Integrating ultrasound into critical care teaching rounds
Overview
Significant advancements have been made in the use of ultrasound technology since its inception in the early 1950s.1 These advances have revolutionized the practice of critical care medicine. Currently, clinicians in the intensive care unit (ICU) have at their disposal a tool at the bedside that augments the physical examination and is safe, portable, noninvasive, and readily available.
Safety
Ultrasound technology uses sound waves, and through reflection, refraction, and attenuation of these ultrasound beams, image acquisition occurs, thus limiting the patient’s exposure to radiation, unlike chest radiography and computed tomography.2,3 The lack of radiation exposure also allows multiple sites to be evaluated repeatedly with minimal risk to the patient (Chapter 1).
Time efficiency
Bedside ultrasound enables immediate integration between the clinical scenario that requires investigation and the operator. This allows a more focused, goal-directed evaluation of hemodynamically unstable patients with immediate interpretation of data and implementation of personalized therapy.4
On review of the literature, the time needed to obtain information during bedside ultrasound evaluation is very brief given the magnitude of information obtained during each evaluation (Table 59-1). Appropriate, focused examinations can be performed as part of routine practice on teaching rounds.
TABLE 59-1
Time Needed to Obtain Information during Bedside Ultrasound
| Investigation | Average Time |
| Lung ultrasound | 2-3 min5,6 |
| Internal jugular vein cannulation (mean start until vessel located) | 9.5-13.8 sec7–10 |
| Subclavian vein cannulation (mean start until vessel located) | 26.8 sec11 |
| Doppler ultrasound | 11.7-15 min12–14 |
| Transthoracic echocardiography | 6-10.5 min15–17 |
| Inferior vena cava measurements | 5-5.8 min18,19 |
Critical care ultrasound is clinically useful
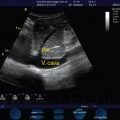
Goal-directed echocardiography enables qualitative assessment of left and right ventricular size and function, identification of pericardial effusion and evaluation for tamponade physiology, measurement of internal vena cava (IVC) diameter, and assessment of respiratory variation to determine preload responsiveness.17,20,21 Critical care ultrasound (CCU) facilitates bedside detection of pleural fluid and pneumothorax and can be used to guide thoracocentesis for diagnostic-therapeutic purposes, as well as other invasive procedures (e.g., placement of thoracostomy tubes, paracentesis, abscess drainage). CCU guides arterial and central venous cannulation and facilitates the placement of peripherally inserted central catheters and hemodialysis catheters.22 CCU detects venous thrombosis14 and free intraperitoneal and pericardial fluid (focused assessment with sonography for trauma) and facilitates the evaluation of patients for acute renal dysfunction23–25 and volume status.
Teaching tool
CCU can be used in conjunction with the physical examination to improve accuracy and clinical utility and enhance the teaching of various physiologic principles.26 It can provide immediate feedback to confirm or refute the physical findings. For example, within a few minutes of using CCU the cause of life-threatening conditions triggering hypotension can be elucidated or excluded from the differential diagnosis. If a resident hears a typical murmur of mitral regurgitation on physical examination, this can be confirmed within minutes with bedside echocardiography. Immediate feedback enhances learning.
Pearls and highlights
• Focused CCU can answer clinically relevant questions quickly.
• Focused CCU has great value as a teaching tool for confirming or refuting the findings on physical examination and for reviewing basic physiology and anatomy.
• Focused CCU can aid in bedside teaching of the differential diagnosis of common reasons for admission to the ICU, such as hypoxemia, shock, or renal failure.
References
1. Kendall, JL, Hoffenberg, SR, Smith, RS, History of emergency and critical care ultrasound: the evolution of a new imaging paradigm. Crit Care Med. 2007;35(5 suppl):S126–S130.
2. Aldrich, J. Basic physics of ultrasound imaging. Crit Care Med. 2007; 35(5 suppl):S126–S130.
3. Brenner, DJ, Elliston, CD. Estimated radiation risk potentially associated with full-body CT screening. Radiology. 2004; 232(3):735–738.
4. Oropello, JM, Manasia, AR, Goldman, M. Goal-directed echocardiography in the ICU. In: Levitov A, Mayo PH, Slonim AD, eds. Critical care ultrasonography. New York: McGraw-Hill; 2009:67.
5. Lichtenstein, DA, Mezière, G, Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol. Chest. 2008;134(1):117–125.
6. Lichtenstein, DA, Mezière, G, Lascois, N, et al. Ultrasound diagnosis of occult pneumothorax. Crit Care Med. 2005; 33(6):1231–1238.
7. Denys, BG, Uretsky, BF, Reddy, PS. Ultrasound-assisted cannulation of the internal jugular vein. A prospective comparison to the external landmark–guided technique. Circulation. 1993; 87(5):1557–1562.
8. Slama, M, Novara, A, Safavian, A, et al. Improvement of internal jugular vein cannulation using an ultrasound-guided technique. Intensive Care Med. 1997; 23(8):916–919.
9. Karakitsos, D, Labropoulos, N, De Groot, E, et al, Real-time ultrasound-guided catherisation of the internal jugular vein: a prospective comparison with the landmark technique in critical care patients. Crit Care Med. 2006;10(6):R162.
10. Leung, J, Duffy, M, Finckh, A, Real-time ultrasonographically-guided internal jugular vein catheterization in the emergency department increases success rates and reduces complications: a randomized, prospective study. Ann Emerg Med. 2006;48(5):540–547.
11. Fragou, M, Gravvanis, A, Dimitriou, V, et al, Real time ultrasound-guided subclavian vein cannulation versus the landmark method in critical care patients: a prospective randomized study. Crit Care Med. 2011;29(7):1607–1612.
12. Jang, T, Docherty, M, Aubin, C, et al, Resident-performed compression ultrasonography for the detection of proximal deep vein thrombosis: fast and accurate. Acad Emerg Med. 2004;11(3):319–322.
13. Magazzini, S, Vanni, S, Toccafondi, S, et al. Duplex ultrasound in the emergency department for the diagnostic management of clinically suspected deep vein thrombosis. Acad Emerg Med. 2007; 14(3):216–220.
14. Kory, PD, Pellecchia, CM, Shiloh, AL, et al. Accuracy of ultrasonography performed by critical care physicians for the diagnosis of DVT. Chest. 2011; 139(3):538–542.
15. Manasia, AR, Nagaraj, HM, Kodali, RB, et al. Feasibility and potential clinical utility of goal-directed transthoracic echocardiography performed by noncardiologist intensivists using a small hand-carried device (SonoHeart) in critically ill patients. J Cardiothorac Vasc Anesth. 2005; 19(2):155–159.
16. Melamed, R, Sprenkle, MD, Ulstad, VK, et al. Assessment of left ventricular function by intensivists using hand-held echocardiography. Chest. 2009; 135(6):1416–1420.
17. Beaulieu, Y. Specific skill set and goals of focused echocardiography for critical care clinicians. Crit Care Med. 2007; 35(5 suppl):S144–S149.
18. Jones, AE, Tayal, VS, Sullivan, DM, et al. Randomized, controlled trial of immediate versus delayed goal-directed ultrasound to identify the cause of nontraumatic hypotension in emergency department patients. Crit Care Med. 2004; 32(8):1703–1708.
19. Stawicki, SP, Braslow, BM, Panebianco, NL, et al, Intensivist use of hand-carried ultrasonography to measure IVC collapsibility in estimating intravascular volume status: correlations with CVP. J Am Coll Surg. 2009;209(1):55–61.
20. Sefidbakht, S, Assadsangabi, R, Abbasi, HR, et al. Sonographic measurement of the inferior vena cava as a predictor of shock in trauma patients. Emerg Radiol. 2007; 14(3):181–185.
21. Yanagawa, Y, Sakamoto, T, Okada, Y. Hypovolemic shock evaluated by sonographic measurement of the inferior vena cava during resuscitation in trauma patients. J Trauma. 2007; 63(6):1245–1248.
22. Abboud, PA, Kendall, JL. Ultrasound guidance for vascular access. Emerg Med Clin North Am. 2004; 22(3):749–773.
23. Kirkpatrick, AW. Clinician-performed focused sonography for the resuscitation of trauma. Crit Care Med. 2007; 35(5 suppl):S162–S172.
24. Scalea, T, Rodriquez, A, Chiu, WC, et al, Focused assessment with sonography for trauma (FAST): results from an international consensus conference. J Trauma. 1999;46(3):466–472.
25. Barozzi, L, Valentino, M, Santoro, A, et al. Renal ultrasonography in critically ill patients. Crit Care Med. 2007; 35(5 suppl):S198–S205.
26. Kirkpatrick, AW. Clinician-performed focused sonography for the resuscitation of trauma. Crit Care Med. 2007; 35(5 suppl):S162–S172.