10 Innervation of muscles and joints
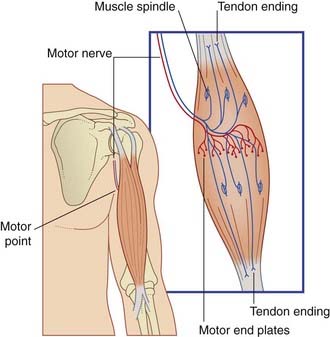
In gross anatomy, the nerves to skeletal muscles are branches of mixed peripheral nerves. The branches enter the muscles about one-third of the way along their length, at motor points (Figure 10.1). Motor points have been identified for all major muscle groups for the purpose of functional electrical stimulation by physical therapists, in order to increase muscle power.
Motor Innervation of Skeletal Muscle
The nerve of supply branches within the muscle belly, forming a plexus from which groups of axons emerge to supply the muscle fibers (Box 10.1 and Figure 10.1). The axons supply single motor end plates placed about halfway along the muscle fibers (Figure 10.2A).
There are three different types of skeletal muscle fiber.
Motor end plates
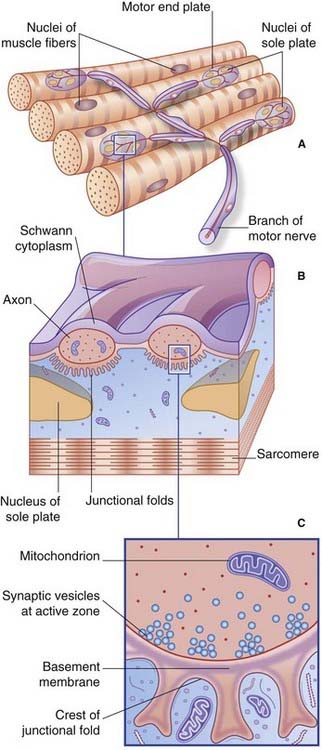
At the myoneural junction, the axon divides into a handful of branchlets that groove the surface of the muscle fiber (Figure 10.2B). The underlying sarcolemma is thrown into junctional folds. The basement membrane of the muscle fiber traverses the synaptic cleft and lines the folds. The underlying sarcoplasm shows an accumulation of nuclei, mitochondria, and ribosomes known as a sole plate.
Each axonal branchlet forms an elongated terminal bouton containing thousands of synaptic vesicles loaded with acetylcholine (ACh). Synaptic transmission takes place at active zones facing the crests of the junctional folds (Figure 10.2C). Vesicular ACh is extruded at great speed by exocytosis into the synaptic cleft. The ACh diffuses through the basement membrane to bind with ACh receptors in the sarcolemma.
Also in terminal boutons are some dense-cored vesicles containing one or more peptides (Figure 12.2C). Best known is calcitonin gene-related peptide, a potent vasodilator.
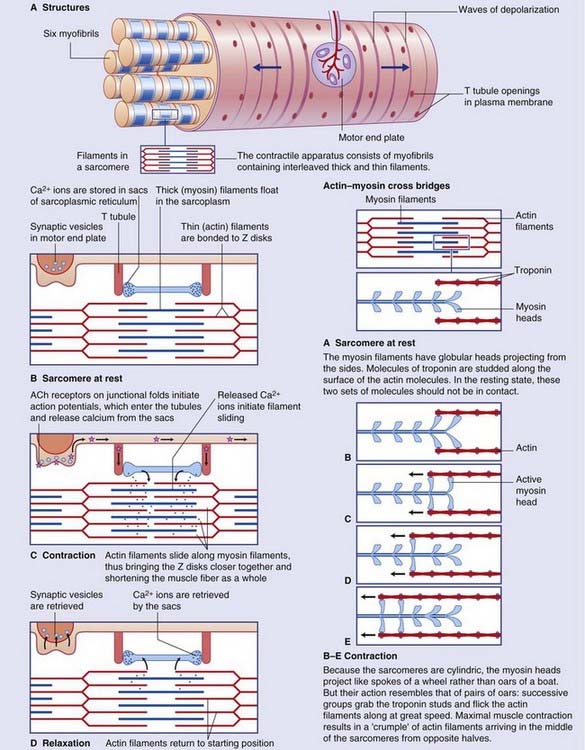
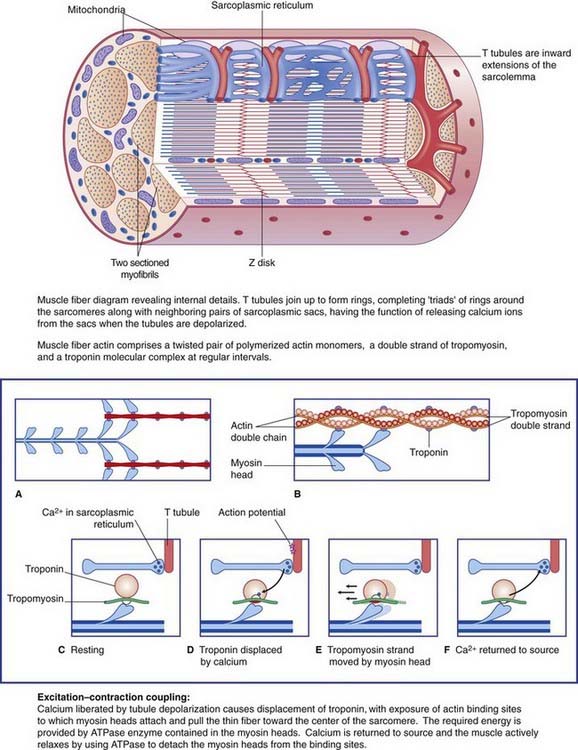
Details of the muscle fiber contraction process are in Box 10.2.
Motor units in the elderly
Progressive wasting of muscles seen in the elderly is mainly due to loss of motor neurons from the spinal cord and brainstem, often due in part to low-grade peripheral neuropathy arising from vascular disease and/or nutritional deficiency. Electromyographic records taken from contracting muscles show giant motor unit potentials during the seventh and eighth decades. The extra-large potentials result from takeover of vacated motor end plates of lost motor neurons by collateral sprouts from the axons of adjacent healthy motor units. Details of electromyography, and of clinical neuromuscular disorders, are in Chapter 12.
Sensory Innervation of Skeletal Muscle
Neuromuscular spindles
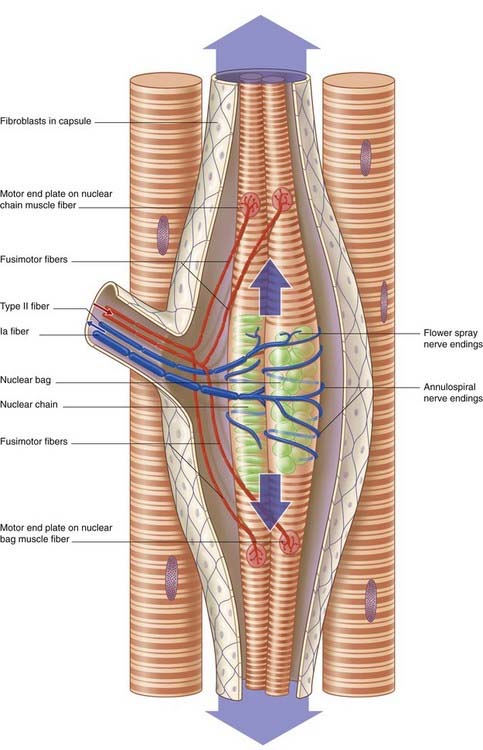
Muscle spindles contain up to a dozen intrafusal muscle fibers (Figure 10.3). (Ordinary muscle fibers are extrafusal in this context.) The larger intrafusal fibers emerge from the poles (ends) of the spindles and are anchored to connective tissue (perimysium). Smaller ones are anchored to the collagenous spindle capsule. At the spindle equator (middle), the sarcomeres are replaced almost entirely by nuclei, in the form of ‘bags’ (in wide fibers) or ‘chains’ (in slender fibers).
Innervation
Muscle spindles have both a motor and a sensory nerve supply. The motor fibers, called fusimotor, are in the Aγ size range, in contrast to the Aα fibers supplying extrafusal muscle. The fusimotor axons divide to supply the striated segments at both ends of the intrafusal muscles (Figure 10.3). A single primary sensory fiber of type Ia caliber applies annulospiral wrappings around the bag or chain segments of the intrafusal muscle fibers. Secondary, ‘flower spray’ sensory endings on one or both sides of the primary are supplied by type II fibers.
Passive stretch
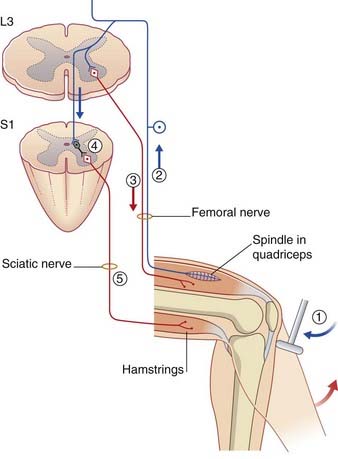
Passive stretch of muscle spindles occurs when an entire muscle belly is passively lengthened. For example, in eliciting a tendon reflex such as the knee jerk, the spindles in the belly of the quadriceps muscle are passively stretched when the tendon is struck. The type Ia and type II fibers discharge to the spinal cord, where they synapse on the dendrites of α motor neurons (Figure 10.4). (α Motor neurons are so called because they give rise to axons of Aα diameter.) The response to the positive feedback from spindles is a twitch of contraction in the extrafusal muscle fibers of quadriceps. The spindles, because they lie in parallel with the extrafusal muscle, are passively shortened; they are described as being unloaded.
In addition to exciting homonymous motor neurons (i.e. motor neurons supplying the same muscles), the spindle afferents inhibit the α motor neurons supplying the antagonist muscles, through the medium of inhibitory internuncial (interposed) neurons (Figure 10.4). This effect is called reciprocal inhibition. The inhibitory neurons involved are called Ia internuncials.
Active stretch
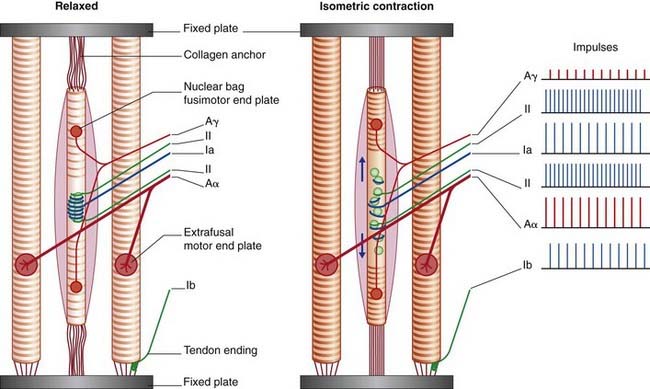
Active stretch is produced by the fusimotor neurons, which elicit contraction of the striated segments of the intrafusal muscle fibers. Because the connective tissue attachments are relatively fixed, the intrafusal fibers stretch the spindle equators by pulling them in the direction of the spindle poles (Figure 10.5). (This could be called a ‘Christmas cracker’ effect.)
Tendon endings
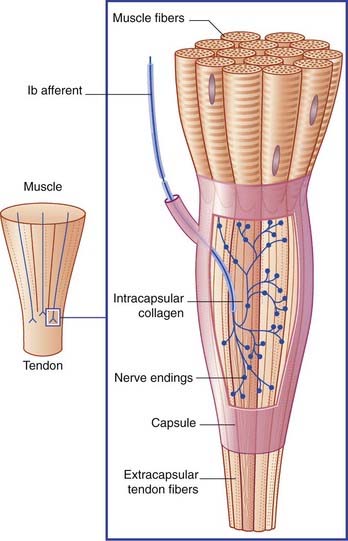
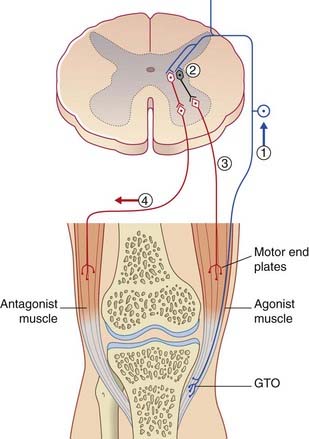
Golgi tendon organs are found at muscle–tendon junctions (Figure 10.6). A single Ib caliber nerve fiber forms elaborate sprays that intertwine with tendon fiber bundles enclosed within a connective tissue capsule.
The Ib afferents exert negative feedback on to the homonymous motor neurons, in contrast to the positive feedback exerted by muscle spindle afferents. The effect is called autogenetic inhibition, and the reflex arc is disynaptic because of the interpolation of an inhibitory neuron (Figure 10.7). If need be, there follows reciprocal excitation of motor neurons supplying antagonist muscles.
An important function of tendon organ afferents is to dampen (restrict) the inherent tendency of moving limb segments to oscillate (sway to and fro). Dampening introduces an element known to physiologists as joint stiffness. Paradoxically, when 1b afferents are allowed too much freedom, as in Parkinson’s disease (Ch. 28), they reinforce the inherent tendency to oscillate, and contribute to the characteristic resting tremor that is most obvious in the forearm (pronation–supination) and in the fingers (‘pill-rolling’ of the thumb pad by adjacent fingers).
Free nerve endings
Muscles are rich in freely ending nerve fibers, distributed to the intramuscular connective tissue and investing fascial envelopes. They are responsible for pain sensation caused by direct injury or by accumulation of metabolites including lactic acid. See also Clinical Panel 10.1.
Clinical Panel 10.1 Myofascial pain syndrome
Over time, pain may become more widespread or severe due to sensitization of dorsal horn neurons. Release of the peptide called substance P by other branches of sensitized neurons (see Clinical Panel 11.1) may initiate creation of new MTPs in the same or an adjacent muscle.
Innervation of Joints
Encapsulated nerve endings in and around joint capsules include Ruffini endings that signal tension, lamellated endings responsive to pressure, and Pacinian corpuscles responsive to vibration (see Ch. 11).
Burke D, Gandevia SC. Peripheral motor system. In: Paxinos G, editor. The human nervous system. San Diego: Academic Press; 1990:125-142.
McCloskey DI. Human proprioceptive sensation. J Clin Neurosci. 1994;1:173-177.
Proske E. Two enigmas in proprioception: abundance and location of muscle spindles. Brain Res Rec. 2007;75:495-496.