Chapter 72 Integration of Genetics into Pediatric Practice
Genetic testing involves analyzing genetic material to obtain information related to a person’s health status using chromosomal (cytogenetic) analysis (Chapter 76) or DNA-based testing.
Diagnostic Testing
Diagnostic genetic testing helps explain a set of signs and/or symptoms of a disease. The list of disorders for which specific genetic tests is available is extensive. The website www.genetests.org provides a database of available tests.
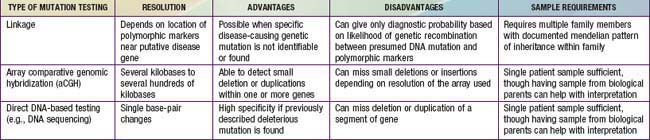
Single-gene disorders can be tested by at least 3 different approaches: linkage analysis, array comparative genomic hybridization (aCGH), and direct mutation (DNA sequence-based) analysis, usually by DNA sequencing (Table 72-1). Linkage analysis is used if the responsible gene is mapped but not yet identified, or if it is impractical to find specific mutations, usually because of the large size and larger number of different mutations in some genes. aCGH can be used to detect large multigene deletions or duplications (copy number variations). However, with increasing resolution, single gene or smaller intragenic deletions or duplications can be detected. Direct DNA mutation analysis is preferred and is possible with the availability of the complete human genome sequence.
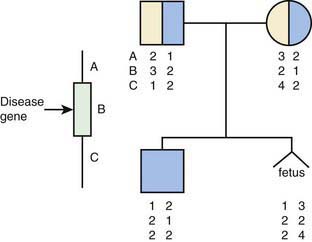
Linkage testing involves tracking a genetic trait through a family using closely linked polymorphic markers as a surrogate for the trait (Fig. 72-1). It requires testing an extended family and is vulnerable to several pitfalls, such as genetic recombination, genetic heterogeneity, and incorrect diagnosis in the proband. Genetic recombination occurs between any pair of loci, the frequency being proportional to the distance between them. This problem can be ameliorated by using very closely linked markers and, if possible, using markers that flank the specific gene. Genetic heterogeneity can be problematic for a linkage-based test if there are multiple distinct genomic loci that can cause the same phenotype, resulting in the risk that the locus tested for is not the one responsible for disease in the family. Incorrect diagnosis in the proband also leads to tracking the wrong gene. Linkage testing remains useful for several genetic conditions, though it is increasingly being superseded by the availability of direct DNA sequencing. It is critically important that genetic counseling be provided to the family to explain the complexities of interpretation of test results.
aCGH (Chapter 76) can detect copy number variation in a patient’s DNA by comparing it to a standard control DNA. In so doing, it provides a level of genetic resolution between what is available with DNA sequencing and what is available with chromosome analysis. Whereas earlier technologies could only identify large deletions or duplications that might encompass multiple genes, aCGH can resolve deletions or duplications of several kilobases within one gene. In theory, this approach can detect deletion and duplication mutations that would be missed by either chromosome analysis or direct mutation testing by DNA sequencing. However, because the specific resolution and coverage of different aCGH platforms can vary tremendously for different gene regions, the sensitivity for detecting deletions and duplications can vary for different diseases and laboratories.
Predictive Testing
A major caution with predictive testing is that the presence of a gene mutation does not necessarily mean that the disease will develop. Many of the disorders with age-dependent penetrance display incomplete penetrance. A person who inherits a mutation might never develop signs of the disorder. There is concern that a positive DNA test could result in stigmatization of the person and might not provide information that will guide medical management. Stigmatization might include psychological stress, but it could also include discrimination, including denial of health, life, or disability insurance or employment (Chapter 73).
Predispositional Testing
It is expected that genetic tests will become available that will predict risk of disease. Common disorders are multifactorial in etiology; there may be many different genes that contribute to risk of any specific condition (Chapter 77). Most of the genetic variants that have been found to correlate with risk of a common disease add small increments of relative risk, probably in most cases too little to guide management. It is possible that further discovery of genes that contribute to common disorders will reveal examples of variants that convey more significant levels of risk. It is also possible that testing several genes together will provide more information about risk than any individual gene variant would confer. The rationale for predispositional testing is that the results would lead to strategies aimed at risk reduction as part of a personalized approach to health care maintenance. This might include avoidance of environmental exposures that would increase risk of disease, medical surveillance, or, in some cases, pharmacologic treatment. The value of predispositional testing will need to be critically appraised through outcomes studies as these tests are developed.
Pharmacogenetic Testing
Polymorphisms in drug metabolism genes can result in distinctive patterns of drug absorption, metabolism, excretion, or effectiveness (Chapters 56 and 77). Knowledge of individual genotypes will guide pharmacologic therapy, allowing customization of choice of drug and dosage to avoid toxicity and provide a therapeutic response. An example of this is testing for polymorphisms within the methylenetetrahydrofolate reductase (MTHFR) gene for susceptibility of potentially increased toxicity to methotrexate antimetabolite therapy for treatment of acute lymphoblastic leukemia.
72.1 Genetic Counseling
Genetic counseling is a communication process in which the genetic contribution to health is explained, along with specific risks of transmission of a trait and options to manage the condition and its inheritance (Table 72-2). The counselor is expected to present information in a neutral, nondirective manner and to provide support to the individual and family to cope with decisions that are made.
Genetic counseling has evolved from a model of care that was developed in the context of prenatal diagnosis and pediatrics (see Table 72-2). For prenatal diagnosis, the task is to assess risk to a couple of having a child with a genetic condition and to advise the couple about options to manage that risk, including reproductive options such as artificial insemination and prenatal or preimplantation genetic diagnosis. In pediatrics, the task is to establish a diagnosis in a child, provide longitudinal care for the child, and advise the parents about risk of recurrence as well as options to deal with that risk.
Genetic Counseling
Providing accurate information to families requires
Prenatal Diagnosis and Prevention
Many different methods of prenatal diagnosis are available, depending on the specific genetic disorder (Chapter 90). The use of ultrasonography allows prenatal diagnosis of anatomic abnormalities such as congenital heart defects. Amniocentesis and chorionic villus sampling are used to obtain fetal tissue for analysis of chromosomal abnormalities, biochemical disorders, and DNA studies. Maternal blood or serum sampling is used for some types of screening. Fetal cells can be retrieved from the umbilical cord or from maternal blood (free fetal DNA) for testing, although mothers might harbor cells from all previous pregnancies.
72.2 Management and Treatment of Genetic Disorders
Resources for patients include the National Organization of Rare Disorders (www.rarediseases.org), the Genetic Alliance (www.geneticalliance.org), the National Library of Medicine (www.nlm.nih.gov/medlineplus/geneticdisorders.html#specificconditions), and a large number of disease-specific websites. A curre2nt listing of federally and privately funded clinical trials, including many for genetic diseases, is available at ClinicalTrials.gov.
Physiologic Therapies
Physiologic therapies attempt to ameliorate the phenotype of a genetic disorder by modifying the physiology of the affected individual. The underlying defect itself is not altered by treatment. Physiologic therapies are used in the treatment of inborn errors of metabolism (Chapter 78). These include dietary manipulation, such as avoiding phenylalanine by persons with phenylketonuria; coenzyme supplementation for some patients with methylmalonic acidemia and mitochondrial diseases; stimulation of alternative pathways to excrete ammonia for those with urea cycle disorders; bisphosphonate treatment for those with osteogenesis imperfecta to reduce bone fractures; and avoiding cigarette smoking by persons with α1-antitrypsin deficiency. Physiologic treatments can be highly effective, but they usually need to be maintained for a lifetime because they do not affect the underlying genetic disorder. Many of these treatments are most effective when begun early in life before irreversible damage has occurred. This is the rationale for comprehensive newborn screening for inborn errors of metabolism.
Replacement Therapies
Transplantation
Cell and organ transplantation are potentially effective approaches to replacement of a defective gene. Aside from transplantation to replace damaged tissues, transplantation of stem cells, liver, or bone marrow is also used for several diseases, mainly inborn errors of metabolism, and hematologic or immunologic disorders. A successful transplant is essentially curative, though there may be significant risks and side effects (Chapters 129–133). Cell and tissue transplantation are effective in many clinical scenarios, but there is always short-term morbidity, often associated with either surgical (liver) or preparative (bone marrow) regimens, and long-term morbidity related to chronic immunosuppression and graft failure. Bone marrow transplantation is the best example of stem cell therapy, but much effort is focused on identifying, characterizing, expanding, and using other tissue stem cells for regenerative therapies.
Gene-transfer vehicles include viral and nonviral approaches. Most human clinical trials have used viral vectors because of their efficiency of tissue transduction. In some diseases, such as X-linked and adenosine deaminase (ADA)-deficient severe combined immunodeficiency (SCID), clinical gene therapy is a viable and effective option (Chapter 120.1). Preliminary results suggest that gene therapy (intraocular delivery) may be effective for Leber congenital amaurosis.
Aiuti A, Cattaneo F, Galimberti S, et al. Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N Engl J Med. 2009;360:447-458.
Ali-Khan SE, Daar AS, Shuman C, et al. Whole genome scanning: resolving clinical diagnosis and management amidst complex data. Pediatr Res. 2009;66:357-363.
Alkan C, Kidd JM, Marques-Bonet T, et al. Personalized copy number and segmental duplication maps using next-generation sequencing. Nat Genet. 2009;41:1061-1067.
Alliance of Genetic Support Groups: Directory of National Genetic Voluntary Organizations. 35 Wisconsin Circle, Suite 440, Chevy Chase, MD 20815–27015.
Bartels DM, LeRoy BS, McCarthy P, et al. Nondirectiveness in genetic counseling: a survey of practitioners. Am J Med Genet. 1997;72:172-179.
Bowles-Biesecker B, Marteau TM. The future of genetic counseling: an international perspective. Nat Genet. 1999;22:133-137.
Dietz HC. New therapeutic approaches to Mendelian disorders. N Engl J Med. 2010;363:852-863.
Farrell MH, Certain LK, Farrell PM. Genetic counseling and risk communication services of newborn screening programs. Arch Pediatr Adolesc Med. 2001;155:120-126.
GeneTests (website), http://www.ncbi.nlm.nih.gov/sites/GeneTests/?db=GeneTests. Accessed February 8, 2011
Hardy J, Singleton A. Genomewide association studies and human disease. N Engl J Med. 2009;360:1759-1768.
Hirschorn JN. Genomewide association studies—illuminating biologic pathways. N Eng J Med. 2009;360:1699-1701.
Holtzman NA, Watson MS. Promoting safe and effective genetic testing in the United States: final report of the task force on genetic testing. Bethesda, MD: Human Genome Research Institute; 1997.
Li MM, Andersson HC. Clinical application of microarray-based molecular cytogenetics: an emerging new era of genomic medicine. J Pediatr. 2009;155:311-317.
Manolio TA. Cohort studies and genetics of complex disease. Nat Genet. 2009;41:5-6.
O’Donovan MC, Kirov G, Owen MJ. Phenotypic variations on the theme of CNVs. Nat Genet. 2008;40:1392-1393.
Press N, Browner CH. Characteristics of women who refuse an offer of prenatal diagnosis: data from the California maternal serum alpha fetoprotein blood test experience. Am J Med Genet. 1998;78:433-445.
Qasim W, Gaspar HB, Thrasher AJ. Update on clinical gene therapy in childhood. Arch Dis Child. 2007;92:1028-1031.
Sermon K, Van Steirteghem A, Liebaers I. Preimplantation genetic diagnosis. Lancet. 2004;363:1633-1640.
Verlinsky Y, Rechitsky S, Sharapova T, et al. Preimplantation HLA testing. JAMA. 2004;291:2079-2085.
Wain LV, Armour JAL, Tobin MD. Genomic copy number variation, human health, and disease. Lancet. 2009;374:340-350.