | Drugs | Metabolic | Neoplasms | Infections | Trauma | Other |
| Brain | Narcotics Barbiturates Sedatives Poisons Anesthetics |
Hyponatremia Hypocalcemia Hypercapnia Alkalosis Hyperglycemia Myxedema |
Primary Metastatic |
Meningitis Encephalitis Abscess Bulbar polio |
Direct injury Increased pressure |
Central alveolar hypoventilation Obstructive sleep apnea |
| Nerves and muscles | Curariform drugs Arsenic Aminoglycosides |
Hypophosphatemia Hypomagnesemia |
Primary Metastatic |
Polio Tetanus |
Direct injury | Motor neuron disease Myasthenia gravis Multiple sclerosis Muscular dystrophy Guillain-Barré syndrome |
| Upper airway | Tonsillar adenoid hyperplasia Goiter Polyps Malignant tumors |
Epiglottitis Laryngotracheitis |
Vocal cord paralysis Tracheomalacia Cricoarytenoid arthritis Laryngeal edema |
|||
| Chest bellows | Flail chest Burn with keloids |
Scleroderma Pleural interposition (fibrosis, fluid, tumor, air) Spondylitis Scoliosis Kyphosis |
||||
| Contributing factors | Massive obesity Ascites Ileus Pain Recumbency |
|||||
| Lower airway and parenchyma | Viral (bronchiolitis, bronchopneumonia) Bacterial (bronchitis, pneumonia, abscess, bronchiectasis) Fungal Mycoplasma |
Contused lung | Bronchospasm Heart failure: congestive, restrictive, obstructive COPD Respiratory distress syndrome Interstitial lung disease Atelectasis Cystic fibrosis Pulmonary emboli |
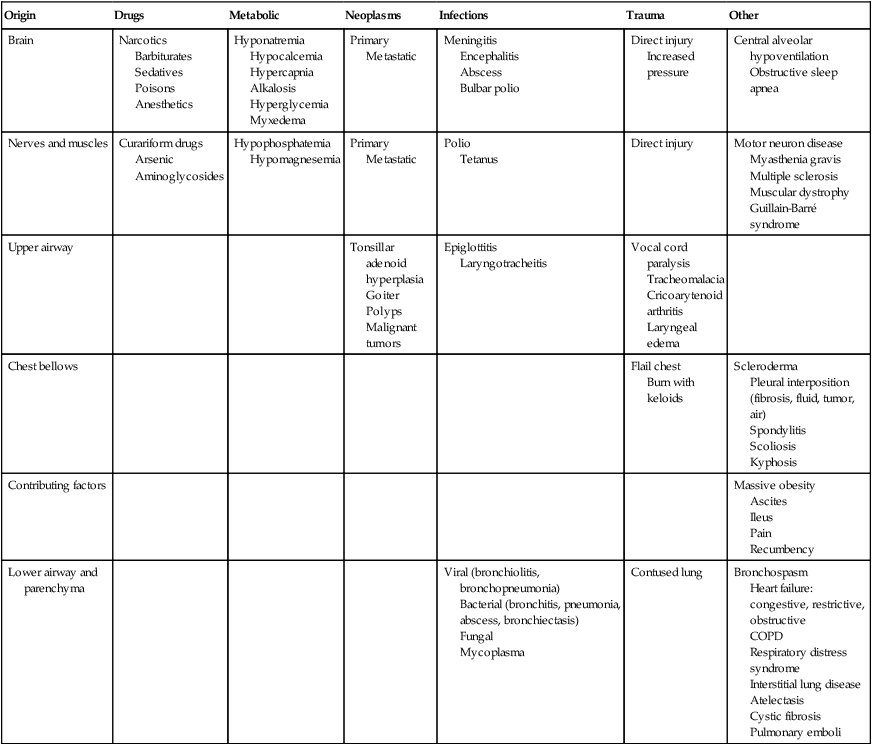
From Civettia, J.M. Taylor, R.W. & Kirby, R.R (1988). Critical care, Philadelphia: JB Lippincott.
Obstructive Lung Disease
Pathophysiology and Medical Management
Obstructive lung disease can result in ventilatory failure and admission of the patient to the ICU, or it can complicate management if the patient is admitted for other reasons.2 If conservative management fails or is unlikely to improve critically impaired oxygen transport and gas exchange and to adequately remove copious and tenacious secretions, intubation and mechanical ventilation are indicated (see Chapter 43). Complicating factors include impaired oxygen delivery, polycythemia, impaired respiratory mechanics secondary to lung damage and increased time constants impairing optimal inhalation and exhalation, flattened hemidiaphragms, rigid barrel-shaped chest wall, increased accessory muscle use and work of breathing, reduced diffusing capacity, impaired mucociliary transport, secretion accumulation, ineffective cough mechanism, increased oxygen consumption, increased work of the heart, and general debility and weakness.
The goal of intubation and mechanical ventilation is to support breathing by providing an airway and adequate alveolar ventilation and is based on arterial blood gas analysis. A tidal volume and a respiratory rate that provide satisfactory blood gas and pH values are established and maintained unless the clinical condition changes. The precise regulation of mechanical ventilation helps to restore adequate blood gases and cardiovascular and pulmonary function, reduce the work of breathing, rest fatigued ventilatory muscles, and provide an optimal fraction of inspired oxygen (FIO2) and humidification (Figure 34-1).
Positive end-expiratory pressure (PEEP) is useful in promoting greater opportunity for gas exchange at end-expiration in mechanically ventilated patients. Venous return, myocardial perfusion, and cardiac output, however, may be impaired during positive-pressure ventilation with PEEP administration.3 Excessive stimulation to cough in these ventilated patients should be avoided because this accentuates the cardiovascular side effects of PEEP. Continuous positive airway pressure (CPAP) can maintain airway patency during spontaneous ventilation. This mode of ventilation, however, seems to be preferred in children, whereas PEEP is used more commonly in adults.
Suctioning can be performed frequently in a patient with an artificial airway and is less traumatic. Patients should be suctioned only as indicated because this procedure can produce significant desaturation (up to 60%), particularly in the ventilated patient.4 Administration of 100% oxygen for 3 minutes before and after suctioning (i.e., hyperoxygenation) minimizes this desaturation effect. This can be accomplished by manual bagging before treatment (i.e., manual hyperventilation) or by presetting of the mechanical ventilator. Risk of aspiration of gastric contents is reduced by the use of a nasogastric tube.
A common cause of acute respiratory failure is advanced chronic airflow limitation.5 The pathophysiological deficits include significant loss of alveolar tissue, increased compliance of alveolar tissue, hyperinflated chest wall, impaired respiratory mechanics, flattened hemidiaphragms, impaired breathing efficiency, and reduced diffusing capacity. Proportional changes in lung volumes and capacities in patients with chronic airway limitation compared with healthy persons are presented in Figure 9-5. The primary abnormalities are significantly increased residual volume and inspiratory reserve volume and hence total lung capacity. Failure of oxygen transport ensues secondary to ventilation and perfusion mismatch, ventilatory muscle fatigue, reactive pulmonary hypertension, and right ventricular failure. Correcting the complications of respiratory failure, however, is often more problematic than treating the specific cause. Hypoxemia and hypercapnia are often present. Hypoxemia is usually improved with supplemental oxygen in the absence of significant diffusion defect or shunt.
Cardiovascular complications are among the most prevalent observed in ventilatory failure. Marked hypercapnia (increased arterial PCO2) with acidemia (reduced pH) can produce extreme vasodilatation and hypotension resulting from the local action on blood vessels.2 Mild hypercapnia can produce reflex vasoconstriction and hypertension. Occasionally, systemic hypertension is observed during weaning from the ventilator with the presence of a moderate degree of hypercapnia.
End-stage respiratory failure results in a progressive increase in airway resistance, work of breathing, oxygen consumption, and carbon dioxide production. In areas of bronchial obstruction, marked alveolar hypoventilation results and ventilation and perfusion are severely mismatched. Hypoxemia and respiratory acidosis produce reactive pulmonary hypertension and further ventilatory failure. Profound carbon dioxide retention, refractory hypoxemia, and respiratory acidemia may terminate in a fatal dysrhythmia.6
Of increasing interest in recent years has been the experience of illness and its contribution to disability. After their first episode of respiratory failure, patients with chronic obstructive pulmonary disease (COPD) report worse cognitive function and overall health status. After several months, however, these conditions may return to levels reported by individuals with similar disease severity who are being conservatively managed and have had no ICU admission.7 The Nottingham Health Profile and Mini-Mental State Examination can be useful tools for ongoing assessment of perceived health and cognition.
Principles of Physical Therapy Management
The principles of management of acute respiratory failure secondary to an exacerbation of COPD are based on interventions that will enhance oxygen transport (i.e., oxygen delivery, oxygen consumption, and oxygen extraction) and facilitate carbon dioxide removal. Thus the steps of the oxygen transport pathway that have been identified as impaired or threatened by the patient’s condition (i.e., restricted mobility, recumbency, and extrinsic and intrinsic factors in addition to the underlying pathophysiology) (see Chapter 2) are the focus of treatment.8 On the basis of a detailed analysis of these factors, treatments are selected, prioritized, and applied to optimize the steps in the pathway that are affected. These may include treatments to maximize the patency of the airways, increase alveolar ventilation, facilitate mucociliary transport, facilitate airway clearance, optimize the mechanical position of the diaphragm, optimize ventilation and perfusion matching, optimize pH, eliminate carbon dioxide, optimize peripheral circulation and tissue perfusion, and reduce the work of breathing and of the heart. To optimize oxygen transport, the primary goals of physical therapy management include the following:
1. Improve or maintain arterial oxygen tension (PaO2) or prevent its deterioration
2. Improve or maintain arterial oxygen saturation (SaO2) or prevent its deterioration
3. Improve or maintain arterial carbon dioxide levels (PaCO2) and pH or prevent their deterioration
4. Optimize oxygen delivery and oxygen consumption relationship
Mobilization: Special Considerations
Mobilization and ambulation are required for normal physiological functioning of the human body (i.e., to stimulate exercise stress and gravitational stress and thereby optimize oxygen transport).9,10 Although patients in the ICU are encouraged to move, exercise, sit up, stand, sit in chairs, take a few steps, and in some circumstances ambulate across the unit even if they are ventilated, therapeutic mobilization is prescribed to exploit its acute effects, long-term cumulative effects, and preventive effects (see Chapter 18). These effects are physiologically distinct and need to be prescribed specifically to address each patient’s problems. With the monitoring capability in the ICU to ensure safety, patients who are critically ill can move and be moved within safe as well as therapeutic limits.
Given that cardiovascular and pulmonary physical therapy is among those activities that are the most metabolically demanding for patients in the ICU,11–14 the patient’s capacity to meet a given increase in oxygen demand must be determined before treatment. Even though cardiovascular and pulmonary physical therapy stresses the oxygen transport system as a means of improving the function and efficiency of this system, unnecessary or excessive energy expenditure is undesirable and thus should be minimized. Interventions that can minimize oxygen demand include relaxation, judicious body positioning to facilitate oxygen transport, coordination of treatments with other interventions, scheduling of treatments at appropriate times, timing of treatments with medications so the maximal effect is achieved, pain control, and coordination of treatments with peak energy periods and around rest periods.
Mobilization and exercise are at the top of the hierarchy of physiological treatments in the management of patients in the ICU15–17; therefore the potent and direct effects of these interventions are exploited first (see Chapter 17). Being moved to (passive) or moving into (active) the upright position promotes improved cardiovascular and pulmonary function and gas exchange. A supported chair should be available beside every ICU bed to provide greater opportunity for the patient to be upright when out of bed. The benefits of the upright sitting position are different from those of sitting propped up in bed. Stretcher chairs are particularly useful for patients who are unable to bear weight (Figure 34-2). There are also beds that are designed to position patients sitting. Even minimal ability of the patient to assist with his or her bed mobility and transferring must be exploited, even if the maneuver takes several minutes and multiple assistants. These minimal efforts must be exploited, or the patient’s oxygen transport system will decondition further, which also will reduce the patient’s tolerance for being mobilized. What justifies this time and personnel cost is the greater therapeutic outcome that can be expected compared with passive interventions. The ultimate goal is enhanced recovery, reduced discomfort, reduced morbidity and mortality, and reduced ICU and hospital stay.
Significant benefit can be gained from standing the ventilated patient, provided there are no absolute contraindications to being upright in terms of cardiovascular and pulmonary function, neuromuscular and musculoskeletal status, and skin integrity. Ambulating the patient who is ventilated is the priority whenever possible.18–20 The potential risks, however, must be recognized. In a prospective study, Bailey and colleagues (2007)21 documented a total of 1449 “activity events” in 103 patients with respiratory failure. These activity events included 233 (16%) instances of sitting on the bed, 454 (31%) instances of sitting in a chair, and 762 (53%) instances of ambulating. In patients with an endotracheal tube, there were 593 activity events reported, of which 249 (42%) were ambulation. Fewer than 1% adverse activity-related events were reported (including fall to the knees without injury, feeding tube removal, systolic blood pressure greater than 200 mm Hg, systolic blood pressure less than 90 mm Hg, and desaturation less than 80%). No patient was extubated during an activity event. Thus activity and mobilization are feasible and safe in patients with respiratory failure. Of note, the majority of survivors (69%) were able to ambulate over 100 feet at discharge from the ICU. These findings have been corroborated by others.22,23 Mobilization and exercise must be prescribed specifically, however, to ensure that the exercise stimulus is therapeutic (i.e., provides an adequate stressor to the oxygen transport pathway yet is not hazardous).
Standing and walking even a few steps can be extremely strenuous with respect to oxygen demand for the patient in the ICU. Such activities need to be introduced gradually and with continuous monitoring to ensure that the patient does not exceed the prescribed therapeutic intensity needed to maximize oxygen transport. Standing and walking are coordinated with other aspects of the patient’s care and should be carried out in several stages (Figure 34-3). Monitoring of the electrocardiogram (ECG) and arterial saturation of the critically ill patient while he or she performs activities such as standing or walking cannot be overemphasized. In anticipation of an increased workload, ventilatory parameters for ventilated patients may require adjusting. A greater concentration of oxygen should be delivered for at least 3 to 5 minutes before the activity and continued afterward for 10 minutes or so until the patient has recovered from the increased exertion and the heart rate and blood pressure have returned to within 5% to 10% of baseline values.
Active movements have greater therapeutic effect on oxygen transport than assisted or passive movements; thus the benefits of active movements are exploited first. Movement recruiting large muscle groups minimizes the disproportionate hemodynamic stress associated with movement of small muscle groups or movements requiring excessive dynamic stabilization. If active movements cannot be performed or excessively stress the oxygen transport system, then active assisted movements are indicated. A recent randomized controlled clinical trial reported evidence supporting that early exercise training (including use of a bedside ergometer for 20 minutes daily either passively or actively) enhanced the recovery of functional exercise capacity in patients in the ICU, their self-perceived functional status, and their muscle force at hospital discharge.24
Passive movements have a role primarily when the patient is paralyzed or so hemodynamically unstable that his or her condition deteriorates with active movement. With respect to cardiovascular and pulmonary benefits, passive movement stimulates changes in ventilatory and circulatory patterns.25 These can be particularly beneficial in patients who have significant mobility restriction; however, they should not substitute for active and active-assisted movements, which are associated with even greater benefit because they are higher-order activities on the physiologically based treatment hierarchy (see Chapter 17).
A study reported on the use of electrical stimulation in combination with active exercise of the limbs in patients with severe COPD who were mechanically ventilated.26 Muscle strength was improved, and the number of days to transfer from bed to chair was reduced. The study’s design precludes electrical stimulation as being superior to extended active exercise and progressive mobilization to the chair and ambulation. Another recent study demonstrated that electrical stimulation is well tolerated and seems to preserve the muscle mass of critically ill patients.27 The use of electrical stimulation as a preventive and rehabilitative tool in patients in the ICU with polyneuromyopathies, however, needs to be further investigated and should not replace mobilization. Deep sedation and bed rest are common in the routine medical care for many mechanically ventilated patients20 and need to be reconsidered in light of rehabilitation initiatives to prevent iatrogenic effects of care and to maximize short- and long-term functional outcomes.
Body Positioning: Special Considerations
Body positioning is a potent therapeutic intervention that promotes optimal oxygen transport and gas exchange in two ways: one, from the physiological benefits accrued from the specific positions themselves, and two, from the physiological benefits accrued from physically changing from one position to another (see Chapter 20). Body position can be used preferentially to augment alveolar volume, alveolar ventilation, ventilation and perfusion matching, respiratory mechanics, cough effectiveness, central and peripheral hemodynamics and fluid shifts, mucociliary transport, and secretion clearance (see Chapter 20). Both ventilation and perfusion are enhanced in the inferior lung fields. Therefore in postural drainage positions the superior lung being treated is neither preferentially ventilated nor perfused. The less-affected lung fields therefore may be contributing more substantially to improving arterial gases. Hence the physical therapist must consider the goals of treatment with respect to pulmonary function in both the involved and less-involved lung fields. During postural drainage, the length of time in a given position needs to be monitored to avoid drainage of secretions into the less-involved, functional, inferior lung fields and to avoid the possibility of compression atelectasis in the inferior lung.
Although positions can be predicted that will optimize ventilation and perfusion matching, each patient will respond differently, depending on such factors as pathology, age, weight, depth of breathing, and mechanical ventilation.28,29 Therefore the patient’s response to specific positioning must be observed, documented, and objectively monitored with respect to the effect on oxygen transport variables.
Another important goal of body positioning is to potentiate position-dependent fluid shifts to optimize cardiovascular function. For this reason the patient should be positioned upright as often as possible commensurate with tolerance and hemodynamic stability. There are other beneficial effects of the upright position on pulmonary function (e.g., maximize lung volumes and capacities, minimize alveolar collapse, decrease airway resistance, increase lung compliance and thereby reduce ventilator system pressure). To maximize the effect of gravity on promoting fluid shifts, the legs should be positioned dependently at frequent intervals. In a patient who is not self-supporting with or without assistance, fluid shifts can be stimulated with a high Fowler position coupled with the use of the bed knee-break. Between sessions of therapeutic body positioning, a schedule of four-point turning (supine, left side, prone, right side) is ideal and should be attempted even in the ventilated patient if not strictly contraindicated.30
Extreme 360-degree body position changes (e.g., randomized positioning in supine, prone, right and left side-lying) have been reported to have beneficial effects on oxygen transport in patients with acute respiratory failure.31 The head-down position has been shown to reduce respiratory distress in some patients with obstructive lung disease.32 The abdominal viscera are displaced cephalad, thereby elevating the typically flattened hemidiaphragms and placing them in a mechanically advantageous position. This effect may be mimicked in other body positions by manual abdominal compression and abdominal binders. In some patients, however, the additional load imposed by the increased intraabdominal pressure on the underside of the diaphragm may inadvertently increase the work of breathing and increase respiratory distress. The prone position has been reported to be beneficial in patients with hypoxemia and acute respiratory failure.33–35 A variant of the prone position, semiprone, may be more beneficial in some patients by reducing intraabdominal pressure. In addition, the semiprone position may be safer and more comfortable for the mechanically ventilated patient. The prescription of any body positioning must be based on its anticipated benefits on oxygen transport. The more extreme positions should be introduced in progressive stages and the patient’s response monitored to ensure the response is favorable.
Time alone is an inadequate parameter for prescribing the duration a patient should remain in one position. Adverse responses including discomfort should serve as guides in addition to the therapeutic goals. Although patients tend to be turned every 2 hours or less across ICUs, there is considerable variability with respect to this practice.36 Positioning is a clinically important evidence-based intervention for maximizing oxygen transport and minimizing multisystemic complications when a patient is unable to be mobilized or between mobilization episodes.
Status Asthmaticus
Pathophysiology and Medical Management
Medical management is aimed at administering drugs and fluids to reduce hypoxemia with oxygen, decrease airway inflammation and resistance, and hence reduce the work of breathing and anxiety. Intravenous sodium bicarbonate helps to reverse respiratory acidosis and possibly metabolic acidosis.5
Principles of Physical Therapy Management
Major problems in status asthmaticus are alveolar hypoventilation, airway obstruction secondary to bronchospasm, mucosal edema, and secretions. Therefore maximizing alveolar ventilation and facilitating mucociliary transport are priorities. Other problems include significantly increased work of breathing because of an inefficient breathing pattern and an ineffective cough. Obtaining a productive cough without augmenting bronchospasm is a challenge. Deep breathing with pursed-lip expiration helps to prolong expiration and maintain the patency of the small airways. Deep, slow, and relaxed breathing is emphasized, along with periodic effective, controlled huffing, and avoidance of forced expiratory maneuvers.37
Restrictive Lung Disease
Pathophysiology and Medical Management
Acute respiratory failure can be associated with primary restrictive lung disease (i.e., interstitial pulmonary fibrosis). This is distinct from restrictive defects secondary to neuromuscular and musculoskeletal diseases (e.g., Guillain-Barré syndrome, myasthenia gravis, and neuromuscular poisonings are common neuromuscular disorders that can precipitate respiratory failure in the absence of underlying primary lung disease) (see Chapter 35).
Restrictive lung dysfunction may complicate the management of patients admitted to the ICU for reasons other than cardiovascular and pulmonary disease. The lung parenchyma, the chest wall, or both may be involved. The underlying cause of respiratory failure may therefore reflect ventilatory pump failure, gas exchange failure, or both. The specific restrictions to cardiovascular and pulmonary function need to be identified and treated individually to optimize treatment. Obstructive and restrictive patterns of cardiovascular and pulmonary function frequently coexist; thus the contribution of both types of defects to a patient’s oxygen transport must be determined. Typically in restrictive lung disease, all lung volumes and capacities are reduced, although tidal volume can be relatively normal (see Figure 9-5). Patients with severe interstitial pulmonary fibrosis have increased pulmonary artery pressures with an associated increase in right ventricular work. These patients are at high risk of desaturating with minimal activity.
Principles of Physical Therapy Management
Restrictive ventilatory dysfunction is commonly associated with both medical and surgical conditions. The principles of management of acute respiratory failure associated with these differ in that medical conditions are associated with underlying irreversible lung damage, whereas in the surgical patient the pulmonary restriction is reversible. The natural course of disease in the medical patient will be determined in part by the patient’s premorbid status. The majority of surgical patients, however, will have had normal lung function before surgery and the development of cardiovascular and pulmonary complications (see Chapter 30). Regardless of whether mechanical ventilation is indicated, tissue oxygenation, carbon dioxide removal, regulation of blood pH, and an effective cardiac output are priorities. Supplemental oxygen is often effective in improving tissue oxygenation in conditions associated with restrictive lung disease in the absence of a right-to-left shunt. In the presence of shunt, supplemental oxygen does not reverse hypoxemia.
Assisted movements are indicated to promote oxygen transport via their effects on ventilatory and circulatory patterns.25 In addition the goal is to maintain joint range of motion and prevent adaptive shortening of periarticular soft tissue in particular. Care must therefore be taken to perform these movements through the complete range of movement for each joint, with special attention to rotary components of joint movement. Lax joints can maintain range with one complete range of motion daily. Lax joints are unprotected and vulnerable to excessive strain. Protection may best be effected in these joints by moving the joint more slowly through the extremes of joint range and just short of complete range. In the presence of spasticity or limb splinting because of discomfort, the involved joints will benefit from two or more excursions through full range daily.
Myocardial Infarction
Pathophysiology and Medical Management
The initial priority of management in the acute phase of MI is the correction of the immediate problems including dysrhythmias, myocardial insufficiency, reduced cardiac output, hypoxemia, chest pain, and anxiety, followed by implementation of a progressive rehabilitation program ranging from the acute medically stable phase to the postdischarge rehabilitation phase.38,39 On admission to the coronary care unit or medical ICU, continuous monitoring of the heart rate and rhythm is established. An intravenous line is routinely started for the administration of medications and fluids. An arterial line may be started for serial blood sampling for blood gases and enzyme measures. Initially pain medications, coronary vasodilators, and diuretics are frequently used to minimize the work of the heart, anginal pain, and discomfort. Drugs such as morphine serve to depress the respiratory drive; thus the physical therapist must be aware of corresponding changes in vital signs. Less-potent sedatives and tranquilizers are more routinely prescribed. Increased pain and anxiety potentially worsen the patient’s cardiac status by increasing myocardial oxygen demand and altering normal breathing pattern and gas exchange.
Myocardial Infarction Conservatively Managed
Principles of Physical Therapy Management
A primary principle of the management of the patient after MI is to reduce myocardial oxygen demand and workload. The myocardium needs rest for optimal healing. Judicious rest of the myocardium is a priority that can often be balanced with gentle rhythmic, nonstatic movements. Box 34-1 illustrates several ways of reducing myocardial workload in patients with cardiovascular and pulmonary conditions.
Depending on the degree of MI and damage, varying lengths of modified mobility may be recommended.38 Shorter initial periods of restricted mobility are safe and promote faster functional recovery.40 During this period the physical therapist concentrates on rhythmic breathing exercises, gentle coughing exercises or huffing, and modified positioning with the bed head elevated at least 30 degrees to facilitate the gravity-dependent mechanical action of the heart and thereby reduce myocardial oxygen demand. Intermittent exposure to orthostatic stress by imposing the upright position is recommended for patients after acute MI.41 The patient is encouraged to perform deep breathing and coughing every hour during the day. Bed exercises including rhythmic, unresisted hip, knee, foot, and ankle exercises are usually performed as frequently as possible by the patient or when the patient turns in bed. The patient is cautioned to exercise one leg at a time, sliding one heel up and down the bed and guarding against lifting the leg off the bed. These exercises, when performed correctly and coordinated with inspiration and expiration, require relatively little effort from and induce little additional physical stress on the individual with MI. Comparable with the management of the postoperative patient, these exercises are performed prophylactically to reduce the risk of venous stasis and formation of thromboemboli. In addition, they may help to regulate more coordinated breathing, encourage deep breaths and mucociliary transport, and reduce atelectasis. The patient is cautioned against performing the Valsalva maneuver and straining because these activities increase intrathoracic pressure and reduce cardiac output.
Electrocardiographic monitoring of the patient with cardiac disease is the responsibility of all members of the health care team involved in the patient’s care. The physical therapist has a special responsibility to be proficient in ECG interpretation in the coronary care unit because physical therapy is one of the most metabolically demanding interventions for patients.11,14 The physical therapist often has the responsibility of initiating new activities with the cardiac patient, which might include sitting over the edge of the bed, engaging in self-care (particularly involving the arms being maintained in a raised position), getting in and out of bed, sitting in a chair, going to the bathroom, walking around the room or in the hallways, and eventually exercising on the treadmill or ergometer. Changes in the ECG must be watched for, particularly in introducing new activities and increasing the intensity of workload in activities. Careful attention to ECG changes and serum enzyme levels will contribute to enhanced physical therapy care of the acute MI patient by optimizing the treatments prescribed and the margin of safety with which activities are performed.
Congestive heart failure may be unavoidable in cases of severe infarction or even milder infarction coupled with lung disease. Fluid intake and output and daily weight measurements promote early detection of congestive heart failure. Signs of imminent and established congestive heart failure are listed in Box 34-2. The work of the heart can be significantly reduced in the upright position.42
The initial rehabilitation program is planned with the long-range rehabilitation goals in mind. The program designed for the cardiac patient is progressive in terms of types of activities, usually beginning with activities of daily living, and with respect to the intensity, duration, and frequency of these activities.39 The patient’s tolerance and changes in ECG and vital signs are used as indicators for establishing and modifying the treatment program. These physiological parameters must be observed carefully as the patient progresses to optimize the potential benefits of the therapeutic regimens as early as possible without endangering the patient.
Open Heart Surgery
Principles of Physical Therapy Management
Patients scheduled for open heart surgery are considered moderately risky surgical candidates because of the nature and invasiveness of this type of surgery (Figure 34-4), regardless of their general level of health before surgery. Whenever possible, patients prepare for surgery in advance by decreasing or stopping smoking, by avoiding exposure to respiratory tract infections, by avoiding stress, by eating a balanced diet, and by getting adequate sleep. Patients can benefit from a modified, prescriptive exercise program before surgery to maximize their aerobic capacity and thereby improve their perioperative course.
Preoperative teaching coupled with a conditioning program before hospitalization has a role in reducing hospital stay and complications (see Chapters 18, 29, and 30). During the preoperative period the physical therapist may spend additional time with patients scheduled for open heart surgery to provide teaching of the basic anatomy and physiology related to the surgery to be performed; the effect of anesthesia; the role of intubation and mechanical ventilation; the incision lines to be expected over the chest, and the legs if veins are to be removed for bypass surgery; the lines, leads, chest tubes, and catheters that will be in place after surgery; interventions beginning during the postanesthesia and recovery period (breathing control and coughing maneuvers, body positioning, foot and ankle exercises, and early mobilization); and the course of recovery the patient might expect, barring complications (see Chapter 17). The emphasis on patient education in most open heart surgery units may contribute to the generally low incidence of complications and mortality.
Some special considerations in physical therapy management of the patient after open heart surgery appear in Table 34-2 and these expand on the concurrent medical course of patients in phase I cardiac rehabilitation (see Chapter 31). Patients are extubated early, and rehabilitation is commenced as soon as possible.43 These guidelines are to be applied thoughtfully and cautiously with regard to each specific patient’s condition and observed recovery. Intermittent orthostatic stress and exercise stress are encouraged.41 These guidelines suggest the upper limit of intensity of physical therapy, which should be applied if all is progressing well initially and reduced if warranted by the patient’s condition. Progression from one stage to the next is based on an optimal and reliable treatment response at each level before proceeding. Different institutions may advocate different practices, depending on their facilities, the experience of the surgical and ICU team, and the incidence of postoperative complications and survival for that institution.
Table 34-2
Stages of and Guidelines for Physical Therapy for the Patient after Open Heart Surgery

*The physical therapist must guard against excessively intense treatment of post–open heart surgery patients as well as other patients receiving prophylactic anticoagulants.
Patients are monitored for complications (see Chapters 16 and 36) and to guide treatment progression and detect untoward responses to treatment. After cardiac surgery, 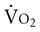 tends to increase owing to increased cardiac output and oxygen delivery.44 The oxygen extraction ratio may increase and
tends to increase owing to increased cardiac output and oxygen delivery.44 The oxygen extraction ratio may increase and 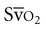 decrease if cardiac function deteriorates.
decrease if cardiac function deteriorates.
Depression has been reported in patients in the ICU, particularly in those admitted for coronary care who are experiencing a physiological loss.45 Signs and symptoms of depression warrant monitoring and reporting to the team to maximize clinical and psychosocial outcomes.
Complications do occur in high-risk ICU settings. Risk assessment is a fundamental component of the assessment (see Chapter 17). Common complications often related to premorbid status include restricted mobility, inability to wean from mechanical ventilation, deep vein thrombosis, pulmonary emboli, and stroke. Other complications are related to surgery (e.g., internal bleeding, overwhelming pneumonia, and lung collapse) or medication reaction. Complications are better managed if anticipated and detected early. Depending on the nature of the complication, physical therapy interventions may be stepped up in terms of intensity, duration, and frequency; modified; or discontinued until the patient is cleared with respect to stability.