Chapter 64 Instrumentation and Stabilization of the Pediatric Spine
Technical Nuances and Age-Specific Considerations
Posterior Spinal Instrumentation in the Pediatric Age Group
Craniocervical Junction (O-C2)
Occipital Screws
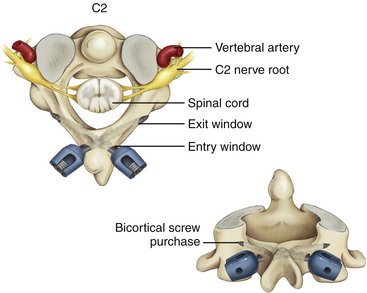
Most of the limitations of occipitocervical fixation systems reside in the cranial part of the construct.1,2 The occiput does not easily accommodate instrumentation.2–4 The slope of the occipital bone and the angle it makes with the cervical spine impose unique geometric constraints. These limitations may lead to poor occipital screw purchase, screw loosening, pullout, breakage, and difficulties with screw insertion,5 culminating in catastrophic hardware failure.
Numerous methods of obtaining occipitocervical stabilization have been described, including the use of methylmethacrylate,6 onlay bone graft with wires,7 contoured rods with wires,8 and metal plates with wires or screws.9–11 Internal fixation is advised to guarantee postoperative stability11 and to enhance the rate of arthrodesis.12
In a biomechanical investigation of occipital screw pullout strength, the bicortical pullout strength was found to be 50% greater than unicortical; wire pullout strength was not significantly different from that of unicortical screws,13 although some authors find a posteriorly wired contoured rod less likely to provide a good fusion environment because of less stabilizing potential and greater potential and greater likelihood of loosening with fatigue.14
Although bicortical screw placement may result in superior holding strength secondary to greater cortical purchase,15 caution is needed to avoid overpenetration and potential neurological injury.16 Bicortical occipital screw insertion risks dural laceration, cerebrospinal fluid (CSF) leakage, dural venous sinus injury/thrombosis,17 and subdural/epidural hematoma formation. CSF leakage and sinus bleeding can be stopped with placement of the occipital screws; if a screw cannot be placed, bone wax may be used to plug the drill hole in the bone.
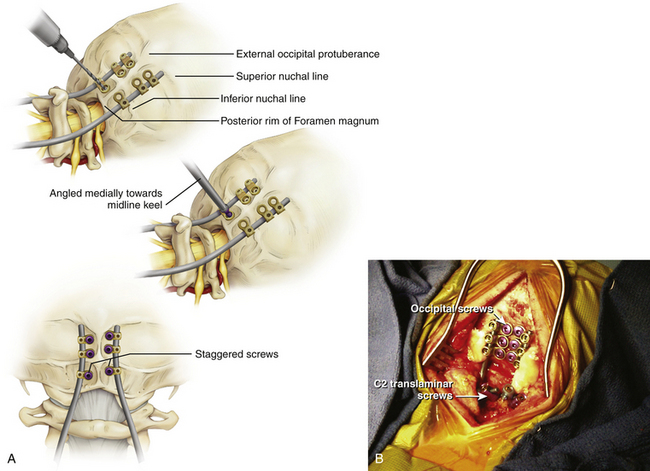
Bicortical screw failure is directly related to screw length, and screw length is dictated by bone thickness.4 Because the occiput is thickest in the region of the midline keel, multiple bicortical fixation points directed towards the midline have been advocated.
The basiocciput below the external occipital protuberance and posterior to the foramen magnum represents the squamous portion and is the site of occipitocervical fusion.2 Screw fixation is more secure in the bone above the inferior nuchal line because bone below this landmark is thin. Screw purchase is improved closer to the superior nuchal line and external occipital protuberance. However, the superior nuchal line does not reflect the location of the transverse sinus accurately, ranging from 15 mm below the superior nuchal line and 17 mm above.4 Unicortical screw purchase at and above the superior nuchal line may be warranted to decrease risk of dural venous sinus penetration.
Careful drilling with triangulation towards midline should be performed millimeter-by-millimeter until the inner table of the occiput is breached; the dura should be palpable with a ball-tipped probe. This “stop-drill” technique is routinely used. With left- and right-sided screws directed towards midline, screw paths may intersect causing even more unwanted difficulty with screw placement. It is useful to stagger the screws on each side of midline, being conscientious to direct the next screw trajectories slightly more cephalad or caudad away from the previous trajectory (Fig. 64-1).
C1 Lateral Mass Screws and C1–C2 Transarticular Screws
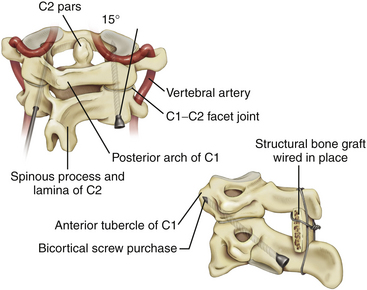
Transarticular C1–C2 screws as described by Magerl13 provide a very rigid and biomechanically sound construct with the incorporation of four cortical surfaces, but the insertion procedure is technically demanding because of the danger of vertebral artery injury especially in cases were atlantoaxial subluxation remains irreducible preoperatively. Although successful transarticular screw fixation of the atlantoaxial complex has been extensively reported in adult series, there have been only a handful of reports in the pediatric population. Analysis of clinical experience in the largest series of pediatric patients10,18,19 suggested a 4% rate of vertebral artery injury during screw placement; none of these injuries resulted in any long-term morbidity or mortality.
Because of the anatomical limitations complicating transarticular screw placement in adults and even more so in children, variations of C1–C2 screw fixation have been reported in adult patients in whom independent C1 lateral mass screws and C2 pars/pedicle screws were connected with either a plate11 or a rod.12 Atlantoaxial screw-rod fixation has been suggested as a safer procedure, and perhaps, as we have found, the technique is applicable in more patients despite anatomic variations, even in the smallest of pediatric patients. It is an ideal technique to fix and reduce occipitoatlantoaxial deformities that remain irreducible with closed reduction (Fig. 64-2).21,22
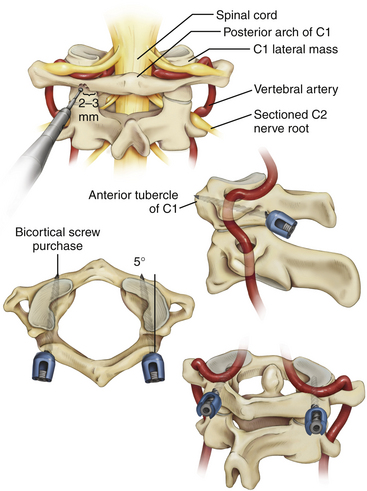
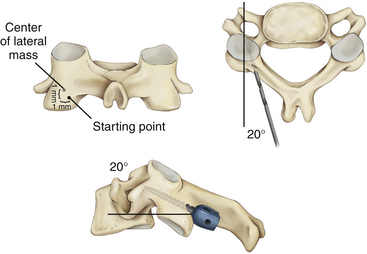
It is imperative that the entry point for the C1 lateral mass screw in the pediatric spine be placed at the confluence of the C1 lamina and the C1 lateral mass. The pediatric spine is unsuitable to accept a C1 lateral mass screw at alternative entry sites, such as the C1 lamina itself14 because of the high risk of violating the superior wall of the lamina and injuring the vertebral artery in the sulcus arteriosus.1
It is also important to note how the pediatric atlas differs from the adult atlas. In the adult atlas, the medial side of the C1 lamina is typically flush with the medial C1 lateral mass. However, in the pediatric atlas, the medial aspect of the C1 lamina is typically 2 to 3 mm laterally stepped-off from the medial surface of the C1 lateral mass. An entry point based on the medial aspect of the C1 lamina will place the entry of the C1 lateral mass screw dangerously lateral in extreme proximity to the vertebral artery. Instead, the medial surface of the C1 lateral mass itself must be used as a landmark for starting the C1 lateral mass screw23,24 (Fig. 64-3).
Although C1 lateral mass screw placement is still technically challenging and the potential risk of vertebral artery injury persists, our initial experience22 is that it is a feasible and efficacious part of an occipitocervical or atlantoaxial screw-rod construct in young patients.
C2 Pars/Pedicle and Translaminar Screws
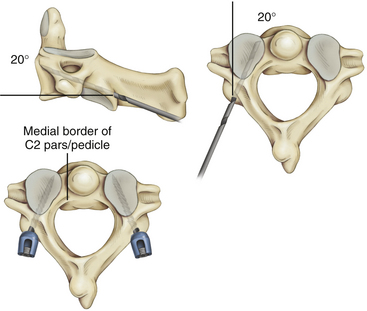
C1–C2 transarticular screw placement is technically demanding because of the close proximity of the vertebral artery to the screw path. Vertebral artery injury has been reported to occur in 2% to 8% in several large series.25–27 Pars/pedicle screws may have a lower incidence of arterial injury,28 but these screws still place the vertebral artery and spinal cord at risk.29
Wright30,31 described a new technique for rigid screw fixation of the axis involving the insertion of polyaxial screws into the laminae of C2 in a bilateral crossing fashion and their feasibility for placement in the general adult population. Because the C2 translaminar screws are not in proximity to the vertebral artery, this technique allows rigid fixation of C2 through a safer technique. Recently, teams of authors32,33 have reported their experience with this technique of crossing and noncrossing screws in small series of children (Fig. 64-4).
Subaxial Cervical Spine (C3–C7)
Lateral Mass Screws
Although lateral mass screw fixation in the cervical spine has been shown to provide excellent stability and high rates of fusion in adult patients,34–36 little has been published about the use of subaxial lateral mass screws in the pediatric age group. Unlike the adult age group, there is limited cadaveric biomechanical analyses of these types of constructs. In addition, based on comparative biomechanical studies of the cervical fixation procedures, there is not much difference in stability between lateral mass screw fixation and conventional nonscrew fixation methods, such as sublaminar wiring.37–41
The two most popular techniques for lateral mass screws are the Roy-Camille and the Magerl techniques. However, nerve roots, vertebral arteries, facet joints, and the dura and spinal cord are at risk during the placement of lateral mass screws. A recent review of the literature42 indicated that the youngest patient where subaxial lateral mass screws were successfully used was 8.2 years old. This correlates with the age at which most authorities agree that the developing spine takes on an “adult” configuration.43,44 Despite this, the authors were only able to place 3.5 × 10 mm screws — the shortest screw length that is manufactured. Although a solid fusion was achieved in this case after 3 months of rigid immobilization, another study36 of predominantly adult patients has suggested that a minimum subaxial lateral mass screw length of 14 mm is needed to confer any degree of biomechanical stability (Fig. 64-5).
Pedicle Screws
Pedicle screw fixation systems have been widely used for reconstruction of the thoracic and lumbar spine because of their biomechanical superiority. Abumi et al.45,46 reported clinical results of pedicle screw fixation for reconstruction of traumatic and nontraumatic lesions of the middle and lower cervical spine. However, the procedure in the upper cervical spine has been criticized due to the potentially high risk to neurovascular structures, except at the C2 level.47–49
Translaminar Screws
Recently, translaminar screws have been promoted as a safe alternative to pedicle screws in the axis and in the upper thoracic spine, as they decrease the risk of violation of the transverse foramen and vertebral artery injury in the cervical spine and the risk of damage to the spinal cord and exiting nerve roots in the upper thoracic spine50,51 without the need for image guidance. We now propose that translaminar screw fixation in the subaxial cervical spine is a consideration in highly selected cases, as an alternative to subaxial lateral mass screws in children with small lateral masses.
Potential drawbacks of this technique must also be noted. Although the dorsal laminar surface is exposed during surgery, the surgeon must be careful to avoid penetration of the nonvisualized ventral laminar wall because penetration could lead to damage to the thecal sac or spinal cord. A modification of Wright’s method of translaminar screw placement with an “exit” window in the dorsal lamina at the laminofacet line can help the surgeon avoid intracanicular violation of the translaminar screw.52 Preoperative fine-cut CT scans with axial, sagittal, and coronal views should be used to estimate the screw length and to determine if the subaxial lamina can accept a minimum 3.5-mm diameter screw. We suggest that the acceptable width of the lamina should be at least 4.0 mm. Clearly, translaminar screws should not be used without intact posterior elements.
Although the biomechanics of lateral mass screw fixation of the subaxial cervical spine are well described, there is no report comparing construct stability or screw pullout strength of translaminar and lateral mass screws in this region. CT morphometric analysis for axial and subaxial translaminar screw placement in the pediatric cervical spine shows the anatomy in 30.4% of patients younger than 16 years of age could accept bilateral C2 translaminar screws; however, the anatomy of the subaxial cervical spine only rarely could accept translaminar screws.53 Nonetheless, feasibility of translaminar screw placement in the subaxial cervical spine should be assessed on a case-by-case basis by careful study of preoperative thin-cut CT and sagittal and coronal reconstructions (Fig. 64-6).
Thoracic and Lumbar Spine
Wires, Hooks, and Pedicle Screws
The history of spinal implants began in Kansas in 1887 when Dr. B.F. Wilkins reduced and fixed a T12–L1 dislocation with silver wires which he passed around the pedicles. Stainless steel wires were developed in the late 1930s. The surgical treatment of spinal deformity was transformed in the 1960s when the Harrington rod instrumentation system was introduced.54 The next major advance in spinal instrumentation came in 1982, when Luque rods and sublaminar wires were introduced. The Luque instrumentation system provided segmental correction and fixation, allowing a more rigid construct that avoided mandatory postoperative external immobilization.55 The Cotrel-Dubousset instrumentation system, developed in Europe in 1978 and introduced in the United States in 1984, has been described as a third-generation system that achieves three-dimensional correction of spinal deformity. The hallmark of this instrumentation system was the segmental nature of the hook attachment that allowed multiple forces to be applied on the same rod to correct the spinal deformity. Since then, other third-generation spinal systems combining the use of longitudinal members with different anchors including pedicle screws in variable positions have been developed.
In the surgical correction of spinal deformities, addition of internal fixation serves the dual function of improving solid arthrodesis by rigid immobilization of the instrumented segments and correcting preexisting deformities by facilitated application of the corrective forces. Since the introduction of spinal pedicle screws by Boucher56 in the late 1950s and the Harrington instrument57 in the early 1960s, spinal internal pedicle screw fixation has gained widespread use and popularity in the correction of spinal deformities.58–61
Noted advantages of pedicle screw fixation include three-column fixation; improved coronal, sagittal, and rotational correction; lower pseudoarthrosis rates, lower implant failures; and few postoperative external orthosis requirements when compared with hook and wire constructs.62–65
Despite their established role at the thoracolumbar, lumbar, and lumbosacral spine,63,66–69 the use of pedicle screws in the thoracic spine (T1 through T10) had limited acceptance among earlier spine surgeons. This is due to concerns related to the small pedicle size and the tight proximity of vascular, nervous, and visceral structures in the thoracic cavity and the thoracic spine itself.70–76 However, since the early 1990s, spine surgeons have been using pedicle screws for the management of thoracic deformities and have not observed any serious vascular or visceral complications65,69,77–81 (Fig. 64-7).
Polyester Bands
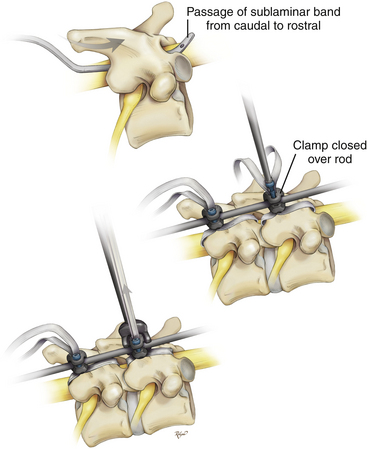
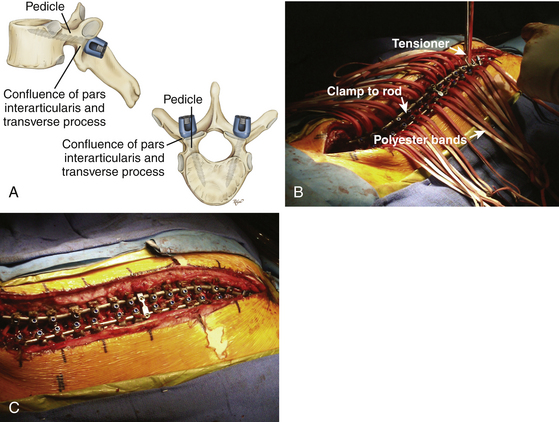
Recently, polyester bands with a locking mechanism to provide rod coupling (Universal Clamp, Zimmer Spine, Warsaw, IN) were developed as an alternative to traditional anchors—wires, hooks, and screws. The material properties of polyester are characterized by its high tensile strength, high resistance to stretch, wet or dry, and resistance to degradation.82 Polyester is biocompatible without an exorbitant inflammatory reaction in surrounding tissue, including the dura. Polyester has been in use for more than 18 years in spinal implants in Europe (K. Mazda, personal communication, October 17, 2007).
Its woven fabric makes it gentle, and its flexibility make it an excellent alternative to implantation into the pediatric spine. This is most applicable when the anatomy is extraordinarily small to accept hooks or screws, or when the anatomy is marked by significant congenital structural abnormalities where free-hand or fluoroscopic-guided hook or screw placement may lead to unacceptably high risk of neurologic injury. The polyester bands and locking mechanism to the rod may be placed at multiple levels, similar to wires, hooks, and screws, to effect segmental control, reduction, and fusion (Fig. 64-8).
Like sublaminar wires, sublaminar polyester bands can directly traumatize the spinal cord.83–89 Meticulous technique can reduce the risk of injury, particularly in the thoracic and thoracolumbar spine.90 Because the metal tip is not visible during the sublaminar passage of the polyester band, the surgeon is unable to appreciate the depth of tip penetration into the spinal canal. Important points to consider when using this technique include: (1) the radius of curvature of the malleable metal tip should be at least equal to the length of the lamina; (2) the bend of the tip should not be greater than 45 degrees; (3) lateral passage of sublaminar polyester bands should be avoided; (4) removal of additional bony lamina is not necessary because it does not significantly decrease the depth of band penetration but potentially weakens the lamina and increases the risk of instrumentation failure; (5) removal of the spinous process is recommended before direct midline passage of the sublaminar band; and (6) maintaining tension on the band throughout passage by using a push-pull technique to prevent bowing of the band into the spinal canal.
Care must be taken not to overtighten the bands, which can cause the bands to pull through or fracture a weak or skeletally immature lamina, as occurred in one of our cases. Excessive laminotomy in preparing for band passage may weaken the lamina; aggressive decortication in preparing the bony bed for arthrodesis may likewise decrease laminar strength91 and increase the risk of laminar fracture in the follow-up period.
Anterior Spinal Instrumentation in the Pediatric Age Group
Advantages of Anterior Instrumentation
Some advantages of anterior instrumentation compared to posterior instrumentation include preservation of motion segments and decreased blood loss with less muscle dissection.92,93 Improved fusion rates and decreased operative times have also been proposed by some authors.93,94 Anterior instrumentation also provides a reduced infection rate compared to posterior procedures.
For skeletally immature patients, circumferential fusion incorporating anterior instrumentation serves to arrest further anterior and posterior column growth and prevents the occurrence of “crankshaft” deformity.95
Disadvantages of Anterior Instrumentation
As with posterior instrumentation, standard spinal instrumentation such as titanium or polyetheretherketone (PEEK) cages may have too large a profile for general pediatric use; careful study of preoperative CT is mandatory to determine if the anatomy of the patient will be able to accept anterior instrumentation. Other potential complications include the risk of nerve root injury; implant loosening and migration; injury to critical vascular structures such as the carotid artery, aorta, or inferior vena cava; injury to the spinal cord or its covering; visceral injury; adjacent level disease especially in the mobile cervical spine;96 stenosis; and possible instability resulting from rigidity of the construct.97
Biomaterials
Autologous bone graft is widely used in the pediatric population. Sources of bone graft include iliac crest, fibula/tibia and rib grafts. Donor site morbidity is no different than what has been described in the adult population. However, iliac crest and rib graft may be cartilaginous in young children and lack sufficient rigidity. Alternative implants include calvarium and allograft which have both been used successfully.98,99 Synthetic materials such as titanium mesh cages and PEEK cages are possible substitutes as well. Titanium mesh cages have been mainly used in the thoracolumbar and lumbar spine for adolescent idiopathic scoliosis correction100 and less so for anterior column reconstruction after total spondylectomy for en bloc tumor resection.101 Because of its size, the titanium cage is mainly used in older adolescents. While its effectiveness in maintaining spine alignment in the adult population has been shown,102 there have been no reports on long-term follow-up in pediatric patients.
An additional problem associated with the titanium cage is its lack of radiolucency, which makes radiographic assessment of bony fusion difficult. A radiolucent alternative is the PEEK cage. PEEK biomaterial is strong, inert and biocompatible, and it is now broadly accepted as an alternative to metallic biomaterials in spine surgery.103 Its use in the pediatric spine, however, remains undefined.
The biomechanical principle and advantages of using anterior cervical plating systems in the pediatric spine are no different than in the adult spine. An anterior plate may maintain cervical alignment, prevent graft extrusion, and kyphotic deformity. The latter may be of particular concern in the pediatric population, as a higher incidence of kyphosis had been noted in pediatric patients after cervical trauma.104,105 Increased posterior ligamentous laxity and the relatively larger head are contributing factors. A particular problem facing the pediatric spine surgeon is that there is no commercially available anterior cervical plate small enough for some infants and young children. A cranial fixation miniplate is sometimes used.106
The bio-absorbable anterior plate is especially attractive in children. It eliminates the possibility of plate migration and imaging degradation associated with implants later in life. Its use in adult patients was first reported in the early 2000s with good clinical and radiographic results.107,108 Use of resorbable material in the form of a macropore craniofacial miniplate was reported in an 18-month-old and a 2-year-old child, both undergoing circumferential fusion.109,110 In these two case reports, both patients were placed in a halo-vest due to the gross instability of the cervical spine. It is not clear whether that is necessary if the pathology is purely anterior. Furthermore, a direct comparison between titanium and resorbable plates in terms of clinical outcome is not yet available either in the adult or pediatric population.
Long-Term Consequences of Fusion in a Growing Spine
A major consideration in pediatric spine fusion is the fact that the pediatric spine continues to grow from childhood into adolescence. For this reason, fusion and stabilization techniques have often been avoided in children and adolescents. Issues include range of motion, limitation on future growth, and development of secondary deformity.
After occipitocervical fusion, three studies had shown that the impact on growth potential and the incidence of secondary spinal malalignment were not significant at a minimum follow-up of 33 months.111–113 Furthermore, vertebral remodeling may even improve sagittal postoperative malalignment to restore the straight, or even lordotic, position of the cervical spine.113,114 With older children, the growth potential of the cervical spine after age 10 is very small, and that may limit the scope of the problem.115 A study by Anderson et al.116 reported on 17 patients aged 6 years or younger who underwent occipitocervical fusion and found no cases of sagittal malalignment or adjacent osteophyte formation or subluxation. In their estimation, based on comparison with radiographic studies of normal cervical spine growth,117 vertical growth potential was not impaired.
The consequence of thoracic and lumbar fusion for scoliosis correction is related to the etiology of the scoliosis and therefore the age at which the fusion is performed. In the largest published series of congenital scoliosis fused posteriorly (291 patients), the most common problem was bending of the fusion mass (crankshaft phenomenon, defined as more of 10 degrees of bending), which occurred in 14% of their patients.118 Patients who were fused without instrumentation before 5 years of age did equally well in terms of maintaining sagittal balance.119 With the addition of newer generation implants that are reduced in size, even better correction could be obtained while eliminating or shortening the periods of cast immobilization.120 While all of these studies have emphasized generally favorable results in maintaining sagittal balance, the effect of long segment fusion at a young age on the overall body height, spine length, and torso-to-leg ratio is seldom reported.121
Anterior column growth after a posterior column fusion may result in the “crankshaft phenomenon”.122–124 Because of the posterior fusion, all longitudinal growth in the posterior elements ceased. The vertebral bodies continue to grow anteriorly and eventually start to pivot on the posterior fusion resulting in progressive angulation and rotation of the spine. It is more of a problem in patients 10 years of age and younger who are skeletally immature,124 and in patients with idiopathic scoliosis than congenital scoliosis. This latter observation may be related to the “sick” anterior growth plate in congenital scoliosis, as healthy growth plates are necessary to produce the crankshaft effect.
One possible solution to crankshafting would be the addition of an anterior fusion.125,126 Various authors have used age, Risser sign of 0, open triradiate cartilage, peak height velocity,127 and a combination of these factors to help to identify those at risk. Others, however, question the indication and the efficacy of additional anterior fusion.128 There are also authors who believe that the newer instrumentation systems that emphasize stiff segmental fixation (e.g., Cotrel-Dubousset, Texas Scottish Rite Hospital, Isola instrumentation) may better prevent this secondary deformity than the older Harrington, Luque or Harri-Luque instrumentation. This is currently an important and active area of research.
Abumi K., Ito H., Taneichi H., Kaneda K. Transpedicular screw fixation for traumatic lesions of the middle and lower cervical spine: description of the techniques and preliminary report. J Spinal Disord. 1994;17:19-28.
Akamaru T., Kawahara N., Tsuchiya H., et al. Healing of autologous bone in a titanium mesh cage used in anterior column reconstruction after total spondylectomy. Spine. 2002;27(13):E329-E333.
Anderson R.C., Ragel B.T., Mocco J., et al. Selection of a rigid internal fixation construct for stabilization at the craniovertebral junction in pediatric patients. J Neurosurg. 2007;107:36-42.
Anderson R.C.E., Kan P., Gluf W.M., Brockmeyer D.L. Long-term maintenance of cervical alignment after occipitocervical and atlantoaxial screw fixation in young children. J Neurosurg. 2006;105(1 Suppl Pediatrics):55-61.
Brockmeyer D.L., York J.E., Apfelbaum R.I. Anatomical suitability of C1-2 transarticular screw placement in pediatric patients. J Neurosurg (Spine 1). 2000;92:7-11.
Chamoun R.B., Relyea K.M., Johnson K.K., et al. Use of axial and subaxial translaminar screw fixation in the management of upper cervical spinal instability in a series of 7 children. Neurosurgery. 2009;64:734-739.
Dickerman R.D., Morgan J.T., Mittler M. Circumferential cervical spine surgery in an 18-month-old female with traumatic disruption of the odontoid and C3 vertebrae. Pediatr Neurosurg. 2005;41:88-92.
Dubousset J., Herring J.A., Shufflebarger H. The crankshaft phenomenon. J Pediatr Orthop. 1989;9:541-545.
Ebraheim N.A., Jabaly G., Xu R., Yeasting R.A. Anatomic relations of the thoracic pedicle to the adjacent neural structures. Spine. 1997;22:1553-1556.
Farey I.D., Nadkarni S., Smith N. Modified Gallie technique versus transarticular screw fixation in C1-C2 fusion. Clin Orthop Relat Res. 1999;359:126-135.
Gluf W.M., Brockmeyer D.L. Atlantoaxial transarticular screw fixation: a review of surgical indications, fusion rate, complications, and lessons learned in 67 pediatric patients. J Neurosurg Spine. 2005;2:164-169.
Goel A., Laheri V. Plate and screw fixation for atlanto-axial subluxation. Acta Neurochir (Wien). 1994;129:47-53.
Harms J., Melcher R.P. Posterior C1-C2 fusion with polyaxial screw and rod fixation. Spine. 2001;26:2467-2471.
Harrington P.R. Treatment of scoliosis: correction and internal fixation by spine instrumentation. J Bone Joint Surg (Am). 1962;44:591.
Jea A., Sheth R.N., Vanni S., et al. Modification of Wright’s technique for placement of bilateral crossing C2 translaminar screws: technical note. Spine J. 2008;8:656-660.
Jea A., Taylor M.D., Dirks P.B., et al. Incorporation of C1 lateral mass screws in occipitocervical and atlantoaxial fusions for children 8 years of age or less: technical note. J Neurosurg. 2007;107:178-183.
Jeanneret B., Magerl F. Primary posterior fusion C1/2 in odontoid fractures: indications, technique, and results of transarticular screw fixation. J Spinal Disord. 1992;5:464-475.
Kretzer R.M., Sciubba D.M., Bagley C.A., et al. Translaminar screw fixation in the upper thoracic spine. J Neurosurg Spine. 2006;5:527-533.
Majid M.E., Castro F.P., Holt R.T. Anterior fusion for idiopathic scoliosis. Spine. 2000;25:696-702.
Parisini P., Di Silvestre M., Greggi T., Bianchi G. C1-C2 posterior fusion in growing patients: long-term follow-up. Spine. 2003;28:566-572.
Roberts D.A., et al. Quantitative anatomy of the occiput and the biomechanics of occipital screw fixation. Spine. 1998;23:1100-1107.
Roy-Camille R., Saillant G., Laville C., Benazet J.P. Treatment of lower cervical spinal injuries—C3 to C7. Spine. 1992;17:S442-S446.
Roy-Camille R., Saillant G., Mazel C. Plating of thoracic, thoracolumbar, and lumbar injuries with pedicle screw plates. Orthop Clin North Am. 1986;17:147-159.
Sasso R.C., et al. Occipitocervical fusion with posterior plate and screw instrumentation. A long-term follow-up study. Spine. 1994;19:2364-2368.
Seitz H., Marlovits S., Schwendenwein I., et al. Biocompatibility of polyethylene terephthalate (Trevira hochfest) augmentation device in repair of the anterior cruciate ligament. Biomaterials. 1998;19:189-196.
Shacked I., Ram Z., Hadani M. The anterior cervical approach for traumatic injuries to the cervical spine in children. Clin Orthop Relat Res. 1993;292:144-150.
Singh H., Rahimi S.Y., Yeh D.J., Floyd D. History of posterior thoracic instrumentation. Neurosurg Focus 16:Article. 11, 2004.
Smith J.L., Ackerman L.L. Management of cervical spine injuries in young children: lesson learned. Report of 2 cases. J Neurosurg Pediatrics. 2009;4:64-73.
Suk S., Kim W.J., Lee S.M., et al. Thoracic pedicle screw fixation in spinal deformities: are they really safe? Spine. 2001;26:2049-2057.
Vaccaro A.R., Balderston R.A. Anterior plate instrumentation for disorders of the subaxial cervical spine. Clin Orthop Relat Res. 1997;335:112-121.
Winter R.B., Moe J.H., Lonstein J.E. Posterior spinal arthrodesis for congenital scoliosis. An analysis of the cases of two hundred and ninety patients, five to nineteen years old. J Bone Joint Surg Am. 1984;66(8):1188-1197.
Winter R.B., Moe J.H. The results of spinal arthrodesis for congenital spine deformity in patients younger than five years old. J Bone Joint Surg Am. 1982;64:419-432.
Wright N.M. Posterior C2 fixation using bilateral, crossing C2 laminar screws: case series and technical note. J Spinal Disord Tech. 2004;17:158-162.
1. Vale F.L., et al. Rigid occipitocervical fusion. J Neurosurg. 1999;91:144-150.
2. Nadim Y., et al. Occipital screws in occipitocervical fusion and their relation to the venous sinuses: an anatomic and radiographic study. Orthopedics. 2000;23:717-719.
3. Papagelopoulos P.J., et al. Biomechanical evaluation of occipital fixation. J Spinal Disord. 2000;13:336-344.
4. Roberts D.A., et al. Quantitative anatomy of the occiput and the biomechanics of occipital screw fixation. Spine. 1998;23:1100-1107.
5. Fehlings M.G., et al. Posterior plates in the management of cervical instability: long-term results in 44 patients. J Neurosurg. 1994;81:341-349.
6. Rea G.L., et al. Occipitocervical fixation in non-traumatic upper cervical spine instability. Surg Neurol. 1993;40:255-261.
7. Elia M., et al. Onlay technique for occipitocervical fusion. Clin Orthop. 1992;280:170-174.
8. Fehlings M.G., et al. Occipitocervical fusion with a five-millimeter malleable rod and segmental fixation. Neurosurgery. 1993;32:198-207.
9. Smith M.D., et al. Occipitocervical arthrodesis using contoured plate fixation. An early report on a versatile fixation technique. Spine. 1993;18:1984-1990.
10. Sasso R.C., et al. Occipitocervical fusion with posterior plate and screw instrumentation. A long-term follow-up study. Spine. 1994;19:2364-2368.
11. Grob D., et al. The role of plate and screw fixation in occipitocervical fusion in rheumatoid arthritis. Spine. 1994;19:2545-2551.
12. Grob D., Schmotzer H. Posterior occipitocervical fusion: spinal biomechanics I. Spine. 1996;2:275-280.
13. Haher T.R., et al. Occipital screw pullout strength. A biomechanical investigation of occipital morphology. Spine. 1999;24:5-9.
14. Bambakidis N.C., et al. Biomechanical comparison of occipitoatlantal screw fixation techniques. J Neurosurg Spine. 2008;8:143-152.
15. Wittenberg R.H., et al. Importance of bone mineral density in instrumented spine fusions. Spine. 1991;16:647-652.
16. Ryken T.C., et al. Assessment of unicortical and bicortical fixation in a quasistatic cadaveric model. Spine. 1995;20:1861-1867.
17. Heywood A.W.B., et al. Internal fixation for occipitocervical fusion. J Bone Joint Surg Br. 1988;70:708-711.
18. Jeanneret B., Magerl F. Primary posterior fusion C1/2 in odontoid fractures: indications, technique, and results of transarticular screw fixation. J Spinal Disord. 1992;5:464-475.
19. Gluf W.M., Brockmeyer D.L. Atlantoaxial transarticular screw fixation: a review of surgical indications, fusion rate, complications, and lessons learned in 67 pediatric patients. J Neurosurg Spine. 2005;2:164-169.
20. Goel A., Laheri V. Plate and screw fixation for atlanto-axial subluxation. Acta Neurochir (Wien). 1994;129:47-53.
21. Harms J., Melcher R.P. Posterior C1-C2 fusion with polyaxial screw and rod fixation. Spine. 2001;26:2467-2471.
22. Jea A., Taylor M.D., Dirks P.B., et al. Incorporation of C1 lateral mass screws in occipitocervical and atlantoaxial fusions for children 8 years of age or less: technical note. J Neurosurg. 2007;107:178-183.
23. Lee M.J., Cassinelli E., Riew K.D. The feasibility of inserting atlas lateral mass screws via the posterior arch. Spine. 2006;31:2798-2801.
24. Aota Y., Honda A., Uesugi M., et al. Vertebral artery injury in C-1 lateral mass screw fixation: case illustration. J Neurosurg Spine. 2006;5:554.
25. Dickman C.A., Sonntag V.K. Posterior C1-C2 transarticular screw fixation for atlantoaxial arthrodesis. Neurosurgery. 1998;43:275-280. discussion 280-271
26. Farey I.D., Nadkarni S., Smith N. Modified Gallie technique versus transarticular screw fixation in C1-C2 fusion. Clin Orthop Relat Res. 1999:126-135.
27. Madawi A.A., Casey A.T., Solanki G.A., et al. Radiological and anatomical evaluation of the atlantoaxial transarticular screw fixation technique. J Neurosurg. 1997;86:961-968.
28. Howington J.U., Kruse J.J., Awasthi D. Surgical anatomy of the C-2 pedicle. J Neurosurg. 2001;95:88-92.
29. Resnick D.K., Lapsiwala S., Trost G.R. Anatomic suitability of the C1-C2 complex for pedicle screw fixation. Spine. 2002;27:1494-1498.
30. Wright N.M. Posterior C2 fixation using bilateral, crossing C2 laminar screws: case series and technical note. J Spinal Disord Tech. 2004;17:158-162.
31. Wright N.M. Translaminar rigid screw fixation of the axis. Technical note. J Neurosurg Spine. 2005;3:409-414.
32. Leonard J.R., Wright N.M. Pediatric atlantoaxial fixation with bilateral, crossing C-2 translaminar screws. Technical note. J Neurosurg. 2006;104:59-63.
33. Chamoun R.B., Relyea K.M., Johnson K.K., et al. Use of axial and subaxial translaminar screw fixation in the management of upper cervical spinal instability in a series of 7 children. Neurosurgery. 2009;64:734-739.
34. Deen H.G., Birch B.D., Wharen R.E., Reimer R. Lateral mass screw-rod fixation of the cervical spine: a prospective clinical series with 1-year follow-up. Spine J. 2003;3:489-495.
35. Roy-Camille R., Saillant G., Laville C., Benazet J.P. Treatment of lower cervical spinal injuries — C3 to C7. Spine. 1992;17:S442-S446.
36. Sekhon L.H. Posterior cervical lateral mass screw fixation: analysis of 1026 consecutive screws in 143 patients. J Spinal Disord Tech. 2005;18:297-303.
37. Coe J.D., Warden K.E., Sutterlin C.E.III, McAfee P.C. Biomechanical evaluation of cervical stabilization methods in a human cadaveric model. Spine. 1989;14:1122-1131.
38. Gill K., Paschal S., Corin J., et al. Posterior plating of the cervical spine. Spine. 1988;13:813-816.
39. Montesano P.X., Jauch E., Jonsson H.Jr. Anatomic and biomechanical study of posterior cervical spine plate arthrodesis: an evaluation of two different techniques of screw placement. J Spinal Disord. 1992;5:301-305.
40. Sutterlin C.E., McAfee P.C., Warden K.E., et al. A biomechanical evaluation of cervical spinal stabilization methods in a bovine model. Spine. 1988;13:795-802.
41. Ulrich C., Woersdoerfer O., Kalff R., et al. Biomechanics of fixation systems to the cervical spine. 1991;16:S4-S9.
42. Anderson R.C., Ragel B.T., Mocco J., et al. Selection of a rigid internal fixation construct for stabilization at the craniovertebral junction in pediatric patients. J Neurosurg. 2007;107:36-42.
43. Bailey D. The normal cervical spine in infants and children. Radiology. 1952;69:712-719.
44. Brockmeyer D.L., York J.E., Apfelbaum R.I. Anatomical suitability of C1-2 transarticular screw placement in pediatric patients. J Neurosurg (Spine 1). 2000;92:7-11.
45. Abumi K., Ito H., Taneichi H., Kaneda K. Transpedicular screw fixation for traumatic lesions of the middle and lower cervical spine: description of the techniques and preliminary report. J Spinal Disord. 1994;7:19-28.
46. Abumi K., Kaneda K. Pedicle screw fixation for nontraumatic lesions of the cervical spine. Spine. 1997;22:1853-1863.
47. Borne G.M., Bedou G.L., Pinaudeau M. Treatment of pedicular fractures of the axis: a clinical study and screw fixation technique. J Neurosurg. 1984;60:88-93.
48. Roy-Camille R., Mazel C., Saillant G., Benazet J.P. Rationale and techniques of internal fixation in trauma of the cervical spine. In: Errico T., Bauer R.D., Waugh T. Spinal Trauma. Philadelphia: JB Lippincott; 1991:163-191.
49. Roy-Camille R., Saillant G., Mazel C. Internal fixation of the unstable cervical spine by a posterior osteosynthesis with plates and screws. In: The Cervical Spine Research Society, editor. The Cervical Spine. 2nd ed. Philadelphia: JB Lippincott; 1989:390-403.
50. Kretzer R.M., Sciubba D.M., Bagley C.A., et al. Translaminar screw fixation in the upper thoracic spine. J Neurosurg Spine. 2006;5:527-533.
51. Wright N.M. Posterior C2 fixation using bilateral, crossing C2 laminar screws: case series and technical note. J Spinal Disord Tech. 2004;17:158-162.
52. Jea A., Sheth R.N., Vanni S., et al. Modification of Wright’s technique for placement of bilateral crossing C2 translaminar screws: technical note. Spine J. 2008;8:656-660.
53. Chern J.J., Chamoun R.B., Whitehead W.E., et al. Computed tomography morphometric analysis for axial and subaxial translaminar screw placement in the pediatric cervical spine. J Neurosurg Pediatr. 2009;3:121-128.
54. Singh H., Rahimi S.Y., Yeh D.J., Floyd D. History of posterior thoracic instrumentation. Neurosurg Focus. 16, 2004. Article 11
55. Luque E.R., Cardoso A. Segmental correction of scoliosis with rigid internal fixation. Orthop Trans. 1977;1:136-137.
56. Boucher H.H. A method of spinal fusion. J Bone Joint Surg (Br). 1959;41:248-259.
57. Harrington P.R. Treatment of scoliosis: correction and internal fixation by spine instrumentation. J Bone Joint Surg (Am). 1962;44:591.
58. Boos N., Webb J.K. Pedicle screw fixation in spinal disorders: a European view. Eur Spine J. 1997;6:2-18.
59. Krag M.H., Beynnon B.D., Pope M.H., et al. An internal fixator for posterior application to short segments of the thoracic, lumbar, or lumbosacral spine: design and testing. Clin Orthop. 1986;203:75-98.
60. Roy-Camille R., Saillant G., Mazel C. Internal fixation of the lumbar spine with pedicle screw plating. Clin Orthop. 1986;203:7-17.
61. Roy-Camille R., Saillant G., Mazel C. Plating of thoracic, thoracolumbar, and lumbar injuries with pedicle screw plates. Orthop Clin North Am. 1986;17:147-159.
62. Belmont P.J.Jr, Klemme W.R., Dhawan A., Polly D.W.Jr. In vivo accuracy of thoracic pedicle screws. Spine. 2001;26:2340-2346.
63. Liljenqvist U.R., Halm H.F., Link T.M. Pedicle screw instrumentation of the thoracic spine in idiopathic scoliosis. Spine. 1997;22:2239-2245.
64. Liljenqvist U.R., Lepsien U., Hackeberg L., et al. Comparative analysis of pedicle screw and hook instrumentation in posterior correction and fusion of idiopathic thoracic scoliosis. Eur Spine J. 2002;11:336-343.
65. Suk S., Kim W.J., Lee S.M., et al. Thoracic pedicle screw fixation in spinal deformities: are they really safe? Spine. 2001;26:2049-2057.
66. Amiot L.P., Lang K., Putzier M., et al. Comparative results between conventional and computer-assisted pedicle screw installation in the thoracic, lumbar, and sacral spine. Spine. 2000;5:606-614.
67. Delorme S., Labelle H., Aubin C.E., et al. A three dimensional radiographic comparison of Cottrel-Dubousset and Colorado instrumentation for the correction of idiopathic scoliosis. Spine. 2000;25:205-210.
68. Jonsson B., Sjostrom L., Olerud C., et al. Outcome after limited posterior surgery for thoracic and lumbar spine metastases. Eur Spine J. 1996;5:36-44.
69. Suk S.I., Lee C.K., Kim W.J., et al. Segmental pedicle screw fixation in the treatment of thoracic idiopathic scoliosis. Spine. 1995;20:1399-1405.
70. Cinotti G., Gumina S., Ripani M., Postacchini F. Pedicle instrumentation in the thoracic spine. A morphometric and cadaveric study for placement of screws. Spine. 1999;24:114-119.
71. Ebraheim N.A., Jabaly G., Xu R., Yeasting R.A. Anatomic relations of the thoracic pedicle to the adjacent neural structures. Spine. 1997;22:1553-1556.
72. Kothe R., O’Holleran J.D., Liu W., Panjabi M.M. Internal architecture of the thoracic pedicle. An anatomic study. Spine. 1996;21:264-270.
73. Krag M.H., Weaver D.L., Beynnon B.D., Haugh L.D. Morphometry of the thoracic and lumbar spine related to transpedicular screw placement for surgical spinal fixation. Spine. 1988;13:27-32.
74. Papin P., Arlet V., Marchesi D., et al. Unusual presentation of spinal cord compression related to misplaced pedicle screws in thoracic scoliosis. Eur Spine J. 1999;8:156-159.
75. Vaccaro A.R., Rizzolo S.J., Allardyce T.J., et al. Placement of pedicle screws in the thoracic spine: I. Morphometric analysis of the thoracic vertebrae. J Bone Joint Surg (Am). 1995;77:1193-1199.
76. Vaccaro A.R., Rizzolo S.J., Balderston R.A., et al. Placement of pedicle screws in the thoracic spine: II. An anatomical and radiographic assessment. J Bone Joint Surg (Am). 1995;7:1200-1205.
77. Bransford R., Bellabarba C., Thompson J.H., et al. The safety of fluoroscopically-assisted thoracic pedicle screw instrumentation for spine trauma. J Trauma. 2006;60:1047-1052.
78. Di Silvestre M., Parisini P., Lolli F., Bakaloudis G. Complications of thoracic pedicle screws in scoliosis treatment. Spine. 2007;32:1655-1661.
79. Kim Y.J., Lenke L.G., Bridwell K.H., et al. Free hand pedicle screw placement in the thoracic spine: is it safe? Spine. 2004;29:333-342.
80. Kuklo T.R., Lenke L.G., O’Brien M.F., et al. Accuracy and efficacy of thoracic pedicle screws in curves more than 90 degrees. Spine. 2005;30:222-226.
81. Suk S.I., Lee C.K., Chung S.S. Comparison of Zielke ventral derotation system and Cotrel-Dubousset instrumentation in the treatment of idiopathic lumbar and thoracolumbar scoliosis. Spine. 1994;19:419-429.
82. Seitz H., Marlovits S., Schwendenwein I., et al. Biocompatibility of polyethylene terephthalate (Trevira hochfest) augmentation device in repair of the anterior cruciate ligament. Biomaterials. 1998;19:189-196.
83. Ben-David B. Spinal cord monitoring. Orthop Clinics North Am. 1988;19:427-448.
84. Burke S.D., Matiko J. Segmental spinal instrumentation in neuromuscular spinal deformity. Orthop Trans. 1983;7:25-26.
85. Carlioz H., Quaknine M. Neurological complications of surgery of the spine in children. Chirurgie. 1994-1995;120:26-30.
86. Girardi F.P., Boachie-Adjei O., Rawlins B.A. Safety of sublaminar wires with Isola instrumentation for the treatment of idiopathic scoliosis. Spine. 2000;25:691-695.
87. Herring J.A., Wenger D.R. Segmental spinal instrumentation. A preliminary report of 40 consecutive cases. Spine. 1982;7:285-298.
88. Johnston C.E.II, Happel L.T., Norris R., et al. Delayed paraplegia complicating segmental spinal instrumentation. J Bone Joint Surg Am. 1986;68:556-563.
89. Lonstein J.E., Winter R.B., Moe J.E., et al. Neurological deficits secondary to spinal deformity: a review of the literature and report of 43 cases. Spine. 1980;5:331-355.
90. Lowe T., Morbidity and Mortality Committee Report. Presented at the Annual Meeting of the Scoliosis Research Society. Canada: Vancouver, British Columbia; 1987.
91. Aydingoz O., Bilsel N., Botanlioglu H., et al. Effect of decortication on laminar strength during sublaminar wiring: an experimental study. J Spinal Disord Tech. 2004;17:498-504.
92. Majid M.E., Castro F.P., Holt R.T. Anterior fusion for idiopathic scoliosis. Spine. 2000;25:696-702.
93. Bernstein R.M., Hall J.E. Solid short rod segment anterior fusion in thoracolumbar scoliosis. J Pediatr Orthop. 1998;7:124-131.
94. Turi M., Johnston C.E., Richards B.S. Anterior correction of idiopathic scoliosis with TSRH instrumentation. Spine. 1993;18:417-422.
95. Herring J.A. Anterior spinal surgery. In: Weinstein S.L., editor. Pediatric Spine Surgery. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2001:239-246.
96. Hilibrand A.S., Carlson G.D., Palumbo M.A., et al. Radiculopathy and myelopathy at segments adjacent to the previous site of a previous anterior cervical arthrodesis. J Bone Joint Surg Am. 1999;81:519-528.
97. Vaccaro A.R., Balderston R.A. Anterior plate instrumentation for disorders of the subaxial cervical spine. Clin Orthop Relat Res. 1997;335:112-121.
98. Casey A.T., Hayward R.D., Harkness W.F., et al. The use of autologous skull bone grafts for posterior fusion of the upper cervical spine in children. Spine. 1995;20:2217-2220.
99. Chadduck W.m., Boop F.A. Use of full-thickness calvarial bone grafts for cervical spine fusions in pediatric patients. Pediatr Neurosurg. 1994;20:107-112.
100. Lenke L.G., Bridwell K.H. Mesh cages in idiopathic scoliosis in adolescents. Clin Orthop Relat Res. 2002;394:98-108.
101. Akamaru T., Kawahara N., Tsuchiya H., et al. Healing of autologous bone in a titanium mesh cage used in anterior column reconstruction after total spondylectomy. Spine. 2002;27(13):E329-E333.
102. Dvorak M.R., Kwon B.K., Fisher C.G., et al. Effectiveness of titanium mesh cylindrical cages in anterior column reconstruction after thoracic and lumbar vertebral body resection. Spine. 2003;28(9):902-908.
103. Kurtz S.M., Devine J.N. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials. 2007;28(32):3834-4869.
104. Shacked I., Ram Z., Hadani M. The anterior cervical approach for traumatic injuries to the cervical spine in children. Clin Orthop Relat Res. 1993;292:144-150.
105. Stauffer E.S., Kelly E.G. Fracture-dislocations of the cervical spine. Instability and recurrent deformity following treatment by anterior interbody fusion. J Bone Joint Surg Am. 1977;59:45-48.
106. Smith J.L., Ackerman L.L. Management of cervical spine injuries in young children: lesson learned. Report of 2 cases. J Neurosurg Pediatrics. 2009;4:64-73.
107. Vaccaro A.R., Carrino J.A., Venger B.H., et al. Use of a bioabsorbable anterior cervical plate in the treatment of cervical degenerative and traumatic disc disruption. J Neurosurg. 2002;97(suppl 4):473-480.
108. Ames CP, Cornwall GB, Crawford NR, et al. Feasibility of a resorbable anterior cervical graft containment plate. J Neurosurg. 97(suppl 4):440-446.
109. Dickerman R.D., Morgan J.T., Mittler M. Circumferential cervical spine surgery in an 18-month-old female with traumatic disruption of the odontoid and C3 vertebrae. Pediatr Neurosurg. 2005;41:88-92.
110. Li V., Lopez D.K., Bennett G.J. Use of a craniofacial miniplate for internal fixation in a young child with cervical instability. Case report. J Neurosurg. 2001;95(suppl 1):128-131.
111. Ahmed R., Traynelis V.C., Menezes A.H. Fusions at the craniovertebral junction. Childs Nerv Syst. 2008;24:1209-1224.
112. Gluf W.M., Brockmeyer D.L. Atlantoaxial transarticular screw fixation: a review of surgical indications, fusion rate, complications, and lessons learned in 67 pediatric patients. J Neurosurg Spine. 2005;2:164-169.
113. Parisini P., Di Silvestre M., Greggi T., Bianchi G. C1-C2 posterior fusion in growing patients: long-term follow-up. Spine. 2003;28:566-572.
114. Toyama Y., Matsumoto M., Chiba K., et al. Realignment of postoperative cervical kyphosis in children by vertebral remodeling. Spine. 1994;19:2565-2570.
115. Baily D. The normal cervical spine in infants and children. Radiology. 1952;69:712-719.
116. Anderson R.C.E., Kan P., Gluf W.M., Brockmeyer D.L. Long-term maintenance of cervical alignment after occipitocervical and atlantoaxial screw fixation in young children. J Neurosurg. 2006;105(suppl 1 Pediatrics):55-61.
117. Wang J.C., Nuccion S.L., Feighan J.E., et al. Growth and development of the pediatric cervical spine documented radiographically. J Bone Joint Surg Am. 2001;83:1212-1218.
118. Winter R.B., Moe J.H., Lonstein J.E. Posterior spinal arthrodesis for congenital scoliosis. An analysis of the cases of two hundred and ninety patients, five to nineteen years old. J Bone Joint Surg Am. 1984;66(8):1188-1197.
119. Winter R.B., Moe J.H. The results of spinal arthrodesis for congenital spine deformity in patients younger than five years old. J Bone Joint Surg Am. 1982;64:419-432.
120. Hedequist D.J., Hall J.E., Emans J.B. The safety and efficacy of spinal instrumentation in children with congenital spine deformities. Spine. 2004;29(18):2081-2086.
121. Winter R.B., Lonstein, John E. Congenital scoliosis with posterior spinal arthrodesis T2-L3 at age 3 years with 41-year follow-up: a case report. Spine. 1999;24(2):194-197.
122. Dubousset J., Herring J.A., Shufflebarger H. The crankshaft phenomenon. J Pediatr Orthop. 1989;9:541-545.
123. Hefti F.L., McMaster M.J. The effect of the adolescent growth spurt on early posterior spinal fusion in infantile and juvenile scoliosis. J Bone Joint Surg (Br). 1983;65:247-254.
124. Lee C.S., Nachemson A.L. The crankshaft phenomenon after posterior Harrington fusion in skeletally immature patients with thoracic or thoracolumbar idiopathic scoliosis followed to maturity. Spine. 1997;22:58-67.
125. Dohin B., Dubousset J.F. Prevention of the crankshaft phenomenon with anterior spinal epiphysiodesis in surgical treatment of severe scoliosis of the younger patient. Eur Spine J. 1994;3:62-67.
126. Lapinksy A.S., Richards B.S. Preventing the crankshaft phenomenon by combining anterior fusion with posterior instrumentation: does it work? Spine. 1995;12:1392-1398.
127. Sanders J.O., Little D.G., Richards B.S. Prediction of the crankshaft phenomenon by peak height velocity. Spine. 1997;22:1352-1357.
128. Burton D.C., Asher M.A., Lai S.M. Scoliosis correction maintenance in skeletally immature patients with idiopathic scoliosis. Is anterior fusion really necessary? Spine. 2000;25:61-68.